Abstract
Aims
Case reports and small case series suggest increased central nervous system (CNS) toxicity, especially convulsions, after overdose of mefenamic acid, compared with other nonsteroidal anti‐inflammatory drugs (NSAIDs), although comparative epidemiological studies have not been conducted. The current study compared rates of CNS toxicity after overdose between mefenamic acid, ibuprofen, diclofenac and naproxen, as reported in telephone enquiries to the UK National Poisons Information Service (NPIS).
Methods
NPIS telephone enquiries related to the four NSAIDs, received between January 2007 and December 2013, were analysed, comparing the frequency of reported CNS toxicity (convulsions, altered conscious level, agitation or aggression, confusion or disorientation) using multivariable logistic regression.
Results
Of 22 937 patient‐specific telephone enquiries, 10 398 did not involve co‐ingestion of other substances (mefenamic acid 461, ibuprofen 8090, diclofenac 1300, naproxen 547). Patients taking mefenamic acid were younger and more commonly female than those using other NSAIDs. Those ingesting mefenamic acid were more likely to experience CNS toxicity than those ingesting the other NSAIDs combined [adjusted odds ratio (OR) 7.77, 95% confidence interval (CI) 5.68, 10.62], especially convulsions (adjusted OR 81.5, 95% CI 27.8, 238.8). Predictors of CNS toxicity included reported dose and age, but not gender.
Conclusions
Mefenamic acid overdose is associated with a much larger and dose‐related risk of CNS toxicity, especially convulsions, compared with overdose of other NSAIDs. The benefit–risk profile of mefenamic acid should now be re‐evaluated in light of effective and less toxic alternatives.
Keywords: CNS toxicity, convulsions, mefenamic acid, nonsteroidal anti‐inflammatory drug, overdose, poisoning
What is Already Known about this Subject
Central nervous system (CNS) toxicity has been reported following severe nonsteroidal anti‐inflammatory drug (NSAID) overdose. Small case series and case reports have suggested that overdose with mefenamic acid is commonly followed by CNS toxicity, especially convulsions.
What this Study Adds
The study demonstrates that mefenamic acid overdose carries a significantly higher risk of dose‐related CNS toxicity compared with other commonly used NSAIDs, and that the risk of convulsion is substantially higher.
Tables of Links
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Mefenamic acid is a fenamate nonsteroidal anti‐inflammatory drug (NSAID), licensed since the early 1960s as a prescription‐only medicine in the UK and Europe 4 and often used for the treatment of dysmenorrhoea and heavy menstrual bleeding (HMB). Although this use was supported by clinical trials 5, 6, 7, and by recommendations in published guidelines 8, 9, more recent evidence has not suggested clinically important benefits for mefenamic acid compared with other NSAIDs for either indication 10, 11, 12. While NSAIDs are cited as treatment options in current guidance, mefenamic acid is not specifically recommended for treating dysmenorrhoea 13 or HMB 14.
The propensity of mefenamic acid overdose to induce central nervous system (CNS) toxicity, particularly convulsions, has been reported in a number of case reports and small case series 15, 16, 17, 18, 19, 20, 21, 22 but the differential neurotoxicity of individual NSAIDs, in overdose and normal use, and the influence of other risk factors on the development of neurotoxicity have not previously been reported.
It is now acknowledged that, when considering the risks and benefits of medicines, the risks produced by overdose should be taken into account, as well as those associated with normal therapeutic use. In the European Union, Directive 2010/84/EU now requires that member states operate a pharmacovigilance system that collects information on suspected adverse reactions from use of medicines outside of (as well as within) the terms of their marketing authorization, including those occurring after overdose 23. One potentially valuable way of doing this is by using data collected by poisons centres when they provide advice on cases of suspected poisoning.
The present study was therefore performed to compare the frequency of neurological toxicity between mefenamic acid and other commonly used NSAIDs following overdose using data collected routinely by poisons centres in the UK, and to examine the effects on this of age, gender and reported ingested dose.
Methods
The National Poisons Information Service (NPIS) is commissioned by Public Health England to provide information and clinical advice for registered healthcare professionals throughout the UK on all aspects of acute and chronic poisoning. For the present study, information was extracted from the clinical records of telephone enquiries to the NPIS, after full anonymization. These data included patient age and gender, reported dose and route of exposure, concomitant medication exposures and clinical features reported during the telephone enquiry. Circumstances of exposure were classified by the information scientist taking the enquiry (e.g. intentional overdose, accidental overdose including therapeutic errors, drug misuse). Where it could be established that more than one enquiry had been made about the same patient and exposure, the clinical information was consolidated into a single record. CNS toxicity was defined as any of the following: convulsions, altered conscious level, agitation, aggression, confusion or disorientation.
