Summary
Background
The aim of this study was to investigate the role of peripheral blood markers as additional diagnostic tools to transvaginal ultrasound (TVU) findings in the diagnosis of endometriosis.
Methods
This study included 40 patients undergoing laparoscopy for suspected endometriosis from January to December 2012. Preoperative levels of serum CA125, CA19-9, CEA and mRNA expression levels for survivin and VEGF were obtained. Real-time PCR was used to determine relative gene expression. A new diagnostic score was obtained by deploying the peripheral blood markers to the TVU findings. Statistical methods used were Chi-square, Fisher’s, Student’s t-test or the Mann – Whitney test.
Results
There was a statistically significant difference in serum CA125, survivin and VEGF levels in patients with endometriosis and those without endometriosis (p<0.001, p=0.025 and p=0.009, respectively). False negative TVU findings were noted in 3/13 patients (23.1%) with peritoneal endometriosis without ovaries involvement. High sensitivity (93.3%), specificity (90.0%), PPV (96.6%), NPV (81.8%) and accuracy (92.5%) were obtained for a diagnostic score based on TVU and significant peripheral blood markers (CA125, survivin and VEGF).
Conclusions
Determination of serum CA125, mRNA expression levels for survivin and VEGF along with TVU can contribute to higher accuracy of the noninvasive diagnostic tools for endometriosis.
Keywords: survivin, VEGF, CA125, endometriosis, laparoscopy, transvaginal ultrasound
Kratak sadržaj
Uvod
Cilj ovog istraživanja je da se ispita značaj određivanja koncentracija serumskih biomarkera (CA125, CA19-9, CEA i ekspresije iRNK za survivin i VEGF) zajedno sa transvaginalnim ultrazvučnim pregledom (TVU) prilikom dijagnostikovanja endometrioze.
Metode
Ova studija obuhvata 40 pacijentkinja kojima je zbog sumnje na postojanje endometrioze urađena dijagnostička laparoskopija sa patohistološkom analizom u jednogodišnjem periodu od januara 2012. do decembra 2013. Preoperativno su određivane koncentracije CA125, CA19-9, CEA kao i nivoi ekspresije iRNK za survivin i VEGF. Real-time PCR je korišćen za određivanje relativne ekspresije gena. Određivanjem vrednosti serumskih biomarkera zajedno sa TVU dobijen je novi dijagnostički skor. Statističke metode koje su korišćene su Hi kvadratni test, Fisherov, Studentov t test ili Mann-Whitney test.
Rezultati
Postoji statistički značajna razlika u serumskoj koncentraciji CA125, survivina i nivoa VEGF kod pacijentkinja sa endometriozom i onih bez endometrioze (p<0,001, p=0,025 i p=0,009). Lažno negativan nalaz TVU zabeležen je kod 3/13 pacijenata (23,1%) sa peritonealnom endometriozom. Visoka osetljivost (93,3%), specifičnost (90,0%), PPV (96,6%), NPV (81,8%) i tačnost (92,5%) postižu se određivanjem koncentracija serumskih biomarkera (CA125, survivin i VEGF) i TVU.
Zaključak
Određivanje serumskih koncentracija CA125, nivoa ekspresije iRNK za survivin i VEGF zajedno sa TVU od velikog je kliničkog značaja u neinvazivnoj dijagnostici endometrioze.
