Abstract
Background
Although statin therapy is associated with reduced stroke and mortality risk, some studies report that higher lipid levels are associated with improved outcomes following ischemic stroke.
Aims
We examined the association of hyperlipidemia (HLD) combined with statin therapy on all-cause mortality in stroke patients.
Methods
All stroke patients in the Greater Cincinnati Northern Kentucky region of ~1.3 million were identified using ICD-9 discharge codes in 2005 and 2010. Stroke patients with and without HLD were categorized based on their reported statin use at baseline or discharge into three groups: no-HLD/no-statins, HLD/no-statins, and HLD/on-statins. Cox proportional hazards model was used to estimate the risk of mortality at 30 days, 1 year, and 3 years poststroke.
Results
Overall, 77% (2953) of the 3813 ischemic stroke patients were diagnosed with HLD and 72% (n = 2123) of those patients were on statin medications. The mean age was 70.0 ± 14.6 years, 56% were women, and 21% were black. In adjusted analyses, the HLD/no-statins group showed 35% (adjusted hazard ratio (aHR) = 0.65, 95% CI: 0.46–0.92), 27% (aHR = 0.73, 95% CI: 0.59–0.90), and 17% (aHR = 0.83, 95% CI: 0.70–0.97) reduced risk of mortality at 30 days, 1 year, and 3 years, respectively, poststroke, compared with no-HLD/no-statins group. The HLD/on-statins group showed an additional 17% significant survival benefit at 3 years poststroke compared with HLD/no-statins group.
Conclusions
A diagnosis of HLD in ischemic stroke patients is associated with reduced short- and long-term mortality, irrespective of statin use. Statin therapy is associated with significant, additional long-term survival benefit.
Keywords: Cerebral infarction, cholesterol, LDL, obesity, outcomes, survival
Introduction
The benefits of statin therapy in primary and secondary prevention of cardiovascular events, stroke, and all-cause mortality are well established.1–4 In a meta-analysis of 27 statin clinical trials that included 174,149 patients, statin therapy produced a highly significant 21% reduction in major vascular events, 24% reduction in both coronary events and coronary revascularization, 15% reduction in any stroke, and 9% reduction in all-cause mortality for each 1 mmol/L reduction in low density lipoprotein (LDL) cholesterol.5
The demonstrated efficacy of statins in reducing the risk of cardiovascular events by reducing serum LDL cholesterol levels is well accepted.5,6 Yet, results from several studies show that high cholesterol levels are associated with improved clinical and functional outcomes following an ischemic stroke.7–18 This paradoxical “protective” effect of higher lipid levels on outcome events seen among ischemic stroke patients raises important questions on (1) the role of hyperlipidemia (HLD) on health outcomes in ischemic stroke patients with pre-existing vascular conditions and (2) the extent of additional protection from mortality gained from statin therapy in ischemic stroke patients. Prior studies examining the association between cholesterol levels and survival in ischemic stroke patients were predominantly hospital- or community-based studies, limiting the generalizability of their results. In addition to the lack of generalizability, findings from previous studies may have been biased due to (1) differing cholesterol levels chosen to define hypercholesterolemia, (2) inadequate adjustment of prior comorbidities, and (3) lack of adjustment of stroke severity, prior or recurrent strokes, statin use, and anticoagulant use that are known to be associated with stroke-related outcomes. Therefore, we investigated the association of HLD with and without statin use on all-cause mortality in ischemic stroke patients from a large population-based study. We hypothesized that ischemic stroke patients with a diagnosis of HLD will have lower risk of short-term and long-term all-cause mortality, irrespective of statin therapy, compared with patients with no evidence of elevated lipid levels.
Methods
This study is a retrospective analysis of black and white adult (age ≥ 20 years) ischemic stroke patients from the 2005 and 2010 study periods of the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS), details of which have been previously published.19–21 Briefly, GCNKSS is a large, bi-racial, population-based epidemiological study of all stroke cases admitted to acute care hospitals among residents of a five-county catchment area of ~1.3 million. In addition, medical records of all stroke-related visits made by patients to the region’s hospital emergency departments, public health clinics, hospital-based outpatient clinics, and family practice centers were identified, and medical records of patients from a randomly selected subset of primary care physicians’ offices and nursing homes within the Cincinnati metropolitan area were screened for stroke events. Quality control check of the patients’ records identified from all sources was performed to prevent duplication, with data from in-hospital ascertainment of a stroke case selected preferentially over out-of-hospital ascertainment. Study research nurses collected all relevant clinical data of the potential cases onto case report forms, including demographics, presenting symptoms, physical and neurological examination findings, medical, family and medication history, and laboratory and imaging testing results. A study physician reviewed each form and all available neuroimaging data to determine whether a stroke or transient ischemic attack (TIA) occurred, in addition to assigning specific stroke type and mechanism to each case. The study was approved by the Institutional Review Board of all participating institutions for both study periods.
