Abstract
Aim
To perform a meta‐analysis of available cohort studies on the association between sertraline use by pregnant women in the first trimester and the findings of congenital anomalies in infants.
Methods
A comprehensive search of articles published from the index date up to 31st December 2015 investigating the aforementioned associations was conducted on PubMed and Web of Science. Mesh headings used included the terms “serotonin reuptake inhibitor,” “sertraline,” “congenital anomalies” and “obstetrical outcome.”
Results
Twelve cohort studies that involved 6 468 241 pregnant women were identified. We summarized odds ratios (ORs) and 95% confidence intervals (CIs) of congenital anomalies using the random‐effects model. Pregnant women who used sertraline in the first trimester had a statistically significant increased risk of infant cardiovascular‐related malformations (OR = 1.36; 95% CI = 1.06–1.74; I2 = 64.4%; n = 12) as well as atrial and/or ventricular septal defects (OR = 1.36, 95% CI = 1.06–1.76; I2 = 62.2%; n = 8). Additionally, positive but nonsignificant associations between sertraline use and congenital anomalies of the nervous system (OR = 1.39; 95% CI = 0.83–2.32; I2 = 0%; n = 5), digestive system (OR = 1.23; 95% CI = 0.76–1.98; I2 = 0%; n = 5), eye, ear, face and neck (OR = 1.08; 95% CI = 0.33–3.55; I2 = 32.1%; n = 3), urogenital system (OR = 1.03; 95% CI = 0.73–1.46; I2 = 0%; n = 5), and musculoskeletal system (OR = 0.97; 95% CI = 0.69–1.36; I2 = 0%; n = 5) were observed.
Conclusion
This meta‐analysis suggested that the use of sertraline use by pregnant women in the first trimester had an increased risk of cardiovascular‐related malformations as well as atrial and/or ventricular septal defects in infants. Meanwhile, nonsignificant associations between sertraline use and other congenital anomalies were found. More cohort studies are warranted to provide detailed results of other congenital anomalies.
Keywords: antidepressive agents, cohort studies, congenital anomalies, meta‐analysis, pregnancy, sertraline
Tables of Links
| TARGETS | |
|---|---|
| G protein‐coupled receptors | |
| 5‐HT2B receptor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2534 |
Introduction
Approximately 10% of pregnant women experience depression 3. Among these women, 20% display signs of depression such as sleep disturbance, guilt and low energy 4. Since exposure to untreated depression during pregnancy might be associated with serious adverse consequences for infants, including premature birth, low birth weight, and future behavioural disturbances 5, 6, prescription rate of antidepressant has showed an increasing trend upward. As first‐generation antidepressants, tricyclics were popular for several decades until there was a drastic shift in the use of tricyclics in the 1980s to selective serotonin (5‐hydroxytryptamine, 5‐HT) reuptake inhibitors (SSRIs) in the 1990s 7. SSRIs (sertraline, fluoxetine, citalopram, paroxetine, fluvoxamine and escitalopram) are more valid and tolerable compared to first‐generation antidepressants 8; consequently, they have become the most frequently prescribed pharmacological treatment for depression during pregnancy 9, 10. Several studies have demonstrated that the prescription rates of SSRIs were 3% and 4–10% in Europe and North America, respectively 11, 12. By comparison, the prescription rates for tricyclics were only 0.14% in Denmark 13.
Among the SSRIs, sertraline is one of the most frequently used antidepressants worldwide 14. In vivo and in vitro studies have suggested that 15 heart defects might be attributed to a mechanism of 5‐HT playing a role in cardiac morphogenesis during endocardial cushion formation. Furthermore, Sari et al. 16 found that serotonin promoted the proliferation of foetal heart cells and abnormal serotonin levels or misuse of the serotonin‐uptake blocker may change the heart development. However, epidemiological studies have provided controversial evidence of the association between sertraline use and the risk of cardiovascular malformations. Several studies 17, 18, 19, 20 have suggested that sertraline could increase the risk of cardiovascular‐related malformations in infants, while other studies 21, 22, 23, 24, 25, 26, 27, 28 found no association at all. A recent meta‐analysis 29 found that sertraline was not associated with the risk of heart defects. However, six cohort studies 18, 19, 20, 23, 26, 27 that reported the outcomes of cardiovascular‐related malformations were excluded in that study. Moreover, several limitations of the previous meta‐analysis were noted: (i) for the authors, journals or institutions of the publications, the process of data extraction and analyses were not blind; and (ii) the study lacked subgroup analyses based on important potential confounders. Except for cardiovascular‐related malformations, very few studies provide evidence of sertraline use and malformations of other systems such as the nervous, digestive, eye, ear, face and neck, urogenital, and musculoskeletal systems.
Given the inconsistency of previous results, as well as to provide the best estimates of the effect of sertraline usage during first trimester of pregnancy, we performed this meta‐analysis of cohort studies to investigate the association between sertraline use during the first trimester of pregnancy and selective congenital anomalies.
Methods
Eligibility criteria, information sources, search strategy
We followed the Meta‐analysis of Observational Studies in Epidemiology guideline 30 and Preferred Reporting Items for Systematic reviews and Meta‐analyses guideline 31 to perform and report our meta‐analysis. A systematic literature search of PubMed (1964 to 31st December 2015) and Web of Science (1992 to 31st December 2015) databases was independently conducted by two investigators, for all correlative studies with respect to the effect of the maternal use of sertraline in the first trimester of pregnancy on the risk of congenital anomalies in infants. We carried out searches using the following keywords and medical subject heading terms including (serotonin reuptake inhibitors OR SSRI OR fluoxetine OR paroxetine OR citalopram OR sertraline OR fluoxamine) AND (malformations OR birth outcomes OR obstetrical outcome OR congenital abnormalities). Additionally, the references cited in retrieved articles were scrutinized by manual search.
Study selection
Studies were considered for inclusion if they: (i) were cohort studies; (ii) defined the exposure period of sertraline as occurring in the first trimester of pregnancy; (iii) defined the nonexposed group as pregnant women who did not use any kind of antidepressants; (iv) reported usable risk estimates (e.g., odds ratio, relative risk or risk ratio with 95% confidence intervals or indispensable data to calculate) of the association between sertraline exposure and congenital anomalies; and (v) were published in English.
Studies were excluded if they met the following criteria: (i) were review articles, systemic reviews and meta‐analyses, commentaries, editorials or meeting abstracts; (ii) used other study designs (e.g., case–control study, descriptive study, randomized controlled trial etc.); (iii) included pregnant women who were exposed to more than two kinds of antidepressant simultaneously; and (iv) defined the exposure period as throughout or another trimester of pregnancy other than the first.
