Abstract
Aim
To determine whether antemortem blood levels of glycated hemoglobin (HbA1c) and glucose predict completed suicide and, by extension, whether markers of glucose metabolism might be associated with a prosuicidal trait or state.
Method
From consecutively performed autopsies, samples of blood and vitreous humor from 17 suicide victims and 27 non-suicide controls were compared with regard to levels of glucose, lactate, and HbA1c.
Results
Mean HbA1c was higher and mean estimated blood glucose was lower among suicide victims, although tests revealed no significant differences (P = 0.171 and P = 0.395, respectively). HbA1c levels exceeding 48.0 mmol/mol, which were indicative of persistent hyperglycemia, were twice as common in suicide victims (59% vs 30%; P = 0.068).
Conclusion
The finding of this pilot study suggest that deranged glucose metabolism may reflect biological events antecedent to, or concomitant with, completed suicide, with the following clinical implications: recurring hyperglycemia due to defective glucose transport, which may give rise to depression and suicidal ideation, and elevated HbA1c levels, which may represent an assayable correlate to neurobiological conditions predisposing to suicide.
Mood disorders are overrepresented in cases of completed suicide (1-4). Observational studies have shown that a diagnosis of diabetes mellitus – a condition characterized by persistent hyperglycemia – is associated with an increased risk of depression and anxiety in both adolescents and adults (5-10). In a recent cohort study of over 1.2 million Korean men and women, a single elevated fasting blood-glucose measurement was found to confer a two- to 3-fold increased risk of completed suicide during a 14-year follow-up (11), while a recent registry-based matched cohort study of the entire Swedish population demonstrated a 3.4-fold risk increase for suicide among subjects afflicted with diabetes (12). However, the mechanism by which deranged glucose metabolism could lead to suicide remains elusive.
Glucose is a ubiquitous source of energy, and under normal metabolic conditions—in the absence of fasting or other states of starvation—it is the human brain’s primary source of cellular fuel. Psychological processes such as self-control, decision making and regulation of emotions depend heavily on intracellular availability of glucose in the brain (13,14).
It has been suggested that dysregulation of emotions—resulting in aggressive impulses, pessimism and impulsivity—occurs more commonly among suicide attempters (15,16). Further, in males, aggressiveness and impulsivity have been shown to be associated to lower blood glucose levels (17). Although previous studies have identified several biochemical markers in completed suicide (18-20), to our knowledge no study has attempted to characterize the state of glucose metabolism in the weeks, hours and minutes prior to the suicidal act.
Hyperglycemia results from a reduced ability of cells to take up circulating glucose, on account of either insulin deficiency or insulin resistance. Elevated blood glucose levels can thus be interpreted as a sign of intracellular glucose deficiency. In the clinic, states of acute and chronic hyperglycemia are diagnosed by way of measurements in blood of glucose and glycosylated hemoglobin (HbA1c), respectively. While blood glucose reflects the current glycemic state, HbA1c—formed by non-enzymatic coupling of glucose and hemoglobin—reflects blood glucose levels over the past one to three months.
In the present pilot study, we compared subjects who had undergone forensic autopsy, grouped according to cause of death, to determine the extent to which glucose metabolism was deranged in the period preceding the suicidal act. We compared the estimated blood glucose and HbA1c concentrations in cases of completed suicide and controls. In addition, we compared frequencies of subjects in each group whose HbA1c concentrations exceeded the threshold for a diagnosis of diabetes mellitus at the time of autopsy.
MATERIAL AND METHODS
Subjects
Beginning in November 2012, in the course of 51 consecutive medico-legal autopsies performed at the Department of Forensic Medicine of the Hjelt Institute of the University of Helsinki, samples were collected post mortem according to standard procedures. All autopsies were conducted by the same forensic team, headed by a single pathologist.
At the time of autopsy, causes of death were determined and coded according to the International Statistical Classification of Disease, 10th revision (ICD-10). Subjects were divided into two groups according to ICD-10 codes: those who had died of suicide (ICD-10 codes X64-X84) and those who had died on account of other causes, including events of undetermined intent (ICD-10 codes Y10- Y34), who made up the control group.
