Abstract
Aim
The aim of the present study was to determine the barriers and motives influencing consumer reporting of adverse drug reactions (ADRs).
Methods
A systematic review, guided by the Cochrane Handbook, was conducted. Electronic searches included MEDLINE, EMBASE, PsycINFO, CINAHL, PubMed and the Cochrane Database of Systematic Reviews from 1964 to December 2014. Eligible studies addressed patients' perceptions and factors influencing ADR reporting. Studies about healthcare professional (HCP) reporting of ADRs were excluded. Studies were appraised for quality, and results were analysed descriptively.
Results
Of 1435 citations identified, 21 studies were eligible. Studies were primarily conducted in the UK, the Netherlands and Australia. The identified barriers to patient reporting of ADRs (n = 15 studies) included poor awareness, confusion about who should report the ADR, difficulties with reporting procedures, lack of feedback on submitted reports, mailing costs, ADRs resolved and prior negative reporting experiences. The identified motives for patients reporting ADRs (n = 10 studies) were: preventing others from having similar ADRs, wanting personal feedback, improving medication safety, informing regulatory agencies, improving HCP practices, responding to HCPs not reporting their ADRs and having been asked to report ADRs by HCPs.
Conclusions
Most patients were not aware of reporting systems and others were confused about reporting. Patients were mainly motivated to make their ADRs known to prevent similar suffering in other patients. By increasing patient familiarity and providing clear reporting processes, reporting systems could better achieve patient reporting of ADRs.
Keywords: adverse drug reaction reporting system, adverse drug reactions, barriers, motives, patient, pharmacovigilance
What is Already Known about this Subject
Little is known about patients reporting of adverse drug reaction. Understanding the barriers and motives for reporting by patients could be of benefit in improving medication safety.
What this Study Adds
Patient reporting of ADRs needs to be actively supported by increasing patient familiarity with available ADR reporting systems, HCPs encouraging them to report, providing clear guidance on using the reporting system as well as providing feedback.
Reporting of ADR by patients is important because it will provide regulators with patients' perspective and because of the under‐reporting by HCPs.
Introduction
Adverse drug reactions (ADRs) cause significant morbidity and mortality across diverse populations worldwide and have an economic impact upon the healthcare system 1. The World Health Organization (WHO) monitors spontaneous ADR reporting in the majority of countries. A common problem is under‐reporting 2. It is estimated that only 5–10% of ADRs are reported 3. Although there is no estimate of patient reporting, 95% of healthcare professionals (HCPs) do not report ADRs 4.
In 1976, a British physician, Inman, was the first to publish reasons for under‐reporting by HCPs 5, including: (i) complacency (believing that serious ADRs are well documented when the drug is released on the market); (ii) fear of being involved in a lawsuit; (iii) guilt for having been responsible for damage observed in a patient; (iv) ambition to publish a case series or financial benefit; (v) lack of awareness of the notification process; (vi) insecurity about reporting suspicions of an ADR; and (vii) indifference. These factors were subsequently confirmed in two systematic reviews of barriers and motives to HCP reporting of ADRs 1, 2. One systematic review concluded that direct reporting from patients may be one way of reducing under‐reporting rates 4.
Patients are often knowledgeable about their health condition and treatments, and are therefore well positioned to participate in reporting ADRs and to improve drug safety 6. However, to our knowledge, there has not been a systematic review reporting the factors influencing patient reporting of ADRs.
To determine strategies for improving voluntary reporting of ADRs by patients, it is important to identify influential factors. In this context, the objective of the present study was to identify the barriers and motives that influence the reporting of ADRs by patients.
Methods
A systematic review, guided by the Cochrane Handbook 7, was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) criteria 8. In accordance with PRISMA guidelines, our systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 4 December, 2014 and was last updated on 19 January, 2015 (registration number CRD42014015310) 8.
Search protocol
A health sciences librarian (E.W.) designed the search strategies. Literature search strategies relied on medical subject headings (MeSH) and text terminology related to patient reporting of ADRs. The following databases were searched without language restriction: MEDLINE, EMBASE and PsycINFO (all Ovid interface; CINAHL; PubMed; Cochrane Database of Systematic Reviews; and Grey Literature). The PROSPERO registry was also searched for ongoing or recently completed pertinent systematic reviews.
