Abstract
Aim
To review the available literature pertaining to fatalities following vaccine administration and, in particular, cases of vaccine-related fatal anaphylaxis.
Method
The MEDLINE database was systematically searched up to March 2016 to identify all relevant articles pertaining to fatal cases of anaphylaxis following vaccine administration.
Results
Six papers pertaining to fatal anaphylaxis following vaccination were found relevant. Mast cell tryptase and total IgE concentration was assessed exclusively in one case. Laryngeal edema was not detected in any of these cases, whereas eosinophil or mast cell infiltration was observed in lymphoid organs. In one case, immunohistochemical investigations using anti-tryptase antibodies allowed pulmonary mast cells and degranulating mast cells with tryptase-positive material outside to be identified.
Conclusion
In any suspected IgE-mediated fatal anaphylactic cases, biochemical investigations should be systematically performed for forensic purposes. Splenic tissue should be routinely sampled for immunohistochemical investigations in all suspected anaphylaxis-related deaths and mast cell/eosinophil infiltrations should be systematically sought out in the spleen, myocardium, and coronary artery wall. The hypothesis of fatal anaphylaxis following vaccination should be formulated exclusively when circumstantial data, available medical records, laboratory investigations, and autopsy or histology findings converge in a consistent pattern. The reasonable exclusion of alternative causes of death after all postmortem investigations is also imperative in order to establish or rule out a cause-and-effect relationship between vaccine administration and any presumptive temporarily-related death.
Vaccination is considered one of the greatest achievements of public health, with vaccination programs contributing to the decline in mortality and morbidity of various infectious diseases. The high rate of childhood vaccination in most developed countries indicates that it remains a widely accepted public measure (1).
Although coverage levels for most childhood vaccines remain high, numerous studies have documented that vaccine-related confidence has been decreasing among parents over the past several years. Vaccine hesitancy refers to concerns regarding vaccine safety and necessity. Nonetheless, most vaccine-hesitant parents do proceed with vaccinations, albeit often with a delay in some or all vaccines that potentially leave their children at risk for vaccine-preventable diseases. Parents who refuse all recommended vaccines are relatively rare (1-5).
Access to vaccine information and misinformation from a wide range of sources significantly influences vaccine decision-making. Parents may hear a multitude of messages regarding vaccination, some of which conflicting or inaccurate. This may lead to misperceptions that can influence vaccine acceptance. Negative media coverage about vaccine safety or vaccine-related illnesses, injuries or death may correlate with an increased incidence of vaccine-preventable diseases (1,5).
Hundreds of millions of vaccinations are administered to children and adults in the world. Serious adverse reactions, including anaphylaxis, are uncommon. However, temporarily associated deaths can occur following immunization and these require careful postmortem assessment. It is essential to establish whether the death is coincidental or causally related to the administered vaccination in order to avoid social concerns that may negatively impact public perception or vaccine acceptance, if not the actual decision to adhere to vaccination programs or not (6).
Even though virtually all vaccines have the potential to trigger anaphylaxis, the risk of anaphylaxis after vaccination is extremely low and varies with different vaccine types (7,8).
Forensic pathologists may occasionally encounter cases of deaths following vaccinations or possibly causally related to vaccination due to the unexplained nature of death and the potential relationship to the adverse effects of the administered vaccine.
There have been very few cases of vaccine-related fatal anaphylaxis described in forensic literature. Of those reported, only a small part had undergone the exhaustive postmortem investigations necessary to correctly formulate the hypothesis of anaphylaxis-related death following vaccine administration. These include total and specific IgE assessment, mast cell tryptase determination and histological/immunohistochemical examination (9).
The aim of this study was to review the available literature pertaining to fatalities following vaccine administration and, in particular, cases of vaccine-related fatal anaphylaxis. These deaths were considered with respect to occurrence, patient characteristics, administered vaccine and performed biochemical or histological investigations as well as any other useful information identified based on clinical history and medical record review.
MATERIAL AND METHODS
The MEDLINE database was systematically searched up to March 2016 to identify all relevant articles pertaining to fatal cases of anaphylaxis following vaccine administration. The search strategy used the keywords and/or mesh-terms “anaphylaxis”, “anaphylactic”, “anaphylactoid”, “shock”, “allergy”, “allergic”, “nonallergic”, “non-allergic”, “hypersensitivity”, “immediate”, “reaction(s)”, “adverse reaction(s)”, “side effect(s)” “adverse effect(s)”, “fatal”, “fatality”, “fatalities”, “lethal”, “death”, “forensic”, “medicolegal”, “medico-legal”, “tryptase”, “beta-tryptase”, “β-tryptase”, “mast cell tryptase”, “postmortem”, “post-mortem” or “autopsy” combined with any combination of the following: “vaccine(s)” (“influenza”, “pertussis”, “measles”, “mumps”, “rubella”, “pneumococcus/pneumococcal”, “meningococcus/meningococcal”, “haemophilus”, “encephalitis”, “tuberculosis”, “hepatitis”, “poliovirus”, “poliomyelitis”, “polio”, “rabies”, “typhoid”, “cholera”, “varicella”, “yellow fever”, “zoster”, “tetanus”, “diphtheria”, “toxoid(s)”, “hexavalent”, “trivalent”, “quadrivalent”, “pentavalent”, “monovalent”, “papillomavirus”, “rotavirus”), “vaccine components”, “vaccination(s)” and “immunization(s)”. The reference lists of the selected articles were then hand-searched for additional, potentially relevant articles. In order to be considered significant for the aim of our study, articles had to report fatal anaphylactic reactions following vaccine administration, patient characteristics (age, gender, medical record availability) and vaccine details. The absence of autopsy/histology findings or postmortem biochemical investigation results was not considered exclusion criteria. Published review articles pertaining to fatal reactions to vaccinations were included when data concerning individual fatal cases could be obtained. Two reviewers [CP and CT] checked all potentially relevant data in order to select pertinent papers. Of all the latter, full text papers were retrieved for further checking of inclusion and exclusion criteria. Articles that did not mention patient characteristics (age, gender, medical records) and vaccine type were excluded. Inclusion and exclusion criteria were checked independently by two reviewers (CT and MPS).