Seven of the 51 subjects in original sample were excluded due to either protracted processes of postmortem decay, which made sampling impossible, or hemolysis of collected blood samples. Thus, a total of 44 individuals were included in the statistical analysis. Clinical and demographic data, as well as results of standard toxicological screening, were available for all 44 subjects.
Samples
Samples of blood and vitreous humor were collected from the femoral vein and vitreous chamber according to standard forensic procedures. Levels of HbA1c in blood were determined by high-pressure liquid chromatography using Mono-S cation-exchange columns, followed by conversion to the unit mmol/mol, as recommended by the International Federation of Clinical Chemistry (21).
Concentrations of glucose and lactic acid were determined by standard enzymatic assays, as described by Sippel and Möttönen (22). In accordance with formula of Traub, the sum of postmortem concentrations in vitreous humor of glucose and lactate was used to estimate antemortem glycemic state. Because levels of lactate in vitreous humor increase linearly with the duration of the postmortem interval (23), Traub-index levels were, for given individuals, not regarded as accurate estimates of antemortem blood glucose levels.
Statistical analysis
Blood HbA1c and Traub-index levels were compared in suicide victims and controls using Wilcoxon rank-sum test and Welch’s t test, respectively. In addition, frequencies of subjects in each group with blood HbA1c levels exceeding the threshold for persistent hyperglycemia, as defined by the World Health Organization (24,25), were compared using Fisher’s exact test. Uncorrected P < 0.05 were regarded as statistically significant. All statistical analyses were performed using R (ver. 3.1.3) (26).
RESULTS
The mean age at death and gender distribution were comparable between groups (Table 1). In the suicide group, physical trauma contributing to death was more common and pre-existing somatic illness less common. Antidepressants were detected in blood at similar rates in both groups. There were differences between groups with regard to the detection in postmortem blood of ethanol, as well as antidiabetic and antipsychotic medications — substances whose antemortem use may affect blood-glucose levels. With the exception of illicit drugs, all other analyzed substances, including alcohol, occurred more commonly in the non-suicide group.
Table 1.
Demographic and toxicological data by cause of death in suicide vs non-suicide subjects
| No. (%) of subjects | ||
|---|---|---|
|
Demographic characteristics |
suicide (n = 17) |
non-suicide (n = 27) |
| Male | 11 (65.0) | 18 (67.0) |
| Age (years; mean±SD; median, range)* | 55.0 ± 19.5 (63.5, 24-86) | 59.3 ± 21.0 (58.3, 21-93) |
| Physical trauma involved | 14 (82.0) | 7 (26.0) |
| Known somatic illness | 1 (6.0) | 20 (74.0) |
| Toxicological characteristics (number positive) | ||
| Ethanol | 4 (24.0) | 11 (41.0) |
| Benzodiazepines | 5 (29.0) | 11 (41.0) |
| Opiates | 2 (12.0) | 9 (33.0) |
| Other narcotic substances† | 3 (18.0) | 2 (7.0) |
| Antidepressants | 4 (24.0) | 8 (30.0) |
| Antipsychotics | 1 (6.0) | 5 (19.0) |
| Antidiabetic medications | 0 (0.0) | 2 (7.0) |
*SD – standard deviation.
†Cannabis, amphetamine.
Of the 44 subjects, 17 were adjudged to have committed suicide and 27 to have died of other causes. Among the suicide victims, specific causes of death were hanging (n = 7), gunshot wounds (n = 5), poisoning (n = 3), exposure to fire (n = 1), and contact with a moving vehicle (n = 1). Among the non- suicide controls, specific causes of death were cardiovascular disease (n = 6), accidental poisoning (n = 6), falling accidents (n = 5), alcohol-related organ damage (n = 3), infectious disease (n = 2), homicide (n = 1), traffic accident (n = 1), Alzheimer disease (n = 1), and unknown causes (n = 2).
Glucose
One subject in the non-suicide group displayed elevated ketone bodies in the absence of hyperglycemia and other findings indicative of alcohol-induced ketoacidosis. Mean values of Traub index, based on glucose and lactate measurements in the vitreous humor, did not differ significantly between the suicide and non-suicide group (35.3 ± 9.8 mmol/L vs 33.4 ± 4.9 mmol/L, respectively, P = 0.395; Table 2). Prior to analysis, the Shapiro-Wilk normality test had revealed that the values were normally distributed, allowing the use of a parametric test.