Introduction
Endometriosis is defined as the presence of ectopic endometrial tissue outside of the uterus (1). According to available data, 10% to 15% of women of reproductive age are affected by endometriosis. In women with infertility issues, the range increases to between 30% and 50%, while in women with chronic pelvic pain the incidence varies from 10% to 70% (2). The diagnosis of endometriosis is very complex. For a definitive diagnosis, a visual inspection of the pelvis at laparoscopy is the gold standard procedure. Considering the invasive nature of the procedure, the interval between the occurrence of the first symptoms and endometriosis diagnosis is often prolonged. Revealing reliable peripheral blood biomarkers for diagnosis of endometriosis should reduce the time from disease onset to disease treatment, improve treatment results and decrease the recurrence of the disease (3). The ideal serum marker should have high sensitivity and specificity, major prognostic significance and good correlation between its concentrations and disease severity. In routine clinical practice, plasma biomarker CA125 is used as a diagnostic tool for endometriosis (4, 5). In recent studies, two serum biomarkers, survivin and the vascular endothelial growth factor (VEGF), were evaluated in terms of diagnostic significance for endometriosis. Survivin is a protein, member of the apoptosis inhibitors family coded by BIRC 5 gene (6). Its function is the inhibition of caspase activation, which leads to a negative regulation of apoptosis. Survivin is highly expressed in most of the tumors in the human body (6). There is also convincing evidence that angiogenesis plays a key role in ectopic implantation of endometrial tissue (7). VEGF is a heparin binding glycoprotein of endothelial cells with a specific mitogen and vascular permeability activity (7).
The objective of this study was to investigate the role of peripheral blood markers (survivin and VEGF) as additional diagnostic tools to TVU findings in the differential diagnosis of endometriosis.
Materials and Methods
Study protocol
This study included 40 patients undergoing laparoscopy for suspected endometriosis at the Department of Gynecology and Obstetrics of the Medical Center Uzice and Clinical Center of Serbia, from January to December 2012. Preoperative levels of serum CA125, CA19-9, CEA and mRNA expression levels for survivin and VEGF were obtained. TVU was done using Logiq P5 (Sony) during preoperative preparation. After laparoscopic surgery, patients were divided into two groups: patients with confirmed diagnosis of endometriosis and a control group of patients without endometriosis.
Ethical consideration
The study was approved by the ethical committee of the study hospital. Disclaimers were obtained from each patient.
Biochemical analysis
Blood samples were obtained to determine serum levels of CA125, CA19-9 and CEA. A chemiluminometric immunoassay was used for the cancer markers on an autoanalyzer (SIEMENS).
RNA extraction, cDNA synthesis and quantitative real-time PCR
RNA was isolated using a TRI Reagent solution (Ambion, USA) and reverse transcribed using (200 U) Moloney Murine Leukemia Virus reverse transcriptase (RevertAid™ H Minus M-MuLV Reverse Transcriptase, Fermentas). A relative gene expression for survivin and VEGF in blood samples was obtained by comparing them to the constitutively expressed gene (»housekeeping« gene i.e. reference gene) glyceraldehyde-3-phosphate dehydrogenase (GADPH) by using a real-time PCR (Realplex2Mastercyclerep gradient S, Eppendorf). Primers used in PCR reactions were designed on the basis of sequences available in ENTEREZ data base by using an appropriate computer program (Primer Express® software v2.0, Applied Biosystems, USA). Sequences, positions in sequence of the whole gene and the final concentration of primers which are used for the amplification and detection of cDNA for survivin, VEGF, and GAPDH are noted in Table I. Primers for survivin and VEGF are synthesized and obtained by Applied Biosystems, and probes for GAPDH by Metabion (Germany). PCR was performed with a »master mix« containing DNA polymerase AmpliTaq Gold®, dNTP and optimized puffer components (2x Universal PCR Master Mix, Applied Biosystems), dye binding DNA (20x EvaGreen, Biotium, USA), primers for gene of interest and total cDNA. Adequate computer software was used for the analysis of obtained results (Mastercyclereprealplex), provided by the manufacturer (Eppendorf) (8).
Table I.
Primers used in PCR reactions.