Search terms used for MEDLINE (see Figure 1) and other databases included: patients, consumers, public, adverse drug reactions, report, reporting, spontaneous, pharmacovigilance and surveillance. Reference lists of eligible studies were also scanned. Citations published from inception of spontaneous ADR reporting in 1964 up to 5 December 5, 2014 were searched.
Figure 1.
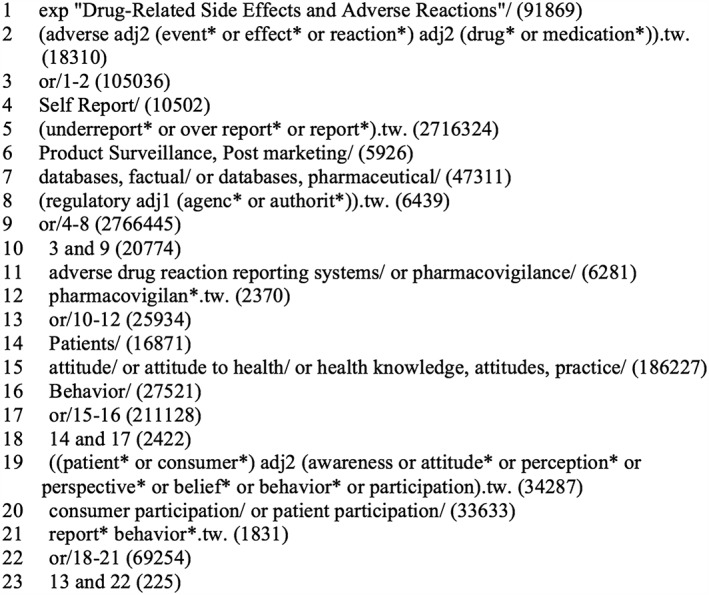
Ovid MEDLINE database search strategy
Selection process
Studies were eligible for inclusion if they: (i) addressed patients' perceptions of ADR reporting and (ii) focused on factors influencing patient reporting of ADRs. No language requirements were imposed, although, owing to resource limitations, only non‐English publications amenable to Google Translate conversion were included (see Table 1).
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Study design | Regardless of methodology, qualifying studies that answered the question ‘what factors influence the reporting of ADRs by the public?’ were included | Letters, editorials and narrative reviews were excluded |
| Participants | All studies addressing patients' perceptions of ADR reporting were included | Studies addressing patient and HCP roles in pharmacovigilance; perception of HCPs on ADR reporting; studies comparing frequencies of reported ADRs by patients vs. HCPs; frequencies of ADRs reported by patients; and studies addressing the role of regulators on pharmacovigilance |
| Language | Studies reported in all languages, if we were able to translate them | Studies unable to translate |
ADR, adverse drug reaction; HCP, healthcare professional
After the identification of studies, duplicates were removed using standard software (ENDNOTE 7). Two independent reviewers (R.D., R.S.) conducted three levels of screening. Level one screening, using citation titles only, determined study relevance to the overall objective of the systematic review. Only citations judged as ‘excludable’ by both reviewers were removed. Level two screening, using title and abstract, determined if the study met the inclusion criteria (see Table 1). Level three screening used the full text to determine eligibility.
The two reviewers independently extracted data, with disagreements resolved through discussion. To extract data from the included studies, reviewers used a modified form based on the Cochrane Effective Practice and Organization of Care Review Group (EPOC) data collection tool. The form was modified to extract data on factors influencing ADR reporting by health consumers, and barriers and motives for health consumers to report ADRs. The modified data collection form was piloted on five randomly selected included studies before its actual use. Using an extraction form ensured a systematic process for data extraction. The following data were extracted: (i) characteristics of studies (country, setting, design and number of participants); (ii) data collection procedures (self‐administered structured questionnaire, focus group, and semi‐structured telephone and face‐to‐face interviews); and (iii) barriers and motives influencing ADR reporting by health consumers. Quality appraisals were conducted by the two reviewers (R.D., R.S.) using the Critical Appraisal Skills Programme (CASP) criteria for the descriptive observational studies 9. Disagreements about data extraction and quality appraisal results were resolved by discussion. Authors of included studies were contacted, as needed, to obtain further information.