Table 2.
Glycemic biomarkers by cause of death in suicide vs non-suicide subjects
| Bimarker concentration (mean±SD; median, range)* | ||||
|---|---|---|---|---|
|
Biomarker |
suicide (n = 17) |
non-suicide (n = 27) |
P |
|
| Vitreous glucose, mmol/L | 0.9 ± 2.1 (0.0, 0.0-7.0) | 1.5 ± 2.9 (0.0, 0.0-11.9) | ||
| Vitreous lactate, mmol/L | 32.5 ± 5.1 (32.4, 23.3-39.1) | 33.9 ± 8.4 (34.9, 16.5-45.8) | ||
| Vitreous Traub†, mmol/L | 33.4 ± 4.9 (32.7; 23.3-41.1) | 35.3 ± 9.8 (34.9, 17.2-56.7) | 0.395‡ | |
| Blood HbA1c, mmol/L | 51.1 ± 14.7 (48.4, 35.6-97.8) | 46.6 ± 13.8 (43.2, 21.9-99.6) | 0.171§ | |
| Blood HbA1c (%)>48.0 mmol/mol (SEM) | 59.0 (5.03) | 30.0 (5.80) | 0.068‖ | |
*SD = standard deviation; SEM = standard error of the mean.
†Formula of Traub = lactate in vitreous humor + glucose in vitreous humor.
‡Welch’s t test.
§Wilcoxon rank-sum test.
‖Fisher’s exact test.
HbA1c
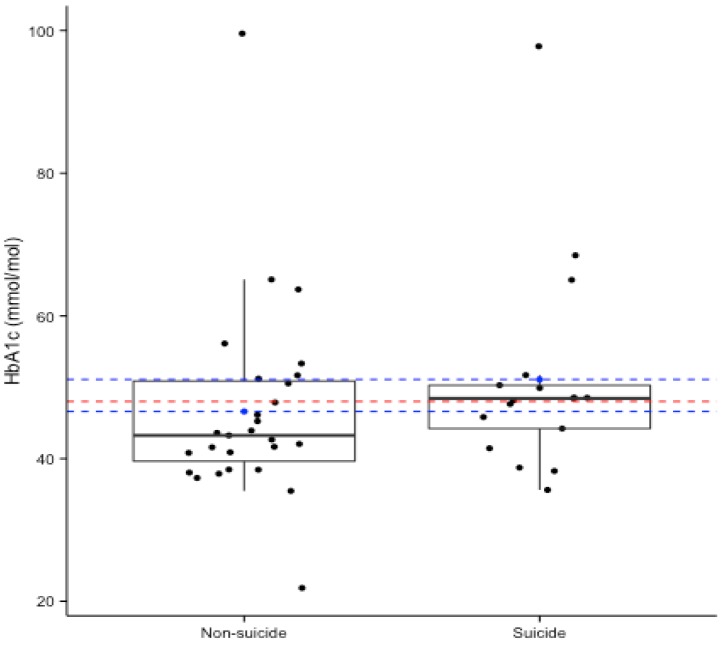
HbA1c levels measured in postmortem blood were, on average, higher among suicide victims than among subjects who had died of other causes (51.1 ± 14.7 mmol/mol vs 46.6 ± 13.8 mmol/mol, respectively; Table 2); however, the Shapiro-Wilk normality test revealed that the values were not normally distributed, necessitating the use of a non-parametric test. When tested using the Wilcoxon rank-sum test, the groups did not differ significantly with regard to HbA1c levels (P = 0.171; Table 2). In addition, HbA1c values exceeding the WHO threshold for dysregulated glucose metabolism (48.0 mmol/mol, recommended for use in diagnosing diabetes mellitus ante mortem) were twice as common in the suicide group, compared to the non- suicide group (59% vs 30%, P = 0.068; Table 2 and Figure 1). The specific causes of death in subjects with HbA1c values exceeding 48.0 mmol/mol were, among suicide victims hanging (n = 4), gunshot wounds (n = 3), poisoning (n = 1), exposure to fire (n = 1) and contact with a moving vehicle (n = 1); and, among non-suicide controls, cardiovascular disease (n = 4), falling accidents (n = 2), accidental poisoning (n = 1) and traffic accident (n = 1).
