Viruses are a recurring threat to human health worldwide. From highly prevalent diseases such as HIV, dengue, and influenza to emerging viruses such as Ebola, SARS, and the Middle East respiratory syndrome coronavirus, viral infections cause serious diseases that require active treatment to prevent morbidity and mortality. Effective antiviral drugs are available for only nine viruses, including HIV, hepatitis B virus, hepatitis C virus, herpesvirus, influenza virus, human cytomegalovirus, varicella-zoster virus, respiratory syncytial virus, and external anogenital warts caused by human papillomavirus; however, most viral diseases lack effective drug treatments (De Clercq and Li, 2016).
The lack of narrow and broad spectrum antivirals is a deficiency in the modern medical formulary. This deficiency is due in large part to the genetic and replication-cycle diversity of viruses and the one-virus, one-drug mindset model used for current antiviral therapies. Most currently marketed antivirals have been developed to interact specifically with a viral protein from a virus of interest (e.g., HIV integrase or protease) and have not been shown to have significant utility against other viruses.
One approach to identifying broad-spectrum antivirals is to investigate host proteins or cofactors shown to be associated with viral replication that can be targeted by small molecules in a way that blocks viral infection. Uncovering host proteins or metabolic pathways that are important for multiple viruses can open the door for developing broad spectrum inhibitors of viral infection.
It is widely recognized that viruses depend on host cellular proteins and machinery at multiple points in their replication cycles, making host factors that are broadly used by many viruses attractive antiviral targets. All viruses use the protein synthesis machinery to drive translation of their mRNA. Identifying components of the translation machinery that are used by multiple viral families offers a significant opportunity to develop a broad spectrum antiviral approach. Inhibiting eukaryotic initiation factor 5A (eIF5A) function has recently emerged as a potential target for this type of intervention.
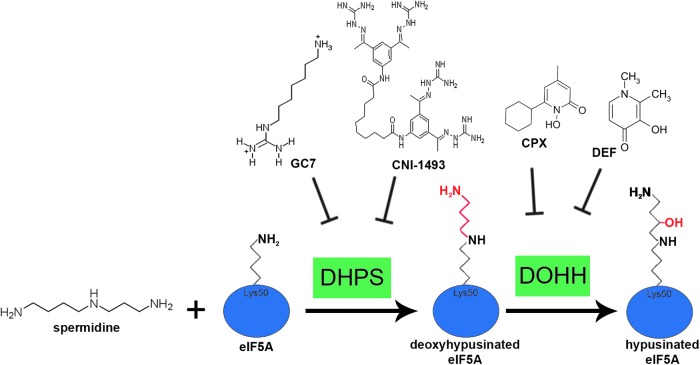
eIF5A is a translation factor that undergoes a unique post-translational modification, called hypusination. Hypusination of eIF5A occurs at Lysine 50 through a two-enzyme cascade, where an aminobutyl moiety from the polyamine spermidine becomes incorporated (by deoxyhypusine synthase [DHPS]) and hydroxylated (by deoxyhypusine hydroxylase [DOHH]), forming the hypusine residue (Fig. 1). eIF5A is the only cellular protein that contains hypusine, and hypusination is critical for its eIF5A function (Park et al., 1981). Hypusinated eIF5A (hyp-eIF5A) has been suggested to be involved in many cellular processes. Originally, hyp-eIF5A was identified as a stimulator of dipeptide synthesis (Kemper et al., 1976; Schreier et al., 1977; Benne and Hershey, 1978), but more recently has been suggested to be important for mRNA nucleocytoplasmic transport (Ruhl et al., 1993; Bevec et al., 1996; Elfgang et al., 1999), mRNA stability (Zuk and Jacobson, 1998; Schrader et al., 2006), and the translation of “hard to translate” regions, such as polyproline stretches (Gutierrez et al., 2013). The cellular functions of eIF5A have been reviewed in detail elsewhere (Dever et al., 2014; Rossi et al., 2014).