Outcome variable
Study outcome was all-cause mortality at 30 days and 1 year following stroke (short-term), and at 3 years following stroke (long-term). Mortality information was ascertained by examination of state death records, obituaries, and the Social Security Death Index for the 3 years following the stroke for all subjects in the study.
Independent variable and covariates
The main independent variable of interest was classification of patients based on HLD and statin use. HLD was defined as having either a medical history of elevated cholesterol or diagnosed with hypercholesterolemia during hospitalization based on an elevated fasting LDL cholesterol level during hospitalization (>70 mg/dL with diabetes, heart disease, or other vascular disease, or >100 mg/dL without these comorbidities). Statin use status was determined as “no-statins” or “on-statins” depending on a patient’s reported history of taking at least one statin drug prior to stroke event or statin treatment documented on patient’s discharge medication list. Accordingly, based on HLD and statin use status, patients were categorized as (1) no-HLD/no-statins, (2) HLD/no-statins, or (3) HLD/on-statins. The presence of other risk factors, specifically hypertension, diabetes, heart disease (established based on prior history of coronary heart disease, myocardial infarction, angina, congestive heart failure, prior cardiac bypass surgery, cardiac vessel angioplasty/stent, or cardiomyopathy), atrial fibrillation, depression, dementia, prior stroke, family history of stroke, and smoking status were obtained from patients’ medical records. Antiplatelet or antithrombotic use prior to stroke event or poststroke was also obtained from patients’ reported history or from their discharge medication list. Smoking status was categorized as current (smoked within past 3 months), former, and never. In addition, prestroke functional status as measured by the modified Rankin Scale (mRS), baseline stroke severity measured retrospectively using National Institutes of Health Stroke Scale (rNIHSS) score (0–42),22,23 and body mass index (BMI: weight in kilograms divided by height in meters-squared) were obtained. BMI was categorized into normal-weight (BMI <25 kg/m2), overweight (BMI 25–29 kg/m2), and obese (BMI ≥ 30 kg/m2) categories based on the National Institutes of Health-recommended BMI cutoff points for US adults.24 Because fewer than 4% (N = 134) of the patients in the study population were underweight (BMI < 18.5 kg/m2), those patients were included in the normal-weight group.
Statistical analysis
In order to have just one stroke per patient in the analysis we decided to use the last stroke event for a patient within the study period as the qualifying event. This was to be consistent with patients with just one stroke within the study period, but a prior stroke outside our study period. Sensitivity analyses were performed using (1) first stroke in the study period and (2) using only patients with incident strokes, that is, no history of prior stroke.
The baseline demographic and clinical characteristics of the study population were compared between the HLD statin use groups using the chi-square (χ2) test for discrete variables and by analysis of variance for continuous variables. A Bonferroni correction was used to adjust for pairwise comparisons among the three groups. Association between HLD statin use and risk of all-cause mortality following stroke was assessed using Cox proportional hazards regression model with no-HLD/no-statins as the reference group. Time to event was defined as the interval between stroke event and death. All patients were followed until 3 years poststroke or death. Mortality status was examined at 30 days, 1 year, and 3 years following stroke. Variables that were significant at p < 0.10 in the univariate analysis were included in the multivariable model as potential confounders. Predefined two-way interactions between HLD statin use and age, sex, race, and BMI categories were considered biologically relevant and were thus evaluated to assess for effect modification. No statistically significant interaction effects were found. In the final multivariable Cox proportional hazard analysis, level of statistical significance was set at p < 0.05. The proportional hazard assumption was validated using Schoenfeld residuals. Adjusted hazard ratios (aHR) and 95% CI for the significant variables are reported. All statistical analyses were performed using SAS® version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The GCNKSS ascertained 7348 strokes and TIAs in black and white adult patients (≥20 years) for the 2005 and 2010 study periods, of which 4461 cases were determined to be of ischemic etiology. For patients with multiple strokes during the study periods, the patient’s last stroke event within the study period was used in the analysis; this resulted in the exclusion of 207 stroke events. After also excluding inpatient deaths (n = 241), and patients with unavailable LDL data (n = 200), 3813 patients were available for final analysis. Of the 2953 (77%) patients diagnosed with HLD in the study 1866 (63%) were diagnosed based on past medical history, 445 (15%) diagnosed during the hospitalization, and 642 (22%) diagnosed based on elevated fasting LDL levels. Of the 2953 HLD patients, 2123 (72%) of them were on statin therapy. The mean ± SD age of the patients was 70.0 ± 14.6 years, 56% were women, and 21% were black.