To standardize for doses between drugs, we calculated the ratio of reported ingested dose to the maximum daily dose, expressed in the present study as ingested‐to‐maximum dose ratio. When available, the maximum recommended daily dose used was that advised by the British National Formulary (BNF) 24 (Table 1). Mefenamic acid is not licensed for children less than 12 years of age, so there is no recommended daily dose for this group. We therefore used a maximum daily dose in mg kg−1, derived from the maximum adult dose and assuming an adult weight of 70 kg.
Table 1.
British National Formulary‐recommended maximum daily doses for mefenamic acid, ibuprofen, diclofenac and naproxen, and the toxic doses for each drug as defined on TOXBASE®. Note: The maximum daily dose for any given indication for a drug was used
| Maximum daily dose | Toxic dose (mg kg −1 ) | ||
|---|---|---|---|
| Adults (mg) | Children | ||
| Mefenamic acid | 1500 | • Less than 12 years: not licensed • 12–18 years: 1500 mg | 40 |
| Ibuprofen | 2400 | • 1–3 months: 20 mg kg−1
• 3 months‐12 years: 30 mg kg−1
• 12–18 years: 2400 mg |
100 |
| Diclofenac | 150 | • 6 months–18 years: 5 mg kg−1 | 7 |
| Naproxen | 1250 | • 1 month–2 years: 15 mg kg−1 • 2–18 years 10 mg kg−1 | 35 |
For children, when the maximum daily dose was expressed in mg kg−1 in the BNF, and when the child's weight was not documented, the weight was assumed using the age and the 50th percentile of the male or female growth charts produced by the World Health Organization and the Royal College of Paediatrics and Child Health 25.
A sensitivity analysis was conducted to assess the robustness of the results, using an alternative approach that employed the toxic threshold doses for each NSAID as listed on TOXBASE®, the online poisons information database provided for UK health professionals by the NPIS (Table 1). When available, reported drug doses were standardized by recorded weight; when this was not documented, the weight of adults was assumed to be the average weight for a male (84 kg) or female (70 kg) from the National Statistics Health Survey for England 2012 and the Welsh Health Survey 2009 26, 27. For children, the weight was calculated as described above. For a 70 kg adult, these toxic threshold doses are 1.9 (mefenamic acid) to 3.3 (diclofenac) times the maximum daily dose as recommended in the BNF. We then standardized for doses between drugs by calculating the ratio of reported ingested dose to the toxic dose, expressed in the present study as ingested‐to‐toxic dose ratio (Table 1).
When analysing the clinical manifestations of toxicity, patients reported to be exposed to other drugs were excluded. Logistic regression models were applied using IBM SPSS v.22 software to compare the odds of developing CNS toxicity between different NSAIDs. Likelihood ratio tests were used to compare models, with drug type included as a covariate to one in which it is excluded, to determine whether drug type was a significant predictor of outcome. Where significant differences were observed, pairwise comparisons were made between each of the pairs of drugs and the P‐values were adjusted using the Bonferroni correction for multiple testing. This model could not be applied to the analysis of reported cases of convulsions as the number of patients who developed convulsions was small and not all NSAIDs were associated with a case of convulsion. Instead, additional models were constructed for the CNS toxicity outcome and for convulsions in which mefenamic acid was compared with all other NSAIDs.
Multivariable logistic regression models were used to test for differences between drugs after adjusting for age, gender and ingested‐to‐toxic dose ratio. To allow for a possible quadratic relationship between age and odds of toxicity, age‐squared was added as a term in the models. Terms allowing for interactions between drug, age, gender and ingested dose were also tested for, but were not statistically significant and so not included in the final models. Patients with no data on age, gender or ingested doses available were excluded in this model.
Ethical approval is not required in the UK for surveillance studies of this type because they involve analysis of anonymized aggregated clinical information that is collected routinely as part of the NPIS clinical record.
Results
Between January 2007 and December 2013, there were 23 144 NPIS telephone enquiries relating to 22 937 separate exposures to the four NSAIDs studied. Exposures were less common for mefenamic acid (925) than for ibuprofen (17 302), diclofenac (3385) or naproxen (1325). The median age of mefenamic acid patients was younger (17 years) than those involved in enquiries about ibuprofen (23 years), diclofenac (29 years) or naproxen (32 years) and there was a significantly higher proportion of female patients in the mefenamic acid group compared with the other groups combined (P < 0.0001, Table 2). A higher proportion of mefenamic acid exposures involved intentional overdose and a lower proportion accidental overdose, including therapeutic errors, compared with the other NSAIDs studied. Acute intentional overdose was the most prevalent exposure type overall (Table 2).
