Summary
Background
In chronic lymphocytic leukemia (CLL), in vivo apoptotic resistance of malignant B lymphocytes results, in part, from the intrinsic defects of their apoptotic machinery. These include genetic alterations and aberrant expression of many apoptosis regulators, among which the Bcl2 family members play a central role.
Aim
The aim of this study was to investigate the association of pro-apoptotic Bax gene expression and Bcl2/Bax ratio with the clinical features of CLL patients as well as with molecular prognostic markers, namely the mutational status of rearranged immunoglobulin heavy variable (IGHV) genes and lipoprotein lipase (LPL) gene expression.
Methods
We analyzed the expression of Bax mRNA and Bcl2/Bax mRNA ratio in the peripheral blood mononuclear cells of 58 unselected CLL patients and 10 healthy controls by the quantitative reverse-transcriptase polymerase chain reaction.
Results
We detected significant Bax gene overexpression in CLL samples compared to non-leukemic samples (p=0.003), as well as an elevated Bcl2/Bax ratio (p=<0.001). Regarding the association with prognostic markers, the Bcl2/Bax ratio showed a negative correlation to lymphocyte doubling time (r=-0.307; p=0.0451), while high-level Bax expression was associated with LPL-positive status (p=0.035). Both the expression of Bax and Bcl2/Bax ratio were higher in patients with unmutated vs. mutated IGHV rearrangements, but this difference did not reach statistical significance.
Conclusions
Our results suggest that dysregulated expression of Bcl2 and Bax, which leads to a high Bcl2/Bax ratio in leukemic cells, contributes to the pathogenesis and clinical course of CLL.
Keywords: apoptosis, Bax, Bcl2/Bax ratio, chronic lymphocytic leukemia, expression analysis
Kratak sadržaj
Uvod
Rezistencija na apoptozu koja karakteriše maligne B limfocite in vivo u hroničnoj limfocitnoj leukemiji (HLL) delimično je uzrokovana unutrašnjim poreme-ćajima apoptotske mašinerije u ovim ćelijama. Ti poremećaji su rezultat genetičkih promena i aberantne ekspresije regulatora procesa apoptoze, među kojima ključnu ulogu imaju članovi Bcl2 familije.
Cilj
Cilj ove studije je bio da se ispita udruženost nivoa ekspresije proapoptotskog Bax gena, kao i Bcl2/Bax odnosa, sa kliničkim karakteristikama bolesnika sa HLL kao i molekularnim prognostičkim markerima, i to mutacionim statusom rearanži-ranih gena za teške lance imunoglobulina (IGHV) i ekspresijom gena za lipoproteinsku lipazu (LPL).
Metode
Analizirana je ekspresija Bax iRNK i Bcl2/Bax iRNK odnos u mononuklearnim ćelijama periferne krvi 58 bolesnika sa HLL i 10 zdravih kontrola metodom reverzne transkripcije i lančane reakcije polimeraze u realnom vremenu (qRT-PCR).
Rezultati
Detektovana je povišena ekspresija Bax gena u HLL uzorcima u odnosu na kontrolne uzorke (p=0,003), kao i povišen Bcl2/Bax odnos (p=<0,001). Kada je u pitanju udruženost sa prognostičkim markerima, Bcl2/Bax odnos je ispoljio negativnu korelaciju sa vremenom udvostručavanja broja limfocita (r=-0,307; p=0,0451), dok je visoka ekspresija Bax bila povezana sa LPL-pozitivnim statusom (p=0,035). I ekspresija Bax gena i Bcl2/Bax odnos su bili viši kod bolesnika sa nemutiranim u odnosu na bolesnike sa mutiranim IGHV genima, ali nije dostignuta statistička značajnost.
Zaključak
Rezultati ove studije ukazuju na moguću ulogu poremećene ekspresije Bcl2 i Bax gena, koja dovodi do visokog Bcl2/Bax odnosa u leukemijskim ćelijama, u patogenezi i kliničkom toku HLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most frequent type of leukemia in Europe and North America, affecting predominantly elderly individuals aged approximately 65–70 years at diagnosis. It is characterized by monoclonal expansion of circulating small, mature CD5+ CD19+ CD23+ sIgMlow B lymphocytes. The most striking feature of CLL is its extremely variable clinical presentation, with diverse therapy requirements and overall survival. In some patients the disease can follow an indolent course for years without developing any symptoms, while in others rapid progression and need of treatment occur soon after diagnosis (1). This fact has led to an extensive search for new cellular and molecular prognostic markers which could predict the clinical course of CLL at the time of initial diagnosis and enable the development of risk-adapted therapeutic strategies (2–4).