Baseline demographic, clinical, and outcome characteristics of the patients by HLD/statin use status are presented in Table 1. After Bonferroni adjustment, individual group comparisons showed that patients diagnosed with HLD, irrespective of their statin use status were significantly younger, more often male, more likely to be obese, more likely to be smokers, more likely to be on antiplatelets, and more often had pre-existing comorbidities of hypertension, diabetes, heart disease, family history of stroke, and dementia compared with patients with no evidence of HLD. Stroke severity and prestroke functional status, however, was worse in patients with no evidence of HLD.
Table 1.
Demographic, clinical, and outcome characteristics of 3813 stroke patients by hyperlipidemia statin use status
| Characteristics | no-HLD/no-statins (n = 860) |
HLD/no-statins (n = 830) |
HLD/on-statins (n = 2123) |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 72.0 ± 17.4 | 70.3 ± 14.9† | 69.1 ± 13.1† |
| Median (25%–75% IQR) | 77.0 (60.0–85.0) | 72.5 (60.0–82.0) | 70.0 (59.0–79.0) |
| Sex (female), n (%) | 550 (64.0) | 463 (55.8)† | 1132 (53.3)† |
| Race (black), n (%) | 171 (19.9) | 161 (19.4) | 461 (21.7) |
| Body mass index, n (%) | |||
| Normal weight | 353 (41.1) | 262 (31.6)† | 576 (27.1)†‡ |
| Overweight | 217 (25.2) | 249 (30.0) | 654 (30.8)† |
| Obese | 144 (16.7) | 217 (26.1)† | 687 (32.4)†‡ |
| Smoking, n (%) | |||
| Never | 479 (55.7) | 393 (47.4)† | 952 (44.8)† |
| Former | 210 (24.4) | 215 (25.9) | 583 (27.5) |
| Current | 171 (19.9) | 222 (26.7)† | 588 (27.7)† |
| History, n (%) | |||
| Hypertension | 618 (71.9) | 708 (85.3)† | 1924 (90.6)†‡ |
| Diabetes mellitus | 226 (26.3) | 279 (33.6)† | 888 (41.8)†‡ |
| Atrial fibrillation | 197 (22.9) | 192 (23.1) | 375 (17.7)†‡ |
| Heart disease | 285 (33.1) | 334 (40.2)† | 993 (46.8)†‡ |
| Prior stroke | 213 (24.8) | 186 (22.4) | 590 (27.8)‡ |
| Family history of stroke | 93 (10.8) | 157 (18.9)† | 438 (20.6)† |
| Depression | 178 (20.7) | 191 (23.0) | 462 (21.8) |
| Dementia | 161 (18.7) | 101 (12.2)† | 194 (9.1)†‡ |
| Medication use | |||
| Antiplatelet | 637 (74.1) | 685 (82.5)† | 1941 (91.4)†‡ |
| Antithrombotic | 248 (28.8) | 239 (28.8) | 595 (28.0) |
| Baseline NIHSS | |||
| Median, (25%–75% IQR) | 4.0 (1.0–8.0) | 3.0 (1.0–6.0)† | 3.0 (1.0–6.0)† |
| Prestroke mRS, n (%) | |||
| (0–2) | 523 (60.8) | 572 (68.9)† | 1600 (75.4)†‡ |
| Mortality, n (%) | |||
| 30 days | 128 (14.9) | 70 (8.4)† | 93 (4.4)†‡ |
| 3 month | 181 (21.1) | 114 (13.7)† | 183 (8.6)†‡ |
| 3 year | 399 (46.4) | 311 (37.5)† | 617 (29.1)†‡ |
p < 0.05 versus no-HLD/no-statins group.
p < 0.05 versus HLD/no-statins group.