Figure 1.
HbA1c levels in postmortem blood by cause of death in suicide vs non-suicide subjects. The red dashed line indicates threshold recommended by the World Health Organization for diagnosis of diabetes mellitus (48.0 mmol/mol). The blue dashed lines indicate the mean value of each group. Groups did not differ significantly with regard to HbA1c levels (P = 0.171); however, HbA1c values exceeding 48.0 mmol/mol were twice as common in the suicide group (P = 0.068).
DISCUSSION
In the present study of glycemic biomarkers in consecutive forensic autopsies, ranked HbA1c levels were found to be higher, albeit nonsignificantly, in subjects who had committed suicide than subjects who had died of other causes. Similarly, in two recent studies, HbA1c levels were associated with suicidal ideation in subjects with either previously diagnosed (27) or undiagnosed (28) diabetes. The authors of the former study speculate that elevated HbA1c levels may reflect poor self-care associated with depression; whereas the authors of the latter study propose that defective glucose transport, resulting in recurring hyperglycemia, in itself may directly give rise to depression and suicidal ideation–may in other words, constitute a persisting, prosuicidal “trait.”
In the present study, we have also investigated whether glucose metabolism might play a role in the prosuicidal “state” – the suicidal act’s concomitant neurobiological substrate. We found that estimated antemortem blood glucose levels were slightly lower in subjects who had committed suicide than in subjects who had died of other causes, including somatic illness – although again the difference was not statistically significant. In the present data set, however, the extent to which the duration of antemortem agony differed between the two groups is unclear. Antemortem agony is, of course, associated with the release of stress hormones resulting in, among other things, transient elevation of blood glucose. By the same token, the reliability of approximations of antemortem blood glucose concentration, using Traub’s formula, is inversely proportionate to the postmortem interval (23), which, in Finland, as has been previously reported, is, on average, relatively long (29). Indeed, in a coming study in a large Swedish autopsy series, we hope to restrict our analysis of estimated blood glucose levels to cases of immediate death.
Acknowledgments
Funding This study wa supported by the Department of Forensic Medicine, University of Helsinki, Helsinki, Finland, and Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
Ethical approval received from the Hjelt Institute Ethical committee, the National Institute of Health and Welfare and the National Supervisory Authority for Welfare and Health (Ethical Approval No. 6112/05.01.00.06/2009).
Declaration of authorship The authors have contributed equally to the conception of the work, acquisition, analysis and interpretation of the data. Further, all authors have taken part of the final drafts of the manuscript and revised it for important intellectual content. Before finally approving the manuscript to be published, questions related to the accuracy or integrity of the work were investigated and resolved by all authors.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work..
References
- 1.Conwell Y, Brent D. Suicide and aging. I: Patterns of psychiatric diagnosis. Int Psychogeriatr. 1995;7:149–64. doi: 10.1017/S1041610295001943. [DOI] [PubMed] [Google Scholar]
- 2.Isometsä ET. Psychological autopsy studies – a review. Eur Psychiatry. 2001;16:379–85. doi: 10.1016/S0924-9338(01)00594-6. [DOI] [PubMed] [Google Scholar]
- 3.Skogman K, Alsén M, Ojehagen A. Sex differences in risk factors for suicide after attempted suicide-a follow-up study of 1052 suicide attempters. Soc Psychiatry Psychiatr Epidemiol. 2004;39:113–20. doi: 10.1007/s00127-004-0709-9. [DOI] [PubMed] [Google Scholar]
- 4.Tidemalm D. Lĺngström N, Lichtenstein P, Runeson B. Risk of suicide after suicide attempt according to coexisting psychiatric disorder: Swedish cohort study with long term follow-up. BMJ. 2008;337:a2205. doi: 10.1136/bmj.a2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, et al. Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics. 2006;117:1348–58. doi: 10.1542/peds.2005-1398. [DOI] [PubMed] [Google Scholar]