Table 2.
Patient demographics and exposure types in cases of mefenamic acid, ibuprofen, diclofenac and naproxen overdose reported in enquiries to the UK National Poisons Information Service
| Mefenamic acid | Ibuprofen | Diclofenac | Naproxen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Total | 925 | 17 302 | 3385 | 1325 | |||||
| Median (interquartile range; min–max) Age, (years) | 17(14–25; 0–83) | 23(2–29; 0–98) | 29 (3–40; 0–94) | 32(20–45: 0–94) | |||||
| Gender | Female | 791 | 85.5% | 9817 | 56.8% | 1844 | 54.5% | 693 | 52.3% |
| Male | 128 | 13.8% | 7324 | 42.3% | 1513 | 44.7% | 626 | 47.2% | |
| Unknown | 6 | 0.7% | 161 | 0.9% | 28 | 0.8% | 6 | 0.5% | |
| Co‐administration | 464 | 50.20% | 9212 | 53.2% | 2085 | 61.6% | 778 | 58.7% | |
| Circumstances | Intentional | 635 | 68.6% | 7955 | 46.0% | 1790 | 52.9% | 724 | 54.6% |
| Accidental (e.g. therapeutic errors) | 244 | 26.4% | 8888 | 51.4% | 1486 | 43.9% | 548 | 41.4% | |
| Drug misuse | 1 | 0.1% | 56 | 0.3% | 6 | 0.2% | 2 | 0.2% | |
| Other/Unknown | 45 | 4.9% | 403 | 2.3% | 103 | 3.0% | 51 | 3.8% | |
| Exposure type | Acute (<1 h) | 643 | 69.5% | 11 602 | 67.1% | 2238 | 66.1% | 761 | 57.4% |
| Staggered (1–24 h) | 89 | 9.6% | 3361 | 19.4% | 447 | 13.2% | 208 | 15.7% | |
| Subacute (1 day to 1 month) | 22 | 2.4% | 1142 | 6.6% | 204 | 6.0% | 97 | 7.3% | |
| Chronic (>1 month) | 1 | 0.1% | 142 | 0.8% | 27 | 0.8% | 22 | 1.7% | |
| Acute on chronic | 5 | 0.5% | 11 | 0.1% | 8 | 0.2% | 4 | 0.3% | |
| Acute on therapeutic | 146 | 15.8% | 844 | 4.9% | 399 | 11.8% | 191 | 14.4% | |
| Other/Unknown | 19 | 2.1% | 200 | 1.1% | 62 | 1.9% | 42 | 3.2% | |
There were 10 398 exposures to one of the studied NSAIDs where co‐exposure to other drugs was not reported. In these, CNS toxicity was recorded in 3% overall (Table 3) and, after adjustment for age, gender and reported dose ingested (ingested‐to‐maximum daily dose ratio), was significantly more common with mefenamic acid than with ibuprofen [adjusted odds ratio (aOR) 11.86], diclofenac (aOR 9.02) or naproxen (aOR 3.80) (Table 4) and with the three comparator NSAIDs combined (aOR 7.77). CNS toxicity was also reported significantly more often after overdose with naproxen compared with ibuprofen (aOR 3.12) and diclofenac (aOR 2.37).
Table 3.
Central nervous system (CNS) toxic effects described by health professionals to the UK National Poisons Information Service after reported overdose of mefenamic acid, ibuprofen, diclofenac and naproxen. Patients with reported co‐exposures have been excluded. Note that some patients may experience more than one feature
| Diclofenac (n = 1300) | Ibuprofen (n = 8090) | Mefenamic acid (n = 461) | Naproxen (n = 547) | |
|---|---|---|---|---|
| CNS toxicity | 35 (2.7%) | 163 (2.0%) | 91 (19.7%) | 33 (6.0%) |
| Confusion | 1 (0.1%) | 12 (0.1%) | 4 (0.9%) | 2 (0.4%) |
| Anxiety | 1 (0.1%) | 4 (0.05%0 | 3 (0.7%) | 1 (0.2%) |
| Convulsions | 1 (0.1%) | 5 (0.06%) | 42 (9.1%) | 0 (0.0%) |
| Reduced conscious level | 18 (1.4%) | 92 (1.1%) | 30 (6.5%) | 23 (4.2%) |
| Dizziness | 15 (1.2%) | 47 (0.6%) | 12 (2.6%) | 5 (0.9%) |
| Agitation/Aggression | 2 (0.2%) | 7 (0.09%) | 16 (3.5%) | 3 (0.5%) |
Table 4.
