Abstract
Purpose: The American Society of Clinical Oncology issued practice guidelines in 2006 to provide critical information about fertility impact to adolescents and young adults (AYA) at the time of cancer diagnosis. Survivors continue to express concerns about their long-term reproductive health after cancer therapy even as treatment options for fertility preservation evolve. An underutilization of fertility preservation methods by cancer patients continues to persist. A review of the literature cites barriers and challenges that limit fertility information and preservation options for AYA cancer patients.
Methods: A review of medical literature was conducted to examine current practice for patients receiving fertility information and the barriers to patients receiving fertility preservation services.
Results: A total of 69 publications were included in this review. The review summarizes (1) patient experiences with receiving fertility information and (2) patient desires, barriers, and challenges to utilizing fertility preservation services.
Conclusions: Despite advances in fertility preservation, there are challenges for patients to utilizing fertility preservation services. Barriers include the following: urgency to initiate treatment, inadequate information, clinic time constraints, and perceptions around patients' gender, age, cost, parity, race, relationship, and sociodemographic status influence whether patients receive fertility preservation consultation. Patients report a lack of adequate information to make informed fertility decisions.
Keywords: : fertility preservation, fertility preservation barriers, fertility preservation utilization, oncofertility
Introduction
The American Cancer Society estimates that more than 140,000 men and women under the age of 45 are diagnosed with cancer annually in the United States alone.1 Cancer is now a disease with a variety of treatment options, which provides survivors an opportunity to lead longer and more productive lives.2 However, challenges remain for cancer survivors striving to return to normalcy. Infertility can be a consequence of many of the more aggressive chemotherapy and radiation regimens that prolong and save lives. In fact, many studies highlight fertility issues as a significant concern among adolescents and young adults (AYA) with cancer.3–6 Further, the psychosocial impact connected to the loss of fertility can affect a survivor's long-term quality of life. Given the importance of this survivorship issue, there is a critical need to provide effective fertility preservation counseling and services.
The American Society of Clinical Oncology (ASCO) is the primary professional organization for all oncology disciplines. ASCO first published guidelines specific to fertility preservation in 2006, recommending that providers advise patients about fertility risks and to refer patients as appropriate to a reproductive specialist.7 ASCO updated these guidelines in 2013 to further advocate the inclusion of fertility issues in the counseling and informed consent process for cancer treatment and to document such discussions with cancer patients.8 Additional guidelines have also been released from similar organizations over the past few years9–12 (Table 1), however, referral to a reproductive medicine specialist has still not been routine for AYA cancer patients.13,14
Table 1.
Comprehensive Literature Review on Fertility Preservation Utilization for Cancer Patients
| Author, year | Methodology | Target audience | Key findings |
|---|---|---|---|
| Current fertility preservation guidelines for providers | |||
| American Society of Reproductive Medicine, (2005)9; (2013)12 | Committee review | Patients receiving gonadotoxic therapies | When damage to reproductive organs due to gonadotoxic therapies is unavoidable, providers should inform patients of options for storing gametes, embryos, or gonadal tissue. |
| Patients undergoing gonadotoxic treatments should be referred to fertility specialist who can provide or counsel patients about preservation services. | |||
| Patients should be offered referral to a mental health specialist or genetic counselor when appropriate. | |||
| Lee et al., (2006)7; Loren et al., (2013)8American Society of Clinical Oncology | Committee review | All cancer patients of reproductive age | Oncologists should address the possibility of infertility and be able to discuss fertility preservation options or refer interested patients to appropriate specialists as part of education and informed consent for cancer treatment. |
| Providers are urged to advise patients regarding potential threats to fertility as early as possible in the treatment process to allow for the widest array of options for fertility preservation. | |||
| Discussions should be documented. | |||
| Sperm and embryos cryopreservation and oocyte cryopreservation are considered standard practice and widely available, other options should be considered experimental and performed only in centers with necessary expertise. | |||
| Kim et al., (2012)10International Society for Fertility Preservation | Committee review | Reproductive age patients with lymphoma, leukemia, and breast cancer | Fertility issues should be addressed to all patients in reproductive age before cancer treatment. |
| For men, cryopreservation of sperm should be offered regardless of risk of gonadal function. | |||
| For women, embryo or oocyte cryopreservation is recommended if there is time for ovarian stimulation. If treatment cannot be delayed or ovarian stimulation is contradicted, ovarian tissue cryopreservation (experimental) should be first option of consideration. | |||
| Pfeifer and Wildra, (2012)11American Society of Reproductive Medicine | Committee review | Young women with cancer who are at risk of infertility | Oocyte freezing is no longer considered experimental. Pregnancy rates and health outcomes of the resulting children are now comparable to those of in vitro fertilization with fresh eggs. |
| Patient challenges | |||
| Bastings et al., (2014)56 | Cross-sectional survey | N = 233 female cancer patients, 0–39 years old from 2001 to 2013; The Netherlands | Approximately 10% of patients were referred for fertility preservation counseling in 2011. Providers reported age, diagnosis, and lack of knowledge as reasons for not referring patients. Patients 20–29 years old and with breast cancer diagnosis were most likely to be referred. |
| Burns et al., (2006)52 | Cross-sectional survey | N = 89 female cancer patients, 10–21 years old and parents; Milwaukee, WI | Patients and parents had similar attitudes toward fertility preservation and considering research protocols but were not willing to delay cancer treatment. |
| Carter et al., (2010)46 | Cross-sectional survey | N = 88 female gynecological cancer survivors, 21–49 years old; New York, NY | Seventy-seven percent of gynecological cancer survivors experienced significant distress related to loss of fertility or impaired function. |
| Chapple A, (2007)23 | Semi-structured interviews | N = 21 male cancer patients; United Kindgom | Male patients had felt unprepared to make decisions about sperm banking and would prefer more counseling regarding procedures and their fertility status. |
| Crawshaw and Sloper, (2010)45 | Exploratory interviews | N = 38 cancer survivors <30 years old, diagnosed as teenagers; United Kingdom | Professional and social network provide little opportunity to ask questions and receive information about fertility issues. Fertility matters affected one's reproductive health one's well-being, identity, and life planning. |
| Duffy et al., (2012)60 | Cross-sectional survey | N = 344 oncologists; United States | Oncologists lack confidence in their knowledge of fertility issues in young women with breast cancer. |
| Friedman et al., (2011)42 | Cross-sectional survey | N = 28 female adult cancer survivors; Palo Alto, CA | Female survivors who attempted fertility preservation felt positively (93%) about their fertility preservation decisions, even if treatment cycle was unsuccessful. |
| Ginsberg et al., (2008)66 | Review | N = 13 publications | Sperm cryopreservation when offered before treatment is an effective fertility preservation method for men. Successful fertility preservation programs must take a team approach to offering sperm banking. Sperm banking should be standard of care for young men diagnosed with cancer. |
