Summary
Background
The activation of NLRP3-inflammasome may contribute to inflammatory processes in rheumatoid arthritis (RA). Functional polymorphisms in the genes coding for its components NLRP3 and CARD8 were associated with a proinflammatory phenotype. Our aim was to investigate the influence of these polymorphisms on RA susceptibility and disease activity at the time of diagnosis and after six months of treatment.
Methods
A group of 128 RA patients treated with methotrexate and 122 healthy controls were genotyped for NLRP3 rs35829419 (p. Q705K) and CARD8 rs2043211 (p. C10X) polymorphisms.
Results
RA susceptibility was not influenced by the investigated polymorphisms or their interaction. The investigated polymorphisms explained 8% of variability in DAS28 at the time of diagnosis. Carriers of NLRP3 rs35829419 or CARD8 rs2043211 polymorphisms had significantly higher DAS28 at the time of diagnosis (p=0.003; p=0.022; respectively). Polymorphic CARD8 rs2043211 TT genotype was also associated with higher DAS28 after six months of treatment (p=0.033).
Conclusions
Genetic variability of inflammasome components may contribute to higher disease activity at the time of diagnosis and after 6 months of methotrexate treatment in RA patients. Better understanding of the immunological mechanisms behind a more active course of RA may suggest novel treatment approaches in a subset of patients with a proinflammatory phenotype.
Keywords: polymorphism, rheumatoid arthritis, inflammasome, BIRC5, NLRP3
Kratak sadržaj
Uvod
Aktivacija NLRP3-inflamazoma može pojačati zapaljenske procese u reumatoidnom artritisu (RA). Funkcionalni polimorfizmi u genima koji kodiraju za njegove komponente NLRP3 i CARD8 dovedeni su u vezu sa proinflamatornim fenotipom. Naš cilj bio je da istražimo uticaj ovih polimorfizama na podložnost RA-u i aktivnost bolesti u vreme dijagnoze i posle šest meseci.
Metode
U grupi od 128 obolelih od RA-a koji su lečeni metotreksatom kao i kod 122 zdravih kontrolnih ispitanika urađena je genotipizacija za polimorfizme NLRP3 rs35829419 (p. Q705K) i CARD8 rs2043211 (p. C10X).
Rezultati
Na podložnost RA-u nisu uticali istraživani polimorfizmi niti njihova interakcija. Istraživani polimorfizmi objašnjavaju 8% varijabilnosti u DAS28 u vreme dijagnoze. Nosioci polimorfizama NLRP3 rs35829419 ili CARD8 rs2043211 imali su značajno viši DAS28 u vreme dijagnoze (p=0,003, odnosno p=0,022). Polimorfni genotip CARD8 rs2043211 TT takođe je bio povezan sa višim DAS28 posle šest meseci lečenja (p=0,033).
Zaključak
Genetska varijabilnost komponenti inflamazoma može dovesti do pojačane aktivnosti bolesti u vreme dijagnoze i posle šest meseci lečenja metotreksatom kod obolelih od RA-a. Bolje razumevanje imunoloških mehanizama koji stoje iza aktivnijeg toka RA-a moglo bi ukazati na nove pristupe u lečenju u podskupu pacijenata sa proinflamatornim fenotipom.
Introduction
The heterogeneity of response observed with rheumatoid arthritis (RA) therapeutics may suggest the existence of differing underlining molecular etiology, albeit presenting as the same disease (1). To achieve better treatment response, more knowledge of the underlying mechanisms and predictive understanding of the response are needed (2, 3). An underlying mechanism that may contribute to the RA pathology, joints inflammation and cartilage destruction in vivo is activation of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3)-inflammasome (4). Its activation results in the caspase-1 mediated cleavage of pro-IL-1β into active IL-1β, a proinflammatory cytokine (5). The nonsynonymous gain-of-function polymorphism NLRP3 rs35829419 (p. Q705K) leads to an overactive NLRP3 inflammasome (6), while caspase recruitment domain-containing protein 8 (CARD8) rs2043211 (p. C10X) polymorphism encodes truncated protein, which does not inhibit the NF-κB stimulation of inflammatory response (7). It was suggested that interaction between NLRP3 rs35829419 and CARD8 rs2043211 increases RA susceptibility (8) and that CARD8 rs2043211 increases early-stage and long-term disease activity (7), but the results are inconclusive (9).
In this study, we investigated the association of NLRP3 and CARD8 polymorphisms with RA susceptibility and disease activity at the time of diagnosis and after six months of methotrexate (MTX) treatment.