Circulating CLL B lymphocytes are arrested in G0/early G1 phase of the cell cycle (5) and their gradual accumulation in blood, bone marrow and secondary lymphoid organs is being attributed primarily to defective apoptosis. Although a growing body of evidence suggests that CLL clone turnover is more dynamic than previously assumed, and that CLL cells proliferate and die at considerable rates (6, 7), impairment of apoptosis remains one of the hallmarks of CLL. The fact that CLL cells undergo spontaneous apoptosis when placed in culture points to the role of microenvironment-derived signals in their survival in vivo (8). On the other hand, marked inter-patient variability in the rate of apoptosis of CLL cells ex vivo implicates the existence of inherent differences in their apoptotic potential (9–11). Indeed, genetic alterations and aberant expression of many apoptotic regulators involved in both intrinsic (mitochondrial) and extrinsic (death receptor) apoptotic pathways have been described in CLL.
Bcl2 family of proteins plays a central role in the regulation of the mitochondria-mediated pathway of apoptosis, and it is generally accepted that their deregulated function is involved in the pathogenesis and/or progression of CLL. Bcl2 family consists of pro-apoptotic (Bax, Bak, Bok, Bim, Bad, Bid, Bik, Bmf, Hrk, Noxa, Puma), and anti-apoptotic proteins (Bcl2, Bcl-Xl, Mcl-1, A1, Bcl-W) (12). Given the functional antagonism between the pro- and anti-apoptotic Bcl2 family members, it is believed that the ratio of their activity levels is a critical determinant of the cells’ susceptibility to apoptosis, rather than the levels of individual proteins.
Leukemic cells of the majority of CLL patients overexpress the anti-apoptotic Bcl2 gene even though translocation (14;18), which juxtaposes Bcl2 to the immunoglobulin heavy chain enhancer, is a very rare event in CLL (13, 14). It has been suggested that the main mechanism underlying Bcl2 upregulation is hypomethylation of its promoter region, detected in a large proportion of patients (15). In addition, miR-15a and miR-16–1, which negatively regulate Bcl2 at the posttranscriptional level, are frequently downregulated or lost by the deletion of 13q14, the most common genomic aberration in CLL (16, 17). Abnormal expression of other Bcl2 family members has also been observed in CLL, namely Mcl1, BclXl, Bag-1, Bax, Bak, Bad (18–20), as well as Bcl2L 12 and Bfl-1 (21, 22). However, the results of different studies regarding the relationship between the expression of Bcl2 family genes and proteins and the disease stage, clinical progression and response to treatment are highly discrepant.
In our previously published paper, we reported a significant overexpression of Bcl2 in a cohort of CLL patients compared to non-leukemic controls, and association of high Bcl2 mRNA levels with adverse prognostic parameters: progressive CLL, high serum β2-microglobulin, shorter lymphocyte doubling time (LDT) and high lipoprotein lipase gene (LPL) expression (21). In the study presented here, we broadened our previous research by analyzing the expression of the pro-apoptotic Bax gene in the same cohort of patients. The aim was to evaluate the association of Bax mRNA levels, as well as Bcl2/Bax ratio, with clinical and molecular prognostic markers in CLL, namely the mutational status of rearranged immunoglobulin heavy chain variable region genes (IGHV) and lipoprotein lipase gene expression. IGHV mutational status is the most powerful and the most stable molecular marker in CLL. Unmutated IGHV rearrangements represent an adverse prognostic factor and are associated with shorter time to progression and overall survival (23–25). Lipoprotein lipase (LPL) is a novel molecular marker whose high-level expression is associated with unfavourable prognostic parameters in CLL, and which has been proposed as a surrogate marker for the IGHV mutational status (26–28).
Materials and Methods
This study enrolled a total of 58 unselected patients from the Hematology Clinic, Clinical Center of Serbia (Belgrade, Serbia), diagnosed as typical B cell CLL based on the clinical criteria and laboratory features. The study was approved by the medical ethic committee of the institution.
The patient group consisted of 45 men and 13 women (male/female ratio = 3.5), with a median age of 63.5 years (range 39–86) at the time of diagnosis. Median white blood cell count was 55×109/L (range 13.5–413), and median lymphocyte count was 42×109/L (range 4.1–371). The distribution of clinical Binet stages was as follows: 22 patients (42.3%) stage A, 7 patients (13.5%) stage B and 23 patients (44.2%) stage C (the staging information was unavailable for 6 patients). Lymphocyte doubling time (LDT) was determined in 43 out of 58 patients; LDT ranged from 1 to 84 months, with a median of 12 months.
Among 52 patients for whom we possessed follow-up information, progressive disease was observed in 40 patients (76.9%), whereas non-progressive disease was observed in 12 patients (23.1%). Patients were considered to have progressive disease based on at least one of the following criteria: lymphocyte doubling time of less than 1 year, progression to a more advanced Binet stage, development of systemic symptoms or Richter syndrome, or a downward trend of hemoglobin/platelet count to below the normal range.