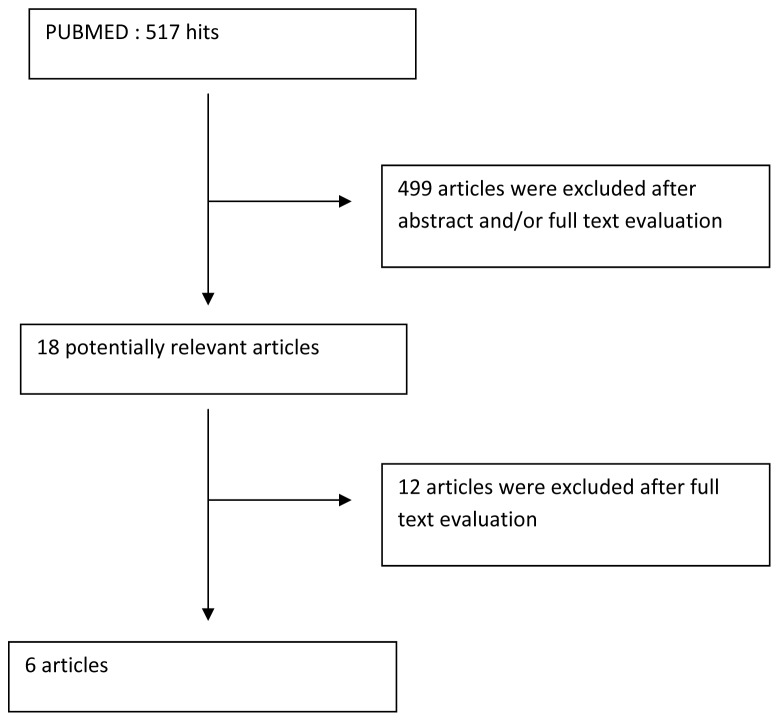
The initial strategy resulted in 517 articles, of which 18 were related to death following vaccination based on the abstracts and/or full texts. After reading the full texts, 6 papers pertaining to fatal anaphylaxis following vaccination were found relevant (Figure 1).
Figure 1.
Search strategy, study selection, and study inclusion.
RESULTS
We collected data on autopsy, histology, toxicology and biochemistry, when available in the selected articles (Table 1)
Table 1.
Fatal presumptive anaphylactic cases following vaccine administration available in the literature
| Authors, year of publication (ref. No.) | Age, gender | Administered vaccine Post-vaccination interval | Autopsy | Histology Immunohistochemistry | Biochemistry | Toxicology |
|---|---|---|---|---|---|---|
| Ziskind and Schattenberg, 1938 (10) |
39 year-old female |
Typhoid vaccine
Second injection
First symptoms 30 min post vaccination
Death occurred shortly post vaccination |
Petechial hemorrhages
of the pleural surfaces
Generalized congestion |
Generalized congestion
Compressed pulmonary alveoli with congested capillaries along with dilated alveoli and ruptured alveolar walls
Eosinophils in the pulmonary vessels. Occasional eosinophils in the hepatic vessels |
Not performed |
Not performed |
| Walker, 1948 (11) |
20 year-old male |
Typhus vaccine
Found dead 26 minutes after receiving typhus vaccine
Allergic to eggs
Yellow fever vaccine administered previously |
No visible local reaction at vaccination location
Generalized congestion
Pulmonary edema |
Generalized congestion
Pulmonary edema |
Not performed |
Not performed |
| Werne and Garrow, 1946 (12) |
10-month-old male identical twins |
Second injection of diphtheria toxoid and pertussis
Death within hours post vaccination |
Petechial hemorrhages
(several organs, including thymus)
Pulmonary edema
Generalized congestion |
Numerous eosinophils in the lymphatic tissues, including the thymus
Slight eosinophilic infiltration in bronchial and pulmonary artery wall |
Not performed |
Not performed |
| Curphey, 1947 (13) |
3˝-year-old female |
Influenza type A and B
First symptoms 4 h post vaccination
Death 7 h ˝ post vaccination |
Generalized congestion
Petechial hemorrhages
(several organs, including thymus) |
Occasional eosinophils in the lungs
No eosinophils in the spleen
Moderate eosinophil infiltration in lymph node |
Not performed |
Not reported |
| Pounder, 1983 (14) |
33-year-old male |
Vaccination against typhoid and cholera
First symptoms 1 h post vaccination
Death 8 h post vaccination |
No visible local reaction at vaccination location
No laryngeal edema
Right atrial and ventricular dilatation |
Occasional eosinophils sparsely scattered in the liver
Cardiac conduction system unremarkable |
Total IgE 720 IU/mL |
Not reported |
| D’Errico et al, 2008 (9) | 3-month-old female | Hexavalent vaccine Death 24 h post vaccination | Cardiac conduction system removed | Immunohistochemistry using anti-tryptase antibodies. Pulmonary mast cells and degranulating mast cell with tryptase-positive material outside identified. Cardiac conduction system unremarkable | Mast cell tryptase 43.3 μg/L | Negative |
Mast cell tryptase was assessed exclusively in one case and revealed a concentration over the clinical reference values in the living. Total IgE concentration was measured in only one case, though without specific IgE and mast cell tryptase. Laryngeal edema was not detected in any of these cases whereas eosinophil or mast cell infiltration was observed in lymphoid organs including the spleen, liver and lungs. In one case, immunohistochemical investigations using anti-tryptase antibodies allowed pulmonary mast cells and degranulating mast cells with tryptase-positive material outside to be identified.
DISCUSSION
An adverse event following vaccine administration is defined as any untoward medical occurrence following its administration and does not necessarily have a causal relationship with its use. Deaths following vaccination, especially when occurring in the first 48 h following vaccine administration, are of particular concern because they may raise community and health provider questions regarding the safety of the administered vaccine as well as the immunization program in general (15,16).
Four categories of suspected adverse events following vaccination have been reported: those induced by the injection process, those relating to a direct action of a vaccine component, those reflecting an immune-mediated process and those due to other mechanisms (17).
Adverse reactions reflecting an immune-mediated process include localized inflammatory responses, systemic inflammatory reactions, allergic (anaphylactic) reactions and other immune-related reactions. Vaccines are a mixture of compounds. Hence, allergic sensitization may occur to any component. Moreover, allergic reactions may follow administration of the first vaccine dose (7,15-18).