Patients and Methods
We performed a retrospective study in RA patients who were older than 18 years, of Central Caucasian origin and initially treated with MTX monotherapy for at least six months at the Department of Rheumatology, University Medical Centre, Ljubljana, Slovenia. Control group included Slovenian healthy blood donors between 18 and 65 years.
The study was approved by the Republic of Slovenia National Medical Ethics Committee and was carried out according to the Declaration of Helsinki. All patients gave their written informed consent to participate in the study.
Demographic and clinical data on RA patients were obtained from the patients′ medical records: data on disease duration before beginning of treatment and Disease Activity Score examined in 28 joints (DAS28) assessed at the time of diagnosis (baseline) (10) and after 6 months of treatment.
Genomic DNA was isolated from peripheral blood leukocytes and genotyped for NLRP3 rs35829419 and CARD8 rs2043211 using a fluorescence-based competitive allele-specific real time polymerase chain reaction (KASPar assay) according to the manufacturer′s instructions (KBiosciences, Herts, UK).
Median was used to present the central tendency with the 25th to 75th percentile range as a measure of variability. Chi-square test was used to assess Hardy-Weinberg equilibrium (HWE) and to compare proportions between groups. Logistic regression was used to assess the influence of interaction between polymorphisms on RA susceptibility. Mann-Whitney U or Kruskal-Wallis H test were used to estimate the differences in DAS28 between genotypes under an additive (NLRP3, CARD8) or recessive (CARD8) genetic model. Because baseline DAS28 was normally distributed, we used a multivariate generalized linear model to analyse the influence of interaction between polymorphisms on baseline DAS28. Multivariate linear regression was performed to test for the association between polymorphisms and DAS28 with adjustment for clinical factors (gender, age and disease duration before beginning of treatment). Adjusted R square was used to evaluate the contribution of both polymorphisms to the variability in baseline DAS28 among RA patients. All significance tests were two-tailed and conducted at the 0.05 level of significance. Analyses were performed using R (11) and SPSS.
Results
Patient characteristics
In total,128 RA patients (102 females and 26 males) with a median (25–75% range) age of 56.9 (45.5–65.3) years and 122 controls (85 females and 43 males) that were 53 (47–60) years old were included in the study. Gender and age did not differ significantly between RA patients and controls (p=0.068, p=0.065, respectively). In RA patients disease duration before beginning of treatment was 2.8 (0–15) weeks. Baseline DAS28 was 5.1 (4.3–5.9) and DAS28 after 6 months of treatment was 3.1 (2.2–4.6).
Association of NLRP3 and CARD8 on RA susceptibility
All the patients and controls were genotyped for NLRP3 rs35829419 and CARD8 rs2043211 polymorphisms. In total, 114 (89%) RA patients were homozygous for wild type NLRP3 CC genotype, while 14 (11%) carried one polymorphic allele. Among the controls, 105 (86%) were carriers of NLRP3 CC genotype while 14 (11%) carried one polymorphic allele.
Altogether 49 (38.3%) RA patients were homozygotes for the wild type CARD8 A allele, 64 (50%) were heterozygotes and 15 (11.7%) carried two polymorphic T alleles. Among the controls, 54 (44.3%) were carriers of CARD8 AA genotype, 57 (46.7%) were heterozygotes and 11 (9%) were homozygotes for polymorphic T allele. Allele frequencies for NLRP3 as well as for CARD8 were in Hardy-Weinberg equilibrium (p = 0.660, p=0.272, respectively).
The allele frequencies of NLRP3 and CARD8 polymorphism did not differ significantly between RA patients and controls (p=0.472, p=0.571, respectively). The interaction between these polymorphisms was also not significantly associated with RA susceptibility (p=0.936; OR=0.910; 95%Cl: 0.093–8.40).
Association of NLRP3 and CARD8 and DAS28
Among RA patients, no homozygotes for polymorphic NLRP3 AA genotype were found, however, carriers of CA genotype had significantly higher baseline DAS28 than the carriers of wild type genotype CC (p=0.003) (Table I, Figure 1). This association remained highly significant after adjustment for clinical factors (p=0.007; B=0.906; 95% Cl: 0.248–1.565). Homozygotes for polymorphic CARD8 T allele had marginally higher baseline DAS28 in the additive model but highly significant in the recessive model (p=0.049; p=0.022, respectively) (Table I, Figure 2). The association in the recessive model remained significant after adjustment for clinical factors (p=0.037; B=0.690; 95%CI: 0.042–1.338). NLRP3 and CARD8 (recessive model) explained altogether 8% of the variability in baseline DAS28 (p=0.006; B = 0.925; 95%CI: 0.274–1.577 and p = 0.029; B = 0.704; 95%CI: 0.072–1.337).
