Abstract
Aims
Elderly transplant recipients have a lower incidence of acute rejection, and a higher risk to die from infectious complications. A potential cause may be differences in the pharmacokinetics (PK) or pharmacodynamics (PD) of the immunosuppressive drugs they are taking. This study was designed to comprehensively evaluate the influence of age on the PK and PD of mycophenolic acid (MPA).
Methods
In this study the PK and PD of MPA was studied in 26 elderly and 54 younger renal transplant recipients treated with mycophenolate mofetil and tacrolimus. Patients were sampled repetitively, both before and during the first 6 months after kidney transplantation. Age‐related variability in MPA PK, baseline IMPDH activity, as well as MPA‐induced IMPDH inhibition were studied.
Results
The IMPDH activity pre‐transplantation did not differ between elderly and younger patients. Neither IMPDH activity pre‐transplantation nor maximum IMPDH inhibition was significantly correlated with the patients' age. The area under the MPA plasma concentration–time curve (AUC0–12h) and the area under the effect (IMPDH activity)–time curve (AEC0–12h) from 0 to 12 h were also not significantly different between the two groups. We found no significant differences in EC50 and E max between elderly and younger patients.
Conclusions
Age did not significantly affect the PK or PD of MPA. It is unlikely that the lower incidence of acute rejection in elderly patients, or the higher risk to die from a severe infection in elderly patients is due to different handling of MPA in the elderly.
Keywords: inosine monophosphate dehydrogenase inhibitor, kidney transplantation, mycophenolic acid, pharmacodynamics, pharmacokinetics
What is Already Known about this Subject
Elderly transplant recipients have a lower incidence of acute rejection and a higher risk to die from infectious complications, compared to younger patients.
The impact of age on the PK and PD of MPA in adult renal transplant patients has not been extensively studied.
What this Study Adds
The IMPDH activity pre‐transplantation (in the absence of MPA treatment) is not different between elderly and younger patients.
Our study shows that the age of the patient does not significantly affect the PK or PD of MPA.
Based on our data, no age‐dependent dose adjustments need to be implemented.
Tables of Links
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
The proportion of elderly patients in the kidney transplant population is increasing, mainly as a consequence of changes in demographics 4, 5. Compared with younger recipients, the elderly have a lower risk of developing acute rejection and are more prone to die from infections 6, 7, 8. There are several, not mutually exclusive, explanations for this observation. First, elderly patients appear to have an impaired effector T‐ and B‐cell response, with altered production of cytokines, chemokines and interferon, and a change in co‐stimulatory molecule expression 9, 10, 11. Apart from age, loss of renal function is associated with premature immunological ageing 12. Both decreased thymic output and increased susceptibility of naive T‐cells for apoptosis may play a role in the loss of naive T‐cells in end‐stage renal disease patients 13. Second, the pharmacokinetics (PK) and pharmacodynamics (PD) of immunosuppressive drugs, such as tacrolimus and cyclosporine, are different in the elderly 14. A clinically relevant impact of age on the pharmacokinetics of immunosuppressive drugs has been shown for calcineurin inhibitors (CNIs). The dose/bodyweight‐normalized trough concentrations of tacrolimus and cyclosporine are more than 50% higher in older recipients than in younger patients 15. A stronger pharmacodynamic effect of cyclosporine in elderly patients has been suggested to be the result of higher intracellular accumulation of the drug, possibly due to lower activity of the efflux pump in the cell membrane of lymphocytes 16.
Mycophenolate mofetil (MMF) is a first‐line drug in the field of solid organ transplantation and has become a consistent member of many different immunosuppressive regimens 17. MMF produces mycophenolic acid (MPA) following exposure to esterase in the body and MPA is the active compound responsible for the reversible inhibition of inosine 5′‐monophosphate dehydrogenase (IMPDH). For MMF there is not a lot of data available on the influence of age on its PK and PD. The PK of MPA is highly variable between individual patients and age may affect MPA disposition through changes in intestinal absorption, protein binding, hepatic glucuronidation, and renal excretion 18, 19. Only a few studies have reported on the PK of MPA in the elderly and results are conflicting and inconsistent 20, 21, 22.
Age‐related changes in the PD of MPA may be even more relevant than PK changes, as PD reflects the susceptibility of an individual to the biological effects of MPA 23. MPA reversibly inhibits IMPDH, which is the rate‐limiting enzyme in the de novo guanine nucleotide synthesis in proliferating T‐ and B‐lymphocytes. Recent studies demonstrated that a high IMPDH activity both before and after transplantation is associated with an increased risk for biopsy‐proven acute rejection 24, 25, 26. However, the effect of age was not investigated in these studies and it is at present unknown if age‐related changes in the PK and/or PD of MPA are one of the reasons for the different outcomes of elderly and younger transplant recipients.
Here, we report a combined PK and PD analysis of MPA performed in a cohort of patients treated with MMF. Patients were followed over time, and sampled repeatedly, both before and during the first 6 months after kidney transplantation. Age‐related variability in MPA PK, baseline IMPDH activity, as well as MPA‐induced IMPDH inhibition were studied. The hypotheses of the present study were: (1) the PK of MPA is different in elderly compared with younger transplant recipients; (2) elderly patients have a lower baseline (i.e. before transplantation) IMPDH activity; and (3) elderly kidney transplant recipients experience a greater degree of IMPDH inhibition following treatment with MMF as compared with younger transplant recipients.