| Ginsberg JP, (2011)3 | Review | N = 49 publications | Infertility is a critical quality of life issue for pediatric cancer survivors. Improvements toward the availability of fertility preservation techniques would alleviate the risk of infertility for pediatric cancer patients. |
| Glaser et al., (2004)18 | Cross-sectional survey | N = 20 pediatric oncology centers; Philadelphia, PA | 70% of pediatric oncology centers in the United Kingdom reported offering fertility preservation services to men who have reached puberty. Only 40% of the centers reported written guidelines for fertility preservation within their institution. |
| Gorman et al., (2011)33 | Semi-structured interviews | N = 20 female breast cancer survivors, diagnosed <40 years old; San Diego, CA | Patients expressed that the importance of fertility, the significant role the oncologist plays in decision making, and the concern for survival. Fertility after cancer is challenging and patients could benefit from fertility preservation at the time of diagnosis. |
| Gorman et al., (2012)37 | Focus groups | N = 22 cancer survivors, 18–34 years old; San Diego, CA | Survivors expressed worry about fertility and parenthood after cancer and desire more control in choices their fertility and better continuity of care. |
| Green et al., (2003)26 | Semi-structured interviews | N = 15 male childhood cancer survivors; United Kingdom | Male childhood cancer survivors expressed being under-informed about fertility risks at diagnosis and were emotionally distraught and had difficulty coping with fertility after cancer. |
| Hammond et al., (2007)63 | Case-control study | N = 120 cancer survivors receiving stem cell transplant and 120 case-matched controls; Seattle, WA | Fertility concerns are higher among stem cell transplant survivors than their matched controls. Younger age at time of transplant and those with no children at time of transplant have greater risk of fertility concerns 10 years after treatment. |
| Hohmann C, (2011)24 | Cross-sectional survey | N = 2754 adult childhood cancer survivors; Germany | Approximately half of childhood cancer survivors do not recall counseling about fertility of cancer treatment. Counseled patients show significantly less concerns about risks to their future children. |
| Jenninga et al., (2008)55 | Retrospective chart review | N = 70 female cancer patients; The Netherlands | Referrals for fertility preservation are minimal. Future research should explore patient's expectations for fertility preservation for referral patterns to be in line with emerging technologies. |
| Karaoz et al., (2010)32 | Semi-structured interviews | N = 20 female breast cancer patients, <50 years old; Turkey | Premenopausal breast cancer patients receive insufficient counseling regarding fertility, contraception, and early menopause. |
| Kim et al., (2013)39 | Cross-sectional survey | N = 52 female cancer patients, 18–43 years old; United States | Seventy-three percent of patients had made decision about fertility preservation after a consult. Financial worries and feeling concerns were not addressed and were found to impair decisional conflict. |
| Klosky et al., (2009)29 | Cross-sectional survey | N = 66 male cancer patients 13–23 years old and 14 pediatric oncologists; Memphis, TN | Rate of sperm banking among male adolescents is low; only 28% of patients informed of their risk went on to pursue sperm banking. Socioeconomic status was significant with lower rates of sperm banking. |
| Klosky et al. (2015)25 | Cross-sectional survey | N = 96 newly diagnosed men, 13–22 years old and 61 parents; Memphis, TN | Having children after cancer was ranked third among priorities of adolescent males with future health ranked as the top priority. Low perception of fertility risk was associated with a higher ranking of future fertility (p = 0.01). Race/ethnicity, relationship status, and cancer diagnosis were not associated with fertility ranking. |
| Kohler et al., (2011)19 | Cross-sectional survey | N = 209 pediatric oncologists; United States | Majority of providers feel fertility threats are a concern to their patients, but utilization of fertility preservation guidelines and referrals are low. |
| Kumar et al., (2012)54 | Retrospective cohort | N = 59 colorectal cancer patients, 18–40 years old | Fertility risk discussion was documented in only 1/3 of colorectal cancer patients. Those receiving radiation therapy and those <35 years of age were more likely to receive discussion. |
| Lee et al., (2011)21 | Focus group | N = 24 women with breast cancer, 18–40 years old | Female breast cancer patients felt they were not provided choices for fertility preservation and would desire a discussion with a fertility specialist to assist in decision making. |
| Letourneau et al., (2012)36 | Cross-sectional survey | N = 1041 female cancer survivors, 18–40 years old at diagnosis | Sixty-one percent recall being counseled on fertility risks at time of treatment, but only 4% pursued fertility preservation. Disparities in access to care for fertility preservation were found among older women, those with previous children, ethnicity and sexual orientation. |
| Linkeviciute et al., (2014)62 | Review | N = 71 publications | Most young cancer survivors desire parenthood. Providing fertility preservations offers these patients a chance to overcome sterility associated with cancer treatment. Providers lack the knowledge to appropriately counsel patients of fertility preservation. Therefore, fertility preservations counseling services should be offered to help patients address these issues. |
| Matthews et al., (2012)41 | Systematic review | N = 68 publications; fertility preservation for women | Fertility preservation methods have made recent advancement, providing a variety of options for women with cancer. Providers need to be informed of the available fertility preservation options to properly counsel patients. |
| Mayor, (2012)17 | Cross-sectional survey | N = 1041 female cancer patients, 18–40 years old | Only 4% of treated for cancer pursued fertility preservation. |
| Murk and Seli, (2011)13 | Review | N = 60 publications; public health concerns | Enhanced provider and patient education and improved coordination of care can contribute to improved access to fertility preservation services for cancer patients. |
| Nahata et al., (2012)43 | Commentary | Urologists | Sperm banking is underutilized and often relies on the referral and knowledge of the oncologist. A multidisciplinary approach incorporating the role of the urologists to develop guidelines and education can improve counseling for patients. |
| Niemasik et al., (2012)59 | Cross-sectional survey | N = 1041 female cancer patients, 18–40 years old | About 1/2 of female patients recall receiving information about reproductive risks and only 12% received a fertility preservation consult. Patients are making uninformed fertility decisions without sufficient information. |
| Oosterhuis et al., (2008)64 | Cross-sectional survey | N = 97 parents of pediatric patients and 37 adolescent oncology patients | Majority of parents and adolescent patients had concerns about fertility-related side effects regardless of treatment received. Only 30% of parents were satisfied with the amount of fertility risk information provided to them. |
| Partridge et al., (2004)4 | Cross-sectional survey | N = 657 young breast cancer survivors | Fertility issues are a significant concern among majority of female breast cancer patients at diagnosis. There is a need to discuss fertility concerns with patients at diagnosis. |
| Peate et al., (2011)68 | Cross-sectional survey | N = 111 young breast cancer patients | Young breast cancer patients have little knowledge about fertility issues at the time of diagnosis. Low knowledge is associated with increased decisional conflict regarding future fertility. Neither relationship status nor a plan for future children predicts whether a patient will pursue fertility preservation. |
| Peddie et al., (2012)38 | Semi-structured interviews | N = 16 cancer patients, 17–49 years old and 15 providers | Communication gaps and the “urgent need for treatment” were barriers to patients pursing fertility preservation. Few women were offered the opportunity for fertility preservation. |
