Abstract
Aims
Acute kidney injury (AKI) is a common and severe complication of cardiac surgery. There is no effective prevention or treatment. Sildenafil citrate (Revatio®, Pfizer Inc.), a phosphodiesterase type 5 inhibitor, prevents post cardiac surgery AKI in pre‐clinical studies, however its use is contraindicated in patients with symptomatic cardiovascular disease. The aim of this study is to assess the safety and pharmacokinetics of intravenous sildenafil in cardiac surgery patients.
Methods
We conducted an open label, dose escalation study with six patients per dose level. The six doses were 2.5 mg, 5 mg or 10 mg as a bolus, either alone or followed by an additional 2 h infusion of 2.5 mg sildenafil.
Results
Thirty‐six patients entered the trial, of which 33 completed it. The mean age was 69.9 years. One patient died during surgery, two others were removed from the trial before dosing (all at dose level 5 mg + 2.5 mg). The pharmacokinetic profile of sildenafil was similar to previously published studies. For a dose of 10 mg administered as a bolus followed by 2.5 mg administered over 2 h the results were AUC∞ 537 ng h ml−1, C max 189.4 ng ml−1 and t 1/2 10.5 h. The drug was well tolerated with no serious adverse events related to drug administration. Higher sildenafil doses stabilized post‐surgery nitric oxide bioavailability.
Conclusions
Pharmacokinetics of sildenafil during cardiopulmonary bypass were comparable to those of other patient groups. The drug was well tolerated at therapeutic plasma levels. These results support the further evaluation of sildenafil for the prevention of AKI in cardiac surgery.
Keywords: cardiac surgery, clinical pharmacology, dose proportionality, nitrite oxide, pharmacokinetics, sildenafil
What is Already Known about this Subject
Acute kidney injury (AKI) is a common complication of cardiac surgery. It increases mortality fourfold and there is no effective prevention or treatment.
Sildenafil citrate, a phosphodiesterase type 5 inhibitor that has clinical efficacy in diseases characterized by endothelial dysfunction prevents post‐cardiac surgery AKI in pre‐clinical studies.
Sildenafil is contraindicated in patients with severe symptomatic cardiovascular disease and intraoperative sildenafil administration has not been reported previously in cardiac surgery.
What this Study Adds
The pharmacokinetics of sildenafil in cardiac surgery patients undergoing cardiopulmonary bypass were similar to those reported in other patient groups.
Plasma levels that are known to have clinical efficacy in other conditions were well tolerated during and after cardiac surgery.
These results support the further evaluation of sildenafil as a renoprotective intervention in cardiac surgery.
Tables of Links
| TARGETS | |
|---|---|
| Enzymes 2 | GPCRs 4 |
| Nitric oxide | Lactate |
| Phosphodiesterase 5A | |
| Other protein targets 3 | |
| Liver fatty acid binding protein |
| LIGANDS |
|---|
| Sildenafil |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3, 4.
Introduction
Acute kidney injury occurs in up to one third of all patients following cardiac surgery 5, 6. It is characterized by an acute decline in kidney function and results in significant increases in postoperative complications as well as an almost fourfold increase in the risk of postoperative death 5, 6. Our understanding of the underlying processes is poor and recent systematic reviews 7, 8 have concluded that there is no effective treatment. Experimental studies have demonstrated that preservation of endogenous nitric oxide (NO) bioavailability is renoprotective in response to a variety of injurious stimuli 9, 10. Endogenous NO activity is increased by administration of the phosphodiesterase (PDE) type 5 inhibitor sildenafil citrate. This is used clinically in the treatment of erectile dysfunction (Viagra®, Pfizer) and more recently as an intravenous formulation (Revatio®, Pfizer) for pulmonary hypertension and acute right ventricular failure 11, 12. We have shown that sildenafil prevents post cardiopulmonary bypass (CPB) mediated acute kidney injury in a preclinical swine model 13, 14. We suggest that the unmet need for effective renoprotective interventions, combined with these pre‐clinical results, support the evaluation of sildenafil in clinical trials in cardiac surgery. However, sildenafil is contraindicated in patients with unstable cardiovascular disease 15. Prior to conducting a safety and efficacy trial, we considered it necessary to first establish a dose of sildenafil that is tolerated by cardiac surgery patients and compare the pharmacokinetics of this dose to the effective dose identified in previous work. We therefore evaluated the pharmacokinetic profile, safety and tolerability of sildenafil in 36 patients undergoing cardiac surgery, in an open label, dose escalation Phase I study. In exploratory analyses the study also evaluated pharmacodynamic outcomes to inform the design of a subsequent randomized Phase IIb efficacy trial.
Methods
Design overview
The REVAKI‐1 study was performed as a parallel, single‐centre, open label dose escalation trial to evaluate the clinical pharmacology of sildenafil and its primary metabolite desmethyl sildenafil in patients undergoing cardiac surgery. The primary aim of the trial was to identify a dose of sildenafil that; (i) could be safely administered to a high risk cardiac surgery population, and (ii) was potentially clinically effective. Dose escalation was performed sequentially to minimize potential risks. After each dose level, the safety profiles of 6 weeks follow‐up were reviewed by an independent Data Safety and Monitoring Committee (DSMC) prior to progression to the next dose. The small sample size and necessary lack of randomization precluded useful assessment of efficacy and therefore the study was performed without placebo control. However, we conducted pre‐specified pharmacodynamic analyses to assess the variance and feasibility of measuring likely outcomes of interest in a subsequent Phase II efficacy trial. The study protocol received ethical (13/SC/0131) and MHRA approval prior to initiation. The trial was registered at ClinicalTrials.gov (NCT02136329). The trial was sponsored by the University of Leicester and supported by Leicester Clinical Trials Unit.
Participants
Adult patients (≥18 years of age) undergoing cardiac surgery with moderately hypothermic CPB (32–34°C) and blood cardioplegia at the Glenfield Hospital in Leicester, who were identified as being at high risk of post‐surgery AKI using a clinical risk score 5, were screened to assess eligibility. The main exclusion criteria were patients in emergency or salvage procedures, patients with ejection fraction of less than 30%, or CKD Stage 5, defined as eGFR < 15 ml min−1, or renal replacement therapy. The screening procedure was performed when patients were listed for surgery. Informed consent was obtained on the day of admission.