Table I.
The influence of NLRP3 and CARD8 polymorphism on DAS28 at the time of diagnosis and after 6 months of treatment.
| Genotype | N (%) | DAS28 at diagnosis | DAS28 after 6 months of treatmenta | |||
|---|---|---|---|---|---|---|
| Median (range) | p-value | Median (range) | p-value | |||
|
NLRP3 rs35829419 |
CC CA AA |
114 (89.1) 14 (10.9) 0 (0) |
5.00 (4.27–5.83) 6.00 (5.26–7.15) NA |
0.003b | 3.00 (2.19–4.60) 3.10 (2.35–4.95) NA |
0.666b |
|
CARD8 rs2043211 |
AA AT TT |
49 (38.3) 64 (50) 15 (11.7) |
5.00 (4.30–5.95) 5.05 (4.13–5.71) 5.80 (5.10–6.40) |
0.049 b 0.022 c |
3.00 (2.00–4.30) 2.60 (2.12–4.35) 4.25 (2.50–6.10) |
0.100b 0.033c |
information on DAS28 is missing for 6 patients
additive genetic model
recessive genetic model; NA – not applicable.
Figure 1.
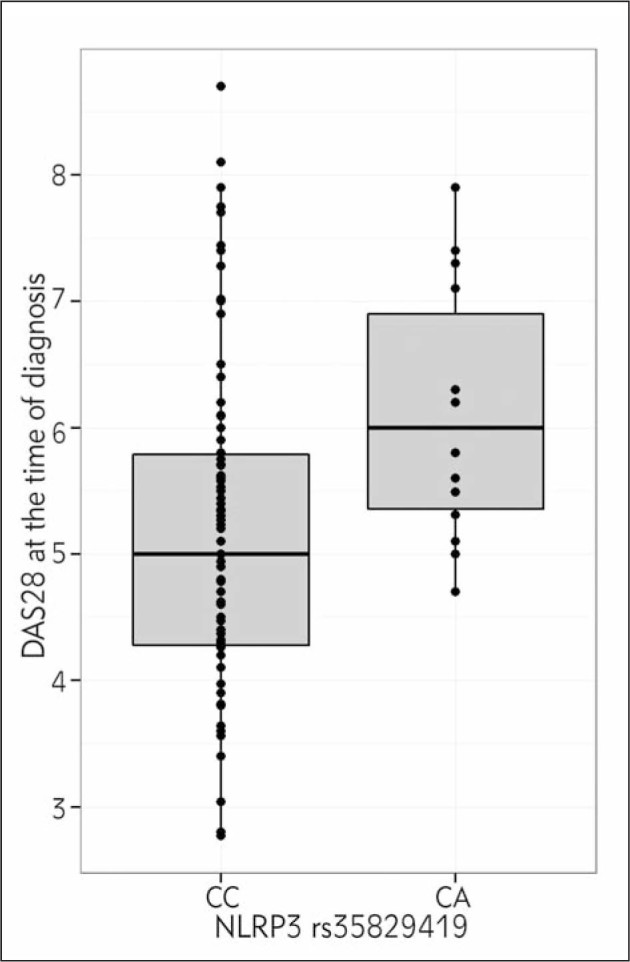
Association between NLRP3 rs35829419 and DAS28 at the time of diagnosis.
Figure 2.