When the results from the same study were reported in different manuscripts, only the newest or most complete study with the largest number of the cohort or cases at the endpoint of our interest was included. The selection and exclusion of studies were previewed by two investigators (T.‐N.Z. and Z.‐Q.S.). Disagreements were resolved by a third author (Q.‐J.W.) through discussion.
A quality assessment of the included studies was conducted by two independent researchers (T.‐N.Z. and S.‐Y.G.) based on the Newcastle–Ottawa Scale for cohort studies 32. The scale consists of eight items, and all of the items were available to our study question. The items are separated into three domains (selection, comparability and outcome). We applied these Newcastle–Ottawa Scale parameters to evaluate the studies rather than scoring them or categorizing them into high or low quality on the basis of the scores.
Data extraction
Data were extracted independently by a standardized form by two reviewers (T.‐N.Z. and Z.‐Q.S.). Dissenting opinions were resolved through discussion. The following data were abstracted from each study: the name of the first author; year of publication; country; number of cases; number of cohorts; study design; exposure time; outcome with their risk estimates and 95% confidence intervals (CIs); plus adjustment confounders. Since the limited number of studies of several outcomes (e.g. conotruncal and major arch anomalies, transposition of great arteries etc.), we only summarized and presented the outcomes of cardiovascular anomalies, cardiac malformations and septal defects. If there were multiple estimates of the association, we extracted the estimate that was adjusted for the largest number of potential confounders. If there was no adjusted estimate in the study, we used the crude estimate.
As for the studies 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 reporting the results of cardiovascular anomalies as other outcomes but with similar definition (e.g., as specific heart anomalies, any cardiac defects, cardiac malformations, congenital cardiovascular defects, congenital heart malformation, all major cardiovascular anomalies and other congenital anomalies of the heart), we extracted these data to calculate the summarized odds ratio (OR) of overall cardiovascular‐related malformations. Similar patterns are also carried out in the analysis of the studies 17, 18, 19, 21, 22, 25, 26, 28 reporting the outcomes as atrial septal defect (ASD), ventricular septal defect (VSD), septal defect, atrioventricular septal defect, in addition to ASD and/or VSD. We extracted these data to calculate summarized OR of ASD and/or VSD events. Additionally, for the studies 18, 21, 23, 25, 26 reporting results of defects of genital organs, defects of external genital organs, defects of internal urinary system, defects of urinary system, and urogenital malformation, the data were extracted to calculate the summarized OR of urogenital malformations 33.
Data synthesis
Since congenital anomaly is a relatively rare event, we assumed that ORs were comparable estimates of the risk ratios (RRs). However, if the study did not provide the estimate, we calculated it through raw data in the study 27. For studies that separately reported the risk estimates of sertraline, we used the effective count method proposed by Harmling et al. 33 to recalculate the effect estimate 34, 35, 36, 37, 38. Random‐effects models by DerSimonian and Laird 39 were applied to obtain summarized OR estimates across the included studies. We calculated the I2 statistic to quantify the magnitude between‐study heterogeneity, and assigned values of 50% or less, 51%–75%, and 76% or more for low‐, moderate‐ and high‐ heterogeneity, respectively 40, 41, 42, 43, 44, 45, 46. Subgroup analysis was carried out based on geographic location (Europe, North America and other regions). Furthermore, a heterogeneity analysis was also conducted to assess the effect of adjustment of confounders, such as maternal age, socioeconomic situations, smoking or drinking situations, body mass index, pregnancy outcomes, and parity. In addition, a sensitivity analysis was conducted and the summarized OR was computed with the omission of one study at a time to detect whether results were strongly influenced by a specific study 42, 47, 48, 49. Finally, we evaluated publication bias through Egger's linear regression 50, Begg's rank‐correlation methods 51 (publication bias considered present if P ≤ 0.10) and visual inspection of funnel plots. All analyses were performed using Stata software, version 12.1 (StataCorp LP, College Station, Texas).
Results
Study selection
We identified a total of 1874 papers (1437 and 437 studies from PubMed and Web of Science, respectively) via the search strategy. Of these studies, 1841 of them were excluded on the basis of titles and abstracts. The remaining 33 studies were considered of interest and full‐text studies were retrieved for detailed evaluation, 21 of these studies were subsequently excluded. Finally, 12 studies 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 were eligible for inclusion in the meta‐analysis, representing a total of 6 468 241 individuals (Figure 1).
Figure 1.
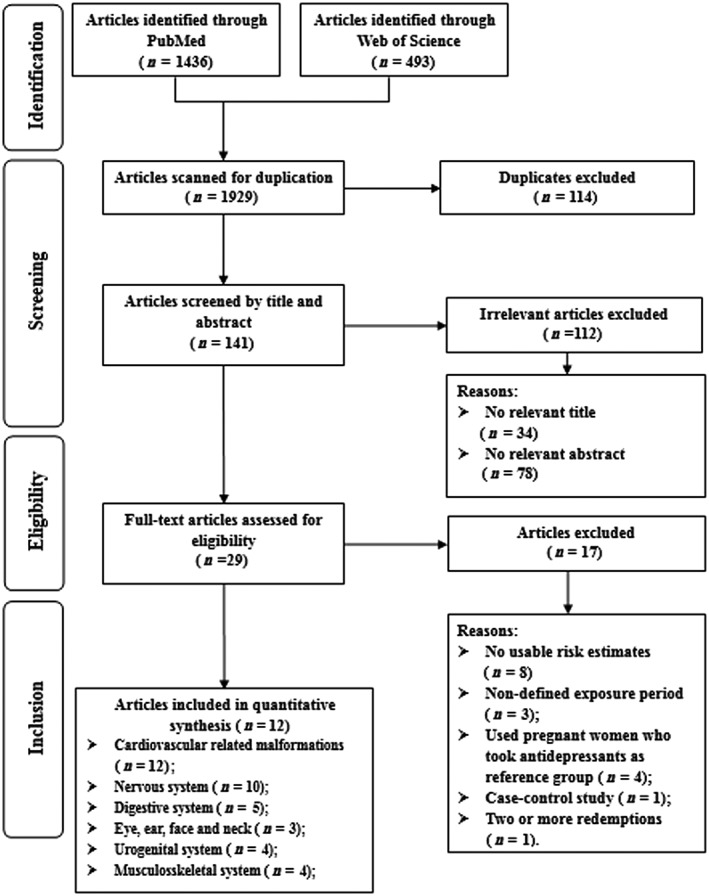
Flow‐chart of study selection
Study characteristics
Characteristics of all 12 studies 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 are shown in Table 1. Among the 12 studies, 10 were prospective cohort studies 17, 18, 19, 20, 21, 22, 23, 25, 27, 28 and two were retrospective cohort studies 24, 26. These studies were published between 2007 28 and 2015 21, 22, which covered a study period from 1990 to 2010. The number of participants in each study ranged from 18 493 21 to 2 303 647 22, and the number of cases ranged from 515 27 to 26 854 22. The studies were generally from European countries (n = 7), North America (n = 3), and one study each were conducted in Australia and Israel. Many studies (n = 9) adjusted for potentially important confounders: maternal age (n = 9); smoking and drinking situation (n = 7); parity (n = 7); pregnancy complication (n = 5); socioeconomic situation (n = 2); and body mass index (n = 1), with the exception of three studies.