Gene Sequence (5′-3′) |
Position in gene |
Conc. (nM) |
|---|---|---|
GAPDH |
||
F - CATCCATGACAACTTTGGTATCG |
564 |
300 |
R - CCATCACGCCACAGTTTCC |
671 |
300 |
Survivin (BIRC-5) |
||
F - GCCAAGAACAAAATTGCAAAGG |
515 |
900 |
R - TCTCCGCAGTTTCCTCAAATTC |
578 |
900 |
VEGF |
||
F - GCCCACTGAGGAGTCCAACAT |
1323 |
900 |
R - TGAGGTTTGATCCGCATAATCTG |
1373 |
900 |
F – Primer complementary to coding (»sense«) chain DNA
R – Primer complementary to non-coding (»antisense«) chain DNA
Statistical methods
Categorical data is presented by absolute numbers with percentages and is analyzed using a Chi-square test or Fisher’s exact test, as appropriate. For continuous variables, the Student’s t test or Mann-Whitney test was used. Sensitivity, specificity, positive and negative predictive values, false positive and negative rates, as well as accuracy of different diagnostic tools (i.e. peripheral blood markers and TVU findings) in patients suspected of suffering from endometriosis and referred for laparoscopy were determined. A ROC (Receiver Operating Characteristics) curve was constructed for VEGF mRNA expression levels. The area under the curve (AUC), which represents a quantitative measure of the predictive value of VEGF mRNA expression levels for diagnosis of endometriosis, was calculated. The cut-off value distinguishing patients with endometriosis from those without endometriosis was also determined. A new diagnostic score was obtained by assigning 1 point to each positive peripheral blood marker or TVU finding, thus making the score ranges between 0 and 4. In all tests, a p value < 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS statistical software (SPSS for Windows, release 21.0, SPSS, Chicago, IL).
Results
Baseline characteristics of patients who underwent laparoscopy due to suspected endometriosis were shown. No difference has been observed in age among patients with endometriosis and controls (32.1±4.8 vs 32.8±2.7, respectively), while patients with endometriosis had higher BMI (23.5±1.8 vs 21.8±1.8) (p=0.015). The range of patients’ age was 24-42 years. Infertility was the most common indication for laparoscopy in both groups (46.6% vs 60%). Chronic pelvic pain was present in 43.3% patients with endometriosis and 30% patients without endometriosis. Dysmenorrhea was indicated in 10% of patients in both groups (Table II).
Table II.
Characteristics of patients referred to laparoscopy.
Characteristic |
Without endometriosis (n = 10) n (%) |
Endometriosis (n=30) n (%) |
|---|---|---|
Age (years)* |
32.80±2.7 |
32.13±4.8 |
Indication for laparoscopic surgery |
||
Chronic pelvic pain |
3 (30%) |
13 (43.3%) |
Infertility |
6 (60%) |
14 (46.6%) |
Dysmenorrhea |
1 (10%) |
3 (10%) |
BMI* |
21.8±1.8 |
23.50±1.8 |
Data are presented as x±sd.
A statistically significant difference has been observed in the serum CA125 concentration (p<0.001), while there was no significant difference in the serum levels of CA19-9 and CEA in patients with endometriosis and those without endometriosis (p>0.05 for both). The survivin expression was noted with 22 (55%) patients, and in 20 patients (90.9%) endometriosis was confirmed. A significant statistical difference has been observed in the survivin expression level in women with endometriosis versus the control group (p=0.025). In patients suffering from endometriosis, the median difference between the expression of the »housekeeping« gene (GAPDH) and the expression of survivin was 13.32 (min 4.20; max 23.68). Patients with endometriosis had significantly higher expressions of mRNA for VEGF (ΔCT8.55 vs 10.17) (p=0.009) (Table III).
Table III.
Peripheral blood markers in patients with and without a definitive diagnosis of endometriosis.
Peripheral blood marker |
Without endometriosis (n = 10) n (%) |
Endometriosis (n=30) n (%) |
p |
|---|---|---|---|
CA125** |
14.4 (16.9) |
38 (4.3) |
<0.001 |
CA125>35† |
1 (10%) |
25 (83.3%) |
<0.001 |
CA19-9* |
16.4±5.0 |
25.7±9.5 |
0.005 |
CA19-9>37† |
0 (0%) |
5 (16.7%) |
0.306 |
CEA* |
1.2±0.8 |
1.4±0.7 |
0.467 |
CEA>3† |
0 (0%) |
0 (0%) |
/ |
Survivin |
2 (20%) |
20 (66.7%) |
0.025 |
ΔCT VEGF** |
10.17 (2.30) |
8.55 (2.99) |
0.009 |
2ΔCT VEGF** |
1156 (1349.5) |
298 (617) |
0.009 |
Data are presented as x±sd.