FIG. 1.
Cartoon representation of the hypusination pathway. eIF5A is hypusinated through a two enzyme cascade, where an aminobutyl group from the polyamine spermidine is first covalently attached to lysine 50 of eIF5A by deoxyhypusine synthase (DHPS) then hydroxylated by deoxyhypusine hydroxylase (DOHH). Small molecule inhibitors for each enzyme are also depicted.
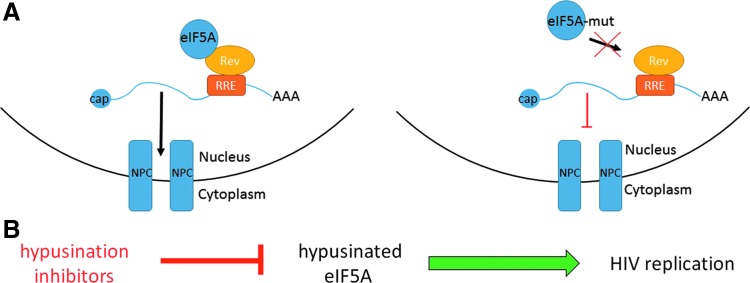
Increasing evidence suggests that eIF5A can play an important role in modulating virus replication. HIV was the first virus suggested to require eIF5A, through dependence of Rev-dependent nucleocytoplasmic transport on eIF5A (Fig. 2). The HIV-1 Rev transactivator protein mediates the translocation of viral mRNAs from the nucleus to the cytoplasm, and as such is essential for the expression of viral structural proteins (Malim et al., 1990). eIF5A specifically binds to Rev (Ruhl et al., 1993) and eIF5A loss-of-function mutants blocked the nuclear export of Rev protein and HIV-1 replication (Bevec et al., 1996). Furthermore, inhibition of the hypusination of eIF5A has also been shown to inhibit HIV replication (Andrus et al., 1998; Hauber et al., 2005; Hoque et al., 2009; Schroeder et al., 2014). Taken together, these data suggest that this pathway may be an interesting target for the development of HIV therapeutics.
FIG. 2.
HIV requires eIF5A for Rev-dependent nucleocytoplasmic transport. (A) Schematic of Rev-dependent nucleocytoplasmic transport with (left) and without (right) eIF5A. Mutant-eIF5A inhibits nucleocytoplasmic transport. (B) Hypusination inhibitors also inhibit HIV replication. NPC, nuclear pore complex; RRE, Rev responsive element.
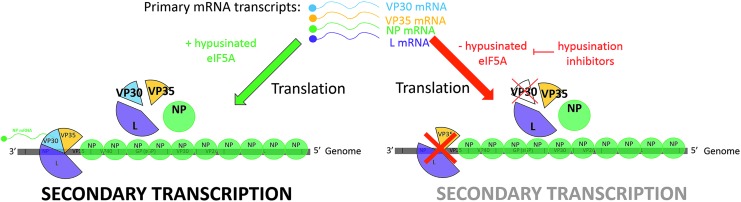
Recent work by our group suggests that hyp-eIF5A also plays a role in the replication of the Filoviruses Ebola virus (EBOV) and Marburg virus (MARV) (Olsen et al., 2016). Depletion of hyp-eIF5A followed by infection with EBOV or MARV resulted in a 3 log reduction of infectious titers of these viruses. Further mechanistic probing utilizing an EBOV transcription and replication minigenome assay revealed a defect in reporter gene expression, indicating that hyp-eIF5A is required for EBOV gene expression and/or genome replication. Furthermore, the accumulation of EBOV protein VP30 was reduced in the presence of hypusination inhibitors. Because VP30 is required for EBOV gene expression, a reduction in VP30 results in reduced reporter gene expression in the minigenome assay, and likely a halt in viral gene expression in viral infection (Fig. 3). These results suggest that targeting the hypusination of eIF5A may provide a therapeutic target for the development of antivirals treating hemorrhagic fever viruses.