For the quality appraisal, the two reviewers independently rated each study for: (i) a clear statement of aims; (ii) methodology; (iii) research design to address research aims; (iv) recruitment strategy; (v) data collected; (vi) whether the relationship between researcher and participants was considered; (vii) ethical issues; (viii) rigor of data analysis; (ix) clear statement of findings; and (x) research value. Disagreements were resolved by consensus. Studies were considered to be of high quality if scores were 80% or above on CASP criteria, medium quality for 60–79.9% and low quality for <60%.
Data analysis
Owing to heterogeneity across study outcomes, data were analysed descriptively. Study comparisons were grouped to answer the research questions. Findings were synthesized based on outcomes. The characteristics of included studies were presented in a narrative format, as recommended by PRISMA 8.
A MeaSurement Tool to Assess Review (AMSTAR) 10 was used to assess the methodological quality of the review. AMSTAR characterizes the quality of systematic reviews at three levels: 8–11 indicating high quality, 4–7 medium quality and 0–3 low quality 11.
Results
Of 1435 citations reviewed, 21 studies, published in 24 papers, were included (see Figure 2). These were published between 2008 and 2014 and used a range of methods. They were conducted in the UK (n = 5 studies), the Netherlands (n = 4), Australia (n = 3) and one each in Italy, Portugal, Romania, Bulgaria, Malaysia, Nepal, Pakistan, Uganda and Saudi Arabia (see Table 2). Overall, quality ratings were medium to high, with only two rated as low quality 9.
Figure 2.
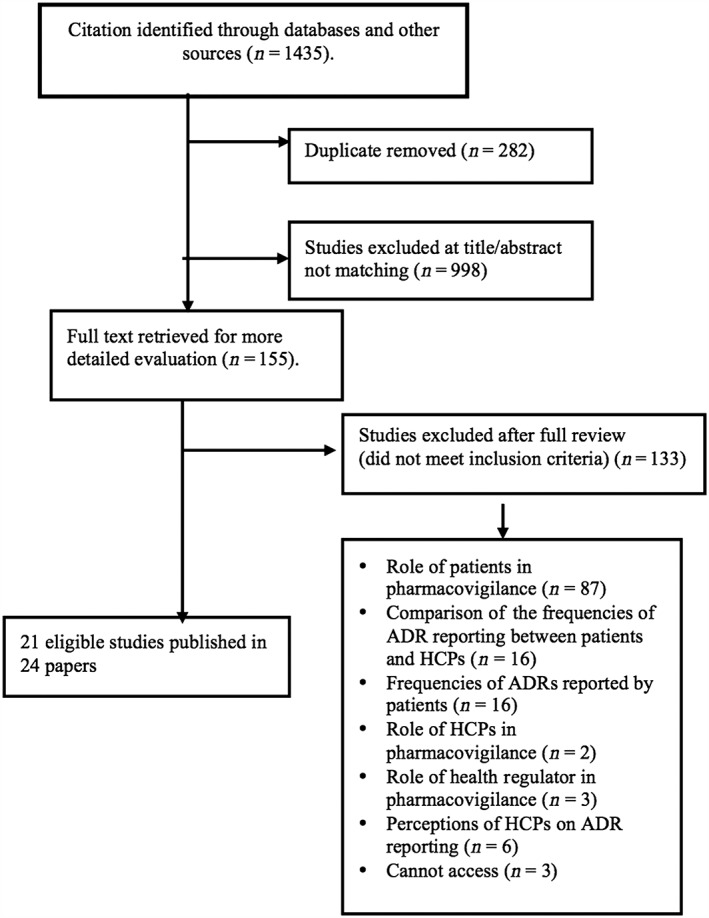
Flow of identified studies through the review process. ADR, adverse drug reaction; HCP, healthcare professional
Table 2.