| Penrose et al., (2012)51 | Semi-structure interviews | N = 25 adult cancer survivors, 24–50 years old | Survivors reported a perceived lack of consideration of their fertility at diagnosis, and that loss of fertility was a concern regardless of the patient's desire to have future children. |
| Quinn et al., (2008)61 | Review | N = 40 publications | Behavioral studies can address the communication barriers between the provider and patient for providing fertility preservation and contribute to the development of tools and resources for shared decision making for treatment. |
| Quinn et al., (2009)58 | Cross-sectional survey | N = 613 oncology providers | Less than 1/2 providers report routinely referring patients for fertility preservation consult. |
| Quinn et al., (2009)22 | Secondary data analysis | N = 54 pediatric and adult oncologists | Majority of providers are not utilizing current fertility preservation guidelines. |
| Quinn and Vadaparampil, (2009)57 | Secondary data analysis | N = 24 pediatric oncologists | Providers need training on how and when to discuss fertility concerns with patients. |
| Quinn et al., (2010)69 | Case report | 27-year-old woman with breast cancer | Cancer survivors continue to suffer the psychological trauma of cancer even after treatments. The loss of fertility is one issue that young cancer patients must deal with. More resources are needed to educate and help patients with fertility preservation decisions including advanced techniques for testing for gene mutations in their offspring. |
| Quinn et al., (2011)70 | Systematic review | N = 29 studies focusing on decision making | Cancer patients desire discussion of their treatment impact on fertility; however, future research is needed to determine personalized approaches to decision making. |
| Reh and Lu, (2011)40 | Cross-sectional survey | N = 29 female cancer patients | Female cancer patients rate fertility of high importance both before and after cancer treatment. The median time from consult to completion of fertility preservation was 25 days. |
| Ruddy et al.34 | Cohort study | N = 620 breast cancer patients, 17–40 years old | Fertility is a major concern among young women diagnosed with breast cancer. In some, their concern for future fertility affects decisions they make about their cancer treatment. Those most concerned about their fertility were younger age, nonwhite, had no children, and receive chemotherapy. Still, only a minority of women pursue fertility preservation. |
| Saito et al., (2005)67 | Cross-sectional survey | N = 51 male cancer patients who froze sperm. | Sperm banking had a positive impact on the patients during and after cancer treatment. Patients who banked sperm (60%) still worried about their future fertility. |
| Schover et al., (1999)44 | Cross-sectional survey | N = 132 cancer survivors <35 years at diagnosis | Fifty-seven percent received information about fertility risks before their treatment, and of those with no children at diagnosis, 76% desired children in the future. |
| Schover et al., (1999)5 | Systematic review | Relevant literature on cancer survivor's concerns about infertility and childbearing | Radiation and chemotherapy impact the reproductive function. Infertility comes as a surprise to many survivors. Survivors have concerns about conceiving because of fear of birth defects/genetic disorders in offspring. Cancer survivors may have higher distress about infertility than otherwise health couples facing infertility. Patients diagnosed in adolescence may be more distressed over infertility than child or adult cancer survivors. Women may be more distressed than men. |
| Schover et al., (2002)48 | Cross-sectional survey | N = 201 male cancer survivors, 14–40 years old | Seventy-seven percent of men without children at diagnosis desire children in the future but only half of male survivors were offered sperm banking and just 24% pursued sperm banking. Lack of information was cited as most common reason for not pursuing sperm banking. |
| Schover, (2009)47 | Review | N = 44 publications | Cancer survivors value parenthood after cancer. Long-term distress is common among survivors who do not become parents. |
| Sheth KR, (2012)14 | Retrospective chart review | N = 4818 male cancer patients, 18–55 years old | The number of men undergoing sperm cryopreservation increased 2.7-fold after implementation of a formalized oncofertility program. |
| Shimizu et al., (2012)28 | Cross-sectional survey | N = 434 breast cancer oncologists | Female and younger oncologists were more likely to refer patients for fertility preservation consult. Barrier to fertility discussion included risk or reoccurrence, lack of collaboration with a reproductive specialist, and time constraints. |
| Stein et al. (2014)30 | Focus-group study | 15 adult male pediatric cancer survivors and 7 parents | Regret that the issue of potential infertility not addressed at time of diagnosis was the most prominent theme among adult survivors and parents. Patients felt it was provider's responsibility to address fertility and that it would of have been impractical for providers to expect them to bring up fertility concerns. |
| Thewes et al., (2005)65 | Cross-sectional survey | N = 228 female cancer patients <40 years old at diagnosis | Seventy-one percent of breast cancer patients discussed fertility-related issues as part of their treatment plan. Women perceived this information more important than menopause-related information at the time of diagnosis. Patients desire to receive information regarding fertility concerns with a fertility specialist. |
| Tschudin and Bitzer, (2009)6 | Systematic review | N = 24 studies; fertility issues in cancer patients | Fertility is an important issue for cancer patients, but current counseling and decision-making support is limited. |
| Tschudin et al., (2010)53 | Cross-sectional survey | N = 80 female cancer survivors | Majority of women (68%) rate the risk of infertility as high but fear safety concerns with fertility preservation methods. Women rely on their spouse and oncologist the most for support in their decisions. |
| West et al., (2009)16 | Review | N = 81 publications | The increasing survival of children and young women with cancer has led to an increased focus on fertility preservation. There are currently several options available for young adults, and some investigational techniques for younger females. |
| Wilkes et al., (2010)31 | Semi-structured interviews | N = 18 cancer survivors, 23–42 years old | Fertility risk information was inconsistent at time of diagnosis. Survivors expressed a desire to explore fertility options at diagnosis and fertility became a greater issue after treatment, and that having children was a positive outcome. |
| Zebrack et al., (2004)49 | Semi-structured interviews | N = 32 childhood cancer survivors, 19–37 years old | Fifty-nine percent of childhood survivors are uncertain about their current fertility status and only half recall being told about potential reproductive risks at the time of diagnosis. |
| Zeltzer et al., (2009)50 | Review | N = 61 publications; health-related outcome measures | Survivors will experience limitations in social and psychological well-being. ongoing concerns related to cancer treatment include physical health, body image, access to insurance, career options, and continuity of care. |
A comprehensive fertility preservation consultation includes informing the patient of fertility risks, counseling about fertility preservation options, and providing treatment for facilitation of reproductive function and ability to conceive as desired. Patients benefit most from counseling before their cancer treatment and should continue to be seen for reproductive care during and after treatment.15 While nearly 70% of young adults report that their diagnosis of cancer did not change their desire for children, future fertility can be easily overlooked at diagnosis.6,16 In fact, less than 5% of women and 43% of men are seen by a reproductive specialist before cancer treatment.14,17 Both men and women of reproductive age are at risk of poor reproductive function after cancer treatment.7 Without adequate clinical services many of these young adults will lose their opportunity to have children in the future. The goal of this review is to summarize (1) the fertility concerns for cancer patients and (2) identify patient desires, barriers, and challenges to utilizing fertility preservation services.