Intervention
The active study drug, Revatio®, is the citrate salt of sildenafil, a selective PDE5 inhibitor manufactured under Good Manufacturing Practice (EU‐GMP) by Pfizer Inc., Surrey, UK. The medication was supplied in single use glass vials and as a clear, colourless, sterile, ready‐to‐use solution containing 10 mg (12.5 ml) of sildenafil. Each ml of solution contained 1.124 mg sildenafil citrate, 50.5 mg dextrose and water for injection. Eligible patients who consented to participate were consecutively allocated to the following groups:
Sildenafil 2.5 mg bolus over 10 min
Sildenafil 2.5 mg bolus over 10 min + 2.5 mg continuous infusion over 2 h
Sildenafil 5.0 mg bolus over 10 min
Sildenafil 5.0 mg bolus over 10 min + 2.5 mg continuous infusion over 2 h
Sildenafil 10.0 mg bolus over 10 min
Sildenafil 10.0 mg bolus over 10 min + 2.5 mg continuous infusion over 2 h
The bolus was started just prior to commencement of CPB. The infusion volume of the bolus dose was kept constant for all dose levels.
Clinical management of study subjects
Eligible patients received standard care preoperatively. A standard anaesthetic protocol was used: patients underwent anaesthetic induction with midazolam/propofol/fentanyl (up to 15 mcg kg−1) and short acting muscle relaxant. Anaesthetic maintenance used isoflurane/sevoflurane until commencement of cardiopulmonary bypass whereupon propofol (1%, Diprivan, 20–40 ml hr−1) maintenance was added until the end of the procedure. Target perfusion pressures (mean 70–80) were maintained initially with incremental metaraminol or phenylephrine boluses (0.5 mg) or vasodilators, and post bypass with inotropic support as necessary. Postoperative analgesia comprised regular paracetamol and morphine PCA for up to 48 h whereupon regular oral tramadol 50–100 mg was commenced.
Cardiopulmonary bypass was managed according to a standard CPB protocol. Normothermic to mild hypothermic non‐pulsatile or pulsatile CPB (32–35°C) was established using a standard venous reservoir, a roller pump, a hollow fibre oxygenator and a non‐heparin bonded circuit with target flows of 2.4–2.7 l min−1 m−2, and mean arterial blood pressure (MABP) maintained between 60 and 80 mmHg. Circuit prime typically included 1000 ml ringers lactate, 500 ml gelofusin, mannitol 20% and 5000 i.u. heparin. Intermittent antegrade/retrograde cold blood cardioplegic arrest was performed. Haematocrit was maintained at >23. Target activated clotting time of >400 s was achieved with heparin (300 μ kg−1 as a loading dose) for bypass. Heparin reversal was achieved with the administration of protamine sulphate in a 1:1 ratio as per standard practice.
Postoperatively, the use of pacing, inotropes or vasopressors was at the discretion of the attending physician. Patients received medications and/or other therapies to treat adverse events as deemed necessary by the clinical team. Concomitant medications and/or therapy that became necessary during the study and any changes in concomitant medication and/or therapy were recorded.
Research procedures
Blood samples for pharmacokinetic analysis were drawn 15, 30, 45 min, 2, 4, 6, 12, 24 and 48 h after start of the intervention. Plasma was separated and stored at −80°C until transported to a central analytical facility (Covance Laboratories, Harrogate, UK) for measurement of sildenafil and its principal metabolite desmethyl sildenafil. Samples for serum NO bioavailability were obtained at start of infusion, end of infusion, 15, 30, 45 and 60 min, 2, 4, 6, 12 h after the end of bolus infusion. MABP, serum creatinine measurements and urine sampling for pharmacodynamics were performed prior to the surgery and 6, 24 and 48 h after start of treatment. Additionally, serum liver function tests and vital signs were recorded to assess safety. Adverse events were monitored up to 6 weeks post discharge.
Pharmacokinetic endpoints
The key pharmacokinetic endpoints were AUC0‐tz (area under the curve until last quantifiable concentration), AUC∞ (AUC extrapolated to infinity using the estimated terminal half‐life) and C max (maximum observed concentration) of sildenafil and desmethyl sildenafil. Additionally, standard kinetic endpoints such as total body clearance and volume of distribution were determined. All endpoints were derived using an algorithm which determines the terminal half‐life using an R 2 optimization 16. If the AUC0‐tz was <80% of AUC∞, the data of pharmacokinetic parameters, which depend on the estimated pharmacokinetic half‐life (such as AUC∞), were removed from subsequent analyses.
Pharmacodynamic endpoints
To assist with the design of any subsequent Phase II trial, we selected pharmacodynamic endpoints that could potentially be measures of the following:
efficacy: serum creatinine measured using Siemens Advia 2400 Chemistry System (Siemens, Frimley, UK), urine neutrophil gelatinase associated lipocalin (NGAL, BioPorto ELISA, Hellerup, DK) and liver fatty acid binding protein (L‐FABP, ELISA Argutusmed, Ireland);
safety: troponin (ENZO life Sciences ELISA, Exeter, UK), lactate measured on the Siemens RapidPoint 500 blood gas analyser, mean arterial blood pressure (MABP) measured via a 12Fr Vygon catheter placed in the left radial artery; and
mechanism: NO bioavailability (R&D Systems colorimetric nitric oxide assay kit, Abingdon, Oxford, UK).
Statistical considerations
The sample size for this trial was not based on a power calculation. Six patients per group was deemed adequate for a preliminary assessment of safety and pharmacokinetics of sildenafil and exploratory evaluation of pharmacodynamics. The primary analysis was the evaluation of dose proportionality of the key pharmacokinetic endpoints. This analysis was first performed separately for doses levels B: 1, 3, 5 and C: 2, 4, 6, because the latter consisted of an additional 2 h continuous infusion. Additionally, a comparison of the dose levels 2 and 3 (2.5 + 2.5 mg vs. 5.0 mg) was performed in exploratory fashion, in order to evaluate whether a combined dose proportionality analysis would be justified. The dose proportionality analyses were performed using a power model on log‐transformed AUC and C max with log‐transformed dose as covariate. In each of these analyses, the slope and its 90% confidence interval was determined 17, 18. The analysis was performed separately for dose levels 1, 3, 5 and 2, 4, 6. In addition, a global model was evaluated for all dose levels, in which a global slope but separate intercepts for levels B and C were estimated.
For pharmacodynamics measurements, summary measures (such as the AUC of the change from baseline, the maximum and the minimum over time) were determined and a potential relationship to increasing doses of sildenafil was explored graphically as well as using a linear model. The statistical model included the actual dose of sildenafil and the baseline value of the measurement. A potential signal for a dose dependency was assumed if P < 0.05 (exploratory significance). No multiplicity adjustment was planned because of the exploratory nature of the trial.