Table 1.
Characteristics of cohort studies in the meta‐analysis
| First author (ref), year, location | Study period | No. of cohort/case | Outcomes | Risk estimates (95% CI) | Adjusted factors |
|---|---|---|---|---|---|
| Furu et al. 22 , 2015, Europe | 1996–2010 | 2 303 647 | Odds ratio | Maternal age, year of birth, birth order, smoking, maternal diabetes, country and use of other prescribed drugs. | |
| 26 854 | Any cardiac defects | 1.13 (0.93–1.38) | |||
| 17 573 | Atrial and ventricular septal defect | 1.05 (0.82–1.35) | |||
| 1079 | Atrioventricular septal defect | 1.81 (0.80–4.06) | |||
| 2231 | Conotruncal and major arch anomalies | 1.02 (0.48–2.15) | |||
| 2599 | Left ventricular outflow tract obstruction | 0.82 (0.37–1.84) | |||
| 2720 | Right ventricular outflow tract obstruction | 1.40 (0.81–2.42) | |||
| Berard et al. 21 , 2015, North America | 1998–2010 | 18 493 | Risk ratio | Maternal age, welfare status, other diabetes, hypertension, asthma, and medication use. | |
| 354 | Cardiac malformations | 1.16 (0.62–2.19) | |||
| 281 | Ventricular/ atrial septal defect | 1.34 (1.02–1.76) | |||
| 122 | Nervous system | 1.67 (0.68–4.13) | |||
| 87 | Eye, ear, face and neck | 0.46 (0.06–3.32) | |||
| 181 | Digestive system | 1.13 (0.46–2.77) | |||
| 164 | Genital organs | 0.97 (0.34–2.75) | |||
| 144 | Urinary system | 0.86 (0.27–2.72) | |||
| 635 | Musculoskeletal system | 1.04 (0.63–1.72) | |||
| Ban et al. 23 , 2014, Europe | 1990–2009 | 349 127 | Odds ratio | Maternal age at the end of pregnancy, year of childbirth, Townsend deprivation quintile, maternal smoking history, body mass index before pregnancy, and maternal diabetes, hypertension, asthma and epilepsy in the year before conception or during pregnancy. | |
| N/A | MCAs | 1.27 (0.85–1.89) | |||
| N/A | Specific heart anomalies | 1.52(0.78–2.96) | |||
| N/A | Genital system | 0.32 (0.04–2.40) | |||
| N/A | Urinary system | 0.54 (0.08–3.76) | |||
| N/A | Nervous system | 1.79 (0.42–7.54) | |||
| N/A | Musculoskeletal system | 2.13 (0.51–8.90) | |||
| N/A | Digestive system | 2.69 (0.67–10.76) | |||
| Huybrechts et al. 17 , 2014, North America | 2000–2007 | 949 504 | Odds ratio | N/A | |
| 6532 | Cardiac malformations | 1.27 (1.07–1.52) | |||
| 3275 | Ventricular septal defect | 1.24 (0.96–1.59) | |||
| 1062 | Right ventricular outflow tract obstruction | 1.03 (0.64–1.66) | |||
| 3280 | Other cardiac defect | 1.39 (1.10–1.76) | |||
| Jimenez‐Solem et al. 18 , 2012, Europe | 1997–2009 | 848 786 | Odds ratio | Mother's age, parity, income, education, smoking and year of conception. | |
| N/A | Congenital malformation of the heart | 2.73 (1.75–4.26) | |||
| N/A | Septal defects | 3.09 (1.82–5.25) | |||
| N/A | Ventricular septal defects | 3.60 (1.86–6.96) | |||
| N/A | Atriventricular septal defects | 2.85 (1.35–5.99) | |||
| N/A | Nervous system | 0.85 (0.12–6.07) | |||
| N/A | Eye | 1.05 (0.15–7.45) | |||
| N/A | Ear, face and neck | 6.13 (0.85–44.05) | |||
| N/A | Digestive system | 1.43 (0.36–5.74) | |||
| N/A | Internal urinary system | 0.44 (0.06–3.11) | |||
| N/A | External genital organs | 0.41 (0.06–2.93) | |||
| N/A | Musculoskeletal system | 0.83 (0.12–5.90) | |||
| Nordeng et al. 24 , 2012, Europe | 1999–2009 | 63 395 | Odds ratio | Maternal depression, maternal age at delivery, parity and use of psychotropic drugs during pregnancy. | |
| 542 | Cardiovasular malformation | 1.15 (0.16–8.28) | |||
| Colvin et al. 25 , 2011, Western Australia | 2002–2005 | 123 405 | Odds ratio | N/A | |
| 672 | Cardiovascular anomalies | 1.74 (0.96–3.17) | |||
| N/A | Ventricular septal defect | 0.49 (0.07–3.50) | |||
| 289–294 | Nervous system | 1.08 (0.35–3.38) | |||
| 224–229 | Ear, face and neck | 0.46 (0.07–3.31) | |||
| 1330 | Urogenital system | 1.27 (0.07–2.09) | |||
| 539 | Digestive system | 0.97 (0.40–2.36) | |||
| 1059 | Musculoskeletal system | 0.69 (0.33–1.46) | |||
| Malm et al. 26 , 2011, Europe | 1996–2006 | 635 583 | Odds ratio | Maternal age at the end of pregnancy, parity, year of pregnancy ending, marital status, smoking any time during pregnancy, other reimbursed psychiatric drug purchases and entitlement for special reimbursement for prepregnancy diabetes. | |
| 8146 | All major cardiovascular anomalies | 0.65 (0.34–1.25) | |||
| 1281 | Atrial septal defect | 0.93 (0.23–3.76) | |||
| 5470 | Ventricular septal defect | 0.53 (0.22–1.29) | |||
| 435 | Conotruncal heart defect | 1.27 (0.18–9.15) | |||
| 239 | Transposition of great arteries | 2.55 (0.35–18.62) | |||
| N/A | Central nervous system | 1.35 (0.50–3.64) | |||
| N/A | Digestive system | 1.09 (0.35–3.41) | |||
| N/A | Urogenital system | 1.22 (0.55–2.74) | |||
| N/A | Musculoskeletal system | 0.97 (0.50–1.88) | |||
| Kornum et al. 19 , 2010, Europe | 1991–2007 | 216 042 | Odds ratio | Maternal smoking status, maternal age, birth order and birth year. | |
| 1410 | Cardiac malformations | 3.0 (1.4–6.4) | |||
| N/A | Septal heart defect | 3.3 (1.5–7.5) | |||
| Merlob et al. 20 , 2009, Israel | 2000–2007 | 67 871 | Relative risk | N/A | |
| 1084 | Congenital heart malformation | 8.78 (1.08–71.42) | |||
| Oberlander et al. 27 , 2008, North America | 1998–2001 | 11 957 | Odds ratio | N/A | |
| 515 | Cardiovascular congenital defects | 1.03 (0.33–3.23) | |||
| Kallen et al. 28 , 2007, Europe | 1995–2004 | 880 431 | Odds ratio | Year of birth, maternal age, parity, smoking and ≥3 previous miscarriages. | |
| 11 384 | Any cardiac defect | 0.76 (0.47–1.23) | |||
| 7174 | VSD and/or ASD | 1.06 (0.63–1.77) | |||
| 1218 | Unspecified cardiac defect | 0.55 (0.01–3.06) |
CI, confidence interval; ASD, atrial septal defect; MCA, major congenital anomalies; N/A, not available; VSD, ventricular septal defect
Quality assessment
Table 2 presents the results of the quality assessment based on the Newcastle–Ottawa Scale. All studies satisfied adequate quality. Meanwhile, in the classification of ‘control for important factor or additional factor’, six studies were not assigned to two scores because they adjusted for fewer than two important confounders. Moreover, in the classification of ‘follow‐up long enough for outcomes to occur’ and ‘adequacy of follow‐up of cohorts’, five studies were not assigned any score because they did not mention follow‐up in their studies.