Materials and methods
Study design and population
A total of 101 patients were recruited for this study from April 2006 to September 2007. Each patient had received a kidney transplant with an uncomplicated immediate post‐operative recovery on the first day after the transplantation and were being treated with MMF. All surgeries were done in the Erasmus MC, University Medical Center, Rotterdam, The Netherlands. For 80 of the 101 patients, a full 12 h curve consisting of six blood samples on day 6.0 was available for measurement of both MPA plasma concentrations and IMPDH activity. The other 21 patients were excluded from the analysis investigating the area under the MPA plasma concentration–time curve (AUC0–12h) and the area under the effect (IMPDH activity)–time curve (AEC0–12h) from 0 to 12 h, because of their incomplete 12 h curve samples. A full area under the curve (AUC0– 12h and AEC0–12h) was obtained at day 6 after surgery, with samples taken at predose, 0.5, 1, 2, 6, and 12 h after oral intake of MMF. On weeks 3, 7 and 20 post‐transplantation, limited sampling AUCs (two samples, obtained at the pre‐dose time point and 120 minutes thereafter) were collected. All blood samples were analysed for MPA plasma concentration and IMPDH activity at the laboratory of the hospital pharmacy.
All patients received triple immunosuppressive therapy, including tacrolimus (Prograft®, Astellas Pharma, Leiderdorp, The Netherlands), mycophenolate mofetil (Cell‐Cept®, Roche Pharma, Woerden, The Netherlands) and prednisone. All patients were treated with 1000 mg MMF b.i.d., except for two patients who received 500 mg MMF b.i.d. at the time of sampling. Doses and predose concentrations of tacrolimus are listed in Table 1. All patients received a fixed‐dose prednisone of 20 mg once daily. Antithymocyte globulin (ATG, Genzyme Corp.) induction therapy was given to 23 patients (Table 1). The study was approved by the Local Ethics Committee of Erasmus MC, Rotterdam, The Netherlands, and complied with the Declaration of Helsinki. All patients gave written informed consent between April 2006 and September 2007. We previously reported data from this cohort of patients on the influence of single nucleotide polymorphisms of the IMPDH type II gene on between‐patient differences in IMPDH activity 27. For this post‐hoc analysis on the data set of the original study, no additional informed consent of patients was obtained.
Table 1.
Patient baseline characteristics and demographics, and laboratory results obtained on day 6 post‐transplantation
| Age group | |||
|---|---|---|---|
| Young (19.2–58.4 years) n = 54 | Elderly (60.1–76.2 years) n = 26 | P‐ value | |
| Age (years) | 43.7 ± 11.0 | 65.8 ± 4.9 | <0.001b |
| Weight (kg) | 78.9 ± 19.0 | 83.5 ± 14.6 | 0.21b |
| Male sex, n (%) | 38 (70.4) | 23 (88.5) | 0.15c |
| Ethnicity, n (%) | |||
| Caucasian | 45 (83.3) | 23 (88.5) | 0.75c |
| Black | 7 (13.0) | 3 (11.5) | |
| Asian | 2 (3.7) | 0 (0) | |
| ATG induction therapy, n (%) | 16 (29.6) | 7 (26.9) | 0.87c |
| DGF, n (%) | 17 (31.4) | 6 (23.1%) | 0.47b |
| MMF daily dose (mg day −1 ) | 1984 ± 170 | 1918 ± 188 | 0.16b |
| Tacrolimus daily dose (mg) | 11.1 ± 3.6 | 11.0 ± 3.3 | 0.88b |
| Tac trough (μg l −1 ) | 10.4 ± 5.5 | 18.5 ± 8.3 | <0.001b |
| Tac trough, dose and weight normalized (ng ml −1 mg −1 ) | 76.8 ± 56.0 | 150.2 ± 93.1 | 0.004 |
| Albumin (g l −1 ) | 34.2 ± 5.9 | 33.8 ± 3.7 | 0.59b |
| Creatinine clearance a (ml min −1 per 1. 73 m 2 ) | 29.3 ± 21.8 | 38.3 ± 24.1 | 0.10b |
| Leucocytes (10 9 l −1 ) | 9.1 ± 4.9 | 8.9 ± 4.5 | 0.91b |
ATG, anti‐thymocyte globulin; DGF, delayed graft function
Estimated using MDRD equation
t‐test
Pearson's χ2 test; data are expressed as mean ± SD
Measurement of MPA plasma concentrations
We used a linear trapezoidal model to calculate manually the MPA–AUC0–12h, including six points (predose, 0.5, 1, 2, 6 and 12 h). The active compound MPA was measured by a validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) method, which consisted of a Waters Acquity Ultra Performance LC coupled to a Quattro Premier XE tandem quadrupole mass spectrometer. The analytical column was an Acquity UPLC BEH C18 2.1*50 mm with 1.7 mm particle size (Waters Inc.). For each sample, 5 μl was injected into the column. Detection was performed by MS with an ESI interface in positive MRM mode 28. The coefficient of variation of the interday and intraday precision of the method used was less than 5 and 3%, respectively. Data were acquired using Masslynx V4.1 and processed using Quanlynx V4.1 software (Waters Inc., Etten‐Leur, The Netherlands).
Measurement of IMPDH activity
We used a linear trapezoidal model to calculate manually the AEC0–12h, including six points (0, 0.5, 1, 2, 6 and 12 h). A validated nonradioactive HPLC method by Glander et al. 29 was used to measure the IMPDH activity. The rate of xanthosine 5′‐monophosphate (XMP) production by IMPDH from peripheral blood mononuclear cells (PBMCs) was measured and was normalized to the measured intra‐cellular adenosine monophosphate (AMP). PBMCs were isolated with Ficoll‐Paque (Greiner Bio‐One, Alphen a/d Rijn, The Netherlands) according to the manufacturer's protocol. The aliquot was resuspended in 250 μl ice‐cold water and stored at −20°C after one washing step. To 50 μl mononuclear cells lysate 130 μl reaction buffer with IMP and β‐NAD+ was added to start the incubation of enzyme reaction. The reaction tubes were placed in a Thermomixer (Eppendorf Ltd, Cambridge, UK) and were incubated at 37°C and 1 × g for 2.5 h. The reaction was stopped by adding 20 μl ice‐cold perchloric acid (4 mol l−1). After centrifugation, 10 μl of 5 mol l−1 potassium carbonate was added to 170 μl supernatant to neutralize the solution. The supernatant was transferred into HPLC for the determination of AMP and of produced XMP. Enzyme activity was expressed as produced XMP (μmol) per time unit (s) per amount of AMP (mol). The precision of the IMPDH activity assay was 6.6–11.9% (quality control samples) and the precision for patient samples ranged from 0.6 to 3.4%.