Characteristics of included studies
| Author/Year | Location | Design | Participants | Data collection | Quality rating |
|---|---|---|---|---|---|
| Aljadhey and Albogami (2013) 12 | Saudi Arabia | Observational, cross‐sectional study | 204 adults | Self‐administered structured questionnaire | Medium |
| Arnott et al. (2013) 20 | UK | Qualitative | 17 parents without previous experience of submitting ADR report and 27 with this experience | Semi‐structured telephone and face‐to‐face interviews | High |
| Avery et al. (2011) 13 | UK | Mixed‐model approach combining qualitative and quantitative research methods | Patients reported ADRs (27 in telephone interviews, 40 in focus group, 1362 in evaluable questionnaire and 2028 in Omnibus survey) | Semi‐structured telephone interviews, questionnaire, focus group, and Omnibus survey | High |
| Braun et al. (2010) 21 | Australia | Observational, cross‐sectional study | 620 patients at 60 community pharmacies and rural pharmacies in three Australian states | Self‐administered structured questionnaire | Medium |
| Bukirwa et al. (2008) 22 | Uganda | Qualitative | 16 adults | Focus group | High |
| Elkalmi et al. (2013) 14 | Malaysia | Qualitative, descriptive | 334 adults | Face‐to‐face interview using a structured questionnaire | High |
| Farcas et al. (2010) 28 | Romania | Qualitative | 50 patients taking antidepressant | Self‐administered structured questionnaire | Medium |
| Gujral et al. (2010) 32 | UK | Cross‐sectional | 154 adults | Self‐administered structured questionnaire | Medium |
| Harmark et al. (2013) 24 | Netherlands | Mixed‐model approach combining qualitative and quantitative research methods | 21 adults | Face‐to‐face interview using a structured questionnaire | High |
| Jha et al. (2014) 23 | Nepal | Cross‐sectional study using qualitative and quantitative methods | 23 adults | Face‐to‐face interview using a semi‐structured questionnaire | High |
| Krska et al. (2011) 15 | UK | Quantitative | 272 adults | Face‐to‐face interview using a semi‐structured questionnaire | High |
| Lebanova and Getov (2014) 16 | Bulgaria | Cross‐sectional study | 211 adults | Self‐administered questionnaire | High |
| Lorimer et al. (2012) 19 | UK | Qualitative | 15 adults | Face‐to‐face interview using a semi‐structured questionnaire | High |
| Matos et al. (2014) 17 | Portugal | A descriptive‐correlational study | 948 adults | Closed‐answer questionnaire | Low |
| Parrella et al. (2014) 33 | South Australia | Cross‐sectional | 2002 adults | Face‐to‐face interview using a structured questionnaire | High |
| Qamar et al. (2014) 34 | Pakistan | Cross‐sectional | 1566 adults | Face‐to‐face interview using a structured questionnaire | Medium |
| Robertson and Newby (2013) 18 | Australia | Cross‐sectional study | 2484 adults | Structured telephone interview and online survey | High |
| Rolfes et al. (2014) 25 | Netherlands | Qualitative | 3 adults | Face‐to‐face interview using a semi‐structured questionnaire | Low |
| Salvo et al. (2013) 35 | Italy | Cross‐sectional study | 744 adults | Structured questionnaire, telephone interview | Medium |
| van Hunsel et al. (2010) 26 | The Netherlands | Cross‐sectional study | 1005 adults | Web‐based questionnaire | High |
| van Hunsel et al. (2010) 27 | The Netherlands | Qualitative | 21 adults | Face‐to‐face interview using a structured questionnaire | Medium |
ADR, adverse drug reaction
Barriers to patient ADR reporting
Of the 21 studies, 15 described barriers to the reporting of ADRs. These included: (i) poor awareness; (ii) confusion as to who reports ADRs, and to whom; (iii) difficulties with ADR reporting procedures and forms; (iv) ADR resolved; (v) lack of feedback on previous ADRs submitted; (vi) mailing costs; and (vii) prior negative experience (see Table 3).
Table 3.
Barriers for patient reporting of ADRs
| Author (year), country | Poor awareness | Confusion as to who reports ADRs, and to whom | Difficulty with ADR reporting procedures and forms | ADRs resolved | Lack of feedback on submitted ADR | Mailing cost | Prior negative experience |
|---|---|---|---|---|---|---|---|
| Aljadhey and Albogami (2013) 12 , Saudi Arabia | 87% | 80% | |||||
| Arnott et al. (2013) 20 , UK | √ | √ | √ | ||||
| Avery et al. (2011) 13 , UK | 74% | √ | 15.9% | √ | √ | ||
| Braun et al. (2010) 21 , Australia | √ | √ | √ | ||||
| Bukirwa et al. (2008) 22 , Uganda | √ | √ | √ | ||||
| Elkalmi et al. (2013) 14 , Malaysia | 65.6% | ||||||
| Gujral and Cairns (2010) 32 UK | √ | √ | |||||
| Jha et al. (2014) 23 Nepal | √ | √ | √ | √ | |||
| Krska et al. (2011) 15 UK | 93.8% | ||||||
| Lebanova et al. (2014) 16 , Bulgaria | 78.7% | √ | |||||
| Lorimer et al. (2012) 19 UK | √ | √ | √ | √ | |||
| Matos et al. (2014) 17 , Portugal | 44% | √ | |||||
| Parrella et al. (2014) 33 Australia | √ | ||||||
| Robertson and Newby (2013) 18 , Australia | 89.7% | √ | |||||
| Salvo et al. (2013) 35 , Italy | √ | ||||||
| Total | 14 | 8 | 4 | 5 | 2 | 2 | 1 |
ADR, adverse drug reaction
Poor awareness
Of the 15 studies, 14 qualitative and quantitative studies cited poor public awareness of ADR reporting systems. Many participants did not know that there was a national regulatory agency. In seven quantitative studies, 75% (range 44.1–93.8%) of participants were not aware of available ADR reporting systems 12, 13, 14, 15, 16, 17, 18.