Methods
A review of the literature was conducted to examine current practice for patients receiving fertility information and the barriers to patients receiving fertility preservation services. The literature search focused on studies published since the initial release of ASCO's fertility preservation clinical guidelines in 2006 to provide a comprehensive synopsis of patient services since the publication of the formal practice guidelines.
The oncofertility literature was searched using the PubMed database for all relevant publications through March 19, 2015. The authors chose PubMed for the literature search given its comprehensive library of biomedical literature from online books and several scientific databases including MEDLINE, and for its availability of studies sponsored by the National Institutes of Health. Specific key word searches focused on patients' utilization and barriers to fertility services: “fertility patient perspective cancer,” “ASCO guidelines fertility,” “qualitative cancer fertility,” “cancer attitudes fertility,” “fertility preservation,” and “fertility preservation utilization.” Studies were limited to those available in English or translated into English, and human studies. In addition, relevant studies cited within reference lists of published articles and in current press were searched to obtain a comprehensive review of available research in the field. Studies of childhood cancer survivors were included in this review, as much of the research conducted in this field has focused on this patient population.
Article titles were reviewed to identify relevant citations. Citations deemed inappropriate for further review included those that focused on other unrelated health conditions (i.e., HPV, HIV, obesity, PCOS, pregnancy, circumcision, and epilepsy), basic science studies such as technology advances and cell processes (i.e., vitrification, stem cell, germ cell, and mitotic cell division), assisted reproduction methods for groups other than with cancer, general sexual function studies, improving palliative care, menopause, and general infertility. In most cases, studies with a focus on a specific rare cancer were excluded if the patients' experiences were not generalizable to AYA cancer patients. Abstracts were then further reviewed to exclude studies that primarily focused on gynecological specific tumors (i.e., ovarian or BRCA gene carriers) in which fertility expectations were not similar to the general cancer population, posthumous reproduction, and disposition of gametes (not in context to fertility preservation practice). Throughout the search, studies identified for inclusion for further review in a prior search were not included a second time. Due to the rapid emergence of studies in this area, reference lists of the included citations, and searches on current studies and/or authors who are known experts in the field were also conducted using the same criteria to yield a comprehensive review.
Results
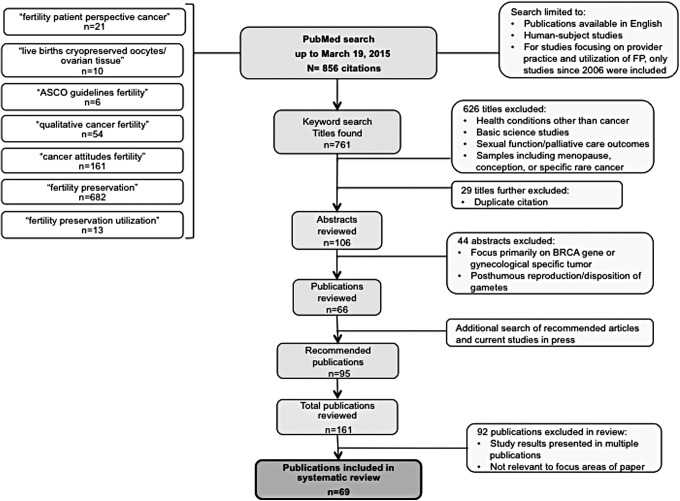
The initial keyword search yielded a total of 761 citations. Based on the title criterion, 626 were excluded and an additional 29 were duplicate citations, leaving 106 abstracts to review. After abstract review, 66 citations remained for inclusion. Specific studies and authors' searches yielded an additional 95 citations for review. Therefore, 161 relevant studies were identified for the literature review, 69 of which presented unique results and relevant to this review (Fig. 1). While mostly observational studies were identified, the search did also yield reviews and cohort studies for inclusion. Table 1 outlines a comprehensive summary of the literature.
FIG. 1.
An illustration of the literature review process conducted for the review of patient challenges to utilizing fertility preservation.
Practice guidance for fertility preservation
The ASCO released practice guidelines in 2006 addressing fertility preservation; these guidelines were most recently updated in 2013. Specifically, they recommend that providers answer basic questions about fertility impact of cancer treatment and refer patients to reproductive and psychosocial specialists.7,8 Although ASCO promoted an open dialog between providers and AYA cancer patients about the risk for future fertility, clinical practice has still been slow to change. The Survey for Preservation of Adolescent Reproduction (SPARE), a national cross-sectional study of pediatric oncology providers conducted in 2010, revealed an overall low utilization of the ASCO guidelines in clinical practice. While 85% of providers surveyed acknowledged that fertility was a significant concern for their patients and families,18 only 39% reported using the ASCO guidelines at least 25% of the time to make decisions regarding the patient's future fertility.19 Providers have identified challenges to providing fertility information to their patients, such as limited education and clinical constraints.20–22 Patients are also not likely to ask for information or initiate the discussion, leaving fertility concerns unaddressed in treatment plans.17,21,22
The patient experience
When facing a cancer diagnosis, fertility concerns may at first be marginal, relative to the life-threatening illness. However, cancer survivors have identified that fertility is not only a major concern but has a psychological and social impact on their quality of life. Overlooking fertility concerns at the time of diagnosis can cause significant distress for cancer survivors.23,24 Adolescent males have ranked future fertility among their top priorities for survivorship25 and noted that becoming infertile after cancer is a loss of their masculinity and self-esteem.26 For young women, the loss of fertility has been said to be as devastating as the diagnosis of cancer itself.27
Men and women differ when it comes to receiving fertility preservation information. While 68% of men are receiving information about their fertility options, only 14% of women are receiving the same information.28 For men, sperm cryopreservation is a relatively simple and affordable procedure; therefore, fertility preservation can be easily integrated into their treatment process. Although even with sperm cryopreservation considered a standard for male fertility preservation the presentation of fertility information to young male cancer patients is not always followed with a referral to a reproductive specialist. A study by Sheth et al. found that only 43% of men are seen by a reproductive specialist before cancer treatment.14 Klosky et al., found similar results demonstrating that while an overwhelming majority of providers discussed fertility risks with adolescent males, patients were not referred to a reproductive specialist and only 28% of adolescent males pursued sperm banking.29 Male survivors have reported regret over not discussing their fertility threat adequately, they did not feel a part of the decision-making process for future fertility, and they were not informed of the impact of their planned treatment regimen on their fertility.30,31
For women, fertility concerns are often overlooked during initial diagnosis.6,23,31–34 Utilization rates of fertility preservation services for women cancer patients in the United States are low, ranging from 4% to 20%.35,36 There is an overwhelming level of frustration among female survivors with the lack of fertility information received. In a study by Gorman et al., participants discussed scenarios in which healthcare providers offered little to no information about fertility issues, or that sufficient information was provided but the women felt they were unable to take advantage of the fertility preservation options presented.37 Similarly, in another study of cancer survivors, females felt the discussion of future fertility was discouraging, leaving an impression that success rates were uncertain, there were risks of reoccurrence, and that delaying cancer treatment was not possible. In addition, there was no formal referral process to a reproductive specialist.38
Patients report a better understanding of their treatment options for future fertility after a fertility consultation with a reproductive specialist.39 For instance, Reh et al. found that 89% of patients proceed to some form of fertility preservation after a formal consultation with a reproductive medicine specialist.40 The lack of referrals to a reproductive specialist ultimately denies the patient of the choice to discuss fertility concerns and to make decisions about their future fertility.