FIG. 3.
Model representation of EBOV requirement of hypusinated-eIF5A for gene expression through a VP30-dependent mechanism. Hypusination inhibitors result in a reduction of VP30 mRNA translation, leading to a defect in EBOV secondary transcription. EBOV, Ebola virus; NP, nucleoprotein; VP30, viral protein 30; VP35, Viral protein 35; L, polymerase.
The appreciation that multiple, diverse virus families require eIF5A for their replication suggests that blocking its function might be a broad spectrum antiviral approach. Several molecules have been developed to target the hypusination of eIF5A. The two-step hypusination pathway offers two enzymes that can be directly targeted. The compounds ciclopirox (CPX) and deferiprone (DEF), which are used clinically as topical antifungal agents, inhibit DOHH (Park et al., 1996). In cell culture experiments, CPX and DEF reduce both HIV (Hoque et al., 2009) and filovirus replication (Olsen et al., 2016), making them strong candidates for drug repurposing. Furthermore, CPX has been tested in vivo in animal models for various human diseases, and has recently been tested in a phase I clinical trial in patients with hematologic malignancies (reviewed by Shen and Huang, 2016). Another compound, CNI-1493 (semapimod), is a guanylhydrazone that efficiently inhibits hypusination through targeting DHPS. CNI-1493 has also been shown to interfere with HIV-1 replication (Hauber et al., 2005).
A second approach to inhibiting hypusination is to add a nonfunctional substrate that inhibits the hypusine synthesis enzymes. N1-guanyl-1,7-diamine-heptane (GC7) is a spermidine analogue and competitive inhibitor of DHS (Jakus et al., 1993). Recent work shows that GC7 is an effective compound to control EBOV replication, suggesting that this compound may be an effective antiviral (Olsen et al., 2016). Another spermidine analogue, 1,8 diaminooctane, has been shown to inhibit HIV replication in vitro (Hart et al., 2002). These findings suggest that inhibition of hypusination through substrate competition at DHPS is possible. GC7 has been extensively studied as an antitumor agent (reviewed by Nakanishi and Cleveland, 2016), as well as in vivo for several applications (Imam et al., 2014; Melis et al., 2016), so animal model testing of GC7 efficacy is a logical next step in investigating the importance of eIF5A for virus replication and pathogenesis.
Virus replication can be attacked by blocking pathways upstream from eIF5A, through alteration of the levels of polyamines such as spermidine, spermine, and putrescine. Inhibition of polyamine synthesis pathways using compounds such as difluoromethylornithine (currently used to reduce trypanosome levels in infected individuals) results in a decrease in viral replication of numerous RNA viruses (Mounce et al., 2016a, 2016b; Olsen et al., 2016). Viruses sensitive to polyamine depletion include flaviviruses (dengue and Zika), bunyaviruses (Rift Valley fever), and picornaviruses (polio, Coxsackievirus) (Mounce et al., 2016a). The exact mechanism for viral sensitivity to polyamine levels is not fully understood, but the requirement for spermidine in the hypusination of eIF5A could offer an explanation and will be important to study moving forward.
Potential Complications of Targeting eIF5A
Nonspecific side effects are always a concern associated when a cellular process is targeted as a way to block virus replication. There are certainly risks with targeting the host translational machinery generally as an antiviral strategy, as the normal synthesis of host proteins is an integral part of cellular and organismal homeostasis. Targeting the hypusination of eIF5A would likely have a more limited off-target effect than one would expect for global translation inhibition, as eIF5A is important for the translation of only a discreet subset of proteins (Fujimura et al., 2015). The current compounds that block hypusination that are available to develop as antivirals may cause additional off-target effects. CPX targets multiple enzymes and pathways, including but not limited to ribonucleotide reductase, Wnt/β-catenin, VEGF, VEGFR-3, mTOR, and cyclin-dependent kinases, which could lead to undesirable off-target effects as an antiviral, although it has been tolerated in animal studies (Shen and Huang, 2016). Similarly, CNI-1493 is a multivalent compound that interferes with several pathways, including the synthesis of TNF and the phosphorylation of p38 MAPK. Furthermore, the clinical applications of using a spermidine analogue, such as 1,8 diaminooctane, are also unclear, given that it could potentially affect other enzymes responsive to spermidine. Toward alleviating some of these issues, novel compounds targeting DHPS, using the crystal structure of DHPS bound to GC7 as a design base, are being synthesized and tested for inhibition of HIV-1 replication (Schroeder et al., 2014).