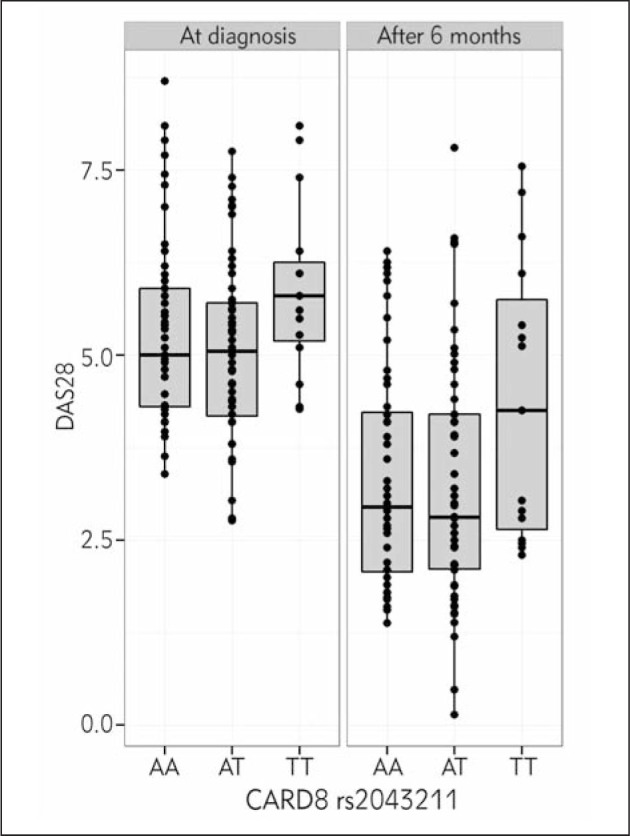
Association between CARD8 rs2043211 and DAS28 at the time of diagnosis and after 6 months of treatment.
After 6 months of MTX treatment, only CARD8 showed association with higher DAS28 under the recessive model (p=0.033) (Table I, Figure 2). This association remained significant after adjustment for clinical factors (p=0.026; B=0.986; 95%Cl=0.120–1.852).
Discussion
We report for the first time on the influence of CARD8 rs2043211 and NLRP3 rs35829419 polymorphisms on a more active course of disease at the time of diagnosis in RA patients. CARD8 rs2043211 remained associated with DAS28 also after 6 months of RA treatment.
In agreement with previous reports, we did not observe any association between NLRP3 rs35829419 or CARD8 rs2043211 and RA susceptibility (7, 9, 12, 13). Moreover, we did not observe any influence of the interaction between both investigated polymorphisms on RA susceptibility, contrary to a previous report (8). It was suggested that 50% to 60% of RA susceptibility can be explained by genetic variation. Major genetic risk was observed within HLA-DRB1 genes, and the second largest was observed within PTPN22, with the effect size of ~1.8 (14), while smaller effects were observed for other genetic variants. In our study, we can assure with 80% of probability that the detected risk factor was greater than 1.7. This is probably too high to detect the genetic variants that influence RA susceptibility, so we could report false negative results.
In our study, heterozygotes for NLRP3 rs35829419 polymorphism had significantly higher baseline DAS28. This could be due to the NLRP3 gain-of-function polymorphism, which leads to an overactive NLRP3 inflammasome (6), and probably to increased activation of IL-1ß (4). Homozygosity for the polymorphic CARD8 rs2043211 T allele strongly influenced DAS28 at the time of diagnosis and after 6 months of treatment. Our results are in agreement with the study that reported the influence of CARD8 rs2043211 on more severe disease course in long-lasting RA (7). Truncated CARD8 resulting from CARD8 rs2043211 may reduce its control over the NF-κB signalling pathway and thus lead to a more active transcriptional factor and an amplification of the inflammatory processes (7). The NF-κB is essential for the expression of both inflammatory cytokines and tissue destructive enzymes in RA (15, 16). The contribution of NLRP3 and CARD8 to active RA was also shown in the gene expression study. Namely, NLRP3 was upregulated and CARD8 was downregulated in the PBMCs of RA patients prior to receiving infliximab therapy in comparison to healthy controls. However, NLRP3 rs35829419 or CARD8 rs2043211 polymorphisms were not associated with baseline expression levels (9).
Our study had some limitations as retrospective data collection could have introduced biases and additional data on the presence of erosions at the beginning of disease and after 6 months of treatment were not available but would be very informative. Although matched cased control analysis would be more appropriate for the analysis of susceptibility to RA, our controls were selected so that they did not significantly differ from cases in gender and age. Our study was also not biased by genetic heterogeneity since all the patients were recruited in a geographic area with an ethnically homogeneous population (17).
Conclusion
This study offers further evidence about the role of inflammasome in RA. Future investigations on bigger cohorts are needed to confirm our results. Investigated polymorphisms may influence more active inflammation and more active course of RA at the time of diagnosis and in early treatment, which may be due to increased activation of NLRP3-inflammasome. Genetic profile of the patient may reveal some of the immunological mechanisms and may provide a clue towards more effective treatment schemes.
Acknowledgments.
This work was financially supported by the Slovenian Research Agency (ARRS Grants No. P1-0170).