Vaccines include active immunizing antigens (complete microorganisms or their fragments, such as capsule polysaccharides), conjugating agents, preservatives, stabilizers, antimicrobial agents, adjuvant and culture media used in the preparation of the vaccine as well as the excipient used in the manufacturing process and inadvertent contaminants introduced during vaccine handling. Almost all vaccine components can be considered potential, allergic reaction triggers. These may include the natural rubber latex that can be contained in the syringe plunger as well as in the tips on prefilled syringes and vial stoppers. However, culture-derived proteins from eggs, gelatin (primarily used in viral vaccines to stabilize virus viability) and yeast are of particular importance. Other sources of allergic reactions are antibiotics and active immunizing antigens (7,9,15,18-23).
The mechanism involved in vaccine-associated allergies is generally considered to be a classical type I immediate hypersensitivity reaction involving an IgE-mediated response against a particular vaccine component. IgE-mediated reactions typically occur within minutes to an hour of exposure to relevant allergens and almost always within 4 hours of causative trigger contact. Type IV hypersensitivity delayed reactions have also been reported, though generally considered harmless. Type IV hypersensitivity reactions start 48 hours after vaccination and peak between 48 and 96 hours. They are typically observed with vaccines containing thimerosal (a mercury-containing preservative used in multidose vials that act as a preservative by inhibiting bacterial contamination), aluminum and antimicrobial agents in sensitized children and adults. Type IV reactions are becoming less frequent as mercury is being removed from modern vaccines. Type III hypersensitivity reactions have been described more rarely. These are attributed to the formation of circulating immune complexes between IgG antibodies and vaccine antigens and may induce a serum sickness-like disease or an exanthematous rash over 6-12 hours. Lastly, some delayed reactions may not be immunologically mediated. For instance, persistent hard nodules at the injection site have been attributed to local inflammatory reactions caused by irritant adjuvants such as aluminum and do not necessarily reflect immunologic hypersensitivity to vaccine constituents, though allergy to aluminum salts has also been proposed as an underlying mechanism (7,17,18,24,25).
Mast cells and basophils are the primary effector cells of classic type I immediate hypersensitivity reactions in humans. On the other hand, clinical and experimental experience has indicated that eosinophils are prominently engaged in allergic conditions and are implicated in the pathogenesis of anaphylaxis. The accumulation of eosinophils and mast cells in the spleen appears to be a hallmark of anaphylaxis, though it is still unclear whether splenic eosinophil infiltration is the consequence of recruitment through locally released chemotactic factors or whether it reflects general eosinophil increase in blood and tissue due to an allergic disposition. While the role of mast cells in human anaphylaxis is rather definite, issues such as the identification of the chemotactic factors that attract eosinophils in the spleen and the major signaling mechanism(s) for eosinophil activation remain poorly understood. Activated mast cells release numerous effector molecules, some of which could act as eosinophil chemotactic factors and consequently be responsible for eosinophil accumulation in the spleen (26-30).
The association between sudden infant death syndrome (SIDS), sudden unexpected death in infancy and vaccination has been discussed frequently. Because of the close temporal association between the first immunizations and the main peak of the SIDS incidence, it has been speculated that vaccinations may cause SIDS or sudden unexpected deaths in infancy. Nevertheless, several case-control studies have suggested that the apparent association between SIDS/sudden unexpected deaths in infancy and vaccinations occurs no more frequently than simply by chance or that immunizations may even be protective against SIDS. These results notwithstanding, the issue arises anew from time to time (16,25,31,32).
In 2003 a German study gave rise to suspicion of a possible association between hexavalent vaccine administration and sudden unexpected death in infancy. The signal was based on the observation of three deaths occurring between November 2000 and June 2003 in toddlers in their second year of life within 48 hours following administration of the fourth vaccine dose. In what became a heatedly discussed and criticized letter, another German team described six cases of sudden infant death shortly after hexavalent vaccination. In March 2011, Japan’s Health Ministry ordered doctors to stop immunizing infants with hexavalent vaccines subsequent to the sudden death of five babies within a short period of time after administration (33-39).
It has been emphasized that the definition itself of sudden infant death or sudden unexpected death in infancy following (hexavalent) vaccination is rather questionable. First, exhaustive postmortem examinations in presumptive vaccination-related death cases are not systematically performed. In addition, if examinations are performed, they may not include in-depth studies of the autonomic nervous system, vital centers of the brainstem or cardiac conduction system on serial sections. Morphological abnormalities in the brainstem and cardiac conduction system (such as arcuate nucleus hypoplasia in the brainstem as well as persistent fetal dispersion and resorptive degeneration in the cardiac conduction system) have been proposed in infants dying suddenly and unexpectedly as possible morphological substrates for sudden reflexogenic deaths, thus highlighting the importance of extensive postmortem investigations in all deaths occurring in infancy and perinatal age shortly after immunization (25,34,40).
As indicated above, vaccines are a mixture of compounds and almost all vaccine components can be considered as potential triggers to an allergic reaction. Anaphylaxis following vaccination is considered very rare with a documented risk in immunization information provided to the public of 1-2 cases per million vaccine dose. However, this becomes less clear when one considers the published literature on reported rates of anaphylaxis following vaccination. Indeed, this varies widely from 0.65 to 120 per million vaccines. The significant variation in the reported rate of anaphylaxis may be accounted for by a myriad of factors. Most importantly of these is case ascertainment and denominator data accuracy (ie, the number of vaccine doses administered). Case ascertainment will vary according to surveillance method (spontaneous reporting or active surveillance) and used case definition. Factors inherent to the target population being vaccinated as well as those inherent to the vaccine composition may also likely contribute to the variable rates of anaphylaxis (8,9,15,22-24,41).
The onus anaphylaxis detection as an adverse event following immunization falls into national, post-market surveillance systems, all of which rely on passive cases reporting. The reporting system of the Medicines and Healthcare Products Regulatory Agency in the UK received 130 reports of anaphylaxis associated with immunization in the six years from 1997 to 2003, suggesting a rate of 1 per million vaccine doses. Likewise, the US Vaccine Adverse Event Reporting System (VAERS) recorded 452 reports of reactions in over 1.9 billion doses of vaccines administrated countrywide over a 10-year period. This yields an estimated incidence of 0.2 cases for million vaccine doses. All post-marketing surveillance systems rely on passive case reporting and are thus prone to underreporting. These incidences are also of overall reaction rates and do not reflect incidences following individual vaccines (8,9,15,19,20).