The crude and adjusted HRs with corresponding 95% CI for short- and long-term mortality by HLD/statin use status are presented in Table 2. The mortality risk (crude) in the HLD/no-statin group was significantly lower by 46% at 30 days, 35% at 1 year, and 28% at 3 years poststroke, compared with patients with no evidence of HLD. The HLD/on-statins group exhibited markedly lower mortality risk (crude) by 72% at 30 days, 56% at 1 year, and 48% at 3 years poststroke, compared with the no-HLD/no-statins group.
Table 2.
Crude and adjusted hazard ratios (HRs) of all-cause mortality at 30 days, 1 year, and 3 years following ischemic stroke
| Crude HRs | 95% CI | Adjusted HRsa | 95% CI | |
|---|---|---|---|---|
| 30 days | ||||
| no-HLD/no-statinsb | 1.00 | 1.00 | ||
| HLD/no-statins | 0.54 | (0.40–0.72) | 0.65 | (0.46–0.92) |
| HLD/on-statins | 0.28 | (0.21–0.36) | 0.51 | (0.37–0.72) |
| 1 year | ||||
| no-HLD/no-statins | 1.00 | 1.00 | ||
| HLD/no-statins | 0.65 | (0.54–0.78) | 0.73 | (0.59–0.90) |
| HLD/on-statins | 0.44 | (0.38–0.52) | 0.62 | (0.51–0.74) |
| 3 years | ||||
| no-HLD/no-statins | 1.00 | 1.00 | ||
| HLD/no-statins | 0.72 | (0.62–0.84) | 0.83 | (0.70–0.98) |
| HLD on-statins | 0.52 | (0.46–0.59) | 0.69 | (0.60–0.80) |
Adjusted for the patient age, sex, race, BMI, NIHSS score, smoking status, hypertension, diabetes, atrial fibrillation, heart disease, dementia, family history of stroke, prior stroke, prestroke functional status, and antiplatelet and antithrombotic use.
Reference group: no-HLD/no-statins.
Multivariable analysis confirmed the inverse relationship between HLD statin use and all-cause mortality following stroke adjusted for age, sex, race, BMI, stroke severity, smoking, hypertension, diabetes, atrial fibrillation, heart disease, dementia, family history of stroke, prestroke functional status, and antiplatelet and antithrombotic use (Table 2). Compared with the no-HLD/no-statins group, the adjusted hazard for mortality in HLD/no-statin group was 35% lower at 30 days (aHR: 0.65, 95% CI: 0.46–0.92), 27% lower at 1 year (aHR: 0.73, 95% CI: 0.59–0.90), and 17% lower at 3 years (aHR: 0.83, 95% CI: 0.70–0.97) poststroke. Likewise, the adjusted hazard for mortality in HLD/on-statin therapy group of patients was lower by 49% at 30 days (aHR: 0.51, 95% CI: 0.37–0.72), 38% lower at 1 year (aHR: 0.62, 95% CI: 0.52–0.75), and 31% lower at 3 years (aHR: 0.69, 95% CI: 0.60–0.80).