For all other endpoints and measurements, descriptive statistics (means + standard deviations (SD) or counts + frequencies) were derived and graphical presentations provided as appropriate.
It was also planned to perform an exploratory comparison to the pharmacokinetic endpoints previously established in healthy volunteer trials 19, 20, 21 and a population pharmacodynamics study 22, 23.
No hypothesis was formally tested in this trial. The analysis was performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The analysis plan was finalized before the data were transferred to the trial statistician.
Results
Participants
In total, 36 patients entered the study of which 33 patients completed the trial as planned, while 3 patients (all in dose level 4) prematurely discontinued. Of these, one died during surgery (haemorrhage) and the other two were withdrawn before start of surgery (one received nitrates on the morning of surgery, the second because of cardiac arrest and stroke before start of treatment). The discontinued patients were not replaced. All patients who completed the trial were followed up for at least 35 days. Hence 34 patients were included in the safety analysis, 33 patients in all other analyses. All patients were Caucasian. The demographic characteristics, baseline and operative characteristics by dose level are presented in Table 1. The majority of the patients were male, with a mean age of 70 years (SD 7.9) and a mean weight of 88 kg (SD 13.2). Medical history included previous cardiac catheter in 28 patients, peripheral vascular disease in 10 patients and unstable angina in 9 patients. Twenty‐nine patients (81%) received concomitant anticoagulants. Twenty‐five received aspirin preoperatively. Six patients had received warfarin, five patients clopidogrel and three patients heparin preoperatively.
Table 1.
Demographic, baseline and operative characteristics of enrolled patients by dose level (mean ± SD or frequencies)
| Dose | 2.5 | 2.5 + 2.5 | 5.0 a | 5.0 + 2.5 | 10.0 | 10.0 + 2.5 | Total |
|---|---|---|---|---|---|---|---|
| Total | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 36 (100%) |
| Male | 5 (83%) | 6 (100%) | 5 (83%) | 4 (67%) | 6 (100%) | 3 (50%) | 29 (81%) |
| Age (years) Mean (SD) | 66.3 (12.8) | 65.2 (6.3) | 74.0 (5.4) | 70.7 (6.3) | 69.7 (7.5) | 73.5 (4.9) | 69.9 (7.9) |
| Weight (kg) Mean (SD) | 90.3 (13.3) | 87.7 (12.7) | 93.4 (12.1) | 86.3 (18.4) | 89.0 (6.7) | 84.3 (17.1) | 88.4 (13.2) |
| BMI (kg m −2 ) Mean (SD) | 31.5 (2.3) | 28.9 (3.3) | 32.2 (5.5) | 29.8 (2.6) | 29.0 (1.8) | 31.1 (6.1) | 30.4 (3.9) |
| AKI risk score (%) Mean (SD) | 28.5 (4.8) | 25.5 (11.6) | 33.5 (14.6) | 27.0 (5.6) | 19.7 (3.5) | 23.8 (7.4) | 26.3 (9.2) |
| Current smoker | 3 (50%) | 2 (33%) | 1 (17%) | 0 | 0 | 0 | 6 (17%) |
| Diabetes | 2 (33%) | 2 (33%) | 0 | 2 (33%) | 0 | 1 (17%) | 7 (19%) |
| Pulmonary disease | 2 (33%) | 1 (17%) | 0 | 1 (17%) | 0 | 0 | 4 (11%) |
| Neurological disease | 3 (50%) | 1 (17%) | 2 (33%) | 1 (17%) | 0 | 0 | 7 (19%) |
| Peripheral vascular disease | 1 (17%) | 3 (50%) | 3 (50%) | 2 (33%) | 1 (17%) | 0 | 10 (28%) |
| Estimated glomerular filtration rate (ml min −1 /1.73 m 2 ) Mean (SD) | 79.2 (17.1) | 78.9 (13.2) | 109.5 (64.2) | 91.4 (25.5) | 77.7 (14.5) | 81.3 (23.0) | 86.3 (31.6) |
| Haemoglobin (g dl −1 ) Mean (SD) | 13.8 (2.0) | 13.0 (2.6) | 13.4 (1.2) | 12.1 (2.0) | 14.3 (0.8) | 13.0 (1.6) | 13.3 (1.8) |
| Serum creatinine (μmol l −1 ) Mean (SD) | 82.0 (12.0) | 86.0 (14.0) | 75.0 (34.0) | 73.0 (25.0) | 87.0 (14.0) | 75.0 (10.0) | 79.7 (20.1) |
| Previous cardiac surgery | 0 | 0 | 0 | 2 (33%) | 0 | 0 | 2 (6%) |
| Previous myocardial infarction | 2 (33%) | 1 (17%) | 0 | 1 (17%) | 1 (17%) | 0 | 5 (14%) |
| NYHA | |||||||
| Class I | 2 (33%) | 3 (50%) | 1 (17%) | 0 | 0 | 2 (33%) | 8 (22%) |
| Class II | 4 (67%) | 2 (33%) | 4 (67%) | 5 (83%) | 6 (100%) | 2 (33%) | 23 (64%) |
| Class III | 0 | 1 (17%) | 1 (17%) | 1 (17%) | 0 | 2 (33%) | 5 (14%) |
| CCS | |||||||
| Class 0 | 2 (33%) | 0 | 4 (80%) | 2 (33%) | 3 (50%) | 3 (50%) | 4 (67%) |
| Class I | 3 (50%) | 2 (33%) | 1 (20%) | 2 (33%) | 2 (33%) | 3 (50%) | 2 (33%) |
| Class II | 1 (17%) | 2 (33%) | 0 | 2 (33%) | 0 | 3 (50%) | 0 |
| Class III | 0 | 2 (33%) | 0 | 0 | 1 (17%) | 3 (50%) | 0 |
| Surgical procedure | |||||||
| CABG | 1 (17%) | 5 (83%) | 4 (67%) | 2 (40%) | 1 (17%) | 2 (33%) | 15 (43%) |
| Valve | 4 (67%) | 0 | 1 (17%) | 2 (40%) | 2 (33%) | 3 (50%) | 12 (34.3%) |
| Valve + CABG | 1 (17%) | 0 | 1 (17%) | 0 | 2 (33%) | 1 (17%) | 5 (14.3%) |
| Major aortic procedure | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 1 (17%) | 0 | 2 (40%) | 1 (17%) | 0 | 4 (11.1%) |
| Cardiopulmonary bypass time (min) | 95.0 (27.2) | 102.0 (35.2) | 100.2 (18.5) | 190.0 (151) | 107.3 (28.7) | 100.8 (16.9) | 114.3 (65.7) |
| Cross clamp time (min) | 82.6 (34.9) | 53.2 (12.1) | 59.7 (22.4) | 71.8 (46.7) | 70.7 (21.0) | 68.2 (26.7) | 67.1 (27.8) |
One patient without CCS classification
Safety
Except for dose level 5 mg + 2.5 mg, all patients completed the trial as planned. In total, 15 adverse events were recorded in 13 patients; seven of these events were serious and with severe intensity (Table 2). Events which have been assessed as being related to or possibly related to the drug are seizures with severe intensity in one patient in the 2.5 mg group (possibly related), and three cases of mild hypotension in the highest dose level (related). In Group 4, two of the patient withdrawals (preoperative administration of nitrates and preoperative stroke) occurred preoperatively and were not drug related. Another patient was withdrawn following severe intraoperative haemorrhage. This was attributed to surgical trauma during redo‐sternotomy and was not drug related.