Sample stability
Investigations with regard to the stability of IMPDH activity in stored samples were carried out for lysate samples stored at −20°C for up to 6 months. No activity was lost when cell extracts were kept at −20°C for up to 6 months (data not shown). There were no significant differences observed in the content of AMP or XMP in completely incubated and neutralized patient samples when they were stored at −20°C for up to 6 months; when compared with initial values, these temperature conditions represented values of 106%. Thus, samples should be analysed within 6 months of storage at −20°C.
Pharmacokinetic and pharmacodynamic analysis
MPA PK parameters were derived from individual plasma concentration–time profiles by using standard non‐compartmental equations. C 0, C 0.5, C 1, C 2, C 6, C 12 values, t Cmax (time of maximum concentration [C max] in a dosing interval), and the AUC0–12h were compared in elderly and younger patients early post‐transplant. C 0 and C 2 values were compared in elderly and younger patients in the maintenance treatment period post‐transplant. For pharmacodynamic analysis, pre‐transplant IMPDH activity (A pre‐tx), A 0, A 0.5, A 1, A 2, A 6, A 12 values, t Amin (time to minimum enzyme activity [A min]), maximum IMPDH inhibition ([1 − A min/A 0] × 100) and AEC0–12h were compared in elderly and younger patients. The AUC0–12h and AEC0–12h were calculated by the linear trapezoidal method. In addition pharmacokinetic and pharmacodynamic analyses were performed using PK Solver 2.0 30 and Microsoft Excel 2003. An inhibitory effect E max model was used to calculate EC50, for each individual patient.
Statistical analysis
Descriptive statistics were calculated to characterize patient demographics, baseline IMPDH activity, pharmacokinetic and pharmacodynamic parameters of various age groups and periods post‐transplant, which included mean, standard deviation (SD) and coefficient of variation, in the case of non‐normal statistical distribution median and range (25–75% quartile). The Shapiro–Wilk test was used to confirm normal distribution of data. Differences between groups were tested for statistical significance by two‐sided Student's t‐test and Mann–Whitney U‐test, and non‐parametrical anova (Kruskal–Wallis test, followed by Schaich–Hammerle post‐hoc analysis), as applicable. Age dependency of IMPDH activity was analysed by non‐linear regression analysis. Descriptive statistics and statistical tests were performed using SPSS software Version 17.0 (SPSS, Chicago, IL, USA); P < 0.05 was considered statistically significant. The power (1 − β) of the study was calculated by a classic formula:
Results
Baseline characteristics
We divided the 80 patients into two groups according to their age (the cutoff value for the elderly group was 60 years). The baseline characteristics of these 80 patients are summarized in Table 1. None of the characteristics showed significant differences between the two groups, except for the tacrolimus predose concentration (C 0). Both the tacrolimus C 0 and dose and weight normalized tacrolimus C 0 were significantly higher in the group of elderly patients compared with the younger age group, while the daily dose was not significantly different.
IMPDH activity pre‐transplantation and maximal IMPDH inhibition
The IMPDH activity pre‐transplantation in both elderly (n = 26) and younger (n = 54) patients displayed a large inter‐individual variability (coefficient of variation, 70.4% vs. 72.1%; Figure 1). The mean IMPDH activity pre‐transplantation did not differ between the two groups: 46.55 (25–75% quartile: 40.43–67.16) vs. 45.37 (25–75% quartile: 29.90–72.74) μmol s−1 mol−1 AMP (P = 0.97; Table 2). All the PK and PD parameters have no significant correlation with the age of recipients in the population of 80 patients (Figure 2). The power values (1 − β) for all parameters were above 0.90: E max: 0.9382, EC50: 0.9699, baseline IMPDH activity: 0.9115, AUC 0–12h: 0.9207 and AEC0–12h: 0.9719.
Figure 1.
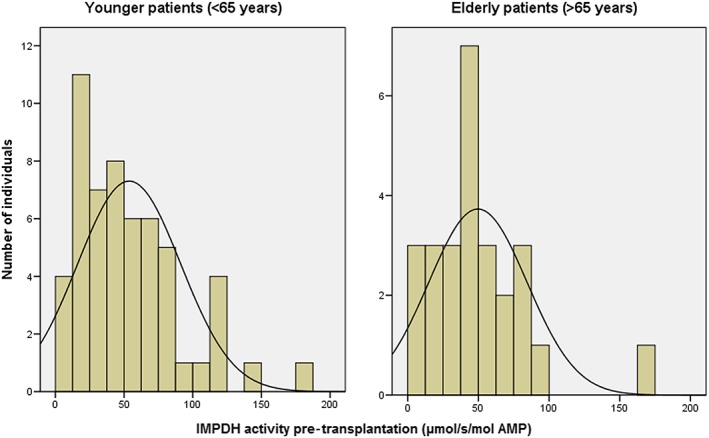
Inter‐individual variability of IMPDH activity in PBMCs of 26 elderly and 54 younger adults
Table 2.