Glossary
Abbreviations
- CARD8
caspase recruitment domain-containing protein 8
- DAS28
Disease Activity Score examined in 28 joints
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- RA
rheumatoid arthritis.
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Karsdal MA, Bay-Jensen AC, Henriksen K, Christiansen C, Genant HK, Chamberlain C. et al. Rheumatoid arthritis: a case for personalized health care? Arthritis Care Res. 2014;66(9):1273–80. doi: 10.1002/acr.22289. [DOI] [PubMed] [Google Scholar]
- 2.Wilson H. Biomarkers in autoimmune rheumatic diseases. Biomarkers in medicine. 2015;9(6):497–8. doi: 10.2217/bmm.15.27. Epub 2015/06/17. [DOI] [PubMed] [Google Scholar]
- 3.Basok IB, Kucur M, Kizilgul M, Yilmaz I, Ekmekci BO, Uzunlulu M, Isman KF. Increased chitotriosidase activities in patients with rheumatoid arthritis: a possible novel marker? J Med Biochem. 2014;33:245–51. [Google Scholar]
- 4.Walle LV, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P. et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014 doi: 10.1038/nature13322. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126(4):659–62. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Verma D, Sarndahl E, Andersson H, Eriksson P, Fredrikson M, Jonsson JI. et al. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1beta and IL-18 production. PloS one. 2012;7(4):e34977. doi: 10.1371/journal.pone.0034977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontalba A, Martinez-Taboada V, Gutierrez O, Pipaon C, Benito N, Balsa A. et al. Deficiency of the NF-kappaB inhibitor caspase activating and recruitment domain 8 in patients with rheumatoid arthritis is associated with disease severity. J Immunol. 2007;179(7):4867–73. doi: 10.4049/jimmunol.179.7.4867. [DOI] [PubMed] [Google Scholar]
- 8.Kastbom A, Verma D, Eriksson P, Skogh T, Wingren G, Soderkvist P. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project) Rheumatology (Oxford, England) 2008;47(4):415–7. doi: 10.1093/rheumatology/kem372. [DOI] [PubMed] [Google Scholar]
- 9.Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Eyre S. et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis. 2014;73(6):1202–10. doi: 10.1136/annrheumdis-2013-203276. [DOI] [PubMed] [Google Scholar]
- 10.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35(4):745–57. doi: 10.1016/j.rdc.2009.10.001. vii-viii. Epub 2009/12/08. [DOI] [PubMed] [Google Scholar]
- 11.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 12.Ben Hamad M, Cornelis F, Marzouk S, Chabchoub G, Bahloul Z, Rebai A. et al. Association study of CARD8 (p.C10X) and NLRP3 (p.Q705K) variants with rheumatoid arthritis in French and Tunisian populations. Int J Immunogenet. 2012;39(2):131–6. doi: 10.1111/j.1744-313X.2011.01070.x. Epub 2011/12/02. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Bermudez M, Lopez-Mejias R, Gonzalez-Juanatey C, Corrales A, Castaneda S, Ortiz AM. et al. CARD8 rs2043211 (p.C10X) polymorphism is not associated with disease susceptibility or cardiovascular events in Spanish rheumatoid arthritis patients. DNA Cell Biol. 2013;32(1):28–33. doi: 10.1089/dna.2012.1836. Epub 2012/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowes J, Barton A. Recent advances in the genetics of RA susceptibility. Rheumatology (Oxford, England) 2008;47(4):399–402. doi: 10.1093/rheumatology/ken005. [DOI] [PubMed] [Google Scholar]
- 15.Andreakos E, Sacre S, Foxwell BM, Feldmann M. The toll-like receptor-nuclear factor kappaB pathway in rheumatoid arthritis. Front Biosci. 2005;10:2478–88. doi: 10.2741/1712. [DOI] [PubMed] [Google Scholar]
- 16.Jančić I, Šefik-Bukilica M, Živojinović S, Damjanov N, Spasovski V, Kotur N, Klaassen K, Pavlović S, Bufan B, Arsenović-Ranin N. Influence of promoter polymorphisms of the tnf-a (-308g/a) and il-6 (-174g/c) genes on therapeutic response to etanercept in rheumatoid arthritis. J Med Biochem. 2015;34:414–21. doi: 10.2478/jomb-2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidan-Jeras B, Jurca B, Dolzan V, Jeras M, Breskvar K, Bohinjec M. Slovenian Caucasian normal. HLA. 1998;1998:180–1. [Google Scholar]