Table 2.
Methodological quality of cohort studies included in the meta‐analysis
| First author (reference), publication year | Representativeness of the exposed cohort | Selection of the unexposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Control for important factor/additional factor a | Assessment of outcome | Follow‐up long enough for outcomes to occur b | Adequacy of follow‐up of cohorts c | Total scores |
|---|---|---|---|---|---|---|---|---|---|
| Furu et al. 22 , 2015 | * | * | * | * | ** | * | * | * | 9 |
| Berard et al. 21 , 2015 | * | * | * | * | * | * | * | * | 8 |
| Ban et al. 23 , 2014 | * | * | * | * | ** | * | ‐ | ‐ | 7 |
| Huybrechts et al. 17 , 2014 | * | * | * | * | ‐ | * | * | * | 7 |
| Jimenez‐Solem et al. 18 , 2012 | * | * | * | * | ** | * | * | * | 9 |
| Nordeng et al. 24 , 2012 | * | * | * | * | * | * | ‐ | ‐ | 6 |
| Colvin et al. 25 , 2011 | * | * | * | * | ‐ | * | * | * | 7 |
| Malm et al. 26 , 2011 | * | * | * | * | ** | * | ‐ | ‐ | 7 |
| Kornum et al. 19 , 2010 | * | * | * | * | ** | * | * | * | 9 |
| Merlob et al. 20 , 2009 | * | * | * | * | ‐ | * | * | * | 7 |
| Oberlander et al. 27 , 2008 | * | * | * | * | ‐ | * | ‐ | ‐ | 5 |
| Kallen et al. 28 , 2007 | * | * | * | * | ** | * | ‐ | ‐ | 7 |
A study could be awarded a maximum of one star for each item except for the item Control for important factor or additional factor. The definition/explanation of each column of the Newcastle–Ottawa Scale is available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
A maximum of two stars could be awarded for this item. Studies that controlled for age received one star, whereas studies that controlled for other important confounders such as smoking and/or alcohol using received an additional star
A cohort study with a follow‐up time > 9 months was assigned one star
A cohort study with a follow‐up rate > 75% was assigned one star
Cardiovascular‐related malformations
Twelve studies 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 evaluated the relationship between sertraline use in the first trimester of pregnancy and any cardiovascular‐related malformations in infants. After summarizing all of the studies, pregnant women exposed to sertraline in the first trimester had a statistically significant increased risk of cardiovascular‐related malformations occurring in their infants (OR = 1.36, 95% CI = 1.06–1.74; Figure 2). Moderate heterogeneity was noted (I2 = 64.4%, P = 0.01). Publication bias was not detected by Egger's tests (P = 0.421), Begg's tests (P = 0.631) and visual inspection of the funnel plot was symmetric. A total of five studies 17, 19, 21, 22, 28 reported the relationship between sertraline use and risk of cardiac malformations. The summarized OR was 1.20 (95% CI = 0.94–1.53; I2 = 59.2; P = 0.04). Eight studies 17, 18, 19, 21, 22, 25, 26, 28 reported ASDs and/or VSDs. The results suggested that exposure to sertraline use had 36% statistically significant increased risk of ASDs and/or VSDs in infants (95% CI = 1.06–1.76; Figure 3). I2 was 62.2%, which suggest a moderate degree of heterogeneity between studies (P < 0.01).
Figure 2.
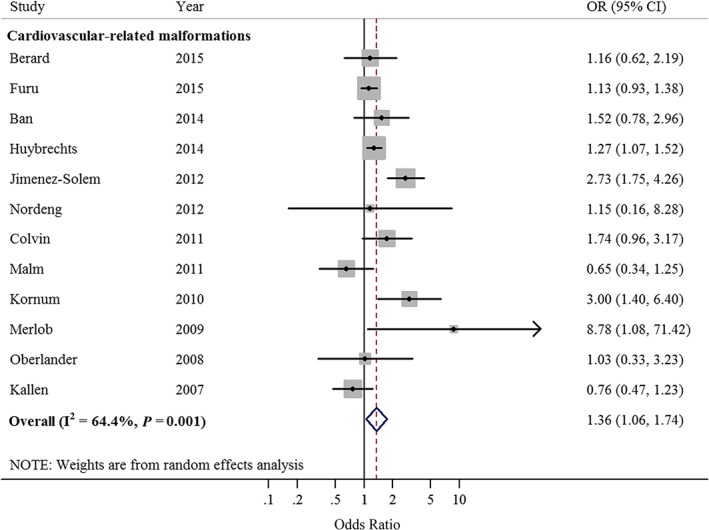
Forest plots of the relationship between sertraline use and risk of cardiovascular‐related malformations. Squares indicate study‐specific risk estimates (size of the square reflects the study‐specific statistical weight); horizontal lines indicate 95% confidence intervals (CIs); diamond indicates the summary odds ratio with its 95% CI. OR: odds ratio
Figure 3.