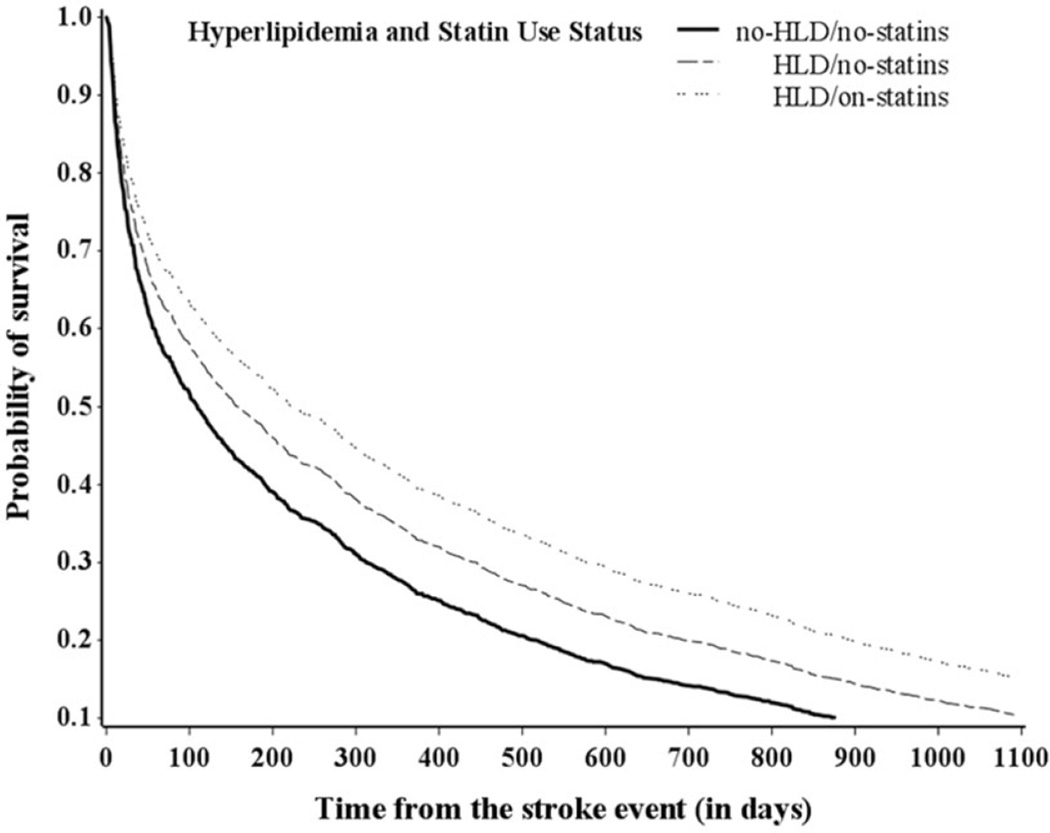
Significant differences in mortality risk were also observed between the two groups of patients diagnosed with HLD at 3 years poststroke, but not at 30 days or 1 year. In multivariable adjusted analyses, the HLD/on-statins group had 18% (HR: 0.82, 95% CI: 0.57–1.18), 15%, (HR: 0.85, 95% CI: 0.70–1.04), and 17% (HR: 0.83, 95% CI: 0.72–0.97) lower risk of mortality at 30 days, 1 year, and 3 years poststroke, respectively, compared with the HLD/no-statins group. Survival curves by HLD-statin use status adjusted for covariates are shown in Figure 1.
Figure 1.
Survival probability of ischemic stroke patients over time by HLD statin use status—multivariable analysis.
Sensitivity analyses on (1) patients with first stroke in the study period (Table S1 online) and (2) patients with incident (first stroke ever) stroke only (Table S2 online) showed similar results. In both groups of patients’ hazard ratios of mortality were marginally attenuated compared to our main analysis, where last stroke was used as the qualifying event. Results are found in the supplementary material.
Discussion
We set out to examine whether a diagnosis of HLD in stroke patients was associated with improved survival following stroke, irrespective of statin use, and whether statin treatment was associated with an additional survival benefit. We found that HLD is statistically significantly associated with lower all-cause mortality at 30 days, 1 year, and 3 years following ischemic stroke independent of statin therapy. The findings of decreased short- and long-term all-cause mortality rates remained significant after controlling for age, sex, race, BMI, stroke severity, smoking, comorbidities, prestroke functional status, and use of antiplatelet and antithrombotic medications. Statin therapy was associated with a significant 17% additional reduction in all-cause mortality after 3 years following stroke.