Table 2.
Adverse Events Summary
| 2.5 | 2.5 + 2.5 | 5.0 | 5.0 + 2.5 a | 10.0 | 10.0 + 2.5 | Total | |
|---|---|---|---|---|---|---|---|
| Total | 6 (100%) | 6 (100%) | 6 (100%) | 4 (100%) | 6 (100%) | 6 (100%) | 36 (100%) |
| Patients with any AE | 4 (67%) | 1 (17%) | 2 (33%) | 1 (25%) | 0 | 5 (83%) | 13 (38%) |
| Patients with serious AEs | 2 (33%) | 1 (17%) | 1 (17%) | 1 (25%) | 0 | 1 (17%) | 6 (18%) |
| Patients with drug related AEs | 1 (17%) | 0 | 0 | 0 | 0 | 3 (50%) | 4 (12%) |
| Fatal outcome | 0 | 0 | 0 | 1 (25%) | 0 | 0 | 1 (3%) |
Two patients were withdrawn before administration of any study treatment
Pharmacokinetics and dose proportionality
The pharmacokinetic profile was in general similar to those in previous studies with intravenous administration of sildenafil. Mean plasma concentrations for sildenafil and desmethyl sildenafil during the course of the study are shown in Figure 1. Individual patient data for these values are shown in Figures S1 and S2. The descriptive statistics of the key pharmacokinetic endpoints are shown in Table 3. The comparison of exposure (AUC) between dose level 2 (2.5 + 2.5 mg) and 3 (5.0 mg) led to similar values, although no definitive conclusion of equivalence could be drawn due to the low sample size. The ratio of AUC∞ between both dose levels was 118.4% (90% CI: 82.4–170.1%) for sildenafil and 100.7% (56.4–179.7%) for desmethyl sildenafil. The C max and AUC0‐tz of both sildenafil and desmethyl sildenafil versus dose of all patients are shown in Figure 2 on the logarithmic scale. It is noted that the C max and AUC of sildenafil following 10 mg + 2.5 mg infusion are unexpectedly lower than those of the 10 mg without infusion. As this finding is not present for the metabolite desmethyl sildenafil, the reason might be that the true C max of sildenafil was missed in this sample.
Figure 1.
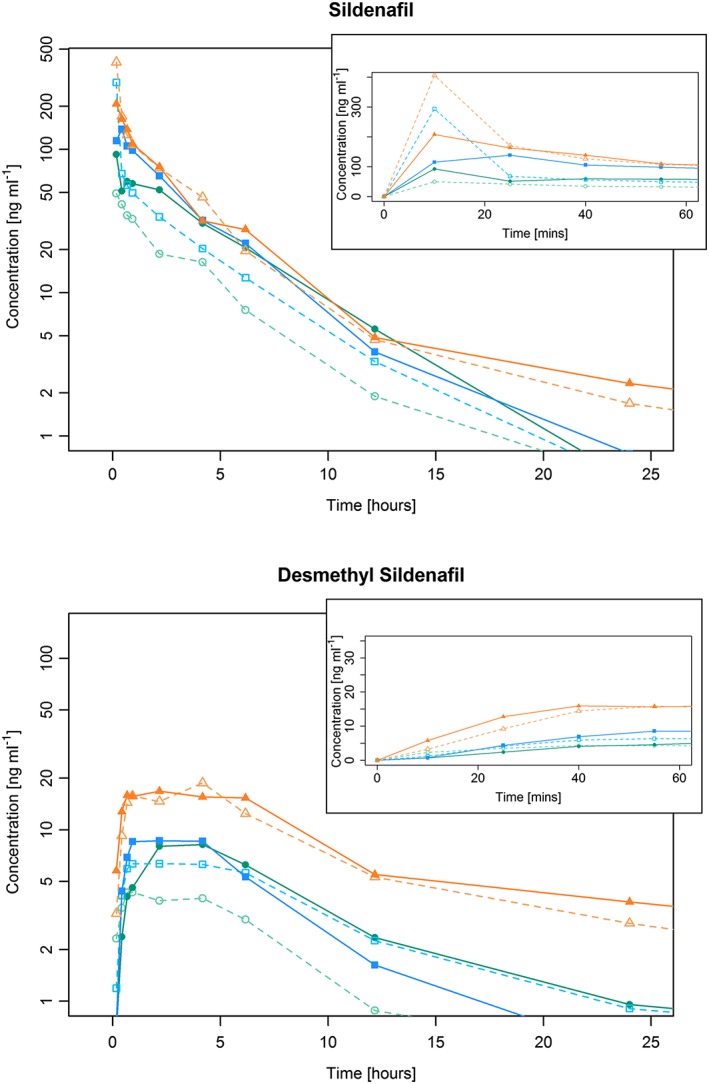
Mean plasma concentrations of (A) sildenafil and (B) desmethyl sildenafil in Groups 1–6
Table 3.