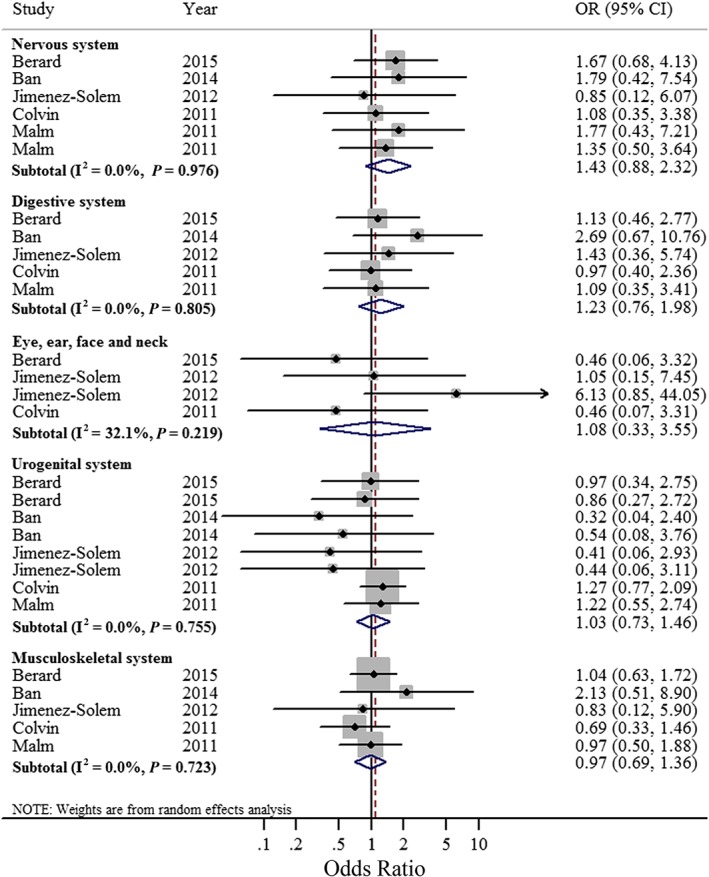
Forest plots of the relationship between sertraline use and congenital anomalies of the nervous system, digestive system, eye, ear, face and neck, urogenital system, and musculoskeletal system. Squares indicate study‐specific risk estimates (size of the square reflects the study‐specific statistical weight); horizontal lines indicate 95% confidence intervals (CIs); diamond indicates the summary odds ratio with its 95% CI
We carried out subgroup analyses stratified by geographic locations in analysis of cardiovascular malformations. Significant results were only observed in North American populations (summarized OR = 1.26; 95% CI = 1.06–1.49). Additionally, when stratified by whether adjustment potential confounders, although the directions of the result of subgroup analyses were consistent with the main findings, not all of them showed statistical significance. Moreover, no statistically significant source of heterogeneity was identified in a metaregression analysis of these subgroups (Table 3).
Table 3.
Summary risk estimates of the association between sertraline use and congenital anomalies
| No. of study | Summary OR 95% CI | I 2 (%) | P* | P** a | |
|---|---|---|---|---|---|
| Cardiovascular‐related malformation | 12 | 1.36 (1.06–1.74) | 64.4 | 0.01 | |
| Atrial and/or ventricular septal defect | 8 | 1.36 (1.06–1.76) | 62.2 | <0.01 | |
| Cardiac malformation | 5 | 1.20 (0.94–1.53) | 59.2 | 0.04 | |
| Nervous system | 5 | 1.39 (0.83–2.32) | 0.0 | 0.95 | |
| Digestive system | 5 | 1.23 (0.76–1.98) | 0.0 | 0.81 | |
| Eye, ear, face and neck | 3 | 1.08 (0.33–3.55) | 32.1 | 0.22 | |
| Urogenital system | 5 | 1.03 (0.73–1.46) | 0.0 | 0.76 | |
| Musuloskeletal system | 5 | 0.97 (0.69–1.36) | 0.0 | 0.72 | |
| Subgroup analysis a | |||||
| Geographic location | 0.53 | ||||
| North America | 3 | 1.26 (1.06–1.49) | 0.0 | 0.91 | |
| Europe | 7 | 1.35 (0.88–2.06) | 77.2 | <0.01 | |
| Others | 2 | 2.83 (0.66–12.08) | 52.8 | 0.15 | |
| Adjustment for potential confounders | |||||
| Age | 0.68 | ||||
| Yes | 8 | 1.32 (0.91–1.91) | 73.4 | <0.01 | |
| No | 4 | 1.43 (0.98–2.07) | 30.6 | 0.23 | |
| Socioeconomic status | 0.32 | ||||
| Yes | 2 | 1.84 (0.80–4.24) | 78.8 | 0.03 | |
| No | 10 | 1.23 (0.98–1.56) | 51.9 | 0.03 | |
| Smoking or alcohol drinking | 0.88 | ||||
| Yes | 6 | 1.36 (0.87–2.11) | 81.0 | <0.01 | |
| No | 6 | 1.30 (1.11–1.53) | 0.0 | 0.48 | |
| Pregnancy body mass index | 0.86 | ||||
| Yes | 1 | 1.52 (0.78–2.96) | N/A | N/A | |
| No | 11 | 1.35 (1.03–1.75) | 67.3 | <0.01 | |
| Pregnancy complications | 0.21 | ||||
| Yes | 4 | 1.10 (0.87–1.38) | 14.3 | 0.32 | |
| No | 8 | 1.62 (1.09–2.42) | 70.4 | <0.01 | |
| Parity | 0.77 | ||||
| Yes | 6 | 1.32 (0.81–2.15) | 80.7 | <0.01 | |
| No | 6 | 1.31 (1.12–1.53) | 0.0 | 0.46 | |
CI, confidence interval; N/A, not available; OR, odd ratio
P* for heterogeneity within each subgroup
P** for heterogeneity between subgroups with metaregression analysis
Subgroup analyses were only carried out for cardiovascular‐related malformation
In the sensitivity analysis, which omitted one study at a time and calculated a summarized OR for the remainder of the studies, the estimated OR in this sensitivity analysis ranged from 1.22 (95% CI = 0.99–1.51) after omission of the study by Jimenez‐Solem et al. 18 to 1.45 (95% CI = 1.13–1.87) after omission of the study by Kallen and Otterblad 28.