Serum markers β2-microglobulin and lactate dehydrogenase were determined in 32/58 and 37/58 patients, respectively. The levels of β2-microglobulin ranged 0.21–13.5 mg/L, with a median of 3.86 mg/L. Twenty-five (67.6%) patients had normal levels of LDH, while in the remaining 12 patients (32.4%) LDH was elevated.
CD38 expression, lipoprotein lipase expression and IGHV mutational status were determined as reported in Karan-Djurasevic et al. (21). CD38 expression was assessed in 38 out of 58 CLL samples and, applying the cut-off level of 30% of CD38 positive cells, 14 patients (36.8%) were classified as CD38-positive, and 24 patients (63.2%) as CD38-negative. Regarding IGHV mutational status, 29 of our patients (50%) belonged to the mutated CLL subset (M-CLL), while the other 29 patients (50%) belonged to the unmutated CLL subset (U-CLL).
In all patients who received treatment (40/58) no therapy had been administered for at least 6 months prior to blood sampling. The control group consisted of 10 healthy individuals, 3 men and 7 women, with a median age of 53 years (range 44–84).
Peripheral blood mononuclear cells (PBMC) of all patients contained >90% of CLL lymphocytes, as confirmed by immunophenotyping. PBMC were isolated by Ficoll density-gradient centrifugation and total RNA was extracted using TRI reagent (Sigma-Alldrich). The isolated RNA was reverse-transcribed using RevertAid M-MuLV Reverse Transcriptase (Fermentas) and random hexamer primers, according to manufacturer’s instructions.
Bax mRNA expression was analysed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) using SYBR Green chemistry in a 7500 Real Time PCR system (Applied Biosystems). The specific primers used for qRT-PCR amplification were: forward 5′-TGGCAGCTGACATGTTTTCTGAC-3′ and reverse 5′-TCACCCAACCACCCTGGTCTT-3′. The amplification of Abl using the following primers: forward 5′-TGGAGATAACACTCTAAGCATAACTAAAGGT-3′ and reverse 5′-GACGTAGTTGCTTGGGACCCA-3′, served as internal control. The reaction mixture contained 50 ng cDNK, 1 × Power SYBR® Green PCR Master Mix (Applied Biosystems) and 0.5 pmol (Bax) or 2 pmol (Abl) of each gene-specific primer, in a final reaction volume of 10 mL. The cycling conditions were as follows: denaturation of the template at 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. Each qRT-PCR reaction was performed in duplicate, in order to evaluate reproducibility of the results. Quantification of target gene expression was made by a comparative ddCt method, using HL-60 cell line as the calibrator.
Statistical analysis
Statistical analyses were performed using Fisher’s exact test, Mann-Whitney rank-sum test, Spearman rank order correlation and receiver operating characteristic (ROC) analysis. All statistical tests were carried out using Sigma Stat 3.5 and SigmaPlot 11.0 software (Systat Software Inc.). Statistical significance was defined as p<0.05.
Results
In this study, we analyzed the expression of Bax gene and Bcl2/Bax ratio in a cohort of 58 unselected patients with chronic lymphocytic leukemia.
Bax expression
qRT-PCR expression analysis of Bax revealed significantly higher levels of Bax mRNA in CLL samples in comparison to non-leukemic samples (p=0.003; Mann-Whitney rank sum test). However, the patient-to-patient variability and the increase of expression level in CLL vs. healthy controls was less prominent than in the case of Bcl2 (Figure 1).
Figure 1.
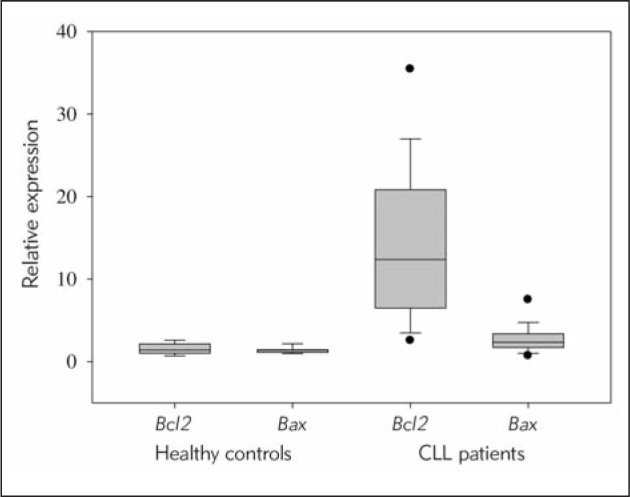
Relative expression of Bcl2 and Bax mRNA in CLL and non-leukemic samples.
qRT-PCR analysis showed a significantly higher expression of both Bcl2 and Bax in mononuclear cells of CLL patients (Bcl2 median 12.374; Bcl2 range 1.168–49.146; Bax median 2.346; Bax range 0.670–10.642) in comparison to healthy controls (Bcl2 median 1.411; Bcl2 range 0.685–2.629; Bax median 1.393; Bax range 0.972–2.243).