Confusion as to who reports ADRs, and to whom
Eight studies reported that patients were not sure who should report an ADR, and to whom. For example, in the UK, some participants considered it their duty to report ADRs, whereas in other countries patients identified the HCPs as most responsible for reporting 13, 19, 20.
Difficulties with ADR reporting procedures and forms
Four quantitative and qualitative studies identified difficulties with procedures and reporting forms. The proportions describing difficulties ranged from 15.9% in the UK to 80% in Saudi Arabia 12, 13. For example, in the UK study, participants described: (i) paper forms as tedious, lengthy, awkwardly constructed, inconsistent with online forms, and available only in English; (ii) telephone reporting being limited to working hours, which was inconvenient and time consuming; and (iii) technical problems with online reporting that often resulted in a loss of information 13.
Anticipating ADRs to resolve
Five qualitative studies reported that patients believed that ADRs would resolve after stopping or completing their treatment, and they did not think there was a benefit to reporting the ADR 13, 18, 19, 21, 22.
Lack of feedback on previously submitted ADRs
Two studies reported patients' concerns over the lack of feedback to submitted ADR reports. For example, in a UK study, 32% of participants expected feedback from the ADR report, and 1.9% felt that a lack of detailed feedback might discourage them from completing an ADR report in the future 13, 20. This problem was not reported in the Netherlands, where the health authority provides customized feedback on each ADR report submitted 13.
Mailing costs
Two studies conducted in Uganda and Nepal identified patients' poor economic status as a barrier to reporting ADRs because they could not afford the cost of mailing their reports 22, 23.
Prior negative experience
One study in Uganda described prior negative experience as a barrier 5. Patients feared that reporting ADRs would be met with disapproval by their HCPs.
Motives for patients to report ADRs
There were 10 qualitative and quantitative studies that identified motives for patients to report ADRs (see Table 4). Motives included: (i) preventing similar ADRs from occurring in others; (ii) improving drug safety; (iii) considering the seriousness of the ADR; (iv) desiring personal feedback; (v) raising awareness of specific ADRs; (vi) improving HCP practices; (vii) responding to HCPs not reporting ADRs; and (viii) having been asked to report ADRs by HCPs.
Table 4.
Motives for patients to report ADRs (n = 10)
| Author (year), country | Preventing similar ADRs in others | Improving drug safety | Seriousness of ADRs | Desire for personal feedback | Informing others | Improving HCPs practice | Responding to HCPs not reporting patient ADRs | Being asked to report ADR by HCPs |
|---|---|---|---|---|---|---|---|---|
| Avery et al. (2011) 13 , UK | 26% | 12.7% | √ | 60.8% | 7% | 12.2% | 7.8% | √ |
| Arnott et al. (2013) 20 , UK | √ | √ | √ | √ | ||||
| Farcas et al. (2010) 28 , Romania | √ | |||||||
| Harmark et al. (2013) 24 , the Netherlands | 89% | √ | √ | 84% | √ | |||
| Krska et al. (2011) 15 , UK | 86% | |||||||
| Lorimer et al. (2012) 19 , UK | 62.4% | |||||||
| Matos et al. (2014) 17 , Portugal | 81.1% | √ | ||||||
| Rolfes et al. (2014) 25 , the Netherlands | √ | |||||||
| van Hunsel et al. (2010) 26 , the Netherlands 26 | √ | √ | √ | √ | √ | |||
| van Hunsel et al. (2010) 27 , the Netherlands | √ | √ | √ | √ | ||||
| Total | 7 | 5 | 5 | 5 | 3 | 3 | 2 | 2 |
ADR, adverse drug reaction; HCP, healthcare professional
Preventing similar ADRs in others
Seven of 10 studies reported that patients wanted to prevent others from suffering similar problems, and perhaps help to find better treatments 13, 20, 24, 25, 26, 27, 28.