Case descriptions of anaphylactic reactions to almost all vaccines exist. The incidence of anaphylactic reactions to the yellow fever vaccine reported to VAERS from 1991 to 1997 was about 1 per 131.000 doses. The reaction often occurred after the first vaccine dose, indicating that ingredients other than the immunizing vaccine antigen itself may have been the cause of the allergic reaction. Nagao et al. (22) demonstrated significantly increased influenza vaccine-specific IgE and vaccine-induced basophil activation in patients with influenza-vaccine induced anaphylaxis (IVA) who were included in the IVA spike in 2011-2012 in Japan. Judging by the number of case reports, the incidence of anaphylactic reactions to vaccines was higher 50 to 60 years ago than it is today. One reason for this may be that diagnostic criteria are more stringent nowadays than in the past. Another explanation can be that many of the reactions were caused by vaccine impurities. Indeed, improved purification processes have reduced the amount of allergenic substances in vaccines. Nevertheless, it can be said that severe systemic allergic reactions to vaccines were rare even 50 years ago (7-9,18,21-24,30,41,42).
Anaphylaxis-related deaths can present a series of unique challenges to forensic pathologists for several reasons. First, most forensic cases have incomplete, unreliable or absent medical records when bodies are admitted to the mortuary. Additionally, macroscopic and microscopic findings may be unspecific or absent. Furthermore, factors consistent with the hypothesis of fatal anaphylaxis include an immediately preceding challenge with an allergen known to cause reactions, clinical features consistent with or suggesting anaphylaxis, a previous history of reactions to similar or cross-reactive allergens, specific IgE antibodies to the allergen suspected of causing the reaction as well as measurable products of mast cell activation (26,43-45).
In the clinical setting, the diagnosis of anaphylaxis is based on consistent symptoms following exposure to potential triggering agents and may be further confirmed by increased levels of histamine and mast cell tryptase in plasma or serum. Unfortunately, practical consequences related to sampling times arise from the differing kinetics of histamine and mast cell tryptase appearance and elimination from blood. Indeed, samples for histamine determination should be obtained within 15 minutes of anaphylaxis onset, which might only be possible in a small proportion of cases and precluded in most of them. Conversely, samples for mast cell tryptase measurement can be obtained up to several hours after the reaction begins (26,44,46-49).
Based on the above, histamine determination is of no value for diagnostic purposes in the forensic setting whereas mast cell tryptase is. Indeed, mast cell tryptase levels can be assessed in postmortem serum even days after death and significantly increased postmortem serum tryptase levels have been reported repeatedly in cases of fatal anaphylaxis (26,44-47,50,51).
Although elevated postmortem serum tryptase concentrations may support the existence of mast cell activation, failure to document its elevation does not refute the diagnosis of anaphylaxis. Indeed, some cases of clinically diagnosed anaphylaxis to orally-ingested food allergens failed to show elevated tryptase levels, though abundant amounts of IgE against the ingested allergen could be detected. Moreover, beyond cases of fatal anaphylaxis, increased levels of postmortem serum tryptase have been observed in subjects with causes of death unrelated to anaphylaxis. This leads to the conclusion that postmortem, straightforward diagnoses of anaphylaxis cannot be exclusively based on mast cell tryptase determination alone (26,44,45,51-58).
Total and specific IgE antibodies appear to be relatively stable in postmortem serum samples and their measurements have proven useful for diagnostic purposes. However, total IgE determination in postmortem serum can only provide information pertaining to atopic disposition in individual cases. This means that increased levels do not prove that death was preceded by an IgE-mediated allergic reaction. In addition, similar to mast cell tryptase, increased total IgE levels have been observed frequently in situations unrelated to anaphylaxis, including diseases characterized by immune deficiencies or significant inflammatory components (26,44,45,59,60).
It has been proposed that combining results from mast cell tryptase determination in postmortem serum with a more specific assay for allergen sensitivity (such as allergen-specific postmortem serum IgE assays, if the identity of the allergen causing anaphylaxis is known or suspected) might support the hypothesis of IgE-mediated fatal anaphylaxis. Nevertheless, high levels of allergen-specific IgE exclusively indicate the degree of specific allergen sensitization in individual cases and do not prove death preceded by anaphylaxis. Hence, increased postmortem serum total and specific IgE levels cannot prove fatal allergic anaphylaxis (45,58,60-62).
Activated mast cell and eosinophil accumulation in the red pulp of the spleen has been demonstrated by several authors in anaphylaxis-related deaths using histochemical methods and specific immunohistochemical staining with monoclonal antibodies (44,54,63-66).
Today, a general consensus exists among researchers in recommending splenic tissue be systematically sampled for immunohistochemical investigations in all suspected anaphylaxis-related deaths. This is suggested in considering eosinophil and mast cell accumulation in splenic red pulp, along with increased postmortem serum tryptase levels, as the most reliable combination of microscopic findings and biochemical results to diagnose fatal anaphylaxis (44,65).
In addition, systematic histological examination of the myocardium and coronary arteries has been recommended by some authors. They suggest traditional and specific staining for mast cell identification in order to identify adventitial eosinophils and mast cells potentially involved in sudden death due to coronary artery spasm (67-72).
Very few cases of presumptive fatal anaphylaxis following immunization (including vaccine details, autopsy and histology findings as well as toxicology and biochemistry results when these analyses were performed) have been reported in the literature (9-14,73). The most exhaustively described case is the one by D’Errico et al. (9) in a 3-month-old baby who was administered hexavalent immunization in 2008. These authors performed not only biochemical analyses, which allowed an increased level of mast cell tryptase (43.3 μg/L) to be measured, but also immunohistochemical investigations using anti-tryptase antibodies, which allowed pulmonary mast cells and degranulating mast cells with tryptase-positive material outside to be identified. Moreover, these authors actually removed the cardiac conduction system during autopsy to carry out its histological examination. Examination of the system proved unremarkable.