The expression of Bax did not show association with gender and Binet staging. Although we observed a tendency towards a negative correlation between Bax expression and the age at diagnosis, statistical significance was not reached (r=-0.26; p=0.0683; Spearman rank order correlation). Bax mRNA level was not associated with the course of the disease (progressive vs. non-progressive) and LDT. In addition, neither correlation to the levels of serum markers β2-microglobulin and LDH, nor association with CD38 status were detected.
Bax was expressed at higher levels in U-CLL vs. M-CLL, but the difference in Bax expression between these two groups of patients did not reach statistical significance (p=0.056; Mann-Whitney rank sum test).
In order to investigate the relationship between LPL expression and the expression of Bcl2, Bax and Bcl2/Bax ratio, we used median LPL mRNA expression as a cut-off level to define LPL status. According to this cut-off level, 29 patients (50%) were LPL-positive and 29 patients (50%) LPL-negative. In addition, LPL-positive status showed strong association with unmutated IGHV genes (p<0.001; Fisher’s exact test), with only 6 discrepant cases (10.3%).
Having divided our cohort into two groups based on LPL status, we then used median expression of Bcl2 and Bax as a cut-off level to discriminate between high and low expressing cases. By applying this approach, we found that high levels of both Bcl2 and Bax expression were associated with an LPL-positive status (p=0.008 and p=0.035, respectively; Fisher’s exact test).
Bcl2/Bax ratio
In our cohort of patients, Bcl2 and Bax expression levels were positively correlated (r=0.6; p=0.00; Spearman rank order correlation). The ratio of Bcl2 and Bax mRNA expression (Bcl2/Bax ratio) was significantly higher in CLL samples in comparison to healthy controls (p<0.001; Mann-Whitney rank sum test) (Figure 2).
Figure 2.
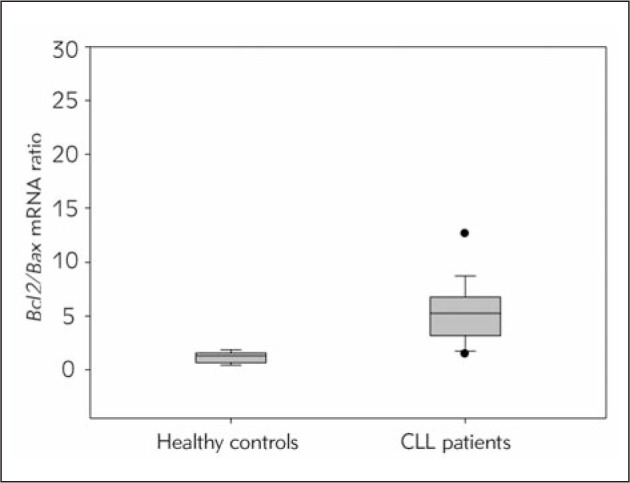
Bcl2/Bax ratio in CLL and non-leukemic samples.
qRT-PCR analysis showed a significantly higher Bcl2/Bax mRNA ratio in mononuclear cells of CLL patients in comparison to healthy controls.
Bcl2/Bax ratio was not found to be significantly associated with either gender, Binet stage or the course of the disease. Similar to Bax expression, the observed trend toward negative correlation to the age at diagnosis was not statistically significant (r=-0.265; p=0.0627; Spearman rank order correlation). On the other hand, there was a significant negative correlation between Bcl2/Bax ratio and LDT (r=-0.307; p=0.0451; Spearman rank order correlation). No association with the levels of β2-microglobulin, LDH and CD38 status was observed.
Bcl2/Bax ratio was higher in U-CLL vs. M-CLL and LPL-positive vs. LPL-negative groups of patients, but the association with either IGHV mutational status or LPL status was not statistically significant.
We performed receiver operating characteristic (ROC) analysis in order to evaluate the discriminatory power of Bcl2 and Bax mRNA expression in CLL. ROC analysis demonstrated that both Bcl2 expression and Bcl2/Bax ratio efficiently distinguished CLL from non-leukemic samples (A=0.98, 95% CI=0.95–1.009, p<0.0001 and A=0.96, 95% CI=0.9230–1.005, p<0.0001, respectively), while Bax expression was found to be less discriminating (A=0.80, 95% CI=0.6920–0.9097, p=0.002514) (Figure 3).
Figure 3.
