Abstract
Background
Rickettsia felis and Rickettsia typhi are emerging arthropod-borne zoonoses causing fever and flu-like symptoms. Seroprevalence and risk factors associated with exposure to these organisms was explored in Australian veterinarians.
Methods
One hundred and thirty-one veterinarians from across Australia were recruited to participate in a cross-sectional survey. Veterinarians provided a single blood sample and answered a questionnaire on potential risk factors influencing their exposure to R. felis and R. typhi. Indirect microimmunofluorescence antibody testing (IFAT) was used to identify evidence of serological exposure of the participants to R. felis and R. typhi. Results were analyzed and a logistical regression model performed to predict risk factors associated with seropositivity.
Results
In total, 16.0% of participants were seropositive to R. felis, 4.6% to R. typhi and 35.1% seropositive to both, where cross-reactivity of the IFAT between R. felis and R. typhi precluded a definitive diagnosis. Veterinarians residing within the south-eastern states of Victoria and Tasmania were at a higher risk of exposure to R. felis or generalised R. felis or R. typhi exposure. Older veterinarians and those that recommended flea treatment to their clients were found to be significantly protected from exposure.
Conclusions
The high exposure to R. felis amongst veterinary professionals suggests that flea-borne spotted fever is an important cause of undifferentiated fever conditions that may not be adequately recognized in Australia.
Keywords: Rickettsia, Rickettsia felis, Rickettsia typhi, flea-borne spotted fever, murine typhus, veterinarian, Australia
Background
Rickettsia felis is a bacterial pathogen and the etiological agent of flea-borne spotted fever (FBSF) or cat flea typhus, cases of which have been described in many parts of the world including Europe [1], the Americas [2], Asia [3] and Oceania [4]. Human infection results from transmission through an infected arthropod vector, typically fleas infecting a bite site with rickettsiae; the resulting infection is typically characterized by a series of non-specific symptoms including pyrexia, maculopapular rash, eschar, myalgia, arthralgia, headache and fatigue [5].
The biological vector for R. felis is the cat flea, Ctenocephalides felis [6], although it has also been found in other arthropods. Rickettsiae are generally maintained within reservoir hosts, typically mammals, and their associated arthropod vectors [7]. Efforts to identify a vertebrate biological reservoir for R. felis have so far remained unresolved. While R. felis DNA has been detected in cat [8], dog [9] and opossum [10] blood, successful culture of the organism from mammalian blood has yet to be achieved. In C. felis, R. felis is maintained for up to 12 generations in the absence of a blood meal [11].
A number of rickettsial organisms have been described in Australia, including Rickettsia australis (causing Queensland tick typhus), Rickettsia honei (causing Flinders Island spotted fever), Rickettsia honei marmionii (causing Australian spotted fever), Rickettsia typhi (causing murine typhus), Orientia tsutsugamushi (causing scrub typhus) and Coxiella burnetti (causing Q fever) [12]. Each can cause fever and flu-like symptoms, are spread by the bite of an infected arthropod and interact with Australian wildlife in sylvatic cycles. Some species, such as R. typhi, display serological cross-reactivity with R. felis, presenting a diagnostic challenge requiring concurrent testing against both R. felis and R. typhi antigen to establish an aetiology [4]. Of the R. felis-like species, the URRWXCal2 (Cal2) variant is predominant in C. felis felis fleas in Australia [13].
Pet ownership is widespread in Australia, with dog and cat ownership estimated at 36% and 23% respectively [14]. Dogs in particular have been implicated in potentially contributing to the life-cycle of R. felis, with molecular detection of the R. felis ompB gene in the blood of 9% of pound dogs in south east Queensland [15] and 2.3% of indigenous community dogs in the Northern Territory [13]. Both dogs and cats are well known to harbor ectoparasites, with C. felis felis being the dominant flea species [16], from which R. felis has also been isolated [17, 18].
The first reported Australian cases of R. felis occurred in Victoria [19] in a family living in metropolitan Melbourne, Victoria, that had received two flea-ridden kittens from a farm in Lara, Victoria. Since then, multiple other clinical cases of human infection in patients in New South Wales, Queensland, South Australia, Tasmania and Western Australia have been confirmed [4]. Risk factors for exposure to R. felis however were not described. In a seroepidemiological study in Spain, R. felis infection was associated with high risk occupations involving working outdoors, contact with animals or potential contact with rodents [20]. In Colombia, gender, home location and age were associated with R. felis exposure [21].