Previous studies that have examined the association between statin use and hyperlipidemia in ischemic stroke patients with clinical outcomes have yielded mixed results.25–29 In a prospective hospital-based stroke registry of 2082 first ever ischemic stroke patients, history of hypercholesterolemia or statin use before stroke was associated with improved functional outcome and reduced in-hospital death.26 Cholesterol levels, however, were not measured in that study, but statin therapy was considered as a surrogate marker of hypercholesterolemia. In the North Dublin population-based prospective cohort study of 448 stroke patients, both prestroke and new acute poststroke statin treatment (≤72 h) was associated with improved rates of early survival at 7 days and 90 days, with benefits persisting up to 1 year following stroke.29 A meta-analysis of 24 observational and 3 randomized control trials (RCTs) showed that both prestroke and acute poststroke statin use was associated with reduced fatality at 90 days and 1 year after stroke in the observational studies, but not in the RCTs.28 A large administrative database study showed that statin use prior to stroke only, and statin use before and during stroke hospitalization are strongly associated with improved rate of survival at 1-year poststroke. The same study reported delay of statin initiation during stroke hospitalization, both among nonstatin users and prior statin users, even for a day, was associated with worse survival.27
Findings of our study add to the existing evidence of potential benefits of statin therapy on clinical outcomes in patients with existing chronic disease such as stroke. But a protective effect on mortality was also seen in our study among patients diagnosed with HLD and not taking any statin medications. Such a paradoxical “protective” effect of hyperlipidemia on clinical outcomes following stroke, independent of statin therapy, is reported in previous studies.7–18 In a retrospective, hospital-based registry study from Sweden, total cholesterol level ≥178 mg/dL at admission was associated with reduced risk of mortality 7 years poststroke. Adjustment for risk factors such as angina and blood pressure, however, attenuated the inverse association and statistical significance.7 In another hospital-based study of ischemic stroke patients, higher cholesterol levels >155 mg/dL at admission was associated with lower risk of stroke severity and improved functional outcome in patients with and without prestroke statin treatment. There were no significant differences, however, in the rates of short- and long-term mortality after multivariate adjustment.8 In a 10-year follow-up of 652 ischemic stroke patients from the Copenhagen stroke study, increase in every 1 mmol/L of total cholesterol was associated with 11% lower risk of mortality.9 This study, however, did not incorporate any data on cholesterol lowering medications.
Studies reporting “lipid-paradox” have predominantly relied upon total cholesterol levels as a biomarker of stroke prognosis. Because total blood cholesterol is a measure of “bad” (LDL cholesterol and triglycerides) and “good” (high density lipoprotein cholesterol or HDL) cholesterol levels, it is difficult to discriminate which component is truly responsible for the observed protective effect. Li14 and Cuadrado-Godia et al.15 examined the effect of total cholesterol, LDL cholesterol, and triglycerides on mortality in ischemic stroke patients. Li observed no significant association between LDL cholesterol and 3-month mortality, while Cuadrado-Godia et al. observed higher total cholesterol and LDL levels associated with better outcome in men. In our study, we could not assess the effect of triglycerides or total cholesterol levels on mortality, but observed a significant positive effect of high LDL levels on survival. It is widely accepted that LDL cholesterol levels best represents the underlying risk of major vascular disease30 and thus choosing LDL cholesterol levels may be the most sensitive marker of stroke-related outcomes.
The biological mechanisms explaining the beneficial effect of statins or hyperlipidemia on stroke-related outcomes, as observed in our study, are yet to be elucidated. It remains unanswered whether statins by its lipid lowering action or cholesterol-independent “pleotropic” effects that include endothelial protection, anti-inflammatory, antithrombotic, and antioxidant properties provide the survival benefit.31,32 Researchers suggest these pleotropic actions of statins may play a role in reduced infarct size and reduced stroke severity thereby improving neurological outcome.33–35 Although we did not adjust for infarct size in our analysis, we did control for the effect of stroke severity and still found HLD was positively associated with improved survival outcome at all the time points. Interestingly, hyperlipidemia is itself purported to have some similar neuroprotective properties of statins such as (1) neutralizing free radicals and thus protecting the tissue and limiting the extent of ischemic injury36—antioxidant property and (2) down regulation of vascular endothelial growth factor which results in preventing cerebral hyperemia, vascular leakage, tissue inflammation or edema37—endothelial protection/antiinflammatory property. Regardless of the underlying mechanisms, our study findings imply that hypercholesterolemia either acts alone or together with statins in exerting a protective effect on subsequent mortality in stroke patients.
Our study has some limitations. First, we could not obtain LDL levels in all patients to diagnose HLD and had to rely on baseline medical history in almost two-thirds of the patients, which may have resulted in overlap of patient groups. However, using a combination of history of elevated cholesterol or LDL levels during hospitalization in addition to cross-checking patient’s reported statin use to define study groups may have limited such misclassification. Second, although we adjusted for several comorbidities in our statistical analysis as potential confounders, we could not account for severity and duration of the hyperlipidemia or duration and intensity of statin treatment due to the retrospective nature of the study design. As a result, we could not investigate whether the beneficial effect of statins on survival in stroke patients is due to mere use of statin therapy or whether the duration of statin therapy makes an impact on clinical stroke outcomes. Third, we have no information on patients’ course of statin treatment (i.e., regular or intermittent) or whether statin treatment was associated with any discernible changes in patients’ LDL levels during the time from discharge to 3-year period. Consequently, we cannot easily explain why statin therapy was associated with additional survival beneficial, between the patient groups diagnosed with HLD, only at 3 years following stroke and not at 30 days or 1 year. Fourth, we did not include stroke mechanism in the model. On examination we found that there was a similar proportion of patients with cardioembolic stroke mechanisms in each group: 25% in the no-HLD, 27% in the HLD no-statins, and 21% in the HLD on-statins.