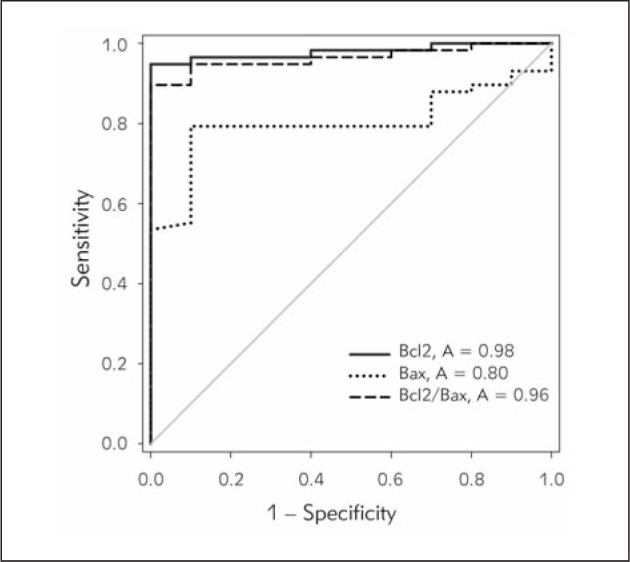
ROC analysis of Bcl2 and Bax expression and Bcl2/Bax ratio in CLL and non-leukemic samples.
Bcl2 mRNA expression and Bcl2/Bax mRNA ratio exert very high discriminatory power between CLL patients and healthy controls.
Bcl2: A=0.98, sensitivity=0.95, specificity=1.00, 95% CI=0.95–1.009, p<0.0001
Bcl2/Bax ratio: A=0.96, sensitivity=0.90, specificity=1.00, 95% CI=0.9230–1.005, p<0.0001
Bax: A=0.80, sensitivity=0.79, specificity=0.90, 95% CI=0.6920–0.9097, p=0.002514
Discussion
Chronic lymphocytic leukemia is considered to be a paradigmatic example of malignancy caused by dysregulation of apoptosis. However, the unique mechanism preventing CLL cells from undergoing apoptosis in vivo is still elusive, as is the significance of their apoptotic resistance for the clinical course of the disease.
Impairment of apoptosis results from the combination of microenvironmental survival signals and inherent genetic and epigenetic alterations of apoptotic machinery in CLL cells, both of which exert high patient-to-patient heterogeneity. Abnormalities in different apoptotic pathways have been described in CLL, namely ATM-p53 pathway (29–31), PI3K/Akt pathway (32–34), NF-κB pathway (32, 35) and Fas/FasL system (36, 37). Aberrant expression and genetic changes of Bcl2 family members, the key regulators of the intrinsic apoptotic pathway, have also been implicated in CLL (38). In addition, various cytokines, notably BAFF (»B-cell activation factor«), APRIL (»a proliferation inducing ligand«), CD40 ligand and interleukin 4, promote survival of CLL cells in both a paracrine and autocrine manner (39–41). Finally, it is noteworthy that the activation of B cell receptor (BcR) also affects apoptotic pathways, although responsiveness of CLL cells to antigenic stimulation and signalling via surface IgM and IgD still remain controversial (42). Even though dysregulation of apoptosis is a distinctive feature of CLL, none of the apoptotic defects has been found to be universally present among CLL patients. This may, at least in part, explain the heterogeneity of the clinical course and response to therapy in CLL.
In our previous research, we investigated the expression of Bcl2 gene, a prototypical anti-apoptotic member of the Bcl2 family, and detected a significant overexpression of Bcl2 in CLL samples, as well as association of high Bcl2 mRNA levels with unfavourable prognostic markers (21). In the present study, we continued our research by analyzing the expression of Bax, a functional antagonist of Bcl2, and the Bcl2/Bax ratio in CLL patients. The level of Bax expression we measured was significantly increased in CLL samples, but it was less heterogeneous and more overlapping with that of healthy controls than in the case of Bcl2. Given its pro-apoptotic role, the overexpression of Bax in CLL cells may seem paradoxical but, interestingly, an overexpression of both pro- and anti-apoptotic proteins has been observed in CLL (43). Moreover, their relative expression levels were positively correlated (44, 45). This is considered to be a compensatory mechanism used by cells in an attempt to regain equilibrium between pro-and anti-apoptotic proteins, which is crucial for apoptosis regulation.
Although in some studies higher expression of Bax mRNA and protein was detected in non-progressive vs. progressive CLL (46, 47), in our cohort no association with the course of the disease, Binet stage or LDT was observed. Regarding the association of Bax with the molecular prognostic markers, we observed higher Bax mRNA levels in U-CLL vs. M-CLL patients but, as was the case with Bcl2, without reaching statistical significance. On the other hand, high expression of both Bcl2 and Bax was associated with an LPL-positive status which, in turn, was an excellent predictor of unmutated IGHV genes. Several studies that investigated differential gene expression in U-CLL vs. M-CLL did not detect a significant association between Bcl2 and Bax expression and the IGHV mutational status (48, 49). However, our results show high Bcl2 and Bax expression in the group of patients defined by LPL positivity and unmutated IGHV rearrangements. In a study of Pallasch et al. (50), it was demonstrated that the LPL inhibitor orlistat has a cytotoxic effect on primary CLL cells through specific and concentration-dependent induction of apoptosis. In addition, the authors found that BcR stimulation significantly increases LPL expression in CLL cells. Thus, it would be important to elucidate the mechanisms by which intracellular pathways of lipid metabolism, apoptotic and BcR signalling are interconnected in CLL.