The other reported cases, though interesting, lack either biochemical analysis or immunohistochemistry. Toxicology was only occasionally performed. Hence, the diagnosis of vaccination-related fatal anaphylaxis, though suggestive, remains (in our opinion) a mere hypothesis.
An analogous consideration may be applied to the case series reported by Zinka et al (35). These authors observed a slight eosinophil infiltration in various areas: in the liver in four out of six cases, in the lung in two out of six cases, in the spleen in just one case and at the cutaneous injection site in one case. Mast cell tryptase was measured in three out of six cases and levels were increased in two out of three. IgE levels were normal and specific IgE were assessed exclusively against tetanus toxoid and latex, both with negative results.
While the forensic literature on this topic is extremely scarce in comparison to other “allergic” or “anaphylactic” fatalities (which could lead to the conclusion that fatal anaphylaxis following vaccination is indeed an extremely rare occurrence, that these cases are only exceptionally investigated in the medico-legal setting or that they are rarely described in the literature as case reports), a very high numbers of papers exist in the clinical setting pertaining to vaccination-related deaths and other serious adverse vaccination-related events reported to various national, post-market surveillance systems. What is surprising “forensically” speaking, is that almost all these papers document a certain number of cases of vaccination-related deaths, though autopsy findings and data from death certificates are only available in a fraction of them (6,74-79).
If, on one hand, it is true that all post-marketing surveillance systems rely on passive case reporting and are prone to underreporting, on the other hand it is true that the number of more or less presumptive vaccination-related death cases (including presumptive fatal anaphylaxis cases following immunization) that had properly undergone in-depth forensic investigations is uncertain. In-depth refers to investigations characterized by histology, immunohistochemistry, biochemistry, toxicology and microbiology as well as microscopic examination of the cardiac conduction system and neuropathology with examination of the autonomic nervous system and vital centers of the brainstem in children. The question then arises, from a forensic point of view, about how many of these presumptive vaccination-related death cases or fatal anaphylaxis cases following immunization are actually caused by the administered vaccine.
Some years ago, the Brighton Collaboration Anaphylaxis Working Group developed case definition and guidelines for data collection, analysis and presentation for anaphylaxis as an adverse event following immunization. This very important paper stipulates that, because of uncertainties regarding the specificity of mast cell tryptase in the diagnosis of anaphylaxis and the absence of vaccine-specific data, mast cell tryptase measurement should be performed in suspected anaphylaxis cases, though this analysis should be considered exclusively as a minor diagnostic criterion. Where the presence of antigen specific serum-IgE does not necessarily predict clinical allergic manifestations, the absence of specific IgE does not rule out anaphylaxis. Hence, though specific IgE may have a useful role in causality assessment, it is not appropriate in case ascertainment. Lastly, the Group emphasizes that since anaphylaxis does not produce pathognomonic postmortem features, the proposed case definition of anaphylaxis should not take autopsy findings into account. On the basis of the conclusions of the Group, data from histology and immunohistochemistry are not considered in the case definition (80).
In conclusion, from a forensic point of view, all cases of sudden unexpected death in both adults and children require as many postmortem investigations as possible to be performed in order to rule out third party involvement and ascertain the cause of death as precisely as possible.
Regarding fatalities following drug administration, the role of forensic investigations is to determine whether the deaths are coincidental or causally related to the administered drug, irrespective of whether the drug is an antibiotic, contrast medium or vaccine. Death causally linked to drug administration but above all to obligatory vaccination may indeed give rise to social concerns, which must be avoided as far as possible, in the absence of reliable scientific evidence.
In suspected IgE-mediated fatal anaphylaxis, including fatal anaphylaxis potentially following vaccination, biochemical investigations should be systematically performed. However, it is worth emphasizing that increased postmortem serum levels of mast cell tryptase, total IgE and specific IgE do not prove (individually considered) that death was preceded by an IgE-mediate event. On the other hand, the absence of increased postmortem serum levels of mast cell tryptase does not allow the hypothesis of fatal anaphylaxis (following immunization) to be ruled out.
The accumulation of activated mast cells and eosinophils in the red pulp of the spleen has been repeatedly demonstrated in anaphylaxis-related fatalities. Consequently, splenic tissue should be routinely sampled for immunohistochemical investigations in all suspected anaphylaxis-related deaths and mast cell/eosinophil infiltrations should be systematically sought out in the spleen, myocardium and coronary artery wall.
Despite a certain number of uncertainties, eosinophil and mast cell accumulation in splenic red pulp, along with increased postmortem serum mast cell tryptase levels, can be considered the most reliable combination of microscopic findings and biochemical results in diagnosing fatal anaphylaxis in the forensic setting. Within the aforementioned limits, this same consideration should be applied to presumptive fatal anaphylaxis following immunization.
Microscopic evaluation of the cardiac conduction system and in-depth neuropathological examination including investigation of the autonomic nervous system and vital centers of the brainstem should always be performed in all cases of sudden unexpected deaths in infancy. This is especially important in situations potentially related to drug/vaccine administration in order to ascertain the role played by the administered medication.
On the other hand, all cases of sudden unexpected death in both adults and children require microbiological, toxicological and biochemical investigations. These are mandatory to exclude infection, intoxication and metabolic disturbances as causes of death.
To conclude, we are of the opinion that the hypothesis of fatal anaphylaxis following vaccination should be formulated exclusively when circumstantial data, medical records when available, and postmortem investigation results (mast cell tryptase, total IgE and specific IgE determination, immunohistochemical research of eosinophil and mast cell accumulation in splenic red pulp) converge in a consistent pattern. In addition, the reasonable exclusion of alternative causes of death is imperative in order to establish or rule out a cause-and-effect relationship between vaccine administration and any presumptive temporarily-related death.
Acknowledgments
Funding None.
Ethical approval was not required.