Data are presented as median (interquartile range).
Cut-off values for a normal laboratory finding.
The performance of survivin and VEGF expression, CA125 serum concentrations and TVU as diagnostic tests for endometriosis is presented in Table IV. Based on the ROC curve, the cut-off value for ΔCT VEGF of 9.5 was established as a value which separates the women with positive from those with negative diagnoses regarding endometriosis (area under the curve 0.733; SE = 0.086; p = 0.010) (Figure 1). Women with endometriosis more often had a difference in the expression of these two genes - less than 9.5 in comparison to women without endometriosis (p=0.006). TVU findings were positive in 27 (67.5%) patients with endometriosis. Negative TVU findings were found in 13 (32.5%) patients, while in 3 (23.1%) patients endometriosis was confirmed. A new diagnostic score was obtained by deploying the peripheral blood markers to the TVU findings. By combining the values of the mRNA expression for survivin and VEGF, levels of CA125 and TVU findings, a score was derived as a sum of all positive findings for the significant diagnostic tools used. High sensitivity, specificity, PPV, NPV and accuracy were obtained for the diagnostic score > 1 (Table IV).
Table IV.
Performance of peripheral blood markers and ultrasound findings as diagnostic tools for endometriosis.
Dg markers |
Sn |
Sp |
PPV |
NPV |
FP |
FN |
Accuracy |
|---|---|---|---|---|---|---|---|
Survivin expression |
66.7 |
80 |
90.9 |
44.4 |
5.0 |
25 |
70 |
ΔCT VEGF<9.5 |
80.0 |
70.0 |
88.9 |
53.8 |
7.5 |
15 |
75 |
CA125>35 |
83.3 |
90.0 |
96.2 |
64.3 |
2.5 |
12.5 |
85 |
TVU findings |
90.0 |
100 |
100 |
76.9 |
0 |
7.5 |
92.5 |
Diagnostic Score>1 |
93.3 |
90.0 |
96.6 |
81.8 |
2.5 |
5.0 |
92.5 |
TVU, Transvaginal Ultrasound
Figure 1.
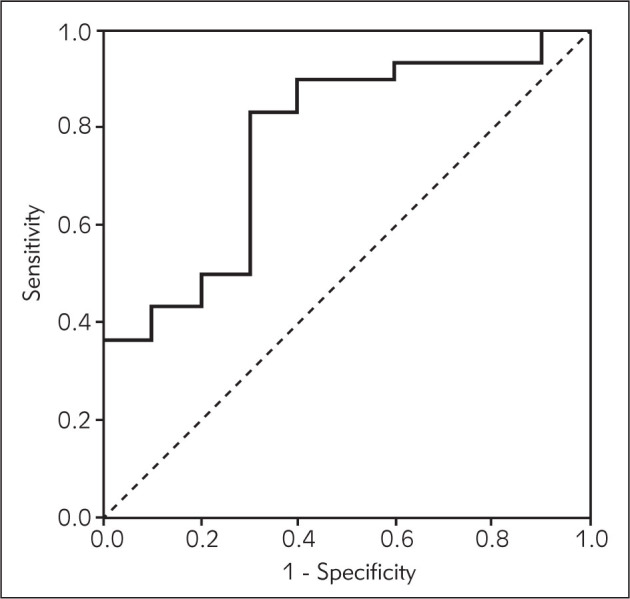
Receiver operating characteristic (ROC) curve for VEGF mRNA expression levels in diagnosis of endometriosis.