Comparison of MPA concentrations and of IMPDH activity in elderly and younger patients on day 6 post‐transplantation
| Age group | |||
|---|---|---|---|
| Young (19.2–58.4 years) n = 54 | Elderly (60.1–76.2 years) n = 26 | P‐ value a | |
| tMPA concentration (mg l −1 ) | |||
| C 0 | 1.77 (1.10–2.83) | 2.01 (1.47–2.76) | 0.55 |
| C 0.5 | 2.37 (1.18–5.65) | 2.69 (1.9–5.13) | 0.37 |
| C 1 | 3.95 (1.78–7.13) | 3.80 (2.58–6.10) | 0.53 |
| C 2 | 4.00 (2.08–6.09) | 4.12 (2.61–5.23) | 0.99 |
| C 6 | 1.75 (1.22–2.72) | 2.22 (1.27–3.49) | 0.35 |
| C 12 | 1.74 (0.94–3.70) | 2.48 (1.26–3.05) | 0.35 |
| t Cmax (h) | 1 (1–2) | 2 (1–2) | 1.00 |
| AUC 0–12h (h.mg l −1 ) | 32.7 (22.6–49.6) | 32.3 (27.1–43.9) | 0.74 |
| IMPDH activity (μmol s −1 mol −1 AMP) | |||
| A 0 | 20.2 (12.1–36.5) | 17.9 (13.0–36.9) | 0.74 |
| A 0.5 | 17.0 (11.4–28.1) | 23.1 (11.9–26.3) | 0.53 |
| A 1 | 13.3 (9.3–21.0) | 14.1 (9.0–23.4) | 0.99 |
| A 2 | 13.0 (10.3–21.0) | 12.7 (9.6–20.0) | 0.94 |
| A 6 | 17.8 (11.4–26.0) | 13.5 (8.81–25.5) | 0.58 |
| A 12 | 24.6 (17.2–47.2) | 27.5 (15.0–45.8) | 0.98 |
| A pre‐Tx | 45.4 (29.9–72.7) | 46.6 (40.4–67.2) | 0.97 |
| t Amin (h) | 2 (1–2) | 1 (1–5) | 0.51 |
| AEC 0–12h (h.μmol s −1 mol −1 AMP) | 237 (165–339) | 212 (168–349) | 0.92 |
| Maximum IMPDH inhibition (%) | 47.0 (35.8–66.0) | 47.1 (31.6–63.3) | 0.74 |
| E max (μmol s −1 mol −1 AMP) | 43.6 (30.5–75.5) | 55.2 (41.7–72.3) | 0.45 |
| EC 50 (mg l −1 ) | 3.17 (0.84–7.81) | 1.54 (0.92–4.50) | 0.63 |
A, IMPDH activity; AEC0–12h, the area under the effect–time curve for the IMPDH activity; AMP, adenosine monophosphate; A pre‐Tx, IMPDH activity measured before transplantation; AUC0–12h, the area under the concentration–time curve of mycophenolic acid; C, mycophenolic acid concentration; E max, the maximal inhibitory effect; EC50, the MPA concentration at half E max; IMPDH, inosine monophosphate dehydrogenase
All data are expressed as median (25–75% quartile)
Mann–Whitney U test
Figure 2.
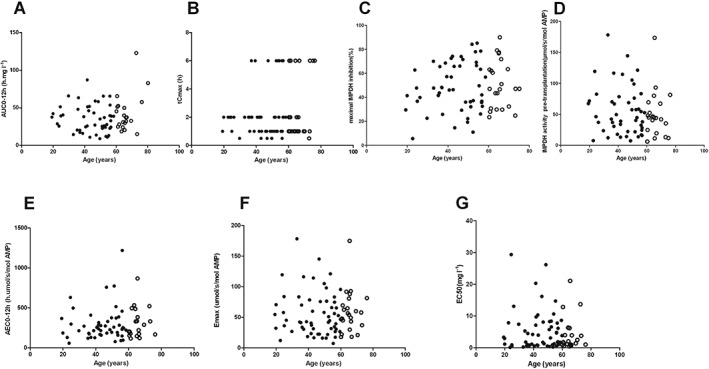
The investigated PK and PD parameters (A–G) as a function of chronological age in 26 elderly (open dots) and 54 younger (solid dots) renal transplant recipients. All the parameters have no significant correlation with the age of recipients: AUC0–12h: r = 0.088, P = 0.36 (A); tCmax: r = −0.065, P = 0.75 (B); Maximum IMPDH inhibition: r = 0.080, P = 0.49 (C); IMPDH activity: r = −0.027; P = 0.81 (D); AEC0–12h: r = −0.022, P = 0.76 (E); E max: r = 0.035, P = 0.52 (F); EC50: r = 0.042, P = 0.66 (G)
MPA concentrations, IMPDH activity and inhibition post‐transplantation
Figure 3 shows the MPA concentrations and the IMPDH activity on day 6 post‐transplantation for the elderly and younger patients groups. The MPA–AUC0–12h and AEC0–12h were not significantly different between the two groups (P = 0.74 and P = 0.92, respectively; Table 2). The MPA concentration and IMPDH activity at each individual time point were also not significantly different between the two groups (Table 2). The MPA concentrations and the IMPDH activity in the stable phase (weeks 3, 7 and 20 post‐transplantation) are summarized in Table 3. With the exception of the MPA C 2 at week 20, the younger group had similar MPA concentrations as the group of elderly patients (P = 0.02; Table 3). The IMPDH activity at weeks 3, 7 and 20 post‐transplantation were also not different between younger and elderly patients (Table 3). There was no difference in MPA exposure nor in IMPDH activity between male (n = 61) and female (n = 19) patients (data not shown).
Figure 3.
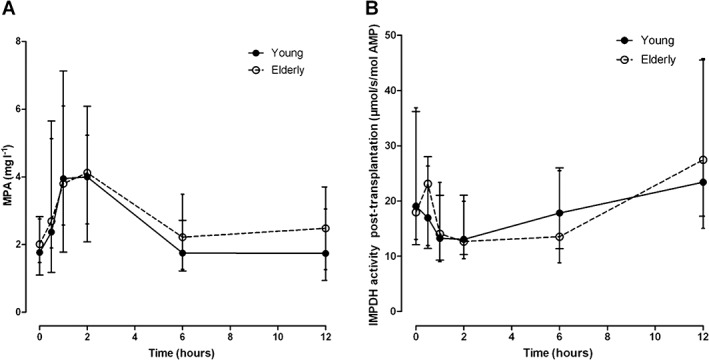
MPA plasma concentration (Figure 3A) and IMPDH activity (Figure 3B) vs. time curve. The MPA exposure (MPA–AUC0–12h) was not significantly different between the two groups and there was also no significant difference at each individual time point between the two groups (Figure 3A). AEC0–12h was not significantly different between the two groups and there was also no significant difference at each time point between the two groups (Figure 3B)
Table 3.