Descriptive statistics of the key pharmacokinetic endpoints of sildenafil and desmethyl sildenafil (geometric means (%gCV))
| Sildenafil | 2.5 mg | 2.5 + 2.5 mg | 5.0 mg | 5.0 + 2.5 mg | 10.0 mg | 10.0 + 2.5 mg | |
|---|---|---|---|---|---|---|---|
| N | 6 | 6 | 6 | 4a | 6 | 6 | |
| AUC ∞ | (ng h ml−1) | 152.1 (23%) | 335.4 (39%) | 283.3 (32%) | 430.3 (55%) | 568.3 (35%) | 537.5 (42%) |
| AUC 0‐tz | (ng h ml−1) | 148.8 (22%) | 331.1 (39%) | 280.0 (32%) | 392.9 (48%) | 562.7 (34%) | 528.6 (42%) |
| C max | (ng ml−1) | 48.1 (24%) | 90.4 (32%) | 175.0 (148%) | 149.5 (24%) | 364.4 (55%) | 189.4 (56%) |
| CL | (l h−1) | 16.4 (23%) | 14.9 (39%) | 17.7 (32%) | 17.4 (55%) | 17.6 (35%) | 223.3 (42%) |
| Vz | (l) | 88.4 (35%) | 76.7 (42%) | 109.2 (69%) | 101.7 (17%) | 131.1 (17%) | 262.7 (40%) |
| t 1/2 | (h) | 3.7 (21%) | 3.6 (17%) | 4.3 (42%) | 4.0 (36%) | 5.2 (45%) | 7.8 (84%) |
| Desmethyl sildenafil | 2.5 | 2.5 + 2.5 | 5.0 | 5.0 + 2.5 | 10.0 | l10.0 + 2.5 | |
|---|---|---|---|---|---|---|---|
| N | 6a | 6 | 6a | 4a | 6a | 6b | |
| AUC ∞ | (ng h ml−1) | 35.3 (40%) | 82.9 (64%) | 82.3 (44%) | 70.7 (35%) | 202.9 (56%) | 210.2 (36%) |
| AUC 0‐tz | (ng h ml−1) | 32.9 (39%) | 76.0 (62%) | 79.5 (42%) | 60.1 (40%) | 200.9 (46%) | 237.6 (47%) |
| C max | (ng ml−1) | 4.7 (24%) | 8.7 (44%) | 7.7 (27%) | 9.6 (83%) | 18.4 (33%) | 19.3 (44%) |
| t 1/2 | (h) | 3.6 (81%) | 5.8 (81%) | 7.9 (35%) | 5.5 (66%) | 8.1 (58%) | 10.5 (20%) |
| MR | 0.221 (24%) | 0.230 (32%) | 0.284 (57%) | 0.153 (86%) | 0.357 (42%) | 0.449 (36%) |
The group names represent the i.v. mg of sildenafil, either as bolus or bolus followed 2 h infusion
t 1/2 = terminal half‐life; MR: metabolic rate = ratio of desmethyl sildenafil AUC0‐tz/sildenafil AUC0‐tz
Except for C max and AUC0‐tz, one patient less (because AUC0‐tz less than 80% of AUC∞)
Except for C max and AUC0‐tz, two patients less (because AUC0‐tz less than 80% of AUC∞)
Figure 2.
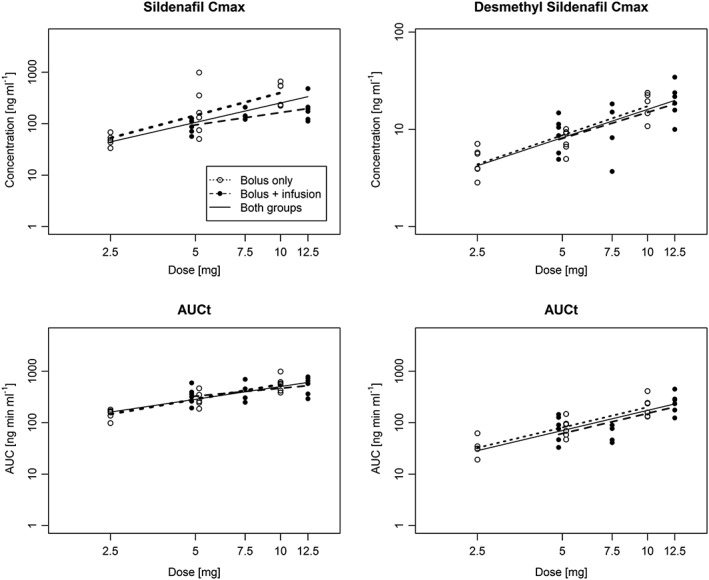
Dose proportionality analysis of AUC0‐tz and C max of sildenafil and desmethyl sildenafil. The slopes have been separately for dose levels with bolus only (open circles) and bolus + infusion (full circles). A common slope (solid line) has been estimated with separate intercepts for both subgroups (only the average intercept is presented)
The results of the dose proportionality analysis are shown in Figure 2 and Table S1. The estimated slopes are consistent with previous findings (slopes in the range of 1), but their confidence intervals are too wide to rule out deviation from proportionality. The elimination rate of sildenafil was in the range of 0.089 and 0.194 h−1, leading to a terminal half‐life between 3.6 and 7.8 h. The metabolic rate (desmethyl sildenafil AUC/sildenafil AUC) was in the range of 0.153 to 0.449. The geometric coefficient of variation (gCV) of all pharmacokinetic endpoints was relatively large in all dose levels. The gCV of the AUC of sildenafil was in the range of 23–48%, for C max even larger values have been estimated. For desmethyl sildenafil, the gCV of AUC ranged between 33 and 62%. In general the gCV was larger in the groups with the additional 2 h infusion compared with the same dose level with bolus only.
Pharmacodynamic outcomes
Results for the principal pharmacodynamics outcomes are shown in Figures 3 and 4 and Table S2. There was no evidence of a dose‐dependent change of serum creatinine or the AKI biomarker urine NGAL. There was strong exploratory evidence of a negative dose relationship of NO bioavailability whereby NO bioavailability was reduced at higher sildenafil concentrations. Further analyses indicated that peak NO bioavailability was reduced by higher doses. Examination of plots of dose vs. AUC of NO bioavailability suggests that the relationship is principally attributable to the highest dose. Without the highest dose, the dose–response was much weaker but still present. Positive dose‐dependent changes were observed for serum troponin and serum lactate levels. This was of marginal statistical significance, however, and was attributable to a wider range of values at the highest dose.
Figure 3.