Adjusted and unadjusted odds ratios (ORs) for the association between central nervous system (CNS) toxicity and drug exposure. Note: to analyse the association between drug exposure and convulsion, a different model was used, in which mefenamic acid was compared with all other nonsteroidal anti‐inflammatory drugs (NSAIDs) as the number of patients who developed convulsions was small and not all NSAIDs were associated with convulsion
| Unadjusted analysis (n = 10 398) | Adjusted analysis (n = 7711) | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower 95% CI | Upper 95% CI | P‐value | OR | Lower 95% CI | Upper 95% CI | P‐value | |
| CNS toxicity outcome | ||||||||
| Mefenamic acid vs. ibuprofen | 11.96 | 9.07 | 15.78 | <0.001 | 11.86 | 8.75 | 16.07 | <0.001 |
| Mefenamic acid vs. diclofenac | 8.89 | 5.92 | 13.35 | <0.001 | 9.02 | 5.73 | 14.20 | <0.001 |
| Mefenamic acid vs. naproxen | 3.83 | 2.52 | 4.54 | <0.001 | 3.80 | 2.40 | 4.49 | <0.001 |
| Naproxen vs. ibuprofen | 3.12 | 2.13 | 4.59 | <0.001 | 3.12 | 2.03 | 4.79 | <0.001 |
| Naproxen vs. diclofenac | 2.32 | 1.43 | 3.77 | 0.007 | 2.37 | 1.37 | 4.09 | 0.002 |
| Diclofenac vs. ibuprofen | 1.35 | 0.93 | 1.95 | 0.116 | 1.32 | 0.86 | 2.01 | 0.204 |
| Mefenamic acid vs. all others | 9.79 | 7.52 | 12.74 | <0.001 | 7.77 | 5.68 | 10.62 | <0.001 |
| Convulsions outcome | ||||||||
| Mefenamic acid vs. all others | 900.0 | 34.7 | 233.2 | <0.001 | 81.5 | 27.8 | 238.8 | <0.001 |
CI, confidence interval
Convulsions were reported in 42 (9.1%) mefenamic acid enquiries, compared with five (0.1%) with ibuprofen, one (0.1%) with diclofenac and none involving naproxen. The risk of convulsions was significantly higher after mefenamic acid than the other three NSAIDs combined (aOR 81.5) (Table 4).
Following mefenamic acid overdose, reported dose was a significant predictor of both CNS toxicity and convulsions, both before and after adjustment for age and gender (P < 0.001) (Table 5). Similarly, age was associated significantly with CNS toxicity in both models, but was not a significant predictor of convulsion after adjustment. The relationship between age and CNS toxicity was quadratic, with the risk initially increasing with age before reaching a peak and decreasing (Figure 1 and Figure S1). For CNS toxicity, the odds were highest at age 21 years in the unadjusted analysis and 22 years in the adjusted analysis. For convulsions, the odds were highest at the ages of 18 years and 17 years in the unadjusted and adjusted analyses, respectively. Gender was not significantly correlated with CNS toxicity or convulsions.
Table 5.
Adjusted and unadjusted odds ratios (ORs) for the association between central nervous system (CNS) toxicity, convulsions and other independent variables in mefenamic acid overdose patients. Patients with no data available on age, gender or ingested doses were excluded. Note: CNS toxicity odds were highest at age 21 years in unadjusted analysis and 22 years in the adjusted analysis. Convulsions odds were highest at age 18 years in unadjusted and 17 years in adjusted analyses
| Unadjusted analysis (n = 461) | Adjusted analysis (n = 405) | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower 95% CI | Upper 95% CI | P‐value | OR | Lower 95% CI | Upper 95% CI | P‐value | |
| CNS toxicity | ||||||||
| Age (years) | 1.02 | 1.01 | 1.03 | <0.001 | 1.14 | 1.01 | 1.02 | 0.014 |
| Age sq (years) | 0.999 | 0.999 | 1.000 | <0.001 | 0.999 | 0.999 | 1.000 | <0.001 |
| Gender – male | (reference) | (reference) | ||||||
| Female | 2.34 | 0.97 | 5.63 | 0.058 | 1.43 | 0.52 | 3.96 | 0.492 |
| ingested‐to‐max dose | 1.10 | 1.06 | 1.14 | <0.001 | 1.09 | 1.06 | 1.13 | <0.001 |
| Convulsions | ||||||||
| Age (years) | 1.02 | 1.00 | 1.03 | 0.012 | 1.01 | 0.996 | 1.03 | 0.119 |
| Age sq (years) | 0.999 | 0.992 | 0.999 | 0.007 | .999 | 0.999 | 1.00 | 0.055 |
| Gender – male | (reference) | (reference) | ||||||
| Female | 3.11 | 0.73 | 13.23 | 0.124 | 1.23 | 0.25 | 6.03 | 0.800 |
| ingested‐to‐max dose | 1.14 | 1.10 | 1.19 | <0.001 | 1.14 | 1.09 | 1.19 | <0.001 |
CI, confidence interval; sq, square
Figure 1.