In small animal clinical practice, exposure to flea-ridden animals is a potential occupational hazard for veterinarians. Approximately 10,000 veterinarians are employed in Australia [22], a proportion of whom will have contact with animals as part of their job. Due to this potential, veterinarians of Australia are the focus of this study which aims to determine the seroprevalence and risk factors for exposure R. felis.
Methods
Participant selection
Veterinarians (n = 131) were recruited at the Australian Veterinary Association Pan-Pacific conference held in Brisbane (May, 2015) and at the University of Melbourne (December, 2015). Selection was opportunistic, with consenting healthy individuals invited to answer a questionnaire and provide a blood sample on a voluntary basis. Serology testing results were made available to the participants.
Survey
A questionnaire was designed to collect information from participants on personal demographics and potential risk factors contributing to R. felis infection; this included age, gender, location, potential for exposure to different animals in the workplace and at home, knowledge on R. felis, attitudes towards flea control in companion animals and any recent disease symptoms. Responses were digitalized, reversibly de-identified, and stored on a password protected computer.
Blood sample collection
Samples were collected by either a registered medical professional (doctor or nurse) or a certified venepuncturist. Approximately 8 ml of blood was taken from the median cubital vein into serum separator tubes which underwent centrifugation at 4000× g for 5 min, and the separated sera stored at -20 °C until processed.
Culture to obtain antigen
Antigen culture and IFAT was performed at the Australian Rickettsial Reference Laboratory, Geelong, Australia. The L929 cell line was selected to establish culture of the rickettsial organisms tested in this study. Once a confluent monolayer was achieved, live R. felis and R. typhi cultures were revived from -80 °C and used to infect separate flasks. Leibovitz-15 media (GIBCO, Rockville, MD, USA) supplemented with 10% foetal calf serum, 2 mM L-glutamine and 5% tryptose phosphate broth was used to maintain R. felis. RPMI media (GIBCO), supplemented with 10% foetal calf serum and 2 mM L-glutamine was used to maintain R. typhi. Infection levels were monitored using a semi-quantitative qPCR, with species confirmation verified using PCR and DNA sequencing (Australian Genomic Research Facility Ltd., Australia); both molecular techniques were based on the citrate synthase (gltA) gene [23].
Infected cell monolayers were harvested by physical detachment and heat inactivated at 56 °C for 30 min. Differential centrifugation at 3000× g for 10 min at room temperature was used to separate the host cell material from the rickettsiae; this pelleted Rickettsia was then resuspended in PBS.
Immunofluorescence antibody testing
The reference method for the diagnosis of R. felis infection is the indirect microimmunofluorescence antibody test (IFAT), a serological test detecting antibodies developed after exposure. Due to shared epitopes, some of which may have been gained from horizontal gene transfer [24], serological cross-reactivity is often noted between R. felis, widely considered to be a spotted fever group (SFG) or transitional group Rickettsia, with others from the closely related typhus group (TG), such as R. typhi [25].
Working concentrations of rickettsial antigen were determined by comparing the fluorescence of serial doubling dilutions of R. felis and R. typhi on the IFAT. Rickettsial antigen of R. felis and R. typhi was spotted on 40-well slides (Scientific Device Laboratory, Des Plaines, IL, USA), air-dried and fixed in 100% acetone for 2 min. Serum samples were diluted in 2% casein in PBS at 1:128, and 2-fold serial dilutions we prepared onward as required until the end-point titer was determined. Positive and negative controls were included in each assay run. Slides were incubated at 35 °C for 40 min in a humidified environment, washed in 1/10 PBS, and air-dried. A fluoresceinisothiocyanate (FITC)-labelled goat anti-human immunoglobulin IgG (H + L) (Kirkegaard & Perry Laboratories, Gaithersburg, USA) diluted at 1:1000 was then spotted on each well, and slides incubated for a further 35 °C for 40 min. Following the final washing, the slides were air-dried, covered and stored in a dark environment at 4 °C until read.
Each well was visualized by fluorescence microscopy to the end-point dilution, with a minimum dilution of 1:128 required to deem a sample as reactive. Readings were repeated by a second independent observer to control bias, with a third independent observer recruited to resolve any discrepancies.