Strengths of our study include large sample size, population-based registry, and meticulous data collection methods minimizing selection bias and thereby supporting our study findings to be generalizable. Although, we cannot exclude the possibility of residual confounding due to unaccounted factors such as alcohol use or cancer, the association between HLD/statin use persisted after adjusting for a large set of covariates (n = 16) including antiplatelet and antithrombotic medication use. We did not adjust for the effect of renal diseases on mortality in our analyses because, fewer than 2% (n = 63) of our study subjects had endstage renal disease. Additionally, we did adjust for the severity of stroke evaluated at the time of stroke event, thus limiting the notion that statins may exert a positive effect on survival in stroke patients due to reduced stroke severity.
Conclusions
In summary, our study shows that ischemic stroke patients who are diagnosed with hyperlipidemia or have elevated LDL levels at the time of stroke event may have longer survival relative to patients who have no evidence of hyperlipidemia, even after adjusting for relevant risk factors. We also observed reduced mortality in stroke patients treated with statins, either before or after the stroke event, as compared with those who were not treated with statins. The most striking observation was that stroke patients who were diagnosed with hyperlipidemia had lower mortality irrespective of statin use. Our study adds to the body of literature on this topic giving the perspective from the viewpoint of a population-based study representative of the United States population.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke Division (R01 NS30678).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 3.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 4.Amarenco P, Goldstein LB, Szarek M, et al. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack – the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke. 2007;38:3198–3204. doi: 10.1161/STROKEAHA.107.493106. [DOI] [PubMed] [Google Scholar]
- 5.Trialists CT. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 6.Yaghi S, Elkind MS. Lipid control and beyond: current and future indications for statin therapy in stroke. Curr Treat Options Cardiovasc Med. 2016;18:1–15. doi: 10.1007/s11936-016-0448-8. [DOI] [PubMed] [Google Scholar]
- 7.Markaki I, Nilsson U, Kostulas K, Sjostrand C. High cholesterol levels are associated with improved long-term survival after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:e47–e53. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Koton S, Molshatzki N, Bornstein NM, Tanne D. Low cholesterol, statins and outcomes in patients with first-ever acute ischemic stroke. Cerebrovasc Dis. 2011;34:213–220. doi: 10.1159/000342302. [DOI] [PubMed] [Google Scholar]
- 9.Olsen TS, Christensen RHB, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and lower all-cause mortality – Ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38:2646–2651. doi: 10.1161/STROKEAHA.107.490292. [DOI] [PubMed] [Google Scholar]
- 10.Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Effects of clinical and laboratory variables at admission and of in-hospital treatment with cardiovascular drugs on short term prognosis of ischemic stroke. The GIFA study. Nutr Metab Cardiovasc Dis. 2013;23:642–649. doi: 10.1016/j.numecd.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Vauthey C, de Freitas GR, van Melle G, Devuyst G, Bogousslavsky J. Better outcome after stroke with higher serum cholesterol levels. Neurology. 2000;54:1944–1948. doi: 10.1212/wnl.54.10.1944. [DOI] [PubMed] [Google Scholar]
- 12.Zuliani G, Cherubini A, Atti AR, et al. Low cholesterol levels are associated with short-term mortality in older patients with ischemic stroke. J Gerontol A Biol Sci Med Sci. 2004;59:293–297. doi: 10.1093/gerona/59.3.m293. [DOI] [PubMed] [Google Scholar]
- 13.Dyker AG, Weir CJ, Lees KR. Influence of cholesterol on survival after stroke: retrospective study. BMJ. 1997;314:1584. doi: 10.1136/bmj.314.7094.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Liu M, Wu B, Liu H, Wang LC, Tan S. Serum lipid levels and 3-month prognosis in Chinese patients with acute stroke. Adv Ther. 2008;25:329–341. doi: 10.1007/s12325-008-0045-7. [DOI] [PubMed] [Google Scholar]