- 7.Hood KK, Lawrence JM, Anderson A, Bell R, Dabelea D, Daniels S, et al. Metabolic and inflammatory links to depression in youth with diabetes. Diabetes Care. 2012;35:2443–6. doi: 10.2337/dc11-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36:480–9. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KJ, Béland M, Clyde M, Gariépy G, Pagé V, Badawi G, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge P, Alonso J, Stein DJ, Kiejna A, Aguilar-Gaxiola S, Viana MC, et al. Associations between DSM-IV mental disorders and diabetes mellitus: a role for impulse control disorders and depression. Diabetologia. 2014;57:699–709. doi: 10.1007/s00125-013-3157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batty GD, Kivimaki M, Park IS, Jee SH. Diabetes and raised blood glucose as risk factors for future suicide: cohort study of 1 234 927 Korean men and women. J Epidemiol Community Health. 2012;66:650–2. doi: 10.1136/jech-2011-200464. [DOI] [PubMed] [Google Scholar]
- 12.Webb RT, Lichtenstein P, Dahlin M, Kapur N, Ludvigsson JF, Runeson B. Unnatural deaths in a national cohort of people diagnosed with diabetes. Diabetes Care. 2014;37:2276–83. doi: 10.2337/dc14-0005. [DOI] [PubMed] [Google Scholar]
- 13.Messer SC, Morris TL, Gross AM. Hypoglycemia and psychopathology: a methodological review. Clin Psychol Rev. 1990;10:631–48. doi: 10.1016/0272-7358(90)90073-J. [DOI] [Google Scholar]
- 14.Gailliot MT, Baumeister RF. The physiology of willpower: linking blood glucose to self-control. Pers Soc Psychol Rev. 2007;11:303–27. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- 15.Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann N Y Acad Sci. 1997;836:158–81. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- 16.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156:181–9. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Svanborg P, Mattila-Evenden M, Gustavsson PJ, Uvnäs-Moberg K, Asberg M. Associations between plasma glucose and DSM-III-R cluster B personality traits in psychiatric outpatients. Neuropsychobiology. 2000;41:79–87. doi: 10.1159/000026637. [DOI] [PubMed] [Google Scholar]
- 18.van Heeringen K. The neurobiology of suicide and suicidality. Can J Psychiatry. 2003;48:292–300. doi: 10.1177/070674370304800504. [DOI] [PubMed] [Google Scholar]
- 19.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–28. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 20.Ernst C, Mechawar N, Turecki G. Suicide neurobiology. Prog Neurobiol. 2009;89:315–33. doi: 10.1016/j.pneurobio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Nordin G, Dybkaer R. Recommendation for term and measurement unit for “HbA1c”. Clin Chem Lab Med. 2007;45:1081–2. doi: 10.1515/CCLM.2007.245. [DOI] [PubMed] [Google Scholar]
- 22.Sippel H, Möttönen M. Combined glucose and lactate values in vitreous humour for postmortem diagnosis of diabetes mellitus. Forensic Sci Int. 1982;19:217–22. doi: 10.1016/0379-0738(82)90081-0. [DOI] [PubMed] [Google Scholar]
- 23.Zilg B, Alkass K, Berg S, Druid H. Postmortem identification of hyperglycemia. Forensic Sci Int. 2009;185:89–95. doi: 10.1016/j.forsciint.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation. Geneva: World Health Organization, IDF; 2006. [Google Scholar]
- 25.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO Consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 26.The R Project for Statistical Computing. R version 3.1.3 (Smooth Sidewalk); 2015. Available from: https://www.r-project.org/. Accessed: February 24, 2017.
- 27.Bot M, Pouwer F, de Jonge P, Tack CJ, Geelhoed-Duijvestijn PH, Snoek FJ. Differential associations between depressive symptoms and glycaemic control in outpatients with diabetes. Diabet Med. 2013;30:e115–22. doi: 10.1111/dme.12082. [DOI] [PubMed] [Google Scholar]
- 28.Lee HY, Hahm MI, Lee SG. Risk of suicidal ideation in diabetes varies by diabetes regimen, diabetes duration, and HbA1c level. J Psychosom Res. 2014;76:275–9. doi: 10.1016/j.jpsychores.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Keltanen T, Sajantila A, Palo JU, Partanen T, Valonen T, Lindroos K. Assessment of Traub formula and ketone bodies in cause of death investigations. Int J Legal Med. 2013;127:1131–7. doi: 10.1007/s00414-013-0917-5. [DOI] [PubMed] [Google Scholar]