Declaration of authorship CP contributed to design of the manuscript, data collection, analysis, and interpretation, and manuscript writing. CT contributed to design of the manuscript, data collection, analysis, and interpretation, and manuscript writing. MPS contributed to design of the manuscript, data collection, analysis, and interpretation, and manuscript writing.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9:1763–73. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Hum Vaccin Immunother. 2013;9:1755–62. doi: 10.4161/hv.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson RM, St Sauver JL, Finney Rutten LJ. Vaccine hesitancy. Mayo Clin Proc. 2015;90:1562–8. doi: 10.1016/j.mayocp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Domachowske JB, Suryadevara M. Practical approaches to vaccine hesitancy issues in the United States: 2013. Hum Vaccin Immunother. 2013;9:2654–7. doi: 10.4161/hv.26783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kestenbaum LA, Feemster KA. Identifying and addressing vaccine hesitancy. Pediatr Ann. 2015;44:e71–5. doi: 10.3928/00904481-20150410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro PL, Arana J, Cano M, Lewis P, Shimabukuro TT. Deaths reported to the vaccine adverse event reporting system, United States, 1997-2013. Clin Infect Dis. 2015;61:980–7. doi: 10.1093/cid/civ423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nokleby H. Vaccination and anaphylaxis. Curr Allergy Asthma Rep. 2006;6:9–13. doi: 10.1007/s11882-006-0003-x. [DOI] [PubMed] [Google Scholar]
- 8.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–78. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Errico S, Neri M, Riezzo I, Rossi G, Pomara C, Turillazzi E, et al. Beta-tryptase and quantitative mast-cell increase in a sudden infant death following hexavalent immunization. Forensic Sci Int. 2008;179:e25–9. doi: 10.1016/j.forsciint.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Ziskind J, Schattenberg HJ. Fatal anaphylactic shock in man. Arch Intern Med (Chic) 1938;62:813–20. doi: 10.1001/archinte.1938.00180160092008. [DOI] [Google Scholar]
- 11.Walker RH. Fatal anaphylaxis following typhus vaccine injection. U S Nav Med Bull. 1948;48:303–5. [PubMed] [Google Scholar]
- 12.Werne J, Garrow I. Fatal anaphylactic shock: occurrence in identical twins following second injection of diphtheria toxoid and pertussis antigen. J Am Med Assoc. 1946;131:730–5. doi: 10.1001/jama.1946.02870260014003. [DOI] [PubMed] [Google Scholar]
- 13.Curphey TJ. Fatal allergic reaction due to influenza vaccine. J Am Med Assoc. 1947;133:1062–4. doi: 10.1001/jama.1947.62880150001007. [DOI] [PubMed] [Google Scholar]
- 14.Pounder DJ. Sudden, unexpected death following typhoid-cholera vaccination. Forensic Sci Int. 1984;24:95–8. doi: 10.1016/0379-0738(84)90157-9. [DOI] [PubMed] [Google Scholar]
- 15.Gold MS, Balakrishnan MR, Amarasinghe A, MacDonald NE. An approach to death as an adverse event following immunization. Vaccine. 2016;34:212–7. doi: 10.1016/j.vaccine.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: What does the evidence show? Vaccine. 2015;33:3288–92. doi: 10.1016/j.vaccine.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007;137(Suppl 1):S46–50. doi: 10.1016/j.jcpa.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Chung EH. Vaccine allergies. Clin Exp Vaccine Res. 2014;3:50–7. doi: 10.7774/cevr.2014.3.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erlewyn-Lajeunesse M, Bonhoeffer J, Ruggeberg JU, Heath PT. Anaphylaxis as an adverse event following immunisation. J Clin Pathol. 2007;60:737–9. doi: 10.1136/jcp.2006.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erlewyn-Lajeunesse M, Hunt LP, Heath PT, Finn A. Anaphylaxis as an adverse event following immunisation in the UK and Ireland. Arch Dis Child. 2012;97:487–90. doi: 10.1136/archdischild-2011-301163. [DOI] [PubMed] [Google Scholar]
- 21.Vanlander A, Hoppenbrouwers K. Anaphylaxis after vaccination of children: review of literature and recommendations for vaccination in child and school health services in Belgium. Vaccine. 2014;32:3147–54. doi: 10.1016/j.vaccine.2014.03.096. [DOI] [PubMed] [Google Scholar]
- 22.Nagao M, Fujisawa T, Ihara T, Kino Y. Highly increased levels of IgE antibodies to vaccine components in children with influenza vaccine-associated anaphylaxis. J Allergy Clin Immunol. 2016;137:861–7. doi: 10.1016/j.jaci.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Coop CA, Balanon SK, White KM, Whisman BA, Rathkopf MM. Anaphylaxis from the influenza virus vaccine. Int Arch Allergy Immunol. 2008;146:85–8. doi: 10.1159/000112507. [DOI] [PubMed] [Google Scholar]
- 24.Martín-Muńoz MF, Pereira MJ, Posadas S, Sánchez-Sabaté E, Blanca M, Alvarez J. Anaphylactic reaction to diphtheria-tetanus vaccine in a child: specific IgE/IgG determinations and cross-reactivity studies. Vaccine. 2002;20:3409–12. doi: 10.1016/S0264-410X(02)00228-1. [DOI] [PubMed] [Google Scholar]
- 25.Ottaviani G, Lavezzi AM, Matturri L. Sudden infant death syndrome (SIDS) shortly after hexavalent vaccination: another pathology in suspected SIDS? Virchows Arch. 2006;448:100–4. doi: 10.1007/s00428-005-0072-6. [DOI] [PubMed] [Google Scholar]
- 26.Palmiere C, Comment L, Mangin P. Allergic reactions following contrast material administration: nomenclature, classification, and mechanisms. Int J Legal Med. 2014;128:95–103. doi: 10.1007/s00414-013-0912-x. [DOI] [PubMed] [Google Scholar]
- 27.Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2006;117(2 Suppl Mini-Primer):S450–6. doi: 10.1016/j.jaci.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kita H, Kaneko M, Bartemes KR, Weiler DA, Schimming AW, Reed CE, et al. Does IgE bind to and activate eosinophils from patients with allergy? J Immunol. 1999;162:6901–11. [PubMed] [Google Scholar]