Comparison of MPA concentrations and of IMPDH activity in elderly and younger patients on week 3, 7 and 20 post‐transplantation
| Age group | ||||
|---|---|---|---|---|
| Young (19.2–58.4 years) n = 54 | Elderly (60.1–76.2 years) n = 26 | P value a | ||
| MPA concentration (mg l −1 ) | ||||
| Week 3 | C 0 | 1.46 (0.93–2.26) | 1.39 (0.73–1.98) | 0.78 |
| C 2 | 4.35 (2.6–5.54) | 3.81 (2.42–4.67) | 0.62 | |
| Dose | 745 ± 287 | 717 ± 253 | 0.58 | |
| Week 7 | C 0 | 1.61 (0.98–2.33) | 1.64 (1.25–2.03) | 0.77 |
| C 2 | 3.98 (2.59–6.11) | 4.41 (3.5–6.29) | 0.65 | |
| Dose | 577 ± 229 | 620 ± 206 | 0.45 | |
| Week 20 | C 0 | 1.65 (0.9–2.72) | 1.24 (0.89–1.42) | 0.18 |
| C 2 | 4.94 (3.17–6.69) | 3.27 (2.92–4.41) | 0.02 | |
| Dose | 520 ± 164 | 577 ± 203 | 0.78 | |
| IMPDH activity (μmol s −1 mol −1 AMP) | ||||
| Week 3 | A 0 | 17.0 (11.1–26.2) | 23.0 (14.1–27.6) | 0.26 |
| A 2 | 13.4 (7.64–18.1) | 13.1 (10.7–19.8) | 0.43 | |
| Week 7 | A 0 | 21.5 (13.3–39.5) | 16.6 (10.5–22.2) | 0.06 |
| A 2 | 14.7 (11.5–21.6) | 16.1 (10.2–25.9) | 0.58 | |
| Week 20 | A 0 | 21.6 (14.7–33.0) | 21.7 (16.2–37.6) | 0.57 |
| A 2 | 16.8 (12.0–23.1) | 15.8 (9.21–21.3) | 0.77 | |
A, IMPDH activity; C, mycophenolic acid concentration
All data are expressed as median (25% and 75% quartile)
Mann–Whitney U test
To further characterize the PD of MPA, we plotted IMPDH values against MPA plasma concentrations and calculated individual E max and EC50 values using inhibitory E max models. We found no significant differences in EC50 between elderly and younger patients (P = 0.63; Table 2), although the median EC50 in younger patients (median 3.17 mg l−1; 25–75% quartile, 0.84–7.81 mg l−1) was about twofold higher than in the elderly (median 1.54 mg l−1; 25–75% quartile, 0.92–4.5 mg l−1). A similar result was also found in the E max calculations (P = 0.45; Table 2). The simple E max model and the sigmoid E max model with full inhibition at high concentrations turned out to be the most appropriate models, showing no difference between younger and elderly patients (Figure 4).
Figure 4.
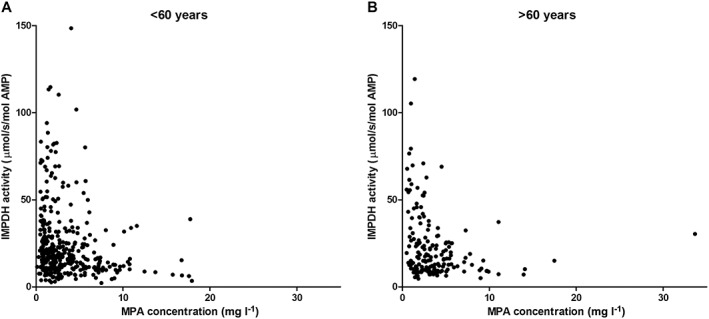
IMPDH activity as a function of MPA plasma concentration in elderly and younger patients on day 6 post‐transplantation. A: Younger patients (<60 years); B: Elderly patients (>60 years)
To further investigate the effect of age on MPA PK and PD, we divided the 80 patients into five groups, according to different ages of patients and keeping the number of every group comparable. Also in this analysis we did not find significant differences in IMPDH activity at baseline, AEC0–12, AUC0–12, maximum IMPDH inhibition, E max and EC50 between the different age groups (Table 4).
Table 4.