Young survivors report a strong desire to have fertility information at the time of diagnosis.37 The exclusion from the decision-making process regarding fertility is associated with poor quality of life, depression, and distress for cancer survivors.33 While men may be more likely to receive fertility information than women, evidence supports that all patients want to be involved in their treatment plan decisions but require guidance from their provider.21,22,33,37 When provided the opportunity to discuss fertility preservation options, women have reported high levels of satisfaction with their fertility decision, even if no fertility preservation is elected and improved coping with their diagnosis.41,42 Furthermore, patients who do not receive support from their provider are likely to not seek fertility preservation and have regret later.43 A fertility preservation consultation provides an environment for the patient to understand the implications to their future fertility, to ask questions and become a part of the decision-making process for their fertility. Both genders have expressed feeling left out of the decision-making process for their future fertility. Men report receiving little information about fertility risks, uses of cryopreserved sperm in the future, and childbearing after cancer therapy.23 Women have reported feeling uninformed, unsupported, and not encouraged to delay treatment to consider fertility preservation options.6,32,33
For many survivors the ability to achieve biological parenthood is essential for a satisfying quality of life.24 Among adult survivors without children at the time of diagnosis, 76% reported a concern about having children after cancer. Further, the cancer diagnosis did not change their initial desire to have children in 71% of women and 68% of men surveyed.44 The psychosocial impact of infertility on cancer survivors is a growing area of investigation.32,33,37,45 The return to “normalcy” after cancer treatment is particularly important for adolescent cancer patients and issues of fertility and sexuality continue to pose challenges well into adulthood. Even among those with no desire to have children, survivors stated a willingness to receive counseling and to discuss expectations about their fertility.32 However, many survivors sense that their providers were uncomfortable discussing cancer-related fertility issues at diagnoses.45 Not providing patients a role in their fertility decision making can lead to poorer quality of life, depression, and distress.33
The psychosocial burden of the loss of fertility can be more profound among cancer patients compared to a general infertility population. AYA experience severe distress when confronted by a cancer diagnosis and the added uncertainty about their long-term reproductive status can be particularly devastating.13,41,46 Despite fears about cancer recurrence, leaving a child behind, or possible pregnancy complications, the desire to have children remained.13 Studies suggest that a personal cancer history increased the importance placed on family and contribute to stronger parent-child relationships in the future.44,47–49
AYA have expressed different concerns than older adults when facing a new cancer diagnosis. Concerns of marriage, intimacy, and fertility are as critical to this group as is educational attainment and employment.50 The late effects of cancer and the fertility issues among these patients stay with them for life.33 Infertility among cancer survivors impacts their self-esteem, identity, sexuality, and self-image.6 The inability to fulfill a desire to have a family and resume normalcy can lead to feelings of emptiness, defeat, and loss. In contrast, maintaining one's fertility is associated with new life, hope, joy, pride, strength, optimism, and a sense of life and growth.6 The loss of fertility is a concern beyond the inability to have children; the loss affects cancer survivors regardless of their desire for children.51 Further contributing to the lack of participation in one's fertility decisions are gender disparities that exist in patients receiving fertility information.38 Since few women are receiving fertility information, females are provided little opportunity to participate in decisions about their future fertility, thus robbing them of their desires for children and normal lives after cancer. Both genders have expressed a poor understanding of their fertility risks and their treatment options; thus all adoloscents would benefit from a fertility preservation consultation. Patients are open to considering alternative treatment options, including investigational protocols, or a delay in treatment to pursue fertility preservation.4,52
Barriers for receiving fertility preservation services
Studies focusing on patient utilization and challenges for accessing fertility preservation have identified several barriers for patients receiving adequate fertility information. Adolescent men and women face personal, cultural, and system barriers that often leave patients with insufficient information to make future fertility decisions.
Patients facing a cancer diagnosis are often influenced by their personal situations in making treatment decisions. The support of their partner, their desire for a future family, and cost can be significant factors contributing to a patient's fertility preservation treatment. In a cross-sectional survey of adult survivors, Tschudin et al. found that partners are most influential to patients' decisions. Partners provide support for the patient in making fertility decisions and contribute to decreased decisional conflict.53 However not having a partner does not change if a patient will utilize fertility preservation. A New York University study found that the majority of patients undergoing fertility preservation treatment were single.40 One's desire for a future family also plays into a patient's treatment decision. Parity (number of children) is often considered as a measure of a patient's desire for children, and it can influence patient referral patterns or decisions to seek additional fertility counseling.14,36 Sheth et al. reported that 90% of those without previous children sought fertility preservation consult versus just 57% of those with two or more children.14
Relationship status and parity not only influence the patients' willingness to seek fertility preservation information, but also have been found to influence fertility discussion by providers as well. Factors including marital status, age, gender, and cancer treatment type have been identified as influences to whether providers discuss fertility information with their patients.14,24,28,54–56 Providers may not be addressing future concerns of adolescent cancer patients at the time of cancer diagnosis. Future studies identifying the priorities for future fertility among adolescent cancer patients will address many of these perceptions providers have in identifying patients appropriate for receiving fertility information.