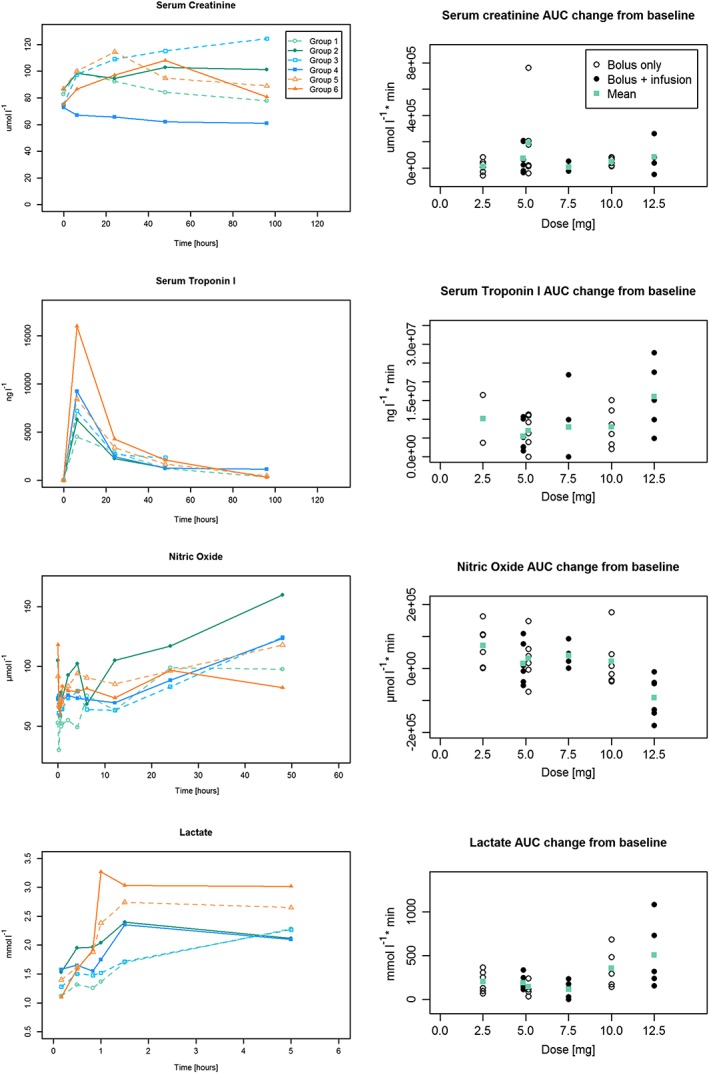
Selected pharmacodynamic outcomes. Left‐hand side: Mean values of serial measures by group. Right‐hand side: Individual AUC change from baseline and mean of each dose level. AUC could not be assessed in all patients due to missing values at individual time points
Figure 4.
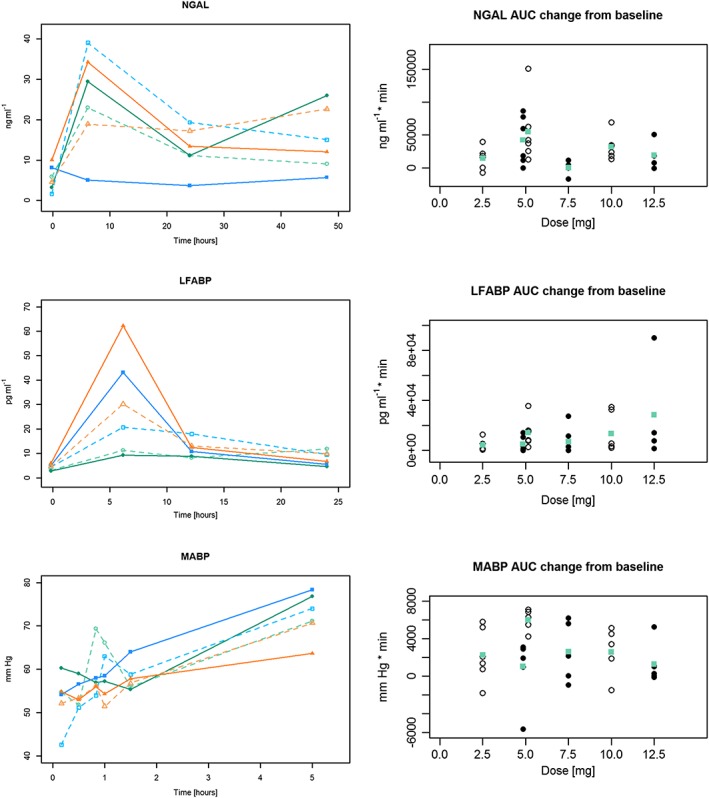
Selected pharmacodynamics outcomes. Presentation as in Figure 3
Discussion
The principal finding of this Phase I study was that sildenafil was safely tolerated up to a dose of 10 mg bolus at the commencement of CPB + 2.5 mg as a 2 h infusion during CPB. Pharmacokinetic analyses for this dose demonstrated an AUC∞ of 537 ng h ml−1 and a t 1/2 of 10.5 h.
Strengths and limitations
This is the first clinical trial to evaluate the safety and tolerability of intraoperative sildenafil administration in cardiac surgery patients. The dose escalation design led to a gradual increase in exposure, safeguarded by an independent DSMC for each escalation step to maximize patient safety. The analysis assumed that two separate escalations were performed; one with the 10 min bolus administration only, and one with a bolus followed by a 2 h infusion. This design allowed for a dedicated assessment of pharmacokinetic dose proportionality, as well as for an exploratory assessment of pharmacodynamics. The principal limitations of the study were the small sample size and the absence of randomization. This precluded any evaluation of efficacy, particularly with respect to our pharmacodynamic analyses, and for these reasons we elected to omit a placebo group. The lack of statistical power restricted our assessment of dose proportionality of pharmacokinetic outcomes due to the wide confidence intervals in each group. Differences between groups for pharmacodynamics outcomes could also have been attributable to clinical events in small numbers of patients. A final limitation was the number of patients in Group 4 (3, 50%) that did not complete the trial. Ideally the number of patients in this group should have been increased to account for these withdrawals. However, an increase in the trial sample size was not anticipated within the trial budget.
These limitations notwithstanding, the trial achieved its aims. It identified a sildenafil dose that resulted in therapeutic plasma levels comparable to those known to have clinical efficacy in other clinical settings. This dose was well tolerated by cardiac surgery patients who by definition have severe symptomatic cardiovascular disease, a conventional contraindication to sildenafil use. There is remaining uncertainty as to the safety of sildenafil in cardiac surgery patients due to the small number that have received the drug, particularly at higher doses. More accurate estimates of safety and efficacy can only be achieved by an adequately powered randomized trial.