Exponentially increasing dilutions were standardized to a linear scale and rickettsial exposure definitively attributed to participants with a preferential serological reactivity at a minimum four-fold dilution difference against one organism compared to the other. Patient samples that tested within this limit were not allocated to a group and were thus classified as indeterminate samples representing mixed infections, reactivity from other related rickettsiae or older infections with lower serological reactivities.
Data analysis
Results were entered and collected using a spreadsheet application (Libreoffice calc). R statistics software was used to read and analyze the data. Animal exposures were grouped into categories (companion, large and exotic) and location was designated metropolitan or rural based on distance of the participant from the centre of a major city (50 km from Sydney, Melbourne, Brisbane, 40 km from Perth, Adelaide, 30 km from Canberra, Hobart, Darwin). The distribution of the participants was compared with that previously determined by the Australian Bureau of Statistics (ABS) in 2015 [22].
Exploratory analyses were performed using the epiR and epitools packages, with comparisons made between patients serologically testing preferentially to R. felis or R. typhi, as well as a category for general rickettsial reactivity including either preferentially reactive sera as well as indeterminate sera. Univariate analyses using probability ratio analyses were compiled from information on risk factors as determined by the questionnaire, and biological risk factors meeting the odds-ratio criteria with a P-value less than 0.2 were selected for inclusion in the multivariate model. Models were developed using the glm function by backwards elimination based on potential risk factors identified in the exploratory data analysis. Graphics were generated using the ggplot2 package, with map data from the GADM database.
Results
A total of 131 veterinarians were recruited for this study (Fig. 1), with a distribution based on age and state approximately representative of the distribution of veterinarians across Australia (Fig. 2) with the exception of the Northern Territory, from which no veterinarians were recruited. Younger participants (20–39 years) and middle-aged participants (40–59 years), on average, spent more hours in private practice (22 and 24 h per week, respectively) compared with 17.5 h per week for older participants.
Fig. 1.
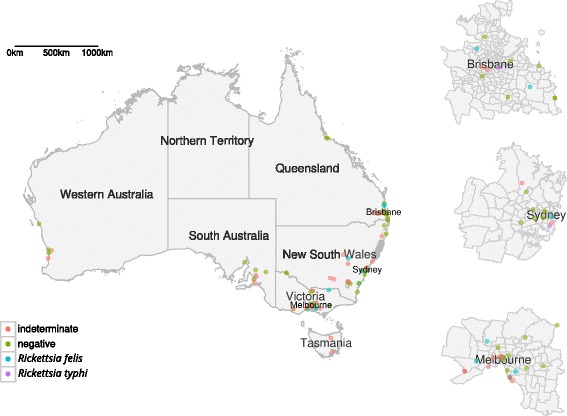
Map of exposures and their associated serological testing exposures
Fig. 2.
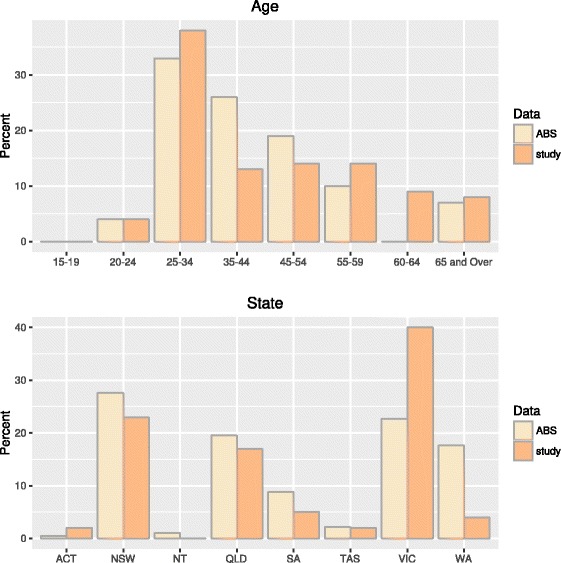
Comparisons of study participants to veterinary population as gathered by the Australian Bureau of Statistics (ABS)
Awareness of R. felis and its associated zoonotic risk was assessed, with only 72 of 127 (55.8%) veterinarians who recorded a response to this question stated they were aware of the organism. While the majority of the 127 respondents to the question (122 or 96.1%) were of the opinion that fleas posed a risk to animals, only 65 (51.2%) thought that fleas also posed a risk to humans. Of the 73 Rickettsia-exposed positive participants, 27 of 71 respondents (38.0%) recorded no recent (within the last 3 months) flea bites.