The high cost of fertility preservation is often cited as a barrier to fertility preservation both by patients and by providers. Misperceptions, or assumptions, about the cost of fertility preservation methods can also contribute to the limited fertility information received by patients.19,20,57 Some providers may assume that cost will be a significant barrier and avoid raising the topic with patients and patient studies suggest cost is a significant factor in decisional conflict surrounding fertility preservation treatment.24,39 Arming providers with accurate financial information and providing patients with financial resources will allow patients to weigh the cost of fertility preservation options in discussions about their future fertility desires.
Patient barriers are also influenced by cultural differences with regard to fertility preservation.57 Letourneau et al. examined sociodemographic characteristics and the association with utilization of fertility preservation among Californian cancer survivors. The authors suggest that racial, socioeconomic, and demographic disparities may limit access to fertility preservation services and that age, desire for children, and educational level may influence treatment decisions in female survivors.36 Alternatively, in men, fertility preservation utilization was not influenced by race or ethnicity, but rather socioeconomic status. Specifically, higher socioeconomic status was associated with greater utilization of sperm cryopreservation.29
Goodman et al. conducted a retrospective cohort study to explore sociodemographic factors that may influence whether a fertility preservation referral is made.35 Although 10% of the study population was Hispanic, none of the patients referred for fertility preservation consultation were of Hispanic ethnicity. Caucasian race and having medical insurance were both associated with fertility preservation referral.35 Again, this is another barrier for these patients that is influenced by misperceptions that a group of patients may not be interested or be able to afford fertility preservation treatment. By not referring all patients to receive fertility information, adolescents are not able to be a part of their future fertility decision.
Providers' discomfort and misinformation about fertility risks may limit the information patients receive. Providers have cited a discomfort in their own knowledge of fertility information and studies have found an association between physician discomfort and fertility discussion practice.19,20,22,24,39,55,58–60 Providers report a concern about causing anxiety for the patient and a pressure to initiate cancer treatment immediately, despite research showing otherwise, that counseling patients increases compliance and decreases their anxiety.24,39 In fact, simply providing the patient with written information about fertility threats may be sufficient enough to initiate a fertility discussion and proper referral to a fertility specialist.21
In 2009, Quinn et al. identified providers' discomforts for discussing fertility with their patients: lack of knowledge and/or training, perceived language or cultural barriers, perception that a fertility discussion would add stress to the patient, uncertainty of fertility preservation success and affordability, and apprehension about discussing the subject with those with advanced disease. The authors identified that the provider's comfort level had a direct effect on whether patients received adequate information about their fertility risks and options.22 For example, providers who reported having little to no training in discussing fertility with their patients while in training or in the field, stated they were without the skills necessary to discuss fertility with their patients.22 Providers also specifically stated challenges with discussing such a topic while the patient is already emotional, while the patient's family was present, or via an interpreter.22 These findings are consistent with other investigators.19,20,24,39,55,58–60
The oncologist's focus on the cancer diagnosis and urgency to start treatment immediately has been cited as a major barrier to discussing fertility with their patients. As news of a cancer diagnosis is often stressful and shocking, many providers perceive fertility as not a priority at the time of diagnosis.19,57,59 This reluctance has led to a significant gap in disclosing and educating patients about fertility risks as a result of their cancer treatment.60 System factors also contribute to inadequate fertility discussions and referrals. For instance, the lack of an integrated referral system, limited resources or oncofertility specialists to refer patients, and time constraints in early cancer treatment consultations have been identified as possible barriers to timely referrals.14,20,21,28,43,57,58,61,62 Few institutions have been able to demonstrate a standardized referral process to fertility services for adolescent cancer patients. Institutional policies and appropriate referral to a specialist will help alleviate the concerns oncology providers have expressed in addressing fertility with their AYA patients.
Adolescent cancer survivors report anxiety and fear at the time of diagnosis, which prevented them from initiating a conversation regarding fertility with their oncology providers. Instead they accepted cancer therapy immediately as they felt that fertility preservation was not a viable option for them.31 The Cancer and Fertility study found that patients have a willingness to undergo fertility preservation but lack information about risks. While patients may seek out information on cancer websites about fertility risks, they do not use this information to make decisions about their future fertility. They require the support from their providers to discuss fertility concerns and would like time to consider their options.53
Only a minority of AYA cancer survivors has reported satisfaction with the fertility information they received from their providers.47,48,63–65 Among those with low satisfaction with fertility information received, there is also a low expectation for quality of life outcomes around relationships and fertility.19 Patients have identified a desire to be told accurate information about their prognosis and their risks and to play a contributing role in their treatment decisions.21,22,33,37 There are significant inconsistencies in dissemination and awareness of fertility information among oncology providers. There is also a lack of support services available to assist patients in making decisions regarding their future fertility. Overcoming many of the current perceptions that influence referrals (age, parity, cost, race, and sociodemographic characteristics), and support for standardize referral to a reproductive specialist, will optimize fertility preservation decisions and utilization.
Conclusions
Challenges to optimizing fertility preservation services are plagued with perceptions and knowledge deficits in regards to availability of services and patient priorities. This review highlights several barriers to the utilization of fertility treatment options for AYA cancer patients: urgency to start cancer treatment, inadequate information provided to patients, clinic time constraints, cost, and perceptions around patients' gender, age, parity, race, relationship, and sociodemographic status influence whether patients received fertility preservation consultation. A review of the literature, focusing on patient utilization and challenges for fertility preservation presented a poor picture of adolescents receiving access to fertility information. Both men and women struggle with initiating discussion of their fertility concerns, and current practice does not leave much time or put much priority into assuring all adolescent cancer patients participate in decisions about their future fertility. There are great opportunities to improve the clinical care of AYA cancer patients thereby removing the barriers to access oncofertility care. The patient's fertility concerns should be prioritized and integrated into the comprehensive cancer care, assuring all AYA patients are provided the opportunity to making informed decisions about their future fertility.
Informed decision making for future fertility is a necessary priority for AYA patients at the time of their cancer diagnosis. Many patients express apprehension to raise the topic of fertility preservation, and do not necessary realize the consequences to having children in the future.23,39 Patients who are left feeling uninformed present decisional conflict over their future fertility decisions and as a result, tend to not choose fertility preservation services. In general, young adults facing cancer feel a fertility consultation is a helpful resource to receiving essential information and having concerns addressed. The majority of patients are able to make a confident decision about their future fertility after a fertility preservation consultation.39 This review has identified the patient barriers to referral and utilization of fertility preservation services. Patient challenges need to be addressed in clinical practice and appropriate discussions about future fertility should be prioritized at the time of cancer diagnosis with a referral to an oncofertility specialist. Supporting adequate referral and counsel of fertility risks will lead to informed decision making and increased utilization of services.