Targeting hypusination and eIF5A as a broad spectrum antiviral approach is an exciting possibility in early stages of development. Challenges remain for this idea in establishing whether this pathway can be an antiviral target, including in vivo testing in relevant animal models and the development of additional small molecule inhibitors/compounds that target the hypusination of eIF5A. Given the importance of hyp-eIF5A for viral replication and cancer biology, there is the opportunity for a multidisciplinary approach to address these concerns. Furthermore, through combining therapies targeting both host and viral targets, antiviral efficacy may improve, reducing the emergence of viral resistance, and minimizing toxicity in the control of viral infection and epidemic viral diseases.
Acknowledgments
We would like to thank Michaela Smith, Alexander Devaux, Emily Speranza, and John Ruedas for helpful comments on the article. This work was funded by NIH R21 AI121933 and NIH RO1 AI1096159-04 grants and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UC6AI058618.
Disclosure Statement
No competing financial interests exist.
References
- Andrus L., Szabo P., Grady R.W., Hanauske A.R., Huima-Byron T., Slowinska B., et al. (1998). Antiretroviral effects of deoxyhypusyl hydroxylase inhibitors: a hypusine-dependent host cell mechanism for replication of human immunodeficiency virus type 1 (HIV-1). Biochem Pharmacol 55, 1807–1818 [DOI] [PubMed] [Google Scholar]
- Benne R., and Hershey J.W. (1978). The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem 253, 3078–3087 [PubMed] [Google Scholar]
- Bevec D., Jaksche H., Oft M., Wohl T., Himmelspach M., Pacher A., et al. (1996). Inhibition of HIV-1 replication in lymphocytes by mutants of the Rev cofactor eIF-5A. Science 271, 1858–1860 [DOI] [PubMed] [Google Scholar]
- De Clercq E., and Li G. (2016). Approved antiviral drugs over the past 50 years. Clin Microbiol Rev 29, 695–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever T.E., Gutierrez E., and Shin B.S. (2014). The hypusine-containing translation factor eIF5A. Crit Rev Biochem Mol Biol 49, 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C., Rosorius O., Hofer L., Jaksche H., Hauber J., and Bevec D. (1999). Evidence for specific nucleocytoplasmic transport pathways used by leucine-rich nuclear export signals. Proc Natl Acad Sci U S A 96, 6229–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K., Choi S., Wyse M., Strnadel J., Wright T., and Klemke R. (2015). Eukaryotic translation initiation factor 5A (EIF5A) regulates pancreatic cancer metastasis by modulating RhoA and Rho-associated kinase (ROCK) protein expression levels. J Biol Chem 290, 29907–29919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E., Shin B.S., Woolstenhulme C.J., Kim J.R., Saini P., Buskirk A.R., et al. (2013). eIF5A promotes translation of polyproline motifs. Mol Cell 51, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart R.A., Billaud J.N., Choi S.J., and Phillips T.R. (2002). Effects of 1,8-diaminooctane on the FIV Rev regulatory system. Virology 304, 97–104 [DOI] [PubMed] [Google Scholar]
- Hauber I., Bevec D., Heukeshoven J., Kratzer F., Horn F., Choidas A., et al. (2005). Identification of cellular deoxyhypusine synthase as a novel target for antiretroviral therapy. J Clin Invest 115, 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M., Hanauske-Abel H.M., Palumbo P., Saxena D., D'Alliessi Gandolfi D., Park M.H., et al. (2009). Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S., Mirmira R.G., and Jaume J.C. (2014). Eukaryotic translation initiation factor 5A inhibition alters physiopathology and immune responses in a “humanized” transgenic mouse model of type 1 diabetes. Am J Physiol Endocrinol Metab 306, E791–E798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus J., Wolff E.C., Park M.H., and Folk J.E. (1993). Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem 268, 13151–13159 [PubMed] [Google Scholar]