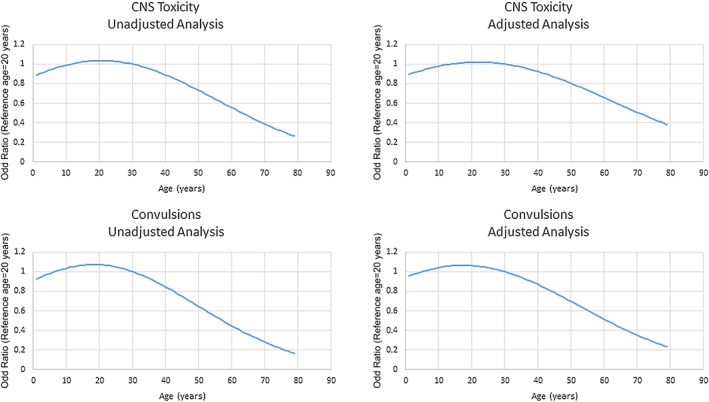
Relationship between age and odds of CNS toxicity and Convulsions. Lines represent the odds at any given age relative to a patient aged 20 years
In sensitivity analyses, the alternative method of dose adjustment, using ingested‐to‐toxic dose ratio as defined by TOXBASE®, produced results consistent with the original analyses (data not shown). Estimated ORs for differences in risk between drugs were almost identical in magnitude, with no changes in terms of statistical significance or interpretation. As in the original analysis, the ingested‐to‐toxic dose ratio was a significant predictor of both CNS toxicity and convulsions, and gender was not related to any of the outcomes. However, age was significantly associated with CNS toxicity in general, but not with convulsions.
Discussion
The study confirmed that mefenamic acid overdose is commonly associated with dose‐related CNS toxicity, especially convulsions, and that this is substantially more common than after overdose with other commonly used NSAIDs. Although intentional overdose formed a larger proportion of mefenamic acid enquiries, the average reported dose taken (as a proportion of the maximum recommended dose) was almost identical for each NSAID studied, and the difference persisted after adjustment for ingested doses.
These results are consistent with previous research in animals and humans. In mice, single large doses of mefenamic acid caused CNS stimulation, followed by incoordination, CNS depression and convulsions 28. Clinical features of CNS toxicity reported in humans range from mild drowsiness and disorientation to convulsions, coma and respiratory arrest 15, 16, 17, 18, 19, 29, 30. Convulsions usually occur 2–7 h after overdose but can occur up to 12 h after ingestion 16, 31. A retrospective review carried out by the Swiss Toxicological Information Centre examined the rates of acute overdose by a single drug between 1997 and 2010 that had resulted in at least one convulsion. Mefenamic acid overdose accounted for 16.3% of all cases. Overall, 11% of mefenamic acid patients developed convulsions, which occurred more frequently in 15–19‐year‐olds (23.9%) than in those 20 years and older (6.0%, P < 0.001) 30.
There is some evidence that the risk of developing convulsions is dose or plasma concentration related 16, 32, 33. In a prospective study of 54 patients with mefenamic acid overdose, mean plasma mefenamic acid concentrations at admission were significantly higher in patients presenting with convulsions than in those without. Most patients developing seizures had plasma mefenamic acid concentrations above a line joining 100 mg l−1 at 2 h with 5 mg l−1 at 15 h, which is substantially higher than those seen during therapeutic dosing (1–10 mg l−1) 16. However, seizures can occur in patients with mefenamic acid concentrations below this threshold line 16, 18, 19, and the lowest 4‐h mefenamic acid concentration at which convulsion has been documented was 21 mg l−1 in a 13‐year‐old girl 18.