Congenital anomalies of the nervous system, digestive system, eye, ear, face and neck, urogenital system, and musculoskeletal system
The association between sertraline exposure in the first trimester and congenital anomalies of the nervous system was explored by five studies 18, 21, 23, 25, 26. The summarized OR was 1.43 (95% CI = 0.88–2.32; P = 0.98 for heterogeneity; I2 = 0.0). The tests for publication bias showed that there was no publication bias (Begg's test = 0.221, Egger's test = 0.195). Sensitivity analyses showed that none of the individual studies greatly influenced the summarized OR (OR range from 1.27 [95% CI = 0.68–2.38] to 1.48 [95% CI = 0.83–2.64]).
Five studies 18, 21, 23, 25, 26 reported the relation of sertraline exposure and congenital anomalies of the digestive system, the summarized OR was 1.23 (95% CI = 0.76–1.98) without heterogeneity (I2 = 0.0%, P = 0.81). The results of the tests for publication bias (Begg's test = 0.086 and Egger's test = 0.166), suggest the existence of some publication bias through Begg's test. To assess whether any one study had a dominant effect on the summarized OR, each study was excluded one at a time and we evaluated the effect on the main summary estimate. The results showed that no study obviously affected the summarized estimate (OR range from 1.10 [95% CI = 0.66–1.84] to 1.35 [95% CI = 0.76–2.39]).
Three studies 18, 21, 25 reported estimates for congenital anomalies of the eye, ear, face and neck; the summarized OR was 1.08 (95% CI = 0.33–3.55; I2 = 32.1%; P = 0.22). We also conducted tests for publication bias (Begg's test = 0.734, Egger's test = 0.883), which suggested no publication bias existed.
Four prospective cohort studies 18, 21, 23, 25 and one retrospective cohort 26 study reported the relation of sertraline exposure and congenital anomalies of the urogenital system. When we summarized these studies, we found that sertraline exposure was not associated with such anomalies (OR = 1.03 95% CI = 0.73–1.46). I2 was 0 (P = 0.76), which suggested a low degree of heterogeneity. In addition, there was indication of a publication bias by using Begg's test (P = 0.002) or Egger's test (P < 0.001). Furthermore, sensitivity analysis presented that none of the individual studies evidently affected the summarized OR (OR range from 0.85 [95%CI = 0.52–1.38] to 1.10 [95% CI = 0.76–1.57]).
Four prospective cohort studies 18, 21, 23, 25 and one retrospective cohort study 26 reported the relation of sertraline exposure and congenital anomalies of the musculoskeletal system. The summarized OR was 0.97 (95%CI = 0.69–1.36; P = 0.72 for heterogeneity; I2 = 0.0%). There was no indication of a publication bias using Begg's test (P = 0.806) or Egger's test (P = 0.685). The tests for sensitivity analysis showed OR range from 0.92 (95%CI = 0.58–1.44) to 1.06 (95% CI = 0.73–1.55).
Discussion
Main findings
To our knowledge, this is the first meta‐analysis to review systematically the relationship between sertraline use in the first trimester of pregnancy and selective congenital anomalies. After summarizing the results from 12 cohort studies, we found that sertraline use had 36% and 35% statistically significant increased risks of the cardiovascular‐related malformations as well as ASDs and/or VSDs, respectively. However, nonsignificant associations between sertraline use and congenital anomalies of the nervous system, digestive system, eye, ear, face and neck, urogenital system, and musculoskeletal system were observed.
The specific biological mechanisms on sertraline use and selective congenital anomalies remain unclear. However, several possible mechanisms may partly explain the aforementioned associations, especially for cardiovascular‐related malformations. In vivo study 52 showed that serotonin might play an important role in cardiac morphogenesis during endocardial cushion formation in the mouse embryo. Besides, sertraline could inhibit proliferation of cardiac mesenchyme, endocardium, and myocardium. Subsequently, Nebigil et al. 53 demonstrated that 5‐HT was a crucial regulator in the process of cardiomyocyte proliferation and differentiation via 5‐HT2B receptor. Additionally, other studies 16 indicated that the blockade of 5‐HT uptake decreased the number of heart cells, which might alter heart development. Considering that there is little information on molecular mechanisms in the cells and tissues level, further experimental studies should be conducted to investigate the potential mechanisms between sertraline and cardiovascular‐related malformations.
Comparing this information to cardiovascular‐related malformations, we failed to find any significant association between sertraline use and other selective congenital anomalies (Table 3), which might be due to the limited number of studies. However, there were several possible mechanisms to explain the potential relevance. A genetic study in mice 54 that focused on the development of the nervous system showed that disruption of serotonin signalling during the period of pre‐ and postnatal development could lead to long‐term behavioural abnormalities. Additionally, an in vitro study 55 found that serotonin could inhibit osteoblast proliferation, differentiation and mineralization at a low concentration. Moreover, as an important transmitter in the gut, serotonin plays an important role in vasodilation, epithelial secretion, stimulation of propulsion and segmentation motility patterns 56. Despite the fact that pre‐existing studies could partly explain the associations between sertraline use and other congenital anomalies, further animal models are needed to investigate the specific role of sertraline on embryo development.
In the subgroup analyses stratified by geographic location, a statistically significant association was only found for populations in North America. This pattern could be partly attributed to the different prescription rates of sertraline among different geographical populations. For example, the average prescription rates were 2.86% (range 1.48–5.10%) and 0.19% (range 0.10–0.31%) in North America and Europe, respectively. By contrast, we could not rule out the possibility of chance finding since there were only three studies in North America and two studies total in Australia and Israel investigating the aforementioned association.
Strengths and limitations
Our study has several strengths. Firstly, to the best of our knowledge, this is the first comprehensive and the most current meta‐analysis that has evaluated the association between sertraline use in the first trimester of pregnancy and congenital anomalies. Secondly, our meta‐analysis included 12 cohort studies with a total number of 6 468 241 participants, which provided sufficient power to detect modest associations. Thirdly, because we only included cohort studies, the influence of biases such as recall bias and selection bias could be minimized. Finally, numerous subgroup and sensitivity analyses were carried out to explore the heterogeneity.
However, several potential limitations of this study also need to be acknowledged. First, the summarized ORs might be overestimated because mothers who have been treated for depression are more likely to receive elaborate examinations, which might lead to the possible detection of some less severe congenital anomalies 57 and thus cause information bias. For instance, infants of women exposed to selective serotonin reuptake inhibitors had approximately twice as many echocardiograms in the first year of life compared with infants of unexposed women 57. Also, more frequent echocadiograms may lead to a higher rate in the detection of heart defects; hence, infants of women who used that drug would more likely be detected. As well, since four studies 17, 19, 25, 27 conducted their investigations by means of record linkage, there was a limitation that drug compliance and length of exposure timing could not be assured, which might result in an overestimation of summarized ORs. However, there have been many studies 58 indicating that the majority of redeemed prescriptions were taken by the pregnant women. When treating chronic illnesses, drug compliance was especially high during pregnancy 59.