Methodological quality of reviewed studies
A comprehensive review of current fertility preservation literature identified some gaps in the current research. Earlier studies have been limited by gender, age, a focus on a single cancer diagnosis, and limited availability of fertility options. Limited evidence is available to support effective fertility preservation programs for all AYA patients with cancer. Effective fertility preservation services can (1) reduce patient uncertainty in the decision-making process, (2) allow the patient to make an informed choice, and (3) contribute to their psychological health for the future.14 Additionally, there is limited information on decisions made at the time of diagnosis and how patients then cope with fertility provided they received adequate information about reproductive threats. There is also limited information that demonstrates effective counseling for fertility preservations for procedures other than sperm cryopreservation.6,38,47,66,67 Much of our understanding in this area relies on retrospective studies exploring patient's desires only after initiation of cancer treatment.3,4,6,14,21,68,69 These past studies identified psychological reactions and suggest informational needs but provide us with little data regarding patients' views on future fertility at the time of diagnosis.
Future directions to enhance fertility preservation services
Cancer care for AYA patients needs to consider patient's priorities and improve access to care for fertility services.57 Fertility is often considered as a secondary issue and providers' are not armed with the necessary knowledge to adequately address patient's concerns.13 The authors of this review have recently completed a study exploring the fertility priorities and desires of young cancer patients at the time of their diagnosis. Results from this research will demonstrate the importance for future fertility, decisional factors for electing fertility preservation, and identify differences among those electing fertility preservation versus those who do not for adolescents facing a cancer diagnosis. Educating providers and standardizing referral practices is critical for patients to receive the appropriate information and services they desire. Future research should continue to identify the benefit of a fertility consultation for all AYA patients and contribute to the understanding of how patients make decisions regarding their fertility. The future of cancer care should consider fertility preservation services as an essential component in the care pathway for AYA cancer patients.
Acknowledgments
Resource support provided by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154 (DMF), NIH WRHR Award 5K12HD001271-13 (LAK). Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.American Cancer Society. Cancer facts & figures 2012. Atlanta: American Cancer Society; 2012 [Google Scholar]
- 2.Nieman CL, Kazer R, Brannigan RE, et al. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol. 2006;4:171–8 [PubMed] [Google Scholar]
- 3.Ginsberg JP. Educational paper: the effect of cancer therapy on fertility, the assessment of fertility and fertility preservation options for pediatric patients. Eur J Pediatr. 2011;170:703–8 [DOI] [PubMed] [Google Scholar]
- 4.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83 [DOI] [PubMed] [Google Scholar]
- 5.Schover LR. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: a review. Med Pediatr Oncol. 1999;33:53–9 [DOI] [PubMed] [Google Scholar]
- 6.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–97 [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Schover LR, Partridge AH, et al. American society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31 [DOI] [PubMed] [Google Scholar]
- 8.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–8 [DOI] [PubMed] [Google Scholar]
- 10.Kim SS, Donnez J, Barri P, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet. 2012;29:465–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeifer S, Wildra E. Egg freezing no longer experimental, American Society for Reproductive Medicine. San Diego, CA: American Society for Reproductive Medicine Practice Committee; 2012 [Google Scholar]
- 12.The Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100:1224–31 [DOI] [PubMed] [Google Scholar]
- 13.Murk W, Seli E. Fertility preservation as a public health issue: an epidemiological perspective. Curr Opin Obstet Gynecol. 2011;23:143–50 [DOI] [PubMed] [Google Scholar]
- 14.Sheth KR, Sharma V, Helfand BT, et al. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol. 2012;187:979–86 [DOI] [PubMed] [Google Scholar]
- 15.Nangia AK, Krieg SA, Kim SS. Clinical guidelines for sperm cryopreservation in cancer patients. Fertil Steril. 2013;100:1203–9 [DOI] [PubMed] [Google Scholar]
- 16.West ER, Zelinski MB, Kondapalli LA, et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53:289–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayor S. Just 4% of young women treated for cancer take up fertility preservation services. BMJ. 2012;344:e2336. [DOI] [PubMed] [Google Scholar]
- 18.Glaser AW, Phelan L, Crawshaw M, et al. Fertility preservation in adolescent males with cancer in the United Kingdom: a survey of practice. Arch Dis Child. 2004;89:736–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohler TS, Kondapalli LA, Shah A, et al. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet. 2011;28:269–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knapp CA, Quinn GP. Healthcare provider perspectives on fertility preservation for cancer patients. Cancer Treat Res 2010;156:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RJ, Wakefield A, Foy S, et al. Facilitating reproductive choices: the impact of health services on the experiences of young women with breast cancer. Psychooncology. 2011;20:1044–52 [DOI] [PubMed] [Google Scholar]
- 22.Quinn GP, Vadaparampil ST, King L, et al. Impact of physicians' personal discomfort and patient prognosis on discussion of fertility preservation with young cancer patients. Patient Educ Couns. 2009;77:338–43 [DOI] [PubMed] [Google Scholar]
- 23.Chapple A, Salinas M, Ziebland S, et al. Fertility issues: the perceptions and experiences of young men recently diagnosed and treated for cancer. J Adolesc Health. 2007;40:69–75 [DOI] [PubMed] [Google Scholar]
- 24.Hohmann C, Borgmann-Staudt A, Rendtorff R, et al. Patient counselling on the risk of infertility and its impact on childhood cancer survivors: results from a national survey. J Psychosoc Oncol. 2011;29:274–85 [DOI] [PubMed] [Google Scholar]
- 25.Klosky JL, Simmons JL, Russell KM, et al. Fertility as a priority among at-risk adolescent males newly diagnosed with cancer and their parents. Support Care Cancer. 2015;23:333–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: a qualitative investigation. Psychooncology. 2003;12:141–52 [DOI] [PubMed] [Google Scholar]
- 27.Dow KH. Having children after breast cancer. Cancer Pract. 1994;2:407–13 [PubMed] [Google Scholar]
- 28.Shimizu C, Bando H, Kato T, et al. Physicians' knowledge, attitude, and behavior regarding fertility issues for young breast cancer patients: a national survey for breast care specialists. Breast Cancer. 2013;20:230–40 [DOI] [PubMed] [Google Scholar]