Improving drug safety
Patients in five studies believed that drug safety could be improved by reporting ADRs 13, 20, 24, 26, 27. For example, in the Netherlands, patients specifically indicated their willingness to invest time in ADR reporting to enhance drug safety 24.
Considering the seriousness of the ADRs
Three quantitative and two qualitative studies indicated that judging the ADR as serious was the main motivation for patients to report 13, 15, 17, 19, 26. For example, in two UK surveys, the majority of respondents (62% and 86%, respectively) declared that only serious ADRs requiring hospital admission or affecting daily life were worthy of reporting 15, 19, 26. Similarly, in Portugal, participants agreed or strongly agreed that severity was the primary reason for reporting ADRs 17.
Desiring personal feedback
One quantitative and four qualitative studies discussed patients' desire for personal feedback. Participants wanted to learn and find others who shared the same experience, to acquire more details about the ADRs and to seek confirmation that the report was received 13, 17, 20, 24, 26.
Raising awareness of specific ADRs
In three quantitative studies, patients indicated that informing regulators, drug manufacturers, HCPs and the public about ADRs was the only way to create awareness of such incidents 13, 26, 27.
Improving HCP practices
In two quantitative studies and one qualitative study, patients raised the view that HCPs need to be informed about ADRs 13, 24, 26. They felt that reporting ADRs would inform HCPs about unknown ADRs, and that this would improve their knowledge and practices.
Responding to HCPs not reporting patients’ ADRs
Two studies cited that failure of HCPs to report ADRs motivated patients to report these themselves. Patients emphasized how HCPs consulted about the ADRs had not taken their concerns seriously 20. This motivated some patients in the UK to self‐report 13. Other patients were motivated to report because they did not think that HCPs would report their ADRs accurately, given that HCPs have limited time and may not be able to provide precise details 13.
Asked to report ADRs by HCPs
In two qualitative studies, patients indicated that HCPs asked them to self‐report ADRs. In the Netherlands, patients were encouraged by pharmacy assistants to self‐report ADRs 24, while in the UK patients were encouraged by pharmacists 13.
Discussion
The present study identified factors influencing patient ADR reporting, with the intention of using these findings to determine approaches that could be used to improve voluntary patient reporting, and ultimately improve patient safety. All of the 21 included studies had been published since 2008, suggesting a growing interest in this area. Fifteen of these studies indicated a range of barriers and 10 identified motives for patient reporting of ADRs. Our findings led us to make the following observations.
Common barriers to patient reporting of ADRs were similar to those previously identified for HCPs 2, 4. Specific barriers both for patients and HCPs included poor awareness about available reporting systems, uncertainty about who should be responsible for submitting the ADR reports, and lack of feedback for submitted reports. Barriers that were unique to patient reporting were mailing costs, prior negative experiences and resolved ADRs. Postal mailing costs for patients to report ADRs was identified in two developing countries; this may not be an issue in other countries, where electronic and telephone reporting are available for patients.
Common motives for patients to report ADRs were altruistic or personal. Examples of altruistic motives were preventing harm to others, feeling responsible for reporting ADRs and reporting their experiences publicly to improve medication safety. Personal motives included the severity of reactions and patients' desire for feedback. The desire for feedback was also a motive for HCPs to report ADRs 2. Another motive that encouraged patients to report ADRs was their concern about HCPs not having time to report ADRs, and this was consistent with the barriers identified by HCPs 2.
Strategies to enhance patient ADR reporting should focus on the most common and feasible barriers to address. If patients are unaware of available reporting systems, how they work and how they are accessed, their contributions will continue to decline. First, strategies are needed to increase patients' awareness of reporting systems, and HCPs' awareness that patients can report on their own. Interestingly, in the two studies in which HCPs encouraged patients to report, the HCPs were limited to those who worked in pharmacies 13, 24. Second, national regulatory agencies could learn from the Netherlands, where responses are provided to patients who report ADRs 24 by letters of acknowledgment. Issuing a response has the potential to reassure patients that their information has been received and may in fact improve subsequent ADR reporting. Finally, increasing patient ADR reporting provides opportunities to promote educational interventions. For example, patients could be directed to websites that provide high‐quality drug information, if the ADRs have already been acknowledged in drug information materials.