IFAT attributed twenty-one (16.0%) participants to R. felis exposure and six (4.6%) to R. typhi exposure from a specifically preferential reaction to the respective antigen. Forty-six (35.1%) veterinarians had a serological reaction to either antigen at a level which was within two serial dilutions to one another, and so a diagnosis was not able to be definitively made based solely on serology. Fifty-eight (44.3%) tested negative by IFAT, suggesting no recent exposure to either organism.
Risk factors
Univariate analyses indicated that sex, metropolitan/rural location and outdoor activity were not significant for exposure to R. felis P > 0.2. Exposure to companion animals was not a significant predictor of R. felis exposure when tested in isolation (P = 1; OR: 1.27, 95% CI: 0.25–6.55). A confounding influence from flea-treatment was noted with Mantel-Haenszel testing: [t = 0.006, P = 0.938 (OR (crude): 1.26, 95% CI: 0.24–6.49; OR (M-H): 1.91, 95% CI: 0.35–10.31; crude: M-H: 0.66)]. Once adjusted, a non-significant but stronger trend was observed amongst veterinarians that had contact with companion animals whilst concurrently not treating their own animals for fleas (P = 0.5; OR: 1.81, 95% CI: 0.43–7.64). Further non-significant positive trends were observed for reported disease symptoms (including rashes, headaches and fevers) potentially associated with rickettsial exposure.
Multivariate logistical regression converged for data on R. felis or R. typhi (including indeterminate) infection and R. felis infection is displayed in Tables 1 and 2; analysis on the R. typhi subset was not successful in producing a model due to low numbers of positives within our study population (Table 3). Participants were divided into categories according to age (20–39, 40–59 (reference category), 60+ years) and region [southeast including Victoria and Tasmania, northeast including Queensland, east including New South Wales and Canberra and south/west including Western Australia and South Australia (reference category)]. Older veterinarians above the age of 60 were at a significantly decreased risk of exposure to R. felis (t = -2.095, P = 0.04; OR: 0.756, 95% CI: 0.582–0.982) or generalized R. felis or R. typhi exposure (t = -2.147, P = 0.034; OR: 0.752, 95% CI: 0.579–0.975). Veterinarians recommending flea treatments to clients were also at a significantly decreased risk of exposure to generalized R. felis or R. typhi exposure (t = -2.034, P = 0.044; OR: 0.611, 95% CI: 0.38–0.982). Conversely, participants working in the southeastern Australian states of Victoria or Tasmania were at an increased risk of R. felis exposure (t = 1.808, P = 0.075; OR: 1.381, 95% CI: 0.973–1.96).
Table 1.
Multivariate risk factor analysis of exposure to Rickettsia felis or R. typhi
| Population | Exposed | Coefficient (SE) | t | P | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Constant | 0.993 (0.291) | 3.41 | 0.001 | |||
| Age | ||||||
| 20–39 years | 64 | 42 | 0.106 (0.101) | 1.051 | 0.295 | 1.112 (0.912–1.356)a |
| 40–59 years | 42 | 24 | Reference | |||
| 60+ years | 23 | 6 | -0.285 (0.133) | -2.147 | 0.034 | 0.752 (0.579–0.975) |
| State | ||||||
| SA or WA | 12 | 5 | Reference | |||
| NSW or ACT | 33 | 16 | -0.040 (0.163) | -0.244 | 0.808 | 0.961 (0.698–1.323) |
| QLD | 22 | 10 | -0.046 (0.175) | -0.263 | 0.793 | 0.955 (0.678–1.346) |
| VIC or TAS | 55 | 36 | 0.093 (0.157) | 0.591 | 0.555 | 1.097 (0.807–1.493) |
| Recommends flea treatment to clients | ||||||
| No | 5 | 5 | Reference | |||
| Yes | 123 | 66 | -0.493 (0.242) | -2.034 | 0.044 | 0.611 (0.38–0.982) |
Abbreviations: CI confidence interval, OR odds ratio, SE standard error
aInterpretation: compared with participants from the reference category (those between the ages of 40–59), after adjusting for the effect of location (state) and whether they recommended flea treatment to clients, participants aged 60+ had a 1.330 (0.752-1; CI: 1.026–1.727) times lower odds of exposure
Table 2.