- 30.Simon HU. Allergic inflammation: focus on eosinophils. Allergy. 2013;68:823–4. doi: 10.1111/all.12231. [DOI] [PubMed] [Google Scholar]
- 31.Vennemann MM, Butterfass-Bahloul T, Jorch G, Brinkmann B, Findeisen M, Sauerland C, et al. Sudden infant death syndrome: no increased risk after immunisation. Vaccine. 2007;25:336–40. doi: 10.1016/j.vaccine.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Törő K, Vörös K, Mészner Z, Váradi-T A, Tóth A, Kovács K. Evidence for infection and inflammation in infant deaths in a country with historically low incidences of sudden infant death syndrome. Front Immunol. 2015;6:389. doi: 10.3389/fimmu.2015.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Kries R, Toschke AM, Strassburger K, Kundi M, Kalies H, Nennstiel U, et al. Sudden and unexpected deaths after the administration of hexavalent vaccines (diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, Haemophilius influenzae type b): is there a signal? Eur J Pediatr. 2005;164:61–9. doi: 10.1007/s00431-004-1594-7. [DOI] [PubMed] [Google Scholar]
- 34.Matturri L, Del Corno G, Lavezzi AM. Sudden infant death following hexavalent vaccination: a neuropathologic study. Curr Med Chem. 2014;21:941–6. doi: 10.2174/09298673113206660289. [DOI] [PubMed] [Google Scholar]
- 35.Zinka B, Rauch E, Buettner A, Ruëff F, Penning R. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine. 2006;24:5779–80. doi: 10.1016/j.vaccine.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 36.von Kries R. Comment on B. Zinka et al., Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine. 2006;24:5783–4, author reply 5785-6. doi: 10.1016/j.vaccine.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt HJ, Siegrist CA, Salmaso S, Law B, Booy R. B. Zinka et al. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine. 2006;24:5781–2, author reply 5785-6. doi: 10.1016/j.vaccine.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 38.Zinka B, Rauch E, Buettner A, Ruëff F, Penning R. Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine. 2006;24:5779–80. doi: 10.1016/j.vaccine.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 39.Maurer W. Death following hexavalent vaccination. Vaccine. 2005;23:5461–3. doi: 10.1016/j.vaccine.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 40.Ottaviani G, Matturri L, Rossi L, James TN. Crib death: further support for the concept of fatal cardiac electrical instability as the final common pathway. Int J Cardiol. 2003;92:17–26. doi: 10.1016/S0167-5273(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 41.Seitz CS, Bröcker EB, Trautmann A. Vaccination-associated anaphylaxis in adults: diagnostic testing ruling out IgE-mediated vaccine allergy. Vaccine. 2009;27:3885–9. doi: 10.1016/j.vaccine.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Kelso JM, Mootrey GT, Tsai TF. Anaphylaxis from yellow fever vaccine. J Allergy Clin Immunol. 1999;103:698–701. doi: 10.1016/S0091-6749(99)70245-9. [DOI] [PubMed] [Google Scholar]
- 43.Khan BQ, Kemp SF. Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11:319–25. doi: 10.1097/ACI.0b013e3283481ab6. [DOI] [PubMed] [Google Scholar]
- 44.Reggiani Bonetti L, Maccio L, Trani N, Radheshi E, Palmiere C. Splenic hypereosinophilia in anaphylaxis-related death: different assessments depending on different types of allergens? Int J Legal Med. 2015;129:97–103. doi: 10.1007/s00414-014-1004-2. [DOI] [PubMed] [Google Scholar]
- 45.Pumphrey RS, Roberts IS. Postmortem findings after fatal anaphylactic reactions. J Clin Pathol. 2000;53:273–6. doi: 10.1136/jcp.53.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa Y, Grant JA. Mediators of anaphylaxis. Immunol Allergy Clin North Am. 2007;27:249–60. doi: 10.1016/j.iac.2007.03.013. . vii. [DOI] [PubMed] [Google Scholar]
- 47.Hogan AD, Schwartz LB. Markers of mast cell degranulation. Methods. 1997;13:43–52. doi: 10.1006/meth.1997.0494. [DOI] [PubMed] [Google Scholar]
- 48.Michalska-Krzanowska G. Tryptase in diagnosing adverse suspected anaphylactic reaction. Adv Clin Exp Med. 2012;21:403–8. [PubMed] [Google Scholar]
- 49.Sala-Cunill A, Cardona V, Labrador-Horrillo M, Luengo O, Esteso O, Garriga T, et al. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol. 2013;160:192–9. doi: 10.1159/000339749. [DOI] [PubMed] [Google Scholar]
- 50.Da Broi U, Moreschi C. Post-mortem diagnosis of anaphylaxis: A difficult task in forensic medicine. Forensic Sci Int. 2011;204:1–5. doi: 10.1016/j.forsciint.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 51.Sravan A, Tse R, Cala AD. A decline in 2 consecutive postmortem serum tryptase levels in an anaphylactic death. Am J Forensic Med Pathol. 2015;36:233–5. doi: 10.1097/PAF.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 52.Edston E, van Hage-Hamsten M. beta-Tryptase measurements post-mortem in anaphylactic deaths and in controls. Forensic Sci Int. 1998;93:135–42. doi: 10.1016/S0379-0738(98)00040-1. [DOI] [PubMed] [Google Scholar]
- 53.Edston E, Eriksson O, van Hage M. Mast cell tryptase in postmortem serum-reference values and confounders. Int J Legal Med. 2007;121:275–80. doi: 10.1007/s00414-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 54.Mayer DE, Krauskopf A, Hemmer W, Moritz K, Jarisch R, Reiter C. Usefulness of post mortem determination of serum tryptase, histamine and diamine oxidase in the diagnosis of fatal anaphylaxis. Forensic Sci Int. 2011;212:96–101. doi: 10.1016/j.forsciint.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Luongo S, Frontalini C, Pesaresi M, Valsecchi M, Tagliabracci A. Histopathological markers for the diagnosis of anaphylactic death. Med Sci Law. 2011;51(Suppl 1):S30–6. doi: 10.1258/msl.2010.010059. [DOI] [PubMed] [Google Scholar]
- 56.Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Ann Allergy Asthma Immunol. 2007;98:252–7. doi: 10.1016/S1081-1206(10)60714-4. [DOI] [PubMed] [Google Scholar]