Comparison of PK and PD parameters in elderly and younger patients
| Age group (years) | ||||||
|---|---|---|---|---|---|---|
| 19.2–37 (n = 15) | 37.3–46.7 (n = 15) | 47.3–56.5 (n = 20) | 56.6–64.1 (n = 15) | 64.3–76.2 (n = 15) | P value a | |
| Age (years) | 29.1 ± 6.4 | 42.3 ± 2.7 | 52.9 ± 2.7 | 60.4 ± 2.4 | 68.9 ± 4.1 | 0.00 |
| IMPDH baseline (μmol s −1 mol −1 AMP) | 46.1 (33.6–77.1) | 38.8 (19.9–72.7) | 55.9 (26.3–74.3) | 42.7 (27.3–49.1) | 44.4 (23.3–76.7) | 0.63 |
| AEC 0–12h (h.μmol s −1 mol −1 AMP) | 213 (152–346) | 238 (199–280) | 255 (184–379) | 178 (111–234) | 289 (186–344) | 0.68 |
| AUC 0–12h (h.mg l −1 ) | 39.0 (23–49.6) | 40.2 (26.1–49.8) | 25.6 (15–37.6) | 33.9 (25.7–48.2) | 32.5 (30.3–38.6) | 0.59 |
| Maximum IMPDH inhibition (%) | 43.7 (38.3–48.2) | 57.2 (46.1–68.6) | 43.3 (33.0–70.9) | 42.7 (31.2–59.6) | 47.1 (43.4–71.8) | 0.56 |
| E max (μmol s −1 mol −1 AMP) | 54.5 (38.8–77.0) | 36.9 (26.9–69.2) | 47.8 (23.5–75.5) | 47.9 (35.1–65.0) | 56.5 (40.1–80.9) | 0.74 |
| EC 50 (mg l −1 ) | 1.76 (0.73–6.32) | 3.94 (0.79–8.40) | 4.72 (0.93–8.20) | 1.87 (1.21–6.20) | 1.36 (0.91–3.81) | 0.84 |
AEC0–12h, the area under the effect–time curve for the IMPDH activity; AUC0–12h, the area under the concentration–time curve of mycophenolic acid; EC50, the MPA concentration at half E max; E max, the maximal inhibitory effect; IMPDH, inosine monophosphate dehydrogenase
non‐parametrical anova (Kruskal–Wallis test, followed by Schaich–Hammerle post hoc analysis)
Anti‐thymocyte globulin induction therapy
Induction therapy with ATG was given to 16 of 54 young patients (29.6%) and to 7 of 26 elderly patients (26.9%). The AEC0–12h of all patients who received ATG induction therapy was not significantly different compared with patients who had not received ATG [203 (141–295) vs. 251 (181–355) h.mmol s−1 mol−1 AMP; P = 0.15]. However, lymphocyte counts were significantly lower in patients who received ATG compared with those who did not (0.17 ± 0.19 vs. 1.15 ± 0.87 × 109 l−1; P < 0.0001). No significant correlation between AEC0–12h and lymphocyte counts was found (r = 0.34; P = 0.32).
Discussion
This is the first study to comprehensively evaluate the influence of age on the PK and PD of MPA in transplanted adults. The results of this study demonstrate that younger and elderly patients have a comparable MPA exposure when treated with similar MMF doses, and that the PD response, namely (inhibition of) IMPDH activity, was similar for both groups.
A large inter‐individual variability of IMPDH activity was found in the patients just prior to transplantation before immunosuppressive drug treatment had started. The lack of a difference in IMPDH baseline activity between elderly and younger patients is in line with previous studies in smaller numbers of adults 26, 31. The large variability in IMPDH activity could be attributed to genetic variation in IMPDH and/or non‐genetic factors 27, 32.
Wang et al. 21 studied the impact of age on PK parameters in Chinese renal transplant recipients and found that the MPA–AUC was significantly lower in the elderly patients. Miura et al. 22 found no impact of age on dose‐adjusted MPA–AUC0–12, C max, C 0, and clearance of MPA. In both studies, MPA PK was assessed at a single time point after transplantation (10–12 weeks and 4 weeks after transplantation, respectively). In our study, the PK of MPA was investigated at multiple time points after transplantation, both early and later after transplantation. We observed that elderly renal transplant recipients have similar MPA PK as younger patients at all time points. In a previous study in another group of patients, we also found that there is no effect of age on MPA clearance 20. Age could theoretically influence MPA disposition through alterations in protein binding, renal function and hepatic glucuronidation capacity 20, 33, 34. Low levels of albumin and impaired renal function are associated with an increased clearance of total MPA. In the present study both levels of albumin and renal function (estimated by creatinine clearance) were comparable in elderly and younger patients (Table 1). MPA is primarily glucuronidated by uridine diphosphate‐glucuronosyl transferases to an inactive MPA glucuronide (MPAG). Phase II glucuronidation is considered to be less affected by ageing 35. Therefore, the PK of MPA may not be significantly affected by ageing. In contrast to MPA we did find a significant effect of age on the PK of tacrolimus (Table 1). While tacrolimus dose was similar, the elderly had much higher tacrolimus dose and weight normalized predose concentrations compared to the younger patients. Jacobson et al. also showed that elderly kidney recipients had higher normalized tacrolimus predose concentrations than middle aged or young adults 15. The most likely explanation for the reduced tacrolimus clearance in elderly patients is a reduced hepatic or intestinal CYP3A enzyme capacity, although the higher drug exposure may also be due to a lower ABCB1 activity, resulting in higher bioavailability of tacrolimus 36.
Previous studies suggested that higher IMPDH activity before and after transplantation is associated with an increased risk of acute rejection 18, 26. Based on these studies there was some debate on whether pre‐dose IMPDH activity or maximal IMPDH inhibition would be superior in identifying patients at risk of acute rejection and MMF‐related side effects 24. Elderly patients have a lower risk of developing acute rejection and are more prone to die from infections. The results of the present study do not offer an explanation for this observation. IMPDH activity before the transplantation and also after initiation of MMF treatment showed no difference between the two age groups (Table 2). Rother et al. 37 also found there were no age‐related differences in IMPDH activity in healthy individuals, but they studied mainly children. In the present study, the degree of maximum IMPDH inhibition after MMF intake was comparable in elderly patients and younger patients (mean maximum inhibition 47.1% and 47.0% respectively), which is less than the mean maximum inhibition reported in some other studies 37, 38.
Inhibitory E max models were used to describe the overall relationship between the MPA concentration and the IMPDH activity and to calculate the estimated MPA concentration associated with half‐maximal inhibitory effect on IMPDH activity in different populations. It has been postulated that the pharmacologic effect of MPA is best described by the free (unbound) MPA (fMPA) concentration 39. Smits et al. investigated effects of fMPA on IMPDH inhibition in paediatric kidney transplant patients and found there was a good correlation between the total and free MPA concentrations 40. In the present PD assessment, the time course of IMPDH activity in mononuclear cells generally mirrored the MPA plasma concentration for both groups. The extent of IMPDH inhibition (E max value) was similar in elderly patients and younger patients in terms of total IMPDH activity over the 12‐h period following administration. As might be expected, the results demonstrated very similar EC50 values for both groups. The median EC50 values in the present study substantially correspond with MPA C 0 levels. The EC50 values are in line with previous studies, both in adult patients 41 and in paediatric patients 42. The EC50 values of all above studies are close to the proposed target MPA trough concentrations for the first months after transplantation 43.