- 29.Klosky JL, Randolph ME, Navid F, et al. Sperm cryopreservation practices among adolescent cancer patients at risk for infertility. Pediatr Hematol Oncol. 2009;26:252–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein DM, Victorson DE, Choy JT, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol. 2014;3:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkes S, Coulson S, Crosland A, et al. Experience of fertility preservation among younger people diagnosed with cancer. Hum Fertil (Camb). 2010;13:151–8 [DOI] [PubMed] [Google Scholar]
- 32.Karaoz B, Aksu H, Kucuk M. A qualitative study of the information needs of premenopausal women with breast cancer in terms of contraception, sexuality, early menopause, and fertility. Int J Gynaecol Obstet. 2010;109:118–20 [DOI] [PubMed] [Google Scholar]
- 33.Gorman JR, Usita PM, Madlensky L, et al. Young breast cancer survivors: their perspectives on treatment decisions and fertility concerns. Cancer Nurs. 2011;34:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32:1151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman LR, Balthazar U, Kim J, et al. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Hum Reprod. 2012;27:2076–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letourneau JM, Smith JF, Ebbel EE, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. 2012;118:4579–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorman JR, Bailey S, Pierce JP, et al. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv. 2012;6:200–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peddie VL, Porter MA, Barbour R, et al. Factors affecting decision making about fertility preservation after cancer diagnosis: a qualitative study. BJOG. 2012;119:1049–57 [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Deal AM, Balthazar U, et al. Fertility preservation consultation for women with cancer: are we helping patients make high-quality decisions? Reprod Biomed Online. 2013;27:96–103 [DOI] [PubMed] [Google Scholar]
- 40.Reh AE, Lu L, Weinerman R, et al. Treatment outcomes and quality-of-life assessment in a university-based fertility preservation program: results of a registry of female cancer patients at 2 years. J Assist Reprod Genet. 2011;28:635–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews ML, Hurst BS, Marshburn PB, et al. Cancer, fertility preservation, and future pregnancy: a comprehensive review. Obstet Gynecol Int. 2012;2012: Article ID 953937:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman BE, O'Leary K, Westphal M. Follow-up survey of cancer survivors who underwent ovarian stimulation for fertility preservation. Fertil Steril. 2011;95:S12–S13 [Google Scholar]
- 43.Nahata L, Cohen LE, Yu RN. Barriers to fertility preservation in male adolescents with cancer: it's time for a multidisciplinary approach that includes urologists. Urology. 2012;79:1206–9 [DOI] [PubMed] [Google Scholar]
- 44.Schover LR, Rybicki LA, Martin BA, et al. Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer. 1999;86:697–709 [DOI] [PubMed] [Google Scholar]
- 45.Crawshaw MA, Sloper P. “Swimming against the tide”—the influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care (Engl). 2010;19:610–20 [DOI] [PubMed] [Google Scholar]
- 46.Carter J, Chi DS, Brown CL, et al. Cancer-related infertility in survivorship. Int J Gynecol Cancer. 2010;20:2–8 [DOI] [PubMed] [Google Scholar]
- 47.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53:281–4 [DOI] [PubMed] [Google Scholar]
- 48.Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–9 [DOI] [PubMed] [Google Scholar]
- 49.Zebrack BJ, Casillas J, Nohr L, et al. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13:689–99 [DOI] [PubMed] [Google Scholar]
- 50.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penrose R, Beatty L, Mattiske J, et al. Fertility and cancer—a qualitative study of Australian cancer survivors. Support Care Cancer. 2012;20:1259–65 [DOI] [PubMed] [Google Scholar]
- 52.Burns KC, Boudreau C, Panepinto JA. Attitudes regarding fertility preservation in female adolescent cancer patients. J Pediatr Hematol Oncol. 2006;28:350–4 [DOI] [PubMed] [Google Scholar]
- 53.Tschudin S, Bunting L, Abraham J, et al. Correlates of fertility issues in an internet survey of cancer survivors. J Psychosom Obstet Gynaecol. 2010;31:150–7 [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Merali A, Pond GR, et al. Fertility risk discussions in young patients diagnosed with colorectal cancer. Curr Oncol. 2012;19:155–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenninga E, Hilders CG, Louwe LA, et al. Female fertility preservation: practical and ethical considerations of an underused procedure. Cancer J. 2008;14:333–9 [DOI] [PubMed] [Google Scholar]
- 56.Bastings L, Baysal O, Beerendonk CC, et al. Referral for fertility preservation counselling in female cancer patients. Hum Reprod. 2014;29:2228–37 [DOI] [PubMed] [Google Scholar]
- 57.Quinn GP, Vadaparampil ST. Fertility preservation and adolescent/young adult cancer patients: physician communication challenges. J Adolesc Health. 2009;44:394–400 [DOI] [PubMed] [Google Scholar]
- 58.Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–7 [DOI] [PubMed] [Google Scholar]
- 59.Niemasik EE, Letourneau J, Dohan D, et al. Patient perceptions of reproductive health counseling at the time of cancer diagnosis: a qualitative study of female California cancer survivors. J Cancer Surviv. 2012;6:324–32 [DOI] [PubMed] [Google Scholar]
- 60.Duffy C, Allen SM, Dube C, et al. Oncologists' confidence in knowledge of fertility issues for young women with cancer. J Cancer Educ. 2012;27:369–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinn GP, Vadaparampil ST, Bell-Ellison BA, et al. Patient-physician communication barriers regarding fertility preservation among newly diagnosed cancer patients. Soc Sci Med. 2008;66:784–9 [DOI] [PubMed] [Google Scholar]
- 62.Linkeviciute A, Boniolo G, Chiavari L, et al. Fertility preservation in cancer patients: the global framework. Cancer Treat Rev. 2014;40:1019–27 [DOI] [PubMed] [Google Scholar]
- 63.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25:3511–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oosterhuis BE, Goodwin T, Kiernan M, et al. Concerns about infertility risks among pediatric oncology patients and their parents. Pediatr Blood Cancer. 2008;50:85–9 [DOI] [PubMed] [Google Scholar]
- 65.Thewes B, Meiser B, Taylor A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23:5155–65 [DOI] [PubMed] [Google Scholar]
- 66.Ginsberg JP, Ogle SK, Tuchman LK, et al. Sperm banking for adolescent and young adult cancer patients: sperm quality, patient, and parent perspectives. Pediatr Blood Cancer. 2008;50:594–8 [DOI] [PubMed] [Google Scholar]
- 67.Saito K, Suzuki K, Iwasaki A, et al. Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer. 2005;104:521–4 [DOI] [PubMed] [Google Scholar]
- 68.Peate M, Meiser B, Friedlander M, et al. It's now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer—an Australian fertility decision aid collaborative group study. J Clin Oncol. 2011;29:1670–7 [DOI] [PubMed] [Google Scholar]
- 69.Quinn GP, Vadaparampil ST, Jacobsen PB, et al. Frozen hope: fertility preservation for women with cancer. J Midwifery Womens Health. 2010;55:175–80 [DOI] [PubMed] [Google Scholar]
- 70.Quinn GP, Murphy D, Knapp C, et al. Who decides? Decision making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health. 2011;49:337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]