- 57.Yunginger JW, Nelson DR, Squillace DL, Jones RT, Holley KE, Hyma BA, et al. Laboratory investigation of deaths due to anaphylaxis. J Forensic Sci. 1991;36:857–65. doi: 10.1520/JFS13095J. [DOI] [PubMed] [Google Scholar]
- 58.McLean-Tooke A, Goulding M, Bundell C, White J, Hollingsworth P. Postmortem serum tryptase levels in anaphylactic and non-anaphylactic deaths. J Clin Pathol. 2014;67:134–8. doi: 10.1136/jclinpath-2013-201769. [DOI] [PubMed] [Google Scholar]
- 59.Randall B, Butts J, Halsey JF. Elevated postmortem tryptase in the absence of anaphylaxis. J Forensic Sci. 1995;40:208–11. doi: 10.1520/JFS15343J. [DOI] [PubMed] [Google Scholar]
- 60.Horn KD, Halsey JF, Zumwalt RE. Utilization of serum tryptase and immunoglobulin e assay in the postmortem diagnosis of anaphylaxis. Am J Forensic Med Pathol. 2004;25:37–43. doi: 10.1097/01.paf.0000113814.56572.de. [DOI] [PubMed] [Google Scholar]
- 61.Ansari MQ, Zamora JL, Lipscomb MF. Postmortem diagnosis of acute anaphylaxis by serum tryptase analysis. A case report. Am J Clin Pathol. 1993;99:101–3. doi: 10.1093/ajcp/99.1.101. [DOI] [PubMed] [Google Scholar]
- 62.Fisher MM, Baldo BA. The diagnosis of fatal anaphylactic reactions during anaesthesia: employment of immunoassays for mast cell tryptase and drug-reactive IgE antibodies. Anaesth Intensive Care. 1993;21:353–7. doi: 10.1177/0310057X9302100321. [DOI] [PubMed] [Google Scholar]
- 63.Fineschi V, Cecchi R, Centini F, Reattelli LP, Turillazzi E. Immunohistochemical quantification of pulmonary mast-cells and post-mortem blood dosages of tryptase and eosinophil cationic protein in 48 heroin-related deaths. Forensic Sci Int. 2001;120:189–94. doi: 10.1016/S0379-0738(00)00469-2. [DOI] [PubMed] [Google Scholar]
- 64.Perskvist N, Edston E. Differential accumulation of pulmonary and cardiac mast cell-subsets and eosinophils between fatal anaphylaxis and asthma death: a postmortem comparative study. Forensic Sci Int. 2007;169:43–9. doi: 10.1016/j.forsciint.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Edston E. Accumulation of eosinophils, mast cells, and basophils in the spleen in anaphylactic deaths. Forensic Sci Med Pathol. 2013;9:496–500. doi: 10.1007/s12024-013-9468-9. [DOI] [PubMed] [Google Scholar]
- 66.Trani N, Reggiani Bonetti L, Gualandri G, Barbolini G. Immediate anaphylactic death following antibiotics injection: splenic eosinophilia easily revealed by pagoda red stain. Forensic Sci Int. 2008;181:21–5. doi: 10.1016/j.forsciint.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med. 2016;54:1545–59. doi: 10.1515/cclm-2016-0010. [DOI] [PubMed] [Google Scholar]
- 68.Kounis NG, Soufras GD. Kounis syndrome: a primary cause for the anaphylactic shock. Cardiol J. 2014;21:102–3. doi: 10.5603/CJ.2014.0012. [DOI] [PubMed] [Google Scholar]
- 69.Kounis NG, Soufras GD, Hahalis G. Anaphylactic shock: Kounis hypersensitivity-associated syndrome seems to be the primary cause. N Am J Med Sci. 2013;5:631–6. doi: 10.4103/1947-2714.122304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kounis NG, Soufras GD, Kounis GN, Hahalis G. Suicidal anaphylactic death: is Kounis anaphylaxis associated syndrome the cause? Forensic Sci Int. 2013;232:e42–3. doi: 10.1016/j.forsciint.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 71.Kounis NG, Kounis GN, Soufras GD, Lianas D, Patsouras N. Postmortem diagnosis of drug-induced anaphylactic death: Kounis syndrome and hypersensitivity myocarditis are the likely culprit in death of severe anaphylactic reactions. J Forensic Leg Med. 2016;40:40–1. doi: 10.1016/j.jflm.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Kounis NG, Soufras GD, Hahalis G. Accumulation of eosinophils, mast cells, and basophils in the spleen and the coronary arteries in anaphylactic deaths: is the Kounis hypersensitivity associated syndrome present? Forensic Sci Med Pathol. 2014;10:150–1. doi: 10.1007/s12024-013-9489-4. [DOI] [PubMed] [Google Scholar]
- 73.Stratton KR, Howe CJ, Johnston RB Jr, editors. Adverse events associated with childhood vaccines. Evidence bearing on causality. National Academy Press, Washington, D.C., 1994. [PubMed] [Google Scholar]
- 74.Johann-Liang R, Josephs S, Dreskin SC. Analysis of anaphylaxis cases after vaccination: 10-year review from the National Vaccine Injury Compensation Program. Ann Allergy Asthma Immunol. 2011;106:440–3. doi: 10.1016/j.anai.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Dong D, Cheng G, Zuo S, Liu D, Du X. Post-marketing surveillance of live-attenuated Japanese encephalitis vaccine safety in China. Vaccine. 2014;32:5875–9. doi: 10.1016/j.vaccine.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Moro PL, Jankosky C, Menschik D, Lewis P, Duffy J, Stewart B, et al. Adverse events following Haemophilus influenzae type b vaccines in the Vaccine Adverse Event Reporting System, 1990-2013. J Pediatr. 2015;166:992–7. doi: 10.1016/j.jpeds.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sukumaran L, McNeil MM, Moro PL, Lewis PW, Winiecki SK, Shimabukuro TT. Adverse events following measles, mumps, and rubella vaccine in adults reported to the vaccine adverse event reporting system (VAERS), 2003-2013. Clin Infect Dis. 2015;60:e58–65. doi: 10.1093/cid/civ061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Traversa G, Spila-Alegiani S, Bianchi C, Ciofi degli Atti M, Frova L, Massari M, et al. Sudden unexpected deaths and vaccinations during the first two years of life in Italy: a case series study. PLoS One. 2011;6:e16363. doi: 10.1371/journal.pone.0016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahajan D, Dey A, Cook J, Harvey B, Menzies R, Macartney K. Surveillance of adverse events following immunisation in Australia annual report, 2013. Commun Dis Intell Q Rep. 2015;39:E369–86. [PubMed] [Google Scholar]
- 80.Rüggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5675–84. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]