Unpublished data from a retrospective study of 241 mefenamic overdose patients showed, the reported dose ingested was related directly to the severity of the toxicity, including CNS toxicity, and the lowest dose at which moderate or severe symptoms developed was 3.5 g 34. The smallest mefenamic acid overdose reported to cause convulsions was 2.5 g 31. Furthermore, mefenamic acid has also been linked to seizures following therapeutic doses 17, 35. While these earlier studies provide evidence of a dose‐related risk of convulsion after mefenamic acid overdose, they do not compare risk with alternative NSAIDs.
The exact mechanism by which NSAIDs induce seizures in overdose is not clear. It has been postulated that NSAIDs reduce the convulsive threshold by inhibiting cerebral prostaglandin and/or thromboxane synthesis 36. Modulation of γ‐aminobutyric acid (GABA) receptors in the CNS has also been suggested as a possible cause for lowering seizure threshold in poisoned patients 37, 38, 39. The propensity of mefenamic acid to be more neurotoxic than other NSAIDs in overdose is currently unexplained; information is lacking on the relative potency of individual NSAIDs for reducing convulsive threshold, and no comparative data are available regarding the efficiency of different NSAIDS in penetrating the blood–brain barrier.
The findings of the present study are important because of the physical risk from convulsions, including the risk of injury, aspiration and hypoxia. Sudden death may occur, although is probably rare in this context. The social impacts that convulsions may have on the individual are also important but will vary between countries. For example, the affected patient may not be able to drive for a period of time and employment may be affected for some occupations,
There were important limitations to the present study that should be considered. The number of enquiries made to the NPIS regarding a particular drug or agent, and reported in the present study, is not the same as the actual number of patients exposed. The NPIS might also be contacted more than once about the same patient, especially those with severe or prolonged clinical features. Identification and consolidation of duplicate enquiries was attempted but was not always possible and some duplicates might have been missed. Not all cases of overdose are referred to the NPIS because the responsible clinician may be confident of management, with or without reference to TOXBASE®. NPIS enquiry numbers are unlikely to correlate directly with patient presentations to hospitals because advice is less likely to be sought for patients with no or mild clinical features. Details of exposure are as initially reported by the patient and then passed on by the enquirer, and this may sometimes be unreliable. Analytical confirmation of exposure and exclusion of other potential toxins is not available as this is not performed as part of the routine care of patients with NSAID overdose. Although the NPIS attempts to follow up episodes of severe poisoning, this is often not possible, so clinical effects occurring after the enquiry may not be captured, including late‐onset convulsions, for example. NPIS data do not always identify important confounding factors, such as past history of epilepsy, alcohol abuse or head injury. These limitations, however, apply to all the NSAIDs studied and it is unlikely that systematic bias in data capture between NSAIDs would occur.
Another limitation was created by the difficulty in comparing doses between drugs. Reported doses in the context of drug overdose may be unreliable. In addition, mefenamic acid is not licensed for children; hence, no recommended daily dose is available for this population group. A further difficulty was that the weight of the patient is not always documented and had to be inferred in many cases. These limitations, however, are very unlikely to have explained the substantial differences between mefenamic acid and other NSAIDs in terms of toxicity and would not have had an effect on studying dose‐toxicity relationship for a specific NSAID. The sensitivity analysis we conducted, standardizing doses between drugs using the toxic dose thresholds provided on TOXBASE®, also gave similar results.
In spite of inherent limitations, the present study demonstrated the potential value of data collected routinely by poisons centres for assessing the safety of medicines when taken in overdose. It confirmed that the risk of CNS toxicity, especially convulsions, is increased after overdose with mefenamic acid compared with other commonly used NSAIDs.
In view of these findings, the balance between benefit and harm for mefenamic acid should now be re‐evaluated. Although previously considered an appropriate therapy for menstrual pain and bleeding 8, 9, more recent evidence does not show that mefenamic acid is more effective than other NSAIDs or that NSAIDs are more effective than alternative interventions 10, 11, 12, 13, 14. Targeting the drug at women with dysmenorrhoea or menorrhagia is of concern because the teenagers and younger adults commonly affected may also be at increased risk of self‐harm 40. As mefenamic acid provides no proven clinical advantages, alternative drugs should be prescribed to manage inflammatory conditions and menstrual problems, especially in those at higher risk of self‐harm. Mefenamic acid should only be considered if alternatives are contraindicated or not tolerated, if used at all. Regulatory authorities should reassess the benefit–risk profile of mefenamic acid, taking into account the available information on CNS toxicity with normal use as well as overdose, and consider if further measures are needed to reduce the public health risk from mefenamic acid toxicity.
