Abstract
Omalizumab is a humanized monoclonal antibody which is an FDA-approved treatment of severe allergic asthma and inhibits IgE binding to FcεRI. According to increasing evidence of IgE inhibition, omalizumab was suggested as a therapeutic approach for bullous pemphigoid (BP). Rituximab has been reported to be effective in various autoimmune diseases, including autoimmune bullous dermatoses. A specific protocol for the use of rituximab to treat BP patients is not yet available. There are only small case series and case reports about the efficacy and safety of rituximab in BP. Here we present a young BP patient who responded well to rituximab therapy and was refractory to conventional and omalizumab therapies although he had elevated IgE levels and eosinophilia. Our case supports the knowledge about the effectiveness and safety of rituximab not only in pemphigus but also in BP. On the other hand, although it did not work in our case, omalizumab may be a potentially effective agent in some carefully selected patients with certain subtypes of BP.
Keywords: Bullous pemphigoid, Omalizumab, Rituximab, Treatment
Background
Bullous pemphigoid (BP) is an autoimmune blistering disorder which is usually characterized by urticarial lesions initially and then tense blisters and erosions. It predominantly affects patients over the age of 65 but sometimes can develop in children and young adults [1]. BP is caused by autoantibodies directed against the hemidesmosomal proteins BP180 and BP230. The pathogenic IgG antibodies are usually directed at the NC16A portion of the BP180 antigen [2]. However, it has recently been shown that IgE antibodies might also be pathogenic in BP [3]. Systemic and/or topical corticosteroids (CS) are still the mainstay of BP treatment. Rarely, systemic immunosuppressive adjuvant therapies are needed. Despite all available effective treatments, the 1-year mortality rate of BP patients is still high, ranging from 13 to 38% [4].
Here we present a case with ichthyosis-associated BP who responded well to rituximab therapy and was refractory to conventional and omalizumab therapies.
Case
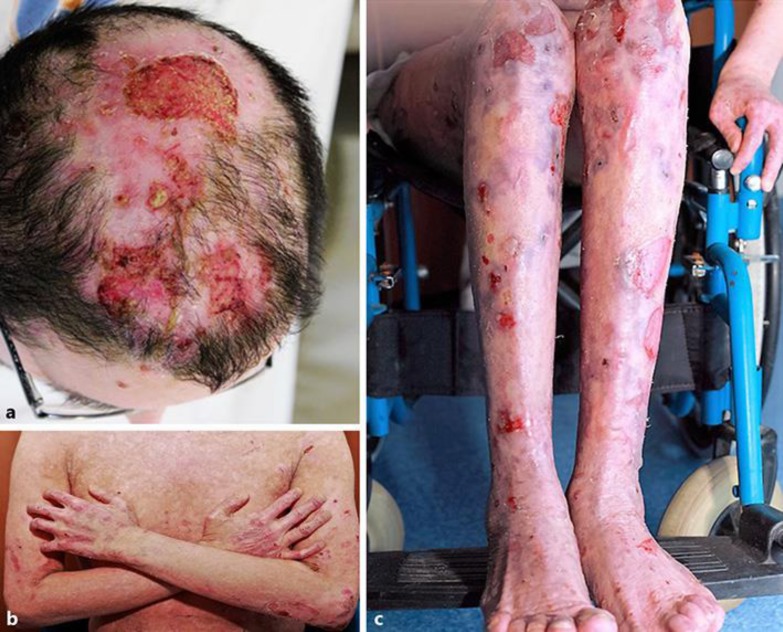
A 25-year-old man was referred to the outpatient clinic's Bullous Disease Unit, at the Department of Dermatology and Venereology of Akdeniz University, with a 3-year history of widespread tense blisters and erosions on his skin and mucous membranes (Fig. 1a–c). He also had ichthyosis vulgaris and a prenatal asphyxia history.
Fig. 1.
a–c Widespread tense blisters and erosions on his skin, especially on the scalp, arms, and legs, as well as erythrodermic ichthyosiform scaly skin due to ichthyosis vulgaris.
Before he was referred to our clinic, he had been diagnosed with linear IgA dermatosis in another center. He was treated with high-dose CS for 3 years and with dapson (100 mg per day) for 1 year without improvement. Due to treatment complications (osteoporosis, myositis, and muscle atrophy) he had become unable to walk and had to use a wheelchair. In our clinical examination, blisters and mostly erosions were observed especially on the trunk, scalp, lower extremities, and oral cavity. These blisters and erosions developed on erythrodermic ichthyosiform scaly skin.