Multivariate risk factor analysis of exposure to Rickettsia felis
| Population | Exposed | Coefficient (SE) | t | P | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Constant | 0.111 (0.177) | 0.625 | 0.534 | |||
| Age | ||||||
| 20–39 years | 34 | 12 | 0.033 (0.112) | 0.294 | 0.769 | 1.033 (0.83–1.286) |
| 40–59 years | 27 | 9 | Reference | |||
| 60+ years | 17 | 0 | -0.28 (0.134) | -2.095 | 0.04 | 0.756 (0.582–0.982) |
| State | ||||||
| SA or WA | 7 | 0 | Reference | |||
| NSW or ACT | 21 | 4 | 0.132 (0.185) | 0.716 | 0.477 | 1.142 (0.794–1.641) |
| QLD | 15 | 3 | 0.151 (0.194) | 0.78 | 0.438 | 1.163 (0.796–1.699) |
| VIC or TAS | 32 | 13 | 0.323 (0.179) | 1.808 | 0.075 | 1.381 (0.973–1.96) |
Abbreviations: CI confidence interval, OR odds ratio, SE standard error
Table 3.
Multivariate risk factor analysis of exposure to Rickettsia typhi
| Population | Exposed | Coefficient (SE) | t | P | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Constant | 0.182 (0.062) | 2.923 | 0.005 | |||
| Age | ||||||
| 20–39 years | 24 | 2 | -0.098 (0.086) | -1.144 | 0.257 | 0.906 (0.765–1.073) |
| 40–59 years | 22 | 4 | Reference | |||
| 60+ years | 17 | 0 | -0.182 (0.094) | -1.93 | 0.058 | 0.834 (0.693–1.003) |
Abbreviations: CI confidence interval, OR odds ratio, SE standard error
Discussion
This study is the first to demonstrate natural exposure to R. felis in 16.0%, R. typhi in 4.6% and potential exposure to either or both in a further 35.1% of Australian veterinarians. This builds upon the evidence that human exposure, as demonstrated in earlier studies in Spain [20] and Colombia [21], where R. felis is ubiquitous in areas where R. felis Cal2 has been detected in fleas, notably C. felis felis. Infection with R. felis in Australia has been previously reported in 20% of companion animal fleas in Brisbane, Sydney and Melbourne [17] and up to 36% in regional centers in Western Australia [18]. Veterinarians in clinical practice are regularly exposed to animals, particularly companion animals (cats and dogs), which may act as potential hosts for ectoparasites. Trends were also seen when observing R. felis and R. typhi reactive participants in combination, which is not surprising as both organisms share vectors and hosts [2].
In this study, R. felis exposure was noted across each of the Australian states tested with proportionally the highest participant count from Victoria. We were able to demonstrate an increased likelihood of exposure in veterinarians working within the states of Victoria and Tasmania (β = 0.323; SE = 0.179; t = 1.808; P = 0.075). Both are coastal south-eastern states and have a temperate climate. While state-based flea infection rates of R. felis have not been thoroughly investigated within Australia, it may be a reflection of higher R. felis-flea infection rates in these cooler climates, as reported previously in other parts of the world [26]. It should be noted that this study was centered on metropolitan areas, whereas previous studies on R. felis infection rates in Australia compared rural and metropolitan flea populations [9, 18].
While epizoonotic associations are typically observed in areas with a warm temperature with precipitation, high temperatures have been noted to affect the survival of fleas as well as vector-borne disease transmission [27]. Given the unusual growth characteristics of R. felis requiring an optimal growth temperature lower than that typical of other rickettsiae at 28 °C [28], a link between Victoria and Tasmania with their moderate and cool temperate climates and R. felis seropositivity is plausible.
Characterization of the epidemiology of FBSF has been complicated by the wide-spread nature of incidental exposure, compounded by a typically long-lived antibody response and non-specific symptoms characteristic of other fever-causing conditions [5]. It is clear from this study that some of these veterinarians had been exposed to R. felis in the past, but no veterinarians had reported clinical symptoms matching the disease syndrome [5] and none had been medically diagnosed. This is supportive of exposure being common but with a mild self-resolving flu-like manifestation rather than severe clinical FBSF [19, 20].