Second, our meta‐analysis did not take malformations leading to an elective termination of pregnancy or miscarriage into consideration. This missing information could disguise a possible teratogenic effect of the sertraline. This results could occur if pregnant women exposed to sertraline had a higher rate of elective abortions or miscarriages due to severe malformation, it would create an underestimation of the risk.
Third, we acknowledge important confounders that may cause similar results; however, we cannot account for unknown confounders. Since previous studies 59 have reported that smoking, alcohol, drug use, poor maternal diet, obesity, and chronic conditions were all frequently seen in patients with depression more so than in those without depression, all of those factors could be considered potential risk factors for congenital anomalies. However, these potential confounders were not consistent in each study. For instance, some studies 17, 20, 25, 27 did not adjust for any confounder, while seven studies 18, 19, 21, 22, 23, 26, 28 adjusted for more than three confounders. Because we did not have access to the primary data of these included studies, future cohort studies are warranted to report analyses stratified by possible risk factors that fully adjust for the potential confounders in order to rule out residual confounders.
Although numerous subgroup and sensitivity analyses were carried out, heterogeneity still existed in our study. Hence heterogeneity could be a concern when interpreting the findings of this study. As suggested previously, significant heterogeneity could potentially be induced by factors such as differences in the assessment of exposure timing, drug compliance, study location or differing covariate adjustment. We conducted many subgroup analyses with the expectation of detecting potential factors for such considerable heterogeneities; however, it appears that in numerous subgroups the heterogeneity remains relatively high. Therefore, further studies are warranted to validate our findings and better characterize the relationship.
Finally, there was a limitation with the review in that there were very few studies focusing on systems other than cardiovascular. For example, just five studies 18, 21, 23, 25, 26 mentioned these anomalies. As for congenital anomalies of the eye, ear, face and neck, the numbers were even fewer 18, 21, 25. Out of the studies, only three reported the risks, and we could not rule out that our results could derive from chance finding. Hence, future studies are still required to investigate the association between sertraline use and congenital anomalies of other systems.
Conclusions
In conclusion, in this original and comprehensive meta‐analysis, we found that pregnant women who were exposed to sertraline during the first trimester of pregnancy had an increased risk of cardiovascular‐related malformations as well as ASDs and/or VSDs in infants. Further investigations are warranted to provide more detailed results of the association between sertraline use and other congenital anomalies.
Competing Interests
There are no competing interests to declare.
This study was supported by grants from the China National Health and Family Planning Commission (No. 201402006 to Cai‐Xia Liu), the funding of the Obstetric Diseases Translational Medicine Research Center Project of Liaoning Province (No.2014225007 to Cai‐Xia Liu), the Natural Science Foundation of China (No. 81402130 for Da Li and No. 81602918 for Qi‐Jun Wu), the Doctoral Start‐up Foundation of Liaoning Province (No. 201501007 for Qi‐Jun Wu and No. 20141045 for Da Li), the Fok Ying Tung Education Foundation (No. 151039 for Da Li), and the Campus Research Fund of China Medical University (No. YQ20160004 for Da Li). Qi‐Jun Wu was supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (D43 TW008313 to Xiao‐Ou Shu).
Contributors
Q.‐J.W. and D.L. designed research; T.‐N.Z., S.‐Y.G., Z.‐Q.S. and Q.‐J.W. conducted literature search; T.‐N.Z., S.‐Y.G., Z.‐Q.S. and Q.‐J.W. analysed data; Z.‐Q.S., S.X.L., D.L. and Q.‐J.W. wrote the draft; all authors read, reviewed and approved the final manuscript. Q.‐J.W. and D.L. had primary responsibility for final content.
Shen, Z.‐Q. , Gao, S.‐Y. , Li, S. X. , Zhang, T.‐N. , Liu, C.‐X. , Lv, H.‐C. , Zhang, Y. , Gong, T.‐T. , Xu, X. , Ji, C. , Wu, Q.‐J. , and Li, D. (2017) Sertraline use in the first trimester and risk of congenital anomalies: a systemic review and meta‐analysis of cohort studies. Br J Clin Pharmacol, 83: 909–922. doi: 10.1111/bcp.13161.
Contributor Information
Qi‐Jun Wu, Email: wuqj@sj-hospital.org.
Da Li, Email: leeda@sina.cn.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Davenport AP, Kelly E, Marrion N, Peters JA, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellfolk M, Malm H. Risks associated with in utero and lactation exposure to selective serotonin reuptake inhibitors (SSRIs). Reprod Toxicol 2010; 30: 249–260. [DOI] [PubMed] [Google Scholar]
- 4. Braithwaite EC, Murphy SE, Ramchandani PG. Effects of prenatal depressive symptoms on maternal and infant cortisol reactivity. Arch Womens Ment Health 2016; 19: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis EP, Glynn LM, Dunkel SC, Hobel C, Chicz‐Demet A, Sandman CA. Corticotropin‐releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci 2005; 27: 299–305. [DOI] [PubMed] [Google Scholar]
- 6. Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod 2009; 24: 146–153. [DOI] [PubMed] [Google Scholar]
- 7. Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry 2002; 159: 2055–2061. [DOI] [PubMed] [Google Scholar]
- 8. Diav‐Citrin O, Ornoy A. Selective serotonin reuptake inhibitors in human pregnancy: to treat or not to treat? Obstet Gynecol Int 2012; 2012: 698947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 2008; 198: 191–194. [DOI] [PubMed] [Google Scholar]
- 10. Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong‐van DBL, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol 2006; 62: 863–870. [DOI] [PubMed] [Google Scholar]
- 11. Kieler H. The Nordic health registers – an important source when evaluating the safety of antidepressants during pregnancy. Clin Epidemiol 2010; 2: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El MH, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry 2012; 69: 706–714. [DOI] [PubMed] [Google Scholar]
- 13. Jimenez‐Solem E, Andersen JT, Petersen M, Broedbaek K, Andersen NL, Torp‐Pedersen C, et al. Prevalence of antidepressant use during pregnancy in Denmark, a nation‐wide cohort study. PLoS One 2013; 8: e63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramos E, Oraichi D, Rey E, Blais L, Berard A. Prevalence and predictors of antidepressant use in a cohort of pregnant women. BJOG 2007; 114: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadler TW. Selective serotonin reuptake inhibitors (SSRIs) and heart defects: potential mechanisms for the observed associations. Reprod Toxicol 2011; 32: 484–489. [DOI] [PubMed] [Google Scholar]
- 16. Sari Y, Zhou FC. Serotonin and its transporter on proliferation of fetal heart cells. Int J Dev Neurosci 2003; 21: 417–424. [DOI] [PubMed] [Google Scholar]