- Kemper W.M., Berry K.W., and Merrick W.C. (1976). Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem 251, 5551–5557 [PubMed] [Google Scholar]
- Malim M.H., Tiley L.S., McCarn D.F., Rusche J.R., Hauber J., and Cullen B.R. (1990). HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell 60, 675–683 [DOI] [PubMed] [Google Scholar]
- Melis N., Rubera I., Cougnon M., Giraud S., Mograbi B., Belaid A., et al. (2016). Targeting eIF5A hypusination prevents anoxic cell death through mitochondrial silencing and improves kidney transplant outcome. J Am Soc Nephrol [Epub ahead of print]; DOI: 10.1681/ASN.2016010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce B.C., Cesaro T., Moratorio G., Hooikaas P.J., Yakovleva A., Werneke S.W., et al. (2016a). Inhibition of polyamine biosynthesis is a broad-spectrum strategy against RNA viruses. J Virol 90, 9683–9692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce B.C., Poirier E.Z., Passoni G., Simon-Loriere E., Cesaro T., Prot M., et al. (2016b). Interferon-induced spermidine-spermine acetyltransferase and polyamine depletion restrict Zika and Chikungunya viruses. Cell Host Microbe 20, 167–177 [DOI] [PubMed] [Google Scholar]
- Nakanishi S., and Cleveland J.L. (2016). Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids 48, 2353–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M.E., Filone C.M., Rozelle D., Mire C.E., Agans K.N., Hensley L., et al. (2016). Polyamines and hypusination are required for ebolavirus gene expression and replication. mBio 7, pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.H., Cooper H.L., and Folk J.E. (1981). Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A 78, 2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.H., Joe Y.A., Kang K.R., Lee Y.B., and Wolff E.C. (1996). The polyamine-derived amino acid hypusine: its post-translational formation in eIF-5A and its role in cell proliferation. Amino Acids 10, 109–121 [DOI] [PubMed] [Google Scholar]
- Rossi D., Kuroshu R., Zanelli C.F., and Valentini S.R. (2014). eIF5A and EF-P: two unique translation factors are now traveling the same road. Wiley Interdiscip Rev RNA 5, 209–222 [DOI] [PubMed] [Google Scholar]
- Ruhl M., Himmelspach M., Bahr G.M., Hammerschmid F., Jaksche H., Wolff B., et al. (1993). Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J Cell Biol 123, 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader R., Young C., Kozian D., Hoffmann R., and Lottspeich F. (2006). Temperature-sensitive eIF5A mutant accumulates transcripts targeted to the nonsense-mediated decay pathway. J Biol Chem 281, 35336–35346 [DOI] [PubMed] [Google Scholar]
- Schreier M.H., Erni B., and Staehelin T. (1977). Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol 116, 727–753 [DOI] [PubMed] [Google Scholar]
- Schroeder M., Kolodzik A., Pfaff K., Priyadarshini P., Krepstakies M., Hauber J., et al. (2014). In silico design, synthesis, and screening of novel deoxyhypusine synthase inhibitors targeting HIV-1 replication. ChemMedChem 9, 940–952 [DOI] [PubMed] [Google Scholar]
- Shen T., and Huang S. (2016). Repositioning the old fungicide ciclopirox for new medical uses. Curr Pharm Des 22, 4443–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk D., and Jacobson A. (1998). A single amino acid substitution in yeast eIF-5A results in mRNA stabilization. EMBO J 17, 2914–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]