In this study blood samples were taken at day 6 after transplantation. Previous studies have shown that already within the first week (as soon as day 3) after surgery, the exposure to MPA is correlated with the incidence of acute rejection 44. Apparently the pharmacodynamic effect is already present shortly after initiation of treatment. Therefore we do not think that the results of this study would be different if samples had been drawn after 2 or 3 weeks of treatment. Induction of IMPDH activity has been observed in whole blood of kidney transplant recipients on MMF therapy. Chiarelli et al. showed that predose IMPDH activity increased over time, with a longer duration of MMF treatment 24. Also in our study, a trend towards an increase in IMPDH activity was observed in the younger age group (Table 3).
In conclusion, MPA plasma concentrations and IMPDH activity displayed a high inter‐individual variability throughout the entire age range studied. However, age did not significantly affect the PK or PD of MPA. Therefore it is unlikely that the lower incidence of acute rejection in elderly patients, or the higher risk to die from a severe infection in elderly patients is due to different handling of MPA in the elderly. Possibly it is the senescence of the immune system that is responsible for the different clinical outcome in elderly transplant patients. There is a need for prospective clinical trials to investigate which immunosuppressive protocols are best for this age group. In elderly patients who fulfil the criteria for frailty, this may be even more relevant 45. Given the fact that the proportion of elderly patients in our population is rising steeply, there is a sense of urgency that should be felt by all of us.
Competing Interests
This study was supported by F. Hoffmann La Roche Ltd, Basel, Switzerland. T. van Gelder has received lecture and consulting fees, as well as grant support from Novartis, TEVA, Astellas, Chiesi and Roche. D.A. Hesselink has received lecture and consulting fees, as well as grant support from Astellas, Chiesi and Bristol‐Myers Squibb. J.‐T. Tang has received grant support from the China Scholarship Council (201 506 245 010). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributors
J‐TT contributed to the research design, analysing data and writing of the article. BCdeW contributed to performing the research, analysing data and writing of the article. DAH contributed to the research design, participated in performing the research, analysing data and writing of the article. FS contributed to performing the research, analysing data and revision of the article. L‐LW contributed to research design and revision of the article. TvanG contributed to research design, participated in performing the research, analysing data and writing of the article.
Tang, J.‐T. , de Winter, B. C. , Hesselink, D. A. , Sombogaard, F. , Wang, L.‐L. , and van Gelder, T. (2017) The pharmacokinetics and pharmacodynamics of mycophenolate mofetil in younger and elderly renal transplant recipients. Br J Clin Pharmacol, 83: 812–822. doi: 10.1111/bcp.13154.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, et al. The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 2015; 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant 2014; 14 (Suppl. 1): 11–44. [DOI] [PubMed] [Google Scholar]
- 5. Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093–2109. [DOI] [PubMed] [Google Scholar]
- 6. Meier‐Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation 2000; 69: 885–889. [DOI] [PubMed] [Google Scholar]
- 7. Karim A, Farrugia D, Cheshire J, Mahboob S, Begaj I, Ray D, et al. Recipient age and risk for mortality after kidney transplantation in England. Transplantation 2014; 97: 832–838. [DOI] [PubMed] [Google Scholar]
- 8. Krenzien F, ElKhal A, Quante M, Rodriguez Cetina Biefer H, Hirofumi U, Gabardi S, et al. A rationale for age‐adapted immunosuppression in organ transplantation. Transplantation 2015; 99: 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tullius SG, Milford E. Kidney allocation and the aging immune response. N Engl J Med 2011; 364: 1369–1370. [DOI] [PubMed] [Google Scholar]
- 10. Denecke C, Bedi DS, Ge X, Kim IK, Jurisch A, Weiland A, et al. Prolonged graft survival in older recipient mice is determined by impaired effector T‐cell but intact regulatory T‐cell responses. PLoS One 2010; 5: e9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metcalf TU, Cubas RA, Ghneim K, Cartwright MJ, Grevenynghe JV, Richner JM, et al. Global analyses revealed age‐related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015; 14: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Betjes MG, Litjens NH. Chronic kidney disease and premature ageing of the adaptive immune response. Curr Urol Rep 2015; 16: 471. [DOI] [PubMed] [Google Scholar]
- 13. Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end‐stage renal disease. Kidney Int 2011; 80: 208–217. [DOI] [PubMed] [Google Scholar]
- 14. Shi YY, Hesselink DA, van Gelder T. Pharmacokinetics and pharmacodynamics of immunosuppressive drugs in elderly kidney transplant recipients. Transplant Rev (Orlando) 2015; 29: 224–230. [DOI] [PubMed] [Google Scholar]
- 15. Jacobson PA, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant 2012; 12: 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falck P, Asberg A, Byberg KT, Bremer S, Bergan S, Reubsaet JL, et al. Reduced elimination of cyclosporine A in elderly (>65 years) kidney transplant recipients. Transplantation 2008; 86: 1379–1383. [DOI] [PubMed] [Google Scholar]
- 17. van Gelder T, Hesselink DA. Mycophenolate revisited. Transpl Int 2015; 28: 508–515. [DOI] [PubMed] [Google Scholar]
- 18. Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol 2014; 88: 1351–1389. [DOI] [PubMed] [Google Scholar]