Older participants (aged 60+) had a 1.323 times lower odds of exposure to R. felis (t = -2.095, P = 0.040; OR: 0.756; CI: 0.582–0.982), 1.202 times lower to R. typhi (t = -1.93, P = 0.058; OR: 0.834, CI: 0.693–1.003), and 1.330 times lower to either R. felis or R. typhi (t = -2.147; P = 0.034; OR: 0.752; CI: 0.579–0.975) exposures, which is consistent with the findings of Hidalgo et al. in a similar study in Spain [21]. In our study, actively working older participants spent less time (17.5 h) in private practice compared with their younger and middle-aged counterparts (22 and 24 h, respectively). This lower clinical exposure is likely reflected in the changing likelihood of rickettsial exposure.
All five veterinarians who indicated that they did not recommend flea-treatment to clients were positive to rickettsial exposure (two to R. felis, three to indeterminate exposure). This result is suggestive of a 1.637 times reduction in odds of exposure to FBSF or MT for veterinarians that recommended their clients treat their pets for fleas ( = -0.493; SE = 0.242; t = -2.034; P = 0.044). Attitudes towards regular flea prophylaxis may effectively function as a reliable predictor for exposure to R. felis with intrinsic ties to the potential for exposure of the general population to flea-borne zoonoses from flea-ridden pets and animals. A potential lack of awareness of recent flea exposure and being bitten by a flea were also trends seen in the data, in which 29 Rickettsia exposed participants reported with certainty that they had not been recently bitten, highlighting that people may not realize that they have been exposed to flea bites or inhaled flea feces [29] and thus the zoonotic vector-borne organisms fleas harbor.
In our study, no statistically significant risk factors were able to be linked between exposure to either R. felis or R. typhi and contact with companion animals or with fleas. This may be a reflection of the widespread, ubiquitous exposure amongst the veterinary populace tested to these factors (e.g. companion animals and the fleas associated with them). There is an ongoing issue with R. felis serology is cross-reactivity with TG rickettsiae (e.g. R. typhi) antibodies [30] impeding acquisition of a definitive serodiagnosis.
A validated protocol used for diagnostic testing [4] was followed where serology was performed concurrently on each sample tested against both R. felis and R. typhi, with a positive result considered only when a sample tested greater or equal to two serial dilutions (a four-fold increase) of one antigen over the other. This ensured rigor of the classification of the R. felis- and R. typhi-exposed patients as high compared to previous serosurveys which utilized protocols that used lower cut-off titers [31] whilst being able to confidently classify the etiological agent [4]. Consequently, the number of participants testing positive for an indeterminate rickettsial infection was produced. It is likely that a proportion of these participants were only exposed to either R. felis or R. typhi infections or alternatively as mixed infections, where a patient may have been exposed to both R. felis and R. typhi. An improvement in the specificity of the test used (e.g. by using cross-adsorption or Western blotting) may result in clearer data on individual exposure status.
Conclusions
In Australia, veterinarians are considered at the forefront of diagnosis, treatment and prevention of zoonotic diseases from companion animals to their owners and the general public. Given the low awareness of R. felis and FBSF amongst our participants, improved education of veterinarians and in turn pet owners is needed. In turn, communication between medical, veterinary and diagnostic laboratory professions is also imperative for the diagnosis and prevention of this common zoonosis. The reported clinical cases of FBSF within the Australian population [4] coupled with high exposure amongst veterinary professionals suggests that FBSF is an important cause of undifferentiated fever conditions that may not be adequately recognized and potentially treated.
Acknowledgements
The authors would like to thank Dr Gemma Vincent (postdoctoral fellow) and Ms Chelsea Nguyen (scientist officer) at the ARRL for assistance with Rickettsia cell culture, serological assay training and IFAT validation. The paper has been sponsored by Bayer Animal Health in the framework of the 12th CVBD World Forum Symposium.
Funding
This research was funded by the Australian Research Council in partnership with Bayer Animal Health Australia and the Australian Rickettsial Reference Laboratory (ARRL).
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author [YTT]. The data are not publicly available as it contains information that could compromise research participant privacy/consent.
Authors’ contributions
YTT administered surveys, performed sample collection, performed culture and IFAT, collated and collected data, analyzed and interpreted data, and drafted the manuscript. SFH, MS and SG assisted with analysis and interpretation of data and revising the article critically for important intellectual content. RR assisted with study design and revising the article critically for important intellectual content. JS and RT assisted with study design, analysis and interpretation of data and revising the article critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent for deidentified data to be published was provided by each participant in this study.