Analysis of the Bcl2/Bax mRNA ratio revealed that it was higher in CLL samples in comparison to control samples and that it efficiently discriminated patients from healthy controls. However, the Bcl2/Bax ratio was not significantly related to either clinical characteristics of CLL (with the exception of LDT), or molecular prognostic markers, although it was slightly higher in LPL-positive vs. LPL-negative and UCLL vs. M-CLL groups of patients. According to the results of some previous studies, an elevated Bcl2/Bax ratio, measured at both the mRNA (46) and protein level (47), is associated with adverse prognostic parameters in CLL and is more relevant for the survival of CLL cells than the expression levels of Bc2 and Bax individually. In other studies, including ours, however, this association was not observed (43). The explanation for these contradictory results may lie in the fact that other members of the Bcl2 family modulate the function of Bcl2 and Bax and, hence, their relative expression and/or activity levels also affect the CLL cells susceptibility to apoptosis. For example, the antiapoptotic protein Mcl1 is overexpressed in CLL and its high expression has been linked with poor prognosis in CLL patients (43, 51, 52). Like Bcl2, Mcl1 forms heterodimers with Bax, so elevated expression of Bcl2 and Mcl1 has an additive or synergistic negative effect on the Bax’s pro-apoptotic function. Saxena et al. (51) suggested that, when it comes to response to apoptosis-inducing chemotherapeutics, a negative effect of high Bcl2/Bax ratio may be overcome by low Mcl1 expression.
In summary, the results of this study further emphasize the complexity of the role that the dysregulated expression of Bcl2 family members plays in prolonged survival of CLL cells and phenotype of the disease. Considerable inter-patient variability in their expression may contribute to the heterogeneity of CLL, but the association with clinical characteristics and molecular prognostic markers remains controversial. Further studies are needed to clarify to what extent the pattern of expression and/or activity of Bcl2 family genes and proteins influences the clinical behaviour of CLL.
Acknowledgement.
This work was supported by grant No. III 41004, Ministry of Education and Science, Republic of Serbia.
Glossary
Abbreviations
- CLL
chronic lymphocytic leukemia
- Bcl2
B-cell lymphoma 2
- Bax
Bcl2-associated X
- IGHV
immunoglobulin heavy chain variable region genes
- LPL
lipoprotein lipase
- mRNA
messenger ribonucleic acid
- miR
micro ribonucleic acid
- PBMC
peripheral blood mononuclear cells
- LDT
lymphocyte doubling time
- LDH
lactate dehydrogenase
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- ROC
receiver operating characteristic
- A
area under the ROC curve
- CI
confidence interval
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Dighiero G. CLL biology and prognosis. Hematology Am Soc Hematol Educ Program. 2005:278–84. doi: 10.1182/asheducation-2005.1.278. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N. Implications of new prognostic markers in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2012:76–87. doi: 10.1182/asheducation-2012.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Sagatys EM, Zhang L. Clinical and laboratory prognostic indicators in chronic lymphocytic leukemia. Cancer Control. 2012;19(1):18–25. doi: 10.1177/107327481201900103. [DOI] [PubMed] [Google Scholar]
- 4.Lekovic D, Mihaljevic B, Kraguljac-Kurtovic N, Perun-cic-Jovanovic M, Bogdanovic A, Colovic M. et al. Prognostic significance of new biological markers in chronic lymphocytic leukaemia. Srp Arh Celok Lek. 2011;139(11–12):753–8. doi: 10.2298/sarh1112753l. [DOI] [PubMed] [Google Scholar]
- 5.Obermann EC, Went P, Tzankov A, Pileri SA, Hofstaedter F, Marienhagen J. et al. Cell cycle phase distribution analysis in chronic lymphocytic leukaemia: a significant number of cells reside in early G1-phase. J Clin Pathol. 2007;60(7):794–7. doi: 10.1136/jcp.2006.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D. et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115(3):755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gent R, Kater AP, Otto SA, Jaspers A, Borghans JA, Vrisekoop N. et al. In vivo dynamics of stable chronic lymphocytic leukemia inversely correlate with somatic hypermutation levels and suggest no major leukemic turnover in bone marrow. Cancer Res. 2008;68(24):10137–44. doi: 10.1158/0008-5472.CAN-08-2325. [DOI] [PubMed] [Google Scholar]