- 19. Gabardi S, Tullius SG, Krenzien F. Understanding alterations in drug handling with aging: a focus on the pharmacokinetics of maintenance immunosuppressants in the elderly. Curr Opin Organ Transplant 2015; 20: 424–430. [DOI] [PubMed] [Google Scholar]
- 20. van Hest RM, Mathot RA, Pescovitz MD, Gordon R, Mamelok RD, van Gelder T. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta‐analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol 2006; 17: 871–880. [DOI] [PubMed] [Google Scholar]
- 21. Wang CX, Meng FH, Chen LZ, Ren B, Li SX, Fei JG, et al. Population pharmacokinetics of mycophenolic acid in senile Chinese kidney transplant recipients. Transplant Proc 2007; 39: 1392–1395. [DOI] [PubMed] [Google Scholar]
- 22. Miura M, Satoh S, Kagaya H, Saito M, Inoue T, Tsuchiya N, et al. No impact of age on dose‐adjusted pharmacokinetics of tacrolimus, mycophenolic acid and prednisolone 1 month after renal transplantation. Eur J Clin Pharmacol 2009; 65: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 23. Gummert JF, Barten MJ, van Gelder T, Billingham ME, Morris RE. Pharmacodynamics of mycophenolic acid in heart allograft recipients: correlation of lymphocyte proliferation and activation with pharmacokinetics and graft histology. Transplantation 2000; 70: 1038–1049. [DOI] [PubMed] [Google Scholar]
- 24. Chiarelli LR, Molinaro M, Libetta C, Tinelli C, Cosmai L, Valentini G, et al. Inosine monophosphate dehydrogenase variability in renal transplant patients on long‐term mycophenolate mofetil therapy. Br J Clin Pharmacol 2010; 69: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raggi MC, Siebert SB, Steimer W, Schuster T, Stangl MJ, Abendroth DK. Customized mycophenolate dosing based on measuring inosine‐monophosphate dehydrogenase activity significantly improves patients' outcomes after renal transplantation. Transplantation 2010; 90: 1536–1541. [DOI] [PubMed] [Google Scholar]
- 26. Glander P, Hambach P, Braun KP, Fritsche L, Giessing M, Mai I, et al. Pre‐transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 2004; 4: 2045–2051. [DOI] [PubMed] [Google Scholar]
- 27. Sombogaard F, van Schaik RH, Mathot RA, Budde K, van der Werf M, Vulto AG, et al. Interpatient variability in IMPDH activity in MMF‐treated renal transplant patients is correlated with IMPDH type II 3757 T>C polymorphism. Pharmacogenet Genomics 2009; 19: 626–634. [DOI] [PubMed] [Google Scholar]
- 28. Brandhorst G, Streit F, Goetze S, Oellerich M, Armstrong VW. Quantification by liquid chromatography tandem mass spectrometry of mycophenolic acid and its phenol and acyl glucuronide metabolites. Clin Chem 2006; 52: 1962–1964. [DOI] [PubMed] [Google Scholar]
- 29. Glander P, Sombogaard F, Budde K, van Gelder T, Hambach P, Liefeldt L, et al. Improved assay for the nonradioactive determination of inosine 5′‐monophosphate dehydrogenase activity in peripheral blood mononuclear cells. Ther Drug Monit 2009; 31: 351–359. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add‐in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 2010; 99: 306–314. [DOI] [PubMed] [Google Scholar]
- 31. Glander P, Hambach P, Braun KP, Fritsche L, Waiser J, Mai I, et al. Effect of mycophenolate mofetil on IMP dehydrogenase after the first dose and after long‐term treatment in renal transplant recipients. Int J Clin Pharmacol Ther 2003; 41: 470–476. [DOI] [PubMed] [Google Scholar]
- 32. Wieland E, Olbricht CJ, Susal C, Gurragchaa P, Bohler T, Israeli M, et al. Biomarkers as a tool for management of immunosuppression in transplant patients. Ther Drug Monit 2010; 32: 560–572. [DOI] [PubMed] [Google Scholar]
- 33. Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation 2007; 84: 285–291. [DOI] [PubMed] [Google Scholar]
- 34. Meier‐Kriesche HU, Kaplan B. Immunosuppression in elderly renal transplant recipients: are current regimens too aggressive? Drugs Aging 2001; 18: 751–759. [DOI] [PubMed] [Google Scholar]
- 35. Liston HL, Markowitz JS, DeVane CL. Drug glucuronidation in clinical psychopharmacology. J Clin Psychopharmacol 2001; 21: 500–515. [DOI] [PubMed] [Google Scholar]
- 36. de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 1999; 37: 485–505. [DOI] [PubMed] [Google Scholar]
- 37. Rother A, Glander P, Vitt E, Czock D, von Ahsen N, Armstrong VW, et al. Inosine monophosphate dehydrogenase activity in paediatrics: age‐related regulation and response to mycophenolic acid. Eur J Clin Pharmacol 2012; 68: 913–922. [DOI] [PubMed] [Google Scholar]
- 38. Budde K, Glander P, Kramer BK, Fischer W, Hoffmann U, Bauer S, et al. Conversion from mycophenolate mofetil to enteric‐coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation 2007; 83: 417–424. [DOI] [PubMed] [Google Scholar]
- 39. Nowak I, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 1995; 41: 1011–1017. [PubMed] [Google Scholar]
- 40. Smits TA, Cox S, Fukuda T, Sherbotie JR, Ward RM, Goebel J, et al. Effects of unbound mycophenolic acid on inosine monophosphate dehydrogenase inhibition in pediatric kidney transplant patients. Ther Drug Monit 2014; 36: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glander P, Sommerer C, Arns W, Ariatabar T, Kramer S, Vogel EM, et al. Pharmacokinetics and pharmacodynamics of intensified versus standard dosing of mycophenolate sodium in renal transplant patients. Clin J Am Soc Nephrol 2010; 5: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fukuda T, Goebel J, Thogersen H, Maseck D, Cox S, Logan B, et al. Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol 2011; 51: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit 2006; 28: 145–154. [DOI] [PubMed] [Google Scholar]
- 44. van Gelder T, Tedesco Silva H, de Fijter JW, Budde K, Kuypers D, Arns W, et al. Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid. Transplantation 2010; 89: 595–599. [DOI] [PubMed] [Google Scholar]
- 45. Exterkate L, Slegtenhorst BR, Seyda M, Schuitenmaker JM, Quante M, Uehara H, et al. Frailty and transplantation. Transplantation 2016; 100: 727–733. [DOI] [PubMed] [Google Scholar]