Discussion
Our study reported that mRNA for survivin and VEGF genes could be used as novel diagnostic biomarkers for endometriosis. High sensitivity, specificity, PPV, NPV and the accuracy of a diagnostic score based on TVU and peripheral blood markers (CA125, mRNA for survivin and VEGF genes) may contribute in distinguishing between patients with endometriosis and those without this disorder. CA125 is a valuable marker in the diagnosis of endometriosis. Previous studies showed significantly higher levels of CA125 in the serum of patients with endometriosis (9, 10). Our results also revealed that the serum level of CA125 was significantly increased in patients with endometriosis. Survivin could be considered as a very attractive candidate gene for endometriosis studies. Survivin is an inhibitor of apoptosis and is expressed during fetal development and in cancer tissues. The expression of the survivin gene in endometriosis is significantly higher in ectopic lesions rather than eutopic endometrium (11, 12), while a reduced number of apoptotic cells in endometrial lesions is a consequence of the overexpression of survivin (11). Survivin expression was found in endometrial ovarian cysts, also, with maximum levels in the micro-focus outside the fibrous capsule (13). It could be expected that survivin is also expressed in ectopic endometriotic foci (14). In our study, we measured the mRNA level of survivin in the peripheral blood in women with and without endometriosis. The results demonstrated that the sensitivity of survivin as a diagnostic test for endometriosis in our study was 66.7%. PPV was 90.9% with a false positive rate of 2/40 (5%). Mabrouk et al. (10) found that the sensitivity of mRNA expression for survivin was 75%, with a false positive rate of 10%. By combining the values of mRNA expressions for survivin, levels of CA125 and CA19-9, they obtained the sensitivity of 87% with 10% of false positive results.
There is convincing evidence that angiogenesis plays an important role in ectopic implantation of the endometrial tissue and in the development of endometric lesions (15). Angiogenesis, the growth of blood vessels from the existing vasculature, implies interactions of a number of hardly regulated molecules, which include a key angiogenesis factor named vascular endothelial growth factor (VEGF). Pellicier et al. (16) noted that there was no significant difference in VEGF levels in the serum of affected patients compared to the control group (15). Mataliotakis registered a discrete but significant difference in the concentration of VEGF in serum compared to the control group (17). These controversial results may be a consequence of the small samples used in studies. Some data indicate that VEGF may be increased in benign gynecological diseases, such as endometrial hyperplasia, abnormal uterus hemorrhage and ovarian cysts (18, 19). In our study, patients with endometriosis had a significantly higher value of mRNA expression for VEGF compared to patients without endometriosis. Our results show that the detection of increased levels of mRNA expression for VEGF in the serum of patients with endometriosis may play a significant role in the diagnosis of endometriosis, as well as in better understanding the etiology of the disease. Taking into account that the diagnosis of endometriosis is very demanding and often delayed because of the nonspecific and late occurrence of the symptoms, the use of laparoscopy as the gold standard tool for definitive diagnosis that is limited by financial resources, availability, surgeon experience, and its invasive nature, using survivin and VEGF as novel biomarkers in the diagnosis of endometriosis could increase the accuracy of TVU findings.
Determination of serum CA125, mRNA expression levels for survivin and VEGF along with TVU can contribute to the higher accuracy of noninvasive diagnostic tools for endometriosis. Laparoscopy could be used only for patients who require immediate surgical treatment.