Results in relation to previous studies
The pharmacokinetics of sildenafil in our cardiac surgery cohort were similar to those reported in previous trials with intravenous and oral administration of sildenafil, when accounting for the demographic parameters. Sildenafil clearance occurs principally in the liver and reduces with age by approximately 3.7% every 10 years 23. This has been shown to result in an increase in AUC and C max of about 80% between a group of young (30 years) and elderly (70 years) patients 21. The absolute bioavailability of oral sildenafil is approximately 40% 19, hence an oral dose of 25 mg could be considered similar to an intravenous dose of 10 mg. In a group of young subjects, 25 mg of oral sildenafil led to an AUC∞ of 361 ng h ml−1 19. When adjusted for the mean age in the current trial, we estimate that this AUC would be about 650 ng h ml−1 for 10 mg intravenous sildenafil. This is only slightly higher than the calculated AUC observed in the 10 mg group in the current study; 568.3 ng h ml−1. Using similar assumptions, based on published data 19, 21, 23, we also estimated that the clearance of 10 mg of intravenous sildenafil in an elderly population would be 22.5 l h−1. The observed values of the clearance ranged between 14.9 and 17.6 l h−1 for dose groups 1–5, slightly less than the model. In dose group 10 + 2.5 mg clearance was estimated as 23.3 l h−1. In addition, the estimated slopes in the dose proportionality analysis using a power model were relatively close to unity, as has been observed in previous studies. The global model, including all six dose levels, accounted for different intercepts, as in particular C max of sildenafil was expected to be smaller when parts of the infusion lasted 2 h. For the AUCs of sildenafil and desmethyl sildenafil, there was virtually no difference in the estimated intercepts between the two subgroups (Figure 1). Given the consistency between our results and those from previous published studies, we conclude that there appears to be only little impact of the medical status of the patients in cardiac surgery and of the surgery itself on the pharmacokinetic profile of sildenafil.
Our results indicate that intravenous sildenafil 10 mg bolus + 2.5 mg as a 2 h infusion is a safe and potentially clinically effective dose for evaluation in later trials. This dose achieved AUC similar to an oral dose of 25 mg, a clinically effective dose for patients with pulmonary hypertension 11. Furthermore, peak plasma levels at this dose were associated with transient hypotension in three patients, indicative of a direct and rapid onset of effect on endothelial function. In each case hypotension was mild, transient and responsive to short‐acting alpha adrenergic agonists. However, to minimize the risk of haemodynamic instability in future studies, we will administer the initial bolus over 20 min to reduce peak values. These were the only adverse events related to drug administration, and suggest that the drug was generally well tolerated. The half‐life for sildenafil at the 10 mg + 2.5 mg dose was 7.8 h. For the active metabolite desmethyl sildenafil t 1/2 is 10.5 h. This rapid offset of action may be important. AKI in the swine model CPB is associated with an initial acute decline in NO bioavailability at 1.5 h post‐surgery, followed by a rebound increase to supra‐normal values at 24 h. The changes correspond with both the loss of endothelial endogenous nitric oxide synthase (eNOS) expression combined with later increased expression of inducible nitric oxide synthase (iNOS) in injured renal tubules 13, 14. In swine, intravenous administration of 10 mg sildenafil at the commencement of CPB prevents AKI by preserving eNOS expression and increasing NO bioavailability immediately post‐surgery 14. In a biphasic manner, sildenafil also inhibits the subsequent expression of iNOS, and attenuates later supra‐normal values of NO 14. Our exploratory pharmacodynamic analyses also suggest a stabilizing effect of sildenafil on NO bioavailability. As with the porcine model, we observed an acute reduction in NO bioavailability in the immediate postoperative period with later increases after 24 h. Dose proportionality suggested that the late rise in NO bioavailability was reduced at higher sildenafil doses.
However, it is important not to over‐interpret these results. We did not adjust for multiple comparisons, the sample size was low, and patients were not randomized. Similar limitations apply to our evaluation of kidney injury (creatinine, NGAL); here, mean baseline AKI risk scores ranged from 19.7% to 33.5%, as well as our analyses of troponin and lactate, where findings of potential dose‐dependent changes were attributable to a small number of patients with high values at the largest dose, and P‐values indicated only marginal statistical significance. The best way to address uncertainty as to the efficacy and safety of sildenafil is to conduct a randomized trial. We propose to evaluate sildenafil at a dose of 10 mg bolus + 2.5 mg as a 2 h infusion for the prevention of AKI in cardiac surgery patients in the REVAKI‐2 trial (ISRCTN18386427).
Competing Interests
The study was funded solely by the British Heart Foundation (grants RG/13/6/29947 and CH/12/1/29419). It was supported by the Leicester Cardiovascular Biomedical Research Unit in Cardiovascular Medicine and the Leicester Clinical Trials Unit. It was not supported or funded by any other organization. Sildenafil (Revatio®) was purchased from University Hospitals of Leicester NHS Trust. GJM has served as a paid member of Advisory Boards for Abbvie (IN, USA) and Thrasos Inc. (MA, USA) in relation to the design and conduct of trials of AKI prevention interventions in cardiac surgery. AR has received research funding and travel grants from Novartis, Roche and Boehringer Ingelheim, unrelated to this trial.
The study was supported by Ms Bejal Ghosai, Miss Pia Nielson (trial coordination) and Mrs Lathishia Joel David (Research Nurse). The Trial Steering Committee comprised: Professor Gavin Murphy, University of Leicester (Chair), Veerle Verheyden, Programme Manager, Department of CV Sciences until January 2014, then Mrs Tracy Kumar, Professor Alison Goodall, Professor of Thrombosis & Haemostasis, Department of Cardiovascular Sciences, University of Leicester, Professor Nigel Brunskill, Professor of Renal Medicine, University of Leicester, Dr Karl Herbert, Senior Lecturer, Department of Cardiovascular Sciences, University of Leicester, Dr Marcin Wozniak, British Heart Funded Research Fellow CH/12/1/29419, Department of Cardiovascular Sciences, University of Leicester, Dr Rakesh Vaja, Clinical Lead for Critical Care and Consultant Anaesthetist, University Hospitals Leicester NHS Trust, Mr Anthony Locke, lay member and Mr Alan Phillips, lay member. Data Safety and Monitoring Committee members were: Professor Derek Hausenloy, University of Singapore (Chair), Professor Chris Imray, University of Warwick, Dr Nick Selby, University of Nottingham and Professor Chris Jennison, University of Bath.