Ethics approval and consent to participate
Ethics approval for this study was granted through the University of Melbourne Research Ethics Committee (ID: 1443412.1). Written informed consent was obtained from participants in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ARRL
Australian Rickettsial Reference Laboratory
- Cal2
Rickettsia felis URRWXCal2
- CI
confidence interval
- FBSF
flea-borne spotted fever
- FITC
fluoroscein isothiocyanate
- GADM
global administrative regions
- IFAT
immunofluorescence antibody testing
- IgG
immunoglobulin G
- Inf
infinity
- M-H
Mantel-Haenszel correction
- MT
murine typhus
- PBS
phosphate buffered saline
- SE
standard error
- SFG
spotted fever group
- TG
Typhus group
- β
beta coefficient
Contributor Information
Yen Thon Teoh, Email: yen.teoh@unimelb.edu.au.
Sze Fui Hii, Email: SzeFui.Hii@barwonhealth.org.au.
Mark A. Stevenson, Email: mark.stevenson1@unimelb.edu.au
Stephen Graves, Email: graves.rickettsia@gmail.com.
Robert Rees, Email: bob.rees@hotmail.com.
John Stenos, Email: johns@barwonhealth.org.au.
Rebecca J. Traub, Email: rebecca.traub@unimelb.edu.au
References
- 1.Oteo JA, Portillo A, Santibanez S, Blanco JR, Perez-Martinez L, Ibarra V. Cluster of cases of human Rickettsia felis infection from southern Europe (Spain) diagnosed by PCR. J Clin Microbiol. 2006;44:2669–71. doi: 10.1128/JCM.00366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreifer ME, Sacci JB, Taylor JP, Higgins JA, Azad AF. Murine Typhus: Updated roles of multiple urban components and a second typhuslike Rickettsia. J Med Entomol. 1994;31:681–5. doi: 10.1093/jmedent/31.5.681. [DOI] [PubMed] [Google Scholar]
- 3.Bhengsri S, Baggett HC, Edouard S, Dowell SF, Dasch GA, Fisk TL, et al. Sennetsu neorickettsiosis, Spotted fever group, and typhus group rickettsioses in three provinces in Thailand. Am J Trop Med Hyg. 2016;95:43–9. doi: 10.4269/ajtmh.15-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teoh YT, Hii SF, Graves S, Rees R, Stenos J, Traub RJ. Evidence of exposure to Rickettsia felis in Australian patients. One Health. 2016;2:95–8. doi: 10.1016/j.onehlt.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S, et al. Rickettsia felis infection in febrile patients, Western Kenya, 2007-2010. Emerg Infect Dis. 2012;18:328–31. doi: 10.3201/eid1802.111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46:723–36. doi: 10.1603/033.046.0402. [DOI] [PubMed] [Google Scholar]
- 7.Abdad MY, Stenos J, Graves S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J. 2011;4:7168. doi: 10.3402/ehtj.v4i0.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed R, Paul SK, Hossain MA, Ahmed S, Mahmud MC, Nasreen SA, et al. Molecular detection of Rickettsia felis in humans, cats, and cat fleas in Bangladesh, 2013-2014. Vector Borne Zoonotic Dis. 2016;16:356–8. doi: 10.1089/vbz.2015.1886. [DOI] [PubMed] [Google Scholar]
- 9.Hii S-F, Abdad MY, Kopp SR, Stenos J, Rees RL, Traub RJ. Seroprevalence and risk factors for Rickettsia felis exposure in dogs from southeast Queensland and the Northern Territory. Australia. Parasit Vectors. 2013;6:159. doi: 10.1186/1756-3305-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peniche-Lara G, Ruiz-PiñA HA, Reyes-Novelo E, Dzul-Rosado K, Zavala-Castro J. Infection by Rickettsia felis in opossums (Didelphis sp.) from Yucatan, Mexico. Rev Inst Med Trop São Paulo. 2016;58:32. doi: 10.1590/S1678-9946201658032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedincamp J, Foil LD. Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouche) J Vector Ecol. 2002;27:96–101. [PubMed] [Google Scholar]
- 12.Graves S, Stenos J. Rickettsioses in Australia. Ann NY Acad Sci. 2009;1166:151–5. [DOI] [PubMed]
- 13.Hii S-F, Kopp SR, Thompson MF, OLeary CA, Rees RL, Traub RJ. Molecular evidence of Rickettsia felis infection in dogs from Northern Territory, Australia. Parasit Vectors. 2011;4:1756–3305. doi: 10.1186/1756-3305-4-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian Companion Animal Council. Contribution of the pet care industry to the Australian economy. Kew East: Rockwell Communications; 2010.