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Adamson GD. Endometriosis classification: an update. Curr Opin Obstet Gynecol. 2011;23:213–20. doi: 10.1097/GCO.0b013e328348a3ba. [DOI] [PubMed] [Google Scholar]
- 2.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17:637–53. doi: 10.1093/humupd/dmr013. [DOI] [PubMed] [Google Scholar]
- 3.Bedaiwy MA, Falcone T. Laboratory testing for endometriosis. Clin Chim Acta. 2004;340:41–56. doi: 10.1016/j.cccn.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Haas D, Chvatal R, Reichert B, Renner S, Shebl O, Binder H. et al. Endometriosis: a premenopausal disease? Age pattern in 42,079 patients with endometriosis. Arch Gynecol Obstet. 2012;286:667–70. doi: 10.1007/s00404-012-2361-z. [DOI] [PubMed] [Google Scholar]
- 5.Santulli P, Streuli I, Melonio I, Marcellin L, M’Baye M, Bititi A. et al. Increased serum cancer antigen-125 is a marker for severity of deep endometriosis. J Minim Invasive Gynecol. 2015;22(2):275–84. doi: 10.1016/j.jmig.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Londero AP, Calcagno A, Grassi T, Marzinotto S, Orsaria M, Beltrami CA. et al. Survivin, MMP-2, MT1-MMP, and TIMP-2: their impact on survival, implantation, and proliferation of endometriotic tissues. Virchows Arch. 2012;461(5):589–99. doi: 10.1007/s00428-012-1301-4. [DOI] [PubMed] [Google Scholar]
- 7.Kianpour M, Nematbakhsh M, Ahmadi SM, Jafarzadeh M, Hajjarian M, Pezeshki Z. et al. Serum and peritoneal fluid levels of vascular endothelial growth factor in women with endometriosis. Int J Fertil Steril. 2013;7(2):96–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Novaković I, Maksimović N, Pavlović A, Žarković M, Rovčanin B, Mirković D, Pekmezović T, Cvetković D. Introduction to molecular genetic diagnostics. J Med Biochem. 2014;33:3–7. [Google Scholar]
- 9.Ozhan E, Kokcu A, Yanik K, Gunaydin M. Investigation of diagnostic potentials of nine different biomarkers in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;178:128–33. doi: 10.1016/j.ejogrb.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Mabrouk M, Elmakky A, Caramelli E, Farina A, Mignemi G, Venturoli S. et al. Performance of peripheral (serum and molecular) blood markers for diagnosis of endometriosis. Arch Gynecol Obstet. 2012;285:1307–12. doi: 10.1007/s00404-011-2122-4. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe A, Taniguchi F, Izawa M, Suou K, Uegaki T, Takai E. et al. The role of survivin in the resistance of endometriotic stromal cells to drug-induced apoptosis. Hum Reprod. 2009;24(12):3172–9. doi: 10.1093/humrep/dep305. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Li M, Zheng X, Sun Y, Wen Z, Zhao X. Endometriotic stromal cells lose the ability to regulate cell-survival signaling in endometrial epithelial cells in vitro. Mol Hum Reprod. 2009;15:653–63. doi: 10.1093/molehr/gap069. [DOI] [PubMed] [Google Scholar]
- 13.Goteri G, Lucarini G, Pieramici T, Filosa A, Pugnaloni A, Montik N. et al. Endothelial cell surviving is involved in the growth of ovarian endometriotic cysts. Anticancer Res. 2005;25:4313–8. [PubMed] [Google Scholar]
- 14.Fujino K, Ueda M, Takehara M, Futakuchi H, Kanda K, Yamashita Y. et al. Transcriptional expression of survivin and its splice variants in endometriosis. Mol Hum Reprod. 2006;12(6):383–8. doi: 10.1093/molehr/gal042. [DOI] [PubMed] [Google Scholar]
- 15.Baranov VS, Ivaschenko TE, Liehr T, Yarmolinskaya MI. Systems genetics view of endometriosis: a common complex disorder. Eur J Obstet Gynecol Reprod Biol. 2015;185:59–65. doi: 10.1016/j.ejogrb.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohí J, Simón C. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70:425–31. doi: 10.1016/s0015-0282(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 17.Matalliotakis IM, Goumenou AG, Koumantakis GE, Neonaki MA, Koumantakis EE, Dionyssopoulou E. et al. Serum concentrations of growth factors in women with and without endometriosis: the action of anti-endometriosis medicines. Int Immunopharmacol. 2003;3:81–9. doi: 10.1016/s1567-5769(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 18.Robati M, Ghaderi A, Mehraban M, Shafizad A, Nasrolahi H, Mohammadianpanah M. Vascular endothelial growth factor (VEGF) improves the sensitivity of CA125 for differentiation of epithelial ovarian cancers from ovarian cysts. Arch Gynecol Obstet. 2013;288(4):859–65. doi: 10.1007/s00404-013-2819-7. [DOI] [PubMed] [Google Scholar]
- 19.Dai H, Zhao S, Xu L, Chen A, Dai S. Expression of Efp, VEGF and bFGF in normal, hyperplastic and malignant endometrial tissue. Oncol Rep. 2010;23(3):795–9. [PubMed] [Google Scholar]