- 15.Cuadrado-Godia E, Jiménez-Conde J, Ois A, Rodríguez-Campello A, Garcia-Ramallo E, Roquer J. Sex differences in the prognostic value of the lipid profile after the first ischemic stroke. J Neurol. 2009;256:989–995. doi: 10.1007/s00415-009-5059-9. [DOI] [PubMed] [Google Scholar]
- 16.Pan SL, Lien IN, Chen TH. Is higher serum total cholesterol level associated with better long-term functional outcomes after noncardioembolic ischemic stroke? Arch Phys Med Rehabil. 2010;91:913–918. doi: 10.1016/j.apmr.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Mizrahi EH, Waitzman A, Arad M, Adunsky A. Functional outcome of elderly survivors of ischemic stroke: a retrospective study comparing non-hypercholesterolemic and hypercholesterolemic patients. Isr Med Assoc J. 2011;13:295–299. [PubMed] [Google Scholar]
- 18.Zhao W, An Z, Hong Y, et al. Low total cholesterol level is the independent predictor of poor outcomes in patients with acute ischemic stroke: a hospital-based prospective study. BMC Neurol. 2016;16:1. doi: 10.1186/s12883-016-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 20.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 21.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsell CJ, Alwell K, Moomaw CJ, et al. Validity of a retrospective National Institutes of Health Stroke Scale scoring methodology in patients with severe stroke. J Stroke Cerebrovasc Dis. 2005;14:281–283. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH stroke scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults; the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 25.Aboa-Eboule C, Binquet C, Jacquin A, et al. Effect of previous statin therapy on severity and outcome in ischemic stroke patients: a population-based study. J Neurol. 2013;260:8. doi: 10.1007/s00415-012-6580-9. [DOI] [PubMed] [Google Scholar]
- 26.Arboix A, Garcia-Eroles L, Oliveres M, Targa C, Balcells M, Massons J. Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: a pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? BMC Neurol. 2010;10:47. doi: 10.1186/1471-2377-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flint AC, Kamel H, Navi BB, et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43:147–154. doi: 10.1161/STROKEAHA.111.627729. [DOI] [PubMed] [Google Scholar]
- 28.Ni Chroinin D, Asplund K, Asberg S, et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–456. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]
- 29.Ni Chroinin D, Callaly EL, Duggan J, et al. Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke: the North Dublin Population Stroke Study. Stroke. 2011;42:1021–1029. doi: 10.1161/STROKEAHA.110.596734. [DOI] [PubMed] [Google Scholar]
- 30.Trialists CT. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JL, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 32.Di Napoli R, Taccardi AA, Oliver M, De Caterina R. Statins and stroke: evidence for cholesterol-independent effects. Eur Heart J. 2002;23:1908–1921. doi: 10.1053/euhj.2002.3236. [DOI] [PubMed] [Google Scholar]
- 33.Endres M, Laufs U, Huang ZH, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholas JS, Swearingen CJ, Thomas JC, Rumboldt Z, Tumminello P, Patel SJ. The effect of statin pretreatment on infarct volume in ischemic stroke. Neuroepidemiology. 2008;31:48–56. doi: 10.1159/000140095. [DOI] [PubMed] [Google Scholar]
- 35.Shook SJ, Gupta R, Vora NA, Tievsky AL, Katzan I, Krieger DW. Statin use is independently associated with smaller infarct volume in nonlacunar MCA territory stroke. J Neuroimaging. 2006;16:341–346. doi: 10.1111/j.1552-6569.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 36.Vatassery GT, Smith WE, Quach HT, Lai JCK. invitro oxidation of vitamin-E, vitamin-C, thiols and cholesterol in rat-brain mitochondria incubated with free-radicals. Neurochem Int. 1995;26:527–535. doi: 10.1016/0197-0186(94)00147-m. [DOI] [PubMed] [Google Scholar]
- 37.Xi L, Ghosh S, Wang XY, Das A, Anderson FP, Kukreja RC. Hypercholesterolemia enhances tolerance to lethal systemic hypoxia in middle-aged mice: possible role of VEGF downregulation in brain. Mol Cell Biochem. 2006;291:205–211. doi: 10.1007/s11010-006-9194-7. [DOI] [PubMed] [Google Scholar]