Interestingly, most included studies were conducted in the UK, the Netherlands and Australia, with none conducted in North America. Patient reporting of ADRs was established in the USA and Canada in 1960 and 1965, respectively 13, and it appears that use of these reporting systems there has not been evaluated to the same extent as in other countries.
Limitations and strengths
One limitation of the present review was that three full‐text articles were not accessible to determine if they were eligible to be included 29, 30, 31. The titles of these articles indicated that they included ADR reporting by patients but there were no authors listed. Although authors of the included studies used a variety of measures to determine factors influencing patient ADR reporting, our systematic review appropriately synthesized the diverse forms of evidence identified. When the methodological quality of our systematic review was appraised using the AMSTAR instrument, it was rated as meeting nine out of 11 criteria. For the two missing items, one was not applicable because there was no meta‐analysis conducted (methods used to combine the findings of studies appropriate) and we did not assess for publication bias.
Conclusion
Several barriers and motives influencing patient reporting of ADRs were identified in 21 studies. Poor patient awareness of available reporting systems was the main barrier to patient reporting of ADRs, and this finding was consistent with studies conducted with HCPs. The main motive for patient reporting of ADRs was altruism, to prevent others from suffering from the same drug reaction. Patient reporting of ADRs should be actively supported by increasing patient familiarity with available ADR reporting systems, HCPs encouraging them to report, providing clear guidance on using the reporting system, as well as providing feedback. Initiating strategies that are informed by these factors has the potential to improve spontaneous patient ADR reporting.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Dr Rana Shash and Erica Wright made important contributions to various stages of developing the systematic review.
Al Dweik, R. , Stacey, D. , Kohen, D. , and Yaya, S. (2017) Factors affecting patient reporting of adverse drug reactions: a systematic review. Br J Clin Pharmacol, 83: 875–883. doi: 10.1111/bcp.13159.
References
- 1. Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol 2009; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varallo FR, Guimarães Sde OP, Abjaude SA, Mastroianni Pde C. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev Esc Enferm USP 2014; 48: 739–747. [DOI] [PubMed] [Google Scholar]
- 3. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA 1995; 274: 29–34. [PubMed] [Google Scholar]
- 4. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf 2006; 29: 385–396. [DOI] [PubMed] [Google Scholar]
- 5. Inman WHW. Assessment drug safety problems In: Epidemiological Issues in Reported Drug‐Induced Illnesses, eds Gent M, Shigmatsu I. Hamilton, ONT: McMaster University Library Press, 1976; 17–24. [Google Scholar]
- 6. van Hunsel F, Talsma A, van Puijenbroek E, de Jong‐van den Berg L, van Grootheest K. The proportion of patient reports of suspected ADRs to signal detection in the Netherlands: case–control study. Pharmacoepidemiol Drug Saf 2011; 20: 286–291. [DOI] [PubMed] [Google Scholar]
- 7. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell, 2008. [Google Scholar]
- 8. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati AD, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 9. Public Health . Critical appraisal of qualitative studies: Public Health Resource Unit, Oxford, UK, 2013.