- 15.Hii SF, Kopp SR, Abdad MY, Thompson MF, O’Leary CA, Rees RL, et al. Molecular evidence supports the role of dogs as potential reservoirs for Rickettsia felis. Vector Borne Zoonotic Dis. 2011;11:1007–12. doi: 10.1089/vbz.2010.0270. [DOI] [PubMed] [Google Scholar]
- 16.Šlapeta J, King J, McDonell D, Malik R, Homer D, Hannan P, et al. The cat flea (Ctenocephalides f. felis) is the dominant flea on domestic dogs and cats in Australian veterinary practices. Vet Parasitol. 2011;180:383–8. doi: 10.1016/j.vetpar.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Barrs V, Beatty J, Wilson B, Evans N, Gowan R, Baral R, et al. Prevalence of Bartonella species, Rickettsia felis, haemoplasmas and the Ehrlichia group in the blood of cats and fleas in eastern Australia. Aust Vet J. 2010;88:160–5. doi: 10.1111/j.1751-0813.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 18.Schloderer D, Owen H, Clark P, Stenos J, Fenwick SG. Rickettsia felis in fleas, Western Australia. Emerg Infect Dis. 2006;12:841. doi: 10.3201/eid1205.051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams M, Izzard L, Graves SR, Stenos J, Kelly JJ. First probable Australian cases of human infection with Rickettsia felis (cat-flea typhus) Med J Aust. 2011;194:41. doi: 10.5694/j.1326-5377.2011.tb04145.x. [DOI] [PubMed] [Google Scholar]
- 20.Bernabeu-wittel M, Del Toro MD, Nogueras MM, Muniain MA, Cardeñosa N, Márquez FJ, et al. Seroepidemiological study of Rickettsia felis, Rickettsia typhi, and Rickettsia conorii infection among the population of southern Spain. Eur J Clin Microbiol Infect Dis. 2006;25:375–81. doi: 10.1007/s10096-006-0147-6. [DOI] [PubMed] [Google Scholar]
- 21.Hidalgo M, Montoya V, Martínez A, Mercado M, De la Ossa A, Vélez C, et al. Flea-borne rickettsioses in the north of Caldas province, Colombia. Vector Borne Zoonotic Dis. 2013;13:289–94. doi: 10.1089/vbz.2012.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian Bureau of Statistics . Labour Force Survey. Canberra: ABS; 2015. [Google Scholar]
- 23.Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am J Trop Med Hyg. 2005;73:1083–5. [PubMed] [Google Scholar]
- 24.Sahni SK, Narra HP, Sahni A, Walker DH. Recent molecular insights into rickettsial pathogenesis and immunity. Future Microbiol. 2013;8:1265–88. doi: 10.2217/fmb.13.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM. Flea-borne rickettsioses: ecologic considerations. Emerg Infect Dis. 1997;3:319–28. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horta MC, Ogrzewalska M, Azevedo MC, Costa FB, Ferreira F, Labruna MB. Rickettsia felis in Ctenocephalides felis felis from five geographic regions of Brazil. Am J Trop Med Hyg. 2014;91:96–100. doi: 10.4269/ajtmh.13-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vectorborne diseases. Am J Prev Med. 2008;35:436–50. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Raoult D, La Scola B, Enea M, Fournier P-E, Roux V, Fenollar F, et al. A flea-associated Rickettsia pathogenic for humans. Emerg Infect Dis. 2001;7:73. doi: 10.3201/eid0701.010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wedincamp J, Foil LD. Infection and seroconversion of cats exposed to cat fleas (Ctenocephalides felis Bouche) infected with Rickettsia felis. J Vector Ecol. 2000;25:123–6. [PubMed] [Google Scholar]
- 30.Adams J, Schmidtmann E, Azad A. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a Rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43:400–9. doi: 10.4269/ajtmh.1990.43.400. [DOI] [PubMed] [Google Scholar]
- 31.Nogueras MM, Pons I, Sanfeliu I, Sala M, Segura F. Serosurvey of Rickettsia typhi and Rickettsia felis in HIV-infected patients: R. typhi and R. felis in HIV-patients. Microbiol Immunol. 2014;58:257–9. doi: 10.1111/1348-0421.12138. [DOI] [PubMed] [Google Scholar]