Competing Interests
The authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organisation for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Figure S1 Relationship between age and log odds of CNS toxicity and Convulsions. Lines represent the odds at any given age relative to a patient age 20 years
Figure S1. Supporting info item
Kamour, A. , Crichton, S. , Cooper, G. , Lupton, D. J. , Eddleston, M. , Vale, J. A. , Thompson, J. P. , and Thomas, S. H. L. (2017) Central nervous system toxicity of mefenamic acid overdose compared with other NSAIDs: an analysis of cases reported to the United Kingdom National Poisons Information Service. Br J Clin Pharmacol, 83: 855–862. doi: 10.1111/bcp.13169.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 2015; 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krause D, Suh H‐S, Cui QL, Durafourt BA, Choi N, Bauman A, et al. The tryptophan metabolite 3‐hydroxyanthranilic acid plays anti‐inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase‐1. Am J Pathol 2011; S179: 1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang WY, Li Wan Po A. Efficacy of minor analgesics in primary dysmenorrhoea: a systematic review. Br J Obstet Gynaecol 1998; 105: 780–789. [DOI] [PubMed] [Google Scholar]
- 6. Fraser IS, McCarron G, Markham R, Robinson M, Smyth E. Long‐term treatment of menorrhagia with mefenamic acid. Obstet Gynecol 1983; 61: 109–112. [PubMed] [Google Scholar]
- 7. Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. Br Med J 1996; 313: 579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centre for Reviews and Dissemination, University of York . The management of menorrhagia. Effect Health Care Bull 1995; 1: 1–14. [Google Scholar]
- 9. National Collaborating Centre for Women's and Children's Health Heavy menstrual bleeding: clinical guideline 2007. Available at https://www.nice.org.uk/guidance/cg44/evidence/full-guideline-195071293 (last accessed 25 November 2016).
- 10. Majoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti‐inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015; 7 [online]. Available at http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001751.pub3/full (last accessed 19 January 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lethaby A, Duckitt K, Farquhar C. Non‐steroidal anti‐inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev 2013; 1: CD000400. [DOI] [PubMed] [Google Scholar]
- 12. Khajehei M, Abdali K, Tabatabaee H. The effect of mefenamic acid and naproxen on heavy menstrual bleeding: a placebo‐controlled study. S Afr J Obstet Gynaecol 2013; 19: 31–34. [Google Scholar]
- 13. National Institute for Health and Care Excellence . Clinical Knowledge Summaries – Dysmenorrhoea 2014 [online]. Available at http://cks.nice.org.uk/dysmenorrhoea (last accessed 19th January 2016).
- 14. National Institute for Health and Care Excellence . Clinical Guideline 44. Heavy menstrual bleeding: assessment and management 2007. [online]. Available at http://www.nice.org.uk/guidance/CG44/chapter/1‐Guidance (last accessed 19 January 2016).
- 15. Young R. Mefenamic acid poisoning and epilepsy. Br Med J 1979; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mood M, Proudfoot A, Critchley J, Prescott L. Mefenamic acid overdosage. Lancet 1981; 317: 1354–1356. [DOI] [PubMed] [Google Scholar]
- 17. Prescott L, Balali‐Mood M, Critchley J, Proudfoot A. Avoidance of mefenamic acid in epilepsy. Lancet 1981; 318: 418. [DOI] [PubMed] [Google Scholar]
- 18. Gössinger H, Hruby K, Haubenstock A, Jung M, Zwerina N. Coma in mefenamic acid poisoning. Lancet 1982; 320: 384. [DOI] [PubMed] [Google Scholar]
- 19. Frank J, Wightkin W, Hubner J. Acute toxicity of nonsteroidal antiinflammatory agents: seizure following a mefenamic acid overdose. Drug Intell Clin Pharm 1983; 17: 204–205. [DOI] [PubMed] [Google Scholar]
- 20. Shipton E, Müller F. Severe mefenamic acid poisoning. A case report. S Afr Med J 1985; 67: 823–824. [PubMed] [Google Scholar]
- 21. Hendrickse M. Mefenamic acid overdose mimicking brainstem stroke. Lancet 1988; 332: 1019. [DOI] [PubMed] [Google Scholar]
- 22. McKillop G, Canning G. A Case of intraveneous and oral mefenamic acid poisoning. Scott Med J 1987; 32: 81–82. [DOI] [PubMed] [Google Scholar]
- 23. European Parliament . Directive 2010/84/EU of the European Parliament and of the council. Available at http://ec.europa.eu/health/files/eudralex/vol‐1/dir_2010_84/dir_2010_84_en.pdf (last accessed 4 January 2016).