- 8.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123(3):380–8. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiorazzi N, Ferrarini M. Evolving view of the in-vivo kinetics of chronic lymphocytic leukemia B cells. Hematology Am Soc Hematol Educ Program. 2006:273–8. doi: 10.1182/asheducation-2006.1.273. 512. [DOI] [PubMed] [Google Scholar]
- 10.Sieklucka M, Pozarowski P, Bojarska-Junak A, Hus I, Dmoszynska A, Rolinski J. Apoptosis in B-CLL: the relationship between higher ex vivo spontaneous apoptosis before treatment in III-IV Rai stage patients and poor outcome. Oncol Rep. 2008;19(6):1611–20. [PubMed] [Google Scholar]
- 11.Kravic-Stevovic T, Bogdanovic A, Bumbasirevic V. Higher percentage of in vitro apoptotic cells at time of diagnosis in patients with chronic lymphocytic leukemia indicate earlier treatment requirement: ten years follow up. Srp Arh Celok Lek. 2014;142(1–2):48–53. doi: 10.2298/sarh1402048k. [DOI] [PubMed] [Google Scholar]
- 12.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43(1):1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 13.Mariano MT, Moretti L, Donelli A, Grantini M, Montagnani G, Di Prisco AU. et al. bcl-2 gene expression in hematopoietic cell differentiation. Blood. 1992;80(3):768–75. [PubMed] [Google Scholar]
- 14.Meijerink JP. t(14;18), a journey to eternity. Leukemia. 1997;11(12):2175–87. doi: 10.1038/sj.leu.2400849. [DOI] [PubMed] [Google Scholar]
- 15.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820–8. [PubMed] [Google Scholar]
- 16.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E. et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M. et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102(39):13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L. et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112(9):3807–17. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 19.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ. et al. Concurrent up-regulation of BCLXL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 20.Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10(3):456–9. [PubMed] [Google Scholar]
- 21.Karan-Djurasevic T, Palibrk V, Zukic B, Spasovski V, Glumac I, Colovic M. et al. Expression of Bcl2L12 in chronic lymphocytic leukemia patients: association with clinical and molecular prognostic markers. Med Oncol. 2013;30(1):405. doi: 10.1007/s12032-012-0405-7. [DOI] [PubMed] [Google Scholar]
- 22.Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. High expression of bfl-1 contributes to the apoptosis resistant phenotype in B-cell chronic lymphocytic leukemia. Int J Cancer. 2005;113(5):730–7. doi: 10.1002/ijc.20614. [DOI] [PubMed] [Google Scholar]
- 23.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54. [PubMed] [Google Scholar]
- 24.Kostareli E, Smilevska T, Stamatopoulos K, Kouvatsi A, Anagnostopoulos A. Chronic lymphocytic leukaemia: an immunobiology approach. Srp Arh Celok Lek. 2008;136(5–6):319–23. doi: 10.2298/sarh0806319k. [DOI] [PubMed] [Google Scholar]
- 25.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL. et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. [PubMed] [Google Scholar]
- 26.Van Bockstaele F, Pede V, Janssens A, Callewaert F, Offner F, Verhasselt B. et al. Lipoprotein lipase mRNA expression in whole blood is a prognostic marker in B cell chronic lymphocytic leukemia. Clin Chem. 2007;53(2):204–12. doi: 10.1373/clinchem.2006.076331. [DOI] [PubMed] [Google Scholar]
- 27.van’t Veer MB, Brooijmans AM, Langerak AW, Verhaaf B, Goudswaard CS, Graveland WJ. et al. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91(1):56–63. [PubMed] [Google Scholar]
- 28.Nuckel H, Huttmann A, Klein-Hitpass L, Schroers R, Fuhrer A, Sellmann L. et al. Lipoprotein lipase expression is a novel prognostic factor in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47(6):1053–61. doi: 10.1080/10428190500464161. [DOI] [PubMed] [Google Scholar]
- 29.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L. et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 30.Pettitt AR, Sherrington PD, Stewart G, Cawley JC, Taylor AM, Stankovic T. p53 dysfunction in B-cell chronic lymphocytic leukemia: inactivation of ATM as an alternative to TP53 mutation. Blood. 2001;98(3):814–22. doi: 10.1182/blood.v98.3.814. [DOI] [PubMed] [Google Scholar]
- 31.Rossi D, Cerri M, Deambrogi C, Sozzi E, Cresta S, Rasi S. et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15(3):995–1004. doi: 10.1158/1078-0432.CCR-08-1630. [DOI] [PubMed] [Google Scholar]