- 17. Huybrechts KF, Hernandez‐Diaz S, Avorn J. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 371: 1168–1169. [DOI] [PubMed] [Google Scholar]
- 18. Jimenez‐Solem E, Andersen JT, Petersen M, Broedbaek K, Jensen JK, Afzal S, et al. Exposure to selective serotonin reuptake inhibitors and the risk of congenital malformations: a nationwide cohort study. BMJ Open 2012; 2: e001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kornum JB, Nielsen RB, Pedersen L, Mortensen PB, Norgaard M. Use of selective serotonin‐reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol 2010; 2: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merlob P, Birk E, Sirota L, Linder N, Berant M, Stahl B, et al. Are selective serotonin reuptake inhibitors cardiac teratogens? Echocardiographic screening of newborns with persistent heart murmur. Birth Defects Res A Clin Mol Teratol 2009; 85: 837–841. [DOI] [PubMed] [Google Scholar]
- 21. Berard A, Zhao JP, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212: 791–795. [DOI] [PubMed] [Google Scholar]
- 22. Furu K, Kieler H, Haglund B, Engeland A, Selmer R, Stephansson O, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350: h1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ban L, Gibson JE, West J, Fiaschi L, Sokal R, Smeeth L, et al. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population‐based cohort study. BJOG 2014; 121: 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard‐Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol 2012; 32: 186–194. [DOI] [PubMed] [Google Scholar]
- 25. Colvin L, Slack‐Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol 2011; 91: 142–152. [DOI] [PubMed] [Google Scholar]
- 26. Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol 2011; 118: 111–120. [DOI] [PubMed] [Google Scholar]
- 27. Oberlander TF, Warburton W, Misri S, Riggs W, Aghajanian J, Hertzman C. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population‐based health data. Birth Defects Res B Dev Reprod Toxicol 2008; 83: 68–76. [DOI] [PubMed] [Google Scholar]
- 28. Kallen BA, Otterblad OP. Maternal use of selective serotonin re‐uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol 2007; 79: 301–308. [DOI] [PubMed] [Google Scholar]
- 29. Wang S, Yang L, Wang L, Gao L, Xu B, Xiong Y. Selective serotonin reuptake inhibitors (SSRIs) and the risk of congenital heart defects: a meta‐analysis of prospective cohort studies. J Am Heart Assoc 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, W64. [DOI] [PubMed] [Google Scholar]
- 32. Wells G, Shea B, O'Connel D. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. 2013. Available at http://www.ohri.ca/programs/clinical_epidemiological/oxford.asp (last accessed 14 October 2016).
- 33. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27: 954–970. [DOI] [PubMed] [Google Scholar]
- 34. Luan NN, Wu QJ, Gong TT, Vogtmann E, Wang YL, Lin B. Breastfeeding and ovarian cancer risk: a meta‐analysis of epidemiologic studies. Am J Clin Nutr 2013; 98: 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gong TT, Wu QJ, Wang YL, Ma XX. Circulating adiponectin, leptin and adiponectin‐leptin ratio and endometrial cancer risk: Evidence from a meta‐analysis of epidemiologic studies. Int J Cancer 2015; 137: 1967–1978. [DOI] [PubMed] [Google Scholar]
- 36. Wu QJ, Wu L, Zheng LQ, Xu X, Ji C, Gong TT. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev 2016; 25: 196–205. [DOI] [PubMed] [Google Scholar]
- 37. Luan NN, Wu L, Gong TT, Wang YL, Lin B, Wu QJ. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta‐analysis of epidemiological studies. Cancer Causes Control 2015; 26: 65–78. [DOI] [PubMed] [Google Scholar]
- 38. Chen J, Gong TT, Wu QJ. Parity and gastric cancer risk: a systematic review and dose–response meta‐analysis of prospective cohort studies. Sci Rep 2016; 6: 18766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 40. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiao YS, Gong TT, Wang YL, Wu QJ. Comorbidity and survival among women with ovarian cancer: evidence from prospective studies. Sci Rep 2015; 5: 11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta‐analysis of observational studies. Ann Oncol 2013; 24: 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hou R, Wu QJ, Gong TT, Jiang L. Dietary fat and fatty acid intake and epithelial ovarian cancer risk: evidence from epidemiological studies. Oncotarget 2015; 6: 43099–43119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang L, Hou R, Gong TT, Wu QJ. Dietary fat intake and endometrial cancer risk: dose–response meta‐analysis of epidemiological studies. Sci Rep 2015; 5: 16693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu QJ, Gong TT, Wang YZ. Dietary fatty acids intake and endometrial cancer risk: a dose–response meta‐analysis of epidemiological studies. Oncotarget 2015; 6: 36081–36097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng TB, et al. Parity and endometrial cancer risk: a meta‐analysis of epidemiological studies. Sci Rep 2015; 5: 14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gong TT, Wu QJ, Vogtmann E, Lin B, Wang YL. Age at menarche and risk of ovarian cancer: a meta‐analysis of epidemiological studies. Int J Cancer 2013; 132: 2894–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei J, Liu CX, Gong TT, Wu QJ, Wu L. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta‐analysis of prospective studies. Oncotarget 2015; 6: 43667–43678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, et al. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta‐analysis. Ann Oncol 2013; 24: 1918–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 52. Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology 1993; 47: 573–584. [DOI] [PubMed] [Google Scholar]
- 53. Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, et al. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A 2000; 97: 9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 2003; 4: 1002–1012. [DOI] [PubMed] [Google Scholar]
- 55. Dai SQ, Yu LP, Shi X, Wu H, Shao P, Yin GY, et al. Serotonin regulates osteoblast proliferation and function in vitro . Braz J Med Biol Res 2014; 47: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mawe GM, Hoffman JM. Serotonin signalling in the gut – functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013; 10: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bar‐Oz B, Einarson T, Einarson A, Boskovic R, O'Brien L, Malm H, et al. Paroxetine and congenital malformations: meta‐analysis and consideration of potential confounding factors. Clin Ther 2007; 29: 918–926. [DOI] [PubMed] [Google Scholar]
- 58. De Jong VDBL, Feenstra N, Sorensen HT, Cornel MC. Improvement of drug exposure data in a registration of congenital anomalies. Pilot‐study: pharmacist and mother as sources for drug exposure data during pregnancy. EuroMAP Group. Europen Medicine and Pregnancy Group. Teratology 1999; 60: 33–36. [DOI] [PubMed] [Google Scholar]
- 59. Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007; 115: 2995–3014. [DOI] [PubMed] [Google Scholar]