- 10. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharif MO, Sharif FNJ, Ali H, Ahmed F. Systematic reviews explained: AMSTAR – how to tell the good from the bad and the ugly. Oral Health Dent Manag 2013; 12: 9–16. [PubMed] [Google Scholar]
- 12. Aljadhey H, Albogami Y. Public perception towards adverse drug reaction reporting in Saudi Arabia. Pharmacoepidemiol Drug Saf 2013; 22: 82–83. [Google Scholar]
- 13. Avery A, Anderson C, Bond C, Fortnum H, Gifford A, Hannaford P, et al. Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 2011; 15: 1–234. [DOI] [PubMed] [Google Scholar]
- 14. Elkalmi R, Hassali MA, Al‐lela OQ, Awadh AIJ, Al‐Shami AK, Jamshed SQ. Adverse drug reactions reporting: knowledge and opinion of general public in Penang, Malaysia. J Pharm Bioallied Sci 2013; 5: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krska J, Jones L, McKinney J, Wilson C. Medicine safety: experiences and perceptions of the general public in Liverpool. Pharmacoepidemiol Drug Saf 2011; 20: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 16. Lebanova H, Getov I. Study of patients' potential as a source for spontaneous reporting systems in Bulgaria. Pharmacoepidemiol Drug Saf 2014; 23 (Suppl. 1): 1–497. [Google Scholar]
- 17. Matos CF, Van Hunsel FP, Joaquim JJ. Are patients ready to take part in the pharmacovigilance system: a Portuguese preliminary study concerning drug reaction reporting. Pharmacoepidemiol Drug Saf 2014; 23: 293. [Google Scholar]
- 18. Robertson J, Newby DA. Low awareness of adverse drug reaction reporting systems: a consumer survey. Med J Aust 2013; 199: 684–686. [DOI] [PubMed] [Google Scholar]
- 19. Lorimer S, Cox A, Langford N. A patient's perspective: the impact of adverse drug reactions on patients and their views on reporting. J Clin Pharm Ther 2012; 37: 148–152. [DOI] [PubMed] [Google Scholar]
- 20. Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol 2013; 75: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braun LA, Tiralongo E, Wilkinson JM, Poole S, Spitzer O, Bailey M, et al. Adverse reactions to complementary medicines: the Australian pharmacy experience. Int J Pharm Pract 2010; 18: 242–244. [DOI] [PubMed] [Google Scholar]
- 22. Bukirwa H, Nayiga S, Lubanga R, Mwebaza N, Chandler C, Hopkins H, et al. Pharmacovigilance of antimalarial treatment in Uganda: community perceptions and suggestions for reporting adverse events. Trop Med Int Health 2008; 13: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 23. Jha N, Rathore DS, Ravi Shankar P, Gyawali S. Pharmacovigilance knowledge among patients at a teaching hospital in Lalitpur district, Nepal. J Clin Diagn Res 2014; 8: 32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harmark L, Lie‐Kwie M, Berm L, de Gier H, van Grootheest K. Patients' motives for participating in active post‐marketing surveillance. Pharmacoepidemiol Drug Saf 2013; 22: 70–76. [DOI] [PubMed] [Google Scholar]
- 25. Rolfes L, Wilkes S, van Hunsel F, van Puijenbroek E, van Grootheest K. Important information regarding reporting of adverse drug reactions: a qualitative study. Int J Pharm Pract 2014; 22: 231–233. [DOI] [PubMed] [Google Scholar]
- 26. van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K. Motives for reporting adverse drug reactions by patient‐reporters in the Netherlands. Eur J Clin Pharmacol 2010; 66: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Hunsel FP, ten Berge EA, Borgsteede SD, van Grootheest K. What motivates patients to report an adverse drug reaction? Ann Pharmacother 2010; 44: 936–937. [DOI] [PubMed] [Google Scholar]
- 28. Farcas AM, Farah C, Bojita MT. Patients reporting of suspected adverse reactions to antidepressants. A pilot methodological study. Farmacia 2010; 58: 255–263. [Google Scholar]
- 29. Anonymous . Advantages of adverse‐effect reporting by patients. Prescrire Int 2011; 20: 70. [PubMed] [Google Scholar]
- 30. Anonymous . Direct reporting by patients: positive results. Prescrire Int 2007; 16: 71. [PubMed] [Google Scholar]
- 31. Anonymous . Patient reporting improves pharmacovigilance. Prescrire Int 2008; 17: 241–242. [PubMed] [Google Scholar]
- 32. Gujral KK, Cairns C. Public awareness of the yellow card scheme for reporting ADRs. Clin Pharm 2010; 2: S39. [Google Scholar]
- 33. Parrella A, Gold M, Braunack‐Mayer A, Baghurst P, Marshall H. Consumer reporting of adverse events following immunization (AEFI): identifying predictors of reporting an AEFI. Hum Vaccin Immunother 2014; 10: 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qamar S, Noureen A, Naveed M. Reporting of adverse drug reactions by the end users‐patients in Pakistan. Drug Saf 2014; 37: 879. [Google Scholar]
- 35. Salvo F, Miroddi M, Alibrandi A, Calapai F, Cafeo V, Mancari F, et al. Attitudes and opinion about adverse drug events of women living in a city of south Italy. Pharmacology 2013; 91: 173–177. [DOI] [PubMed] [Google Scholar]