- 24. Joint Formulary Committee . British National Formulary [Online]. London: BMJ Group and Pharmaceutical Press. Available at http://www.medicinescomplete.com (last accessed 4 January 2016).
- 25. Royal College of Paediatrics and Child Health . UK‐WHO growth charts [online]. Available at www.rcpch.ac.uk/child‐health/standards‐care/nutrition‐and‐growth/uk‐who‐growth‐charts/uk‐who‐growth‐charts (last accessed 4 January 2016).
- 26. Health and Social Care Information Centre . Health Survey for England 2012. [online]. Available at http://www.hscic.gov.uk/catalogue/PUB13218 (last accessed 4th January 2016).
- 27. Welsh Assembly Government . Welsh Health Survey 2009. Available at http://wales.gov.uk/docs/statistics/2010/100915healthsurvey09en.pdf (last accessed 4 January 2016).
- 28. Winder CV, Kaump DH, Glazko AJ, Holmes EL. Experimental observations on flufenamic, mefenamic, and meclofenamic acids. Rheumatology 1966; 8 (Suppl. 1): 7–49. [DOI] [PubMed] [Google Scholar]
- 29. Smolinske S, Hall A, Vandenberg S, Spoerke D, McBride P. Toxic effects of nonsteroidal anti‐inflammatory drugs in overdose. Drug Saf 1990; 5: 252–274. [DOI] [PubMed] [Google Scholar]
- 30. Reichert C, Reichert P, Monnet‐Tschudi F, Kupferschmidt H, Ceschi A, Rauber‐Lüthy C. Seizures after single‐agent overdose with pharmaceutical drugs: analysis of cases reported to a poison center. Clin Toxicol 2014; 52: 629–634. [DOI] [PubMed] [Google Scholar]
- 31. Court H, Volans G. Poisoning after overdose with non‐steroidal anti‐inflammatory drugs. Adverse Drug React Acute Poisoning Rev 1984; 3: 1–21. [PubMed] [Google Scholar]
- 32. Thundiyil J, Kearney T, Olson K. Evolving epidemiology of drug‐induced seizures reported to a Poison Control Center System. J Med Toxicol 2007; 3: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robson RH, Balali M, Critchley J, Proudfoot A, Prescott L. Mefenamic acid poisoning and epilepsy. Br Med J 1979; 2: 1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laredo PB. Die akute Intoxikation mit Mefenaminsäure. [dissertation]. University Of Zurich; 2007.
- 35. Mines D, Novelli L. The risk of first seizure associated with mefenamic acid in women of reproductive age. Pharmacoepidemiol Drug Saf 2004; 13: S333–S334. [Google Scholar]
- 36. Steinhauser H, Hertting G. Lowering of the convulsive threshold by non‐steroidal anti‐inflammatory drugs. Eur J Pharmacol 1981; 69: 199–203. [DOI] [PubMed] [Google Scholar]
- 37. Woodward R, Polenzani L, Miledi R. Effects of fenamates and other nonsteroidal anti‐inflammatory drugs on rat brain GABAA receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 1994; 268: 806–817. [PubMed] [Google Scholar]
- 38. Yakushiji T, Shirasaki T, Akaike N. Non‐competitive inhibition of GABAA responses by a new class of quinolones and non‐steroidal anti‐inflammatories in dissociated frog sensory neurones. Br J Pharmacol 1992; 105: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halliwell R, Thomas P, Patten D, James CH, Martinez‐Torres A, Miledi R, et al. Subunit‐selective modulation of GABAA receptors by the non‐steroidal anti‐inflammatory agent, mefenamic acid. Eur J Neurosci 1999; 11: 2897–2905. [DOI] [PubMed] [Google Scholar]
- 40. Royal College of Psychiatrists . Self‐harm, suicide, and risk: helping people who self‐harm. Final report of a working group 2010. [online]. Available at http://www.rcpsych.ac.uk/files/pdfversion/cr158.pdf (last accessed 14 July 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Relationship between age and log odds of CNS toxicity and Convulsions. Lines represent the odds at any given age relative to a patient age 20 years
Figure S1. Supporting info item