- 32.Cuni S, Perez-Aciego P, Perez-Chacon G, Vargas JA, Sanchez A, Martin-Saavedra FM. et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18(8):1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 33.Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C. et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100(10):3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 34.Barragan M, Bellosillo B, Campas C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99(8):2969–76. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- 35.Hewamana S, Alghazal S, Lin TT, Clement M, Jenkins C, Guzman ML. et al. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111(9):4681–9. doi: 10.1182/blood-2007-11-125278. [DOI] [PubMed] [Google Scholar]
- 36.Panayiotidis P, Ganeshaguru K, Foroni L, Hoffbrand AV. Expression and function of the FAS antigen in B chronic lymphocytic leukemia and hairy cell leukemia. Leukemia. 1995;9(7):1227–32. [PubMed] [Google Scholar]
- 37.Osorio LM, Aguilar-Santelises M, De Santiago A, Hachiya T, Mellstedt H, Jondal M. Increased serum levels of soluble Fas in progressive B-CLL. Eur J Haematol. 2001;66(5):342–6. doi: 10.1034/j.1600-0609.2001.066005342.x. [DOI] [PubMed] [Google Scholar]
- 38.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114(4):441–9. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V. et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 40.Schattner EJ. CD40 ligand in CLL pathogenesis and therapy. Leuk Lymphoma. 2000;37(5–6):461–72. doi: 10.3109/10428190009058499. [DOI] [PubMed] [Google Scholar]
- 41.Pu QQ, Bezwoda WR. Interleukin-4 prevents spontaneous in-vitro apoptosis in chronic lymphatic leukaemia but sensitizes B-CLL cells to melphalan cytotoxicity. Br J Haematol. 1997;98(2):413–7. doi: 10.1046/j.1365-2141.1997.2113028.x. [DOI] [PubMed] [Google Scholar]
- 42.Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC. et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98(10):3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 43.Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S. et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91(9):3379–89. [PubMed] [Google Scholar]
- 44.Faderl S, Keating MJ, Do KA, Liang SY, Kantarjian HM, O’Brien S. et al. Expression profile of 11 proteins and their prognostic significance in patients with chronic lymphocytic leukemia (CLL) Leukemia. 2002;16(6):1045–52. doi: 10.1038/sj.leu.2402540. [DOI] [PubMed] [Google Scholar]
- 45.Aviram A, Rabizadeh E, Zimra Y, Yeshoron M, Marmelstein M, Shaklai M. et al. Expression of bcl-2 and bax in cells isolated from B-chronic lymphocytic leukemia patients at different stages of the disease. Eur J Haematol. 2000;64(2):80–4. doi: 10.1034/j.1600-0609.2000.90042.x. [DOI] [PubMed] [Google Scholar]
- 46.Aguilar-Santelises M, Rottenberg ME, Lewin N, Mellstedt H, Jondal M. Bcl-2, Bax and p53 expression in B-CLL in relation to in vitro survival and clinical progression. Int J Cancer. 1996;69(2):114–9. doi: 10.1002/(SICI)1097-0215(19960422)69:2<114::AID-IJC8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Molica S, Dattilo A, Giulino C, Levato D, Levato L. Increased bcl-2/bax ratio in B-cell chronic lymphocytic leukemia is associated with a progressive pattern of disease. Haematologica. 1998;83(12):1122–4. [PubMed] [Google Scholar]
- 48.Kienle DL, Korz C, Hosch B, Benner A, Mertens D, Habermann A. et al. Evidence for distinct pathomechanisms in genetic subgroups of chronic lymphocytic leukemia revealed by quantitative expression analysis of cell cycle, activation, and apoptosis-associated genes. J Clin Oncol. 2005;23(16):3780–92. doi: 10.1200/JCO.2005.02.568. [DOI] [PubMed] [Google Scholar]
- 49.Kienle D, Benner A, Krober A, Winkler D, Mertens D, Buhler A. et al. Distinct gene expression patterns in chronic lymphocytic leukemia defined by usage of specific VH genes. Blood. 2006;107(5):2090–3. doi: 10.1182/blood-2005-04-1483. [DOI] [PubMed] [Google Scholar]
- 50.Pallasch CP, Schwamb J, Konigs S, Schulz A, Debey S, Kofler D. et al. Targeting lipid metabolism by the lipoprotein lipase inhibitor orlistat results in apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia. 2008;22(3):585–92. doi: 10.1038/sj.leu.2405058. [DOI] [PubMed] [Google Scholar]
- 51.Saxena A, Viswanathan S, Moshynska O, Tandon P, Sankaran K, Sheridan DP. Mcl-1 and Bcl-2/Bax ratio are associated with treatment response but not with Rai stage in B-cell chronic lymphocytic leukemia. Am J Hematol. 2004;75(1):22–33. doi: 10.1002/ajh.10453. [DOI] [PubMed] [Google Scholar]
- 52.Veronese L, Tournilhac O, Verrelle P, Davi F, Dighiero G, Chautard E. et al. Low MCL-1 mRNA expression correlates with prolonged survival in B-cell chronic lymphocytic leukemia. Leukemia. 2008;22(6):1291–3. doi: 10.1038/sj.leu.2405052. [DOI] [PubMed] [Google Scholar]