Contributors
G.J.M. was the principal investigator. G.J.M., V.V. and N.B. designed the trial and wrote the protocol. M.W., N.S., W.D., V.V., T.K., R.V. and G.J.M. recruited patients, managed the trial and collected the data. M.W. and N.S. analysed samples in the laboratory. A.R., T.M. and G.J.M. wrote the analysis plan, performed the statistical analysis and drafted the manuscript. All authors reviewed and approved the manuscript.
Supporting information
Figure S1 Individual patient plasma concentrations (hatched line) of (A) aildenafil and (B) desmethyl sildenafil in Groups 1–6. Mean values (solid line) are as reported in Figure 1
Figure S2 Log individual patient plasma concentrations (hatched line) demonstrating terminal half‐life of (A) sildenafil and (B) desmethyl sildenafil in Groups 1–6
Table S1 Dose proportionality of pharmacokinetic data. Values represent estimated slopes (adjusted means and standard errors) of the dose proportionality analysis of the key pharmacokinetic endpoints of sildenafil and desmethyl sildenafil
Table S2 Linear relationship between sildenafil dose and pharmacodynamic endpoints. Values represent estimated slopes of the linear model between sildenafil dose and selected pharmacodynamics endpoints (magnitude of change of endpoint per mg sildenafil). The P‐values denote exploratory significance for the slope being different from zero
Table S3 Descriptive statistics of the key pharmacokinetic endpoints of sildenafil and desmethyl sildenafil (geometric means (%gCV))
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Ring, A. , Morris, T. , Wozniak, M. , Sullo, N. , Dott, W. , Verheyden, V. , Kumar, T. , Brunskill, N. , Vaja, R. , and Murphy, G. J. (2017) A Phase I study to determine the pharmacokinetic profile, safety and tolerability of sildenafil (Revatio®) in cardiac surgery: the REVAKI‐1 study. Br J Clin Pharmacol, 83: 709–720. doi: 10.1111/bcp.13162.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, et al. The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 2015; 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birnie K, Verheyden V, Pagano D, Bhabra B, Tilling K, Sterne JAS, et al. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care 2014; 18: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119: 495–502. [DOI] [PubMed] [Google Scholar]
- 7. Zacharias M, Mugawar M, Herbison GP, Walker RJ, Hovhannisyan K, Sivalingam P, et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev 2013; 9: CD003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel NN, Rogers CA, Angelini GD, Murphy GJ. Pharmacological therapies for the prevention of acute kidney injury following cardiac surgery: a systematic review. Heart Fail Rev 2011; 16: 553–567. [DOI] [PubMed] [Google Scholar]
- 9. Guan Z, Miller SB, Greenwald JE. Zaprinast accelerates recovery from established acute renal failure in the rat. Kidney Int 1995; 47: 1569–1575. [DOI] [PubMed] [Google Scholar]
- 10. Lledo‐Garcia E, Rodriguez‐Martinez D, Cabello‐Benavente R, Moncada‐Iribarren I, Tejedor‐Jorge A, Dulin E, et al. Sildenafil improves immediate post transplant parameters in warm‐ischemic kidney transplants: experimental study. Transplant Proc 2007; 39: 1354–1356. [DOI] [PubMed] [Google Scholar]
- 11. Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil use in pulmonary arterial hypertension (SUPER) study group: sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 12. EMA . Orphan designation (EU/3/10/815) for sildenafil citrate for the treatment of postcardiotomy right ventricular failure. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/orphans/2010/12/human_orphan_000866.jsp&mid=WC0b01ac058001d12b (last accessed 27 July 2016).
- 13. Patel NN, Toth T, Jones C, Lin H, Ray P, Sleeman P, et al. Prevention of post cardiopulmonary bypass acute kidney injury by endothelin – a receptor blockade. Crit Care Med 2011; 39: 793–802. [DOI] [PubMed] [Google Scholar]
- 14. Patel NN, Toth T, Jones C, Lin H, Ray P, Sleeman P, et al. Phosphodiesterase‐5 inhibition prevents post cardiopulmonary bypass acute kidney injury in swine. Ann Thorac Surg 2011; 92: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 15. Revatio 0.8mg/ml solution for injection . electronic Medicines Compendium (eMC). Available at http://www.medicines.org.uk/emc/medicine/22707 (last accessed 27 July 2016).
- 16. Matos‐Pita AS, Lillo BM. Non‐compartmental pharmacokinetics and bioequivalence analysis. Pharmasug, 2005; SP07. Available at http://www.lexjansen.com/pharmasug/2005/statisticspharmacokinetics/sp07.pdf (last accessed 27 July 2016).
- 17. Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res 2000; 17: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 18. Hummel J, McKendrick S, Brindley C, French R. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat 2009; 8: 38–49. [DOI] [PubMed] [Google Scholar]
- 19. Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol 2002; 53 (Suppl. 1): 5S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muirhead GJ, Wilner K, Colburn W, Haug‐Pihale G, Rouviex B. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil. Br J Clin Pharmacol 2002; 53 (Suppl. 1): 21S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muirhead GJ, Faulkner S, Harness JA, Taubel J. The effects of steady‐state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers. Br J Clin Pharmacol 2002; 53 (Suppl. 1): 37S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vachiery JL, Huez S, Gillies H, Layton G, Hayashi N, Gao X, et al. Safety, tolerability and pharmacokinetics of an intravenous bolus of sildenafil in patients with pulmonary arterial hypertension. Br J Clin Pharmacol 2011; 71: 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milligan PA, Marshall SF, Karlsson MO. A population pharmacokinetic analysis of sildenafil citrate in patients with erectile dysfunction. Br J Clin Pharmacol 2002; 53 (Suppl. 1): 45S–52S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Individual patient plasma concentrations (hatched line) of (A) aildenafil and (B) desmethyl sildenafil in Groups 1–6. Mean values (solid line) are as reported in Figure 1
Figure S2 Log individual patient plasma concentrations (hatched line) demonstrating terminal half‐life of (A) sildenafil and (B) desmethyl sildenafil in Groups 1–6
Table S1 Dose proportionality of pharmacokinetic data. Values represent estimated slopes (adjusted means and standard errors) of the dose proportionality analysis of the key pharmacokinetic endpoints of sildenafil and desmethyl sildenafil
Table S2 Linear relationship between sildenafil dose and pharmacodynamic endpoints. Values represent estimated slopes of the linear model between sildenafil dose and selected pharmacodynamics endpoints (magnitude of change of endpoint per mg sildenafil). The P‐values denote exploratory significance for the slope being different from zero
Table S3 Descriptive statistics of the key pharmacokinetic endpoints of sildenafil and desmethyl sildenafil (geometric means (%gCV))
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