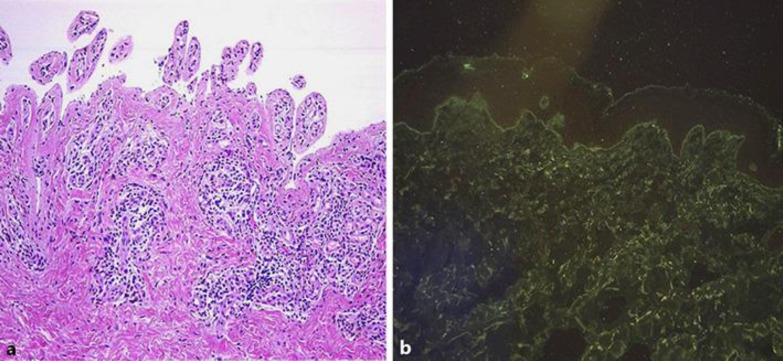
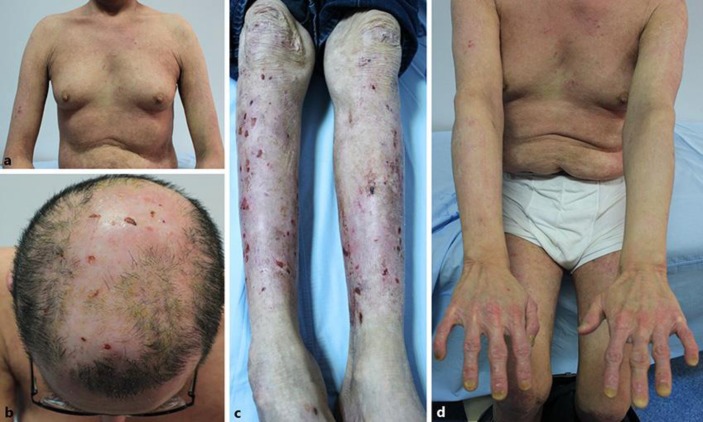
The diagnosis of BP was based on histologic findings showing subepidermal bullae and a mixed infiltrate with eosinophils (Fig. 2a), direct immunofluorescence on basal membrane zone (BMZ) showing linear IgG and complement 3 deposition, serum indirect immunofluorescence (IIF) revealing an IgG-circulating anti-BMZ antibody (BP180 and BP230) (Fig. 2b), serum IIF on salt-split, human skin substrate labeling the epidermal roof of the salt-split skin, and enzyme-linked immunosorbent assay (ELISA) showing that his serum anti-BMZ autoantibody recognized BP180 (>200 U), but not desmogleins. There was a high IgE level (1,598 IU, reference <87 IU) and eosinophilia in peripheral blood. Diagnosis was confirmed as BP, and systemic CS treatment was started at a dose of 0.75 mg/kg/day. After 2 weeks, since there was no satisfactory response to systemic therapy, topical superpotent CS (clobetasol propionate 0.05%) was added. One month later, when he was under poor control on this combined therapy, he was lost to follow-up. When he was referred again after 6 months, a large body surface area was covered with tense blisters and erosions overlying urticarial plaques, involving his upper and lower extremities, chest, and back. He had used both systemic and topical CS therapy in an uncontrolled way during this period. Since osteoporosis, myositis, and widespread skin atrophies were observed due to previous and recent uncontrolled steroid use, we wanted to avoid further steroids. Oral CS were decreased to minimal doses and the decision was made to administer omalizumab (Xolair). The omalizumab dose was calculated according to the asthma dosing nomogram, and he received 525 mg subcutaneously every 2 weeks for the 8-week duration of treatment. One week after his first dose, the patient reported a decrease in the intact blister count, but it did not work after a while. By week 8, his body surface involvement with the tense blisters of BP was nearly the same. No change was seen in IgG antibodies against BP180 (>200 U pretreatment, >200 U posttreatment). Only total serum IgE level had decreased (100 IU) with every omalizumab administration. Then, omalizumab injections were continued as 450 mg subcutaneously every 2 weeks for another 2-month period. After a 4-month treatment period, no significant clinical improvement had occurred. He continued to suffer new bullae formation and erosions. So he was accepted being refractory to omalizumab. In July 2014, he was treated with rituximab in a rheumatoid arthritis protocol (1,000 mg 2 times with 2 weeks apart). Serum autoantibodies of BP180 were finally decreased with rituximab therapy as soon as clinical improvement was seen and systemic CS was finally tapered to under 16 mg/day. Tachycardia was the only side effect due to rituximab and solved with slowing down the rituximab infusion rate. Total IgE level decreased and stayed at a low level during rituximab therapy. In February 2015, 6 months after the first rituximab administration, a second course of 1,000 mg rituximab was given. CS was tapered and discontinued. He has been maintaining his state of complete remission off therapy for 12 months (Fig. 3a–d). He finally became able to walk with the aid of a walker.
Fig. 2.
a Subepidermal bullae and a mixed infiltrate with eosinophils. b Direct immunofluorescence shows linear IgG and complement 3 deposition in the BMZ.
Fig. 3.
a–d Complete resolution of blisters was observed.
Discussion
We report a case successfully treated with rituximab who did not respond to CS and omalizumab, respectively. Omalizumab is a humanized monoclonal antibody which is an FDA-approved treatment of severe allergic asthma and inhibits IgE binding to FcεRI. According to increasing evidence of IgE inhibition, omalizumab may be a therapeutic approach for BP [2]. To date, 10 case reports have been reported with severe recalcitrant BP treated with omalizumab [5, 6, 7]. There is only one open, uncontrolled study of omalizumab for BP. In this trial, 5 of 6 patients had good response to omalizumab with less use of other immunosuppressants, inhibition of new bullae, less pruritus, and dramatic decreases in eosinophil counts [5].
The use of omalizumab in the treatment of BP is based on these observations: (1) IgE levels are elevated in most patients with untreated BP; (2) patients with BP express IgE autoantibodies against BP antigens; and (3) the developing knowledge that IgE autoantibodies can replicate the early phases of lesion development in BP [3, 8, 9]. Owing to having a relatively favorable adverse effect profile compared with other immunosuppressants, omalizumab seems to have benefits in recalcitrant disease [9]. Yu et al. [5] found that peripheral blood eosinophil counts followed disease activity most closely during omalizumab therapy in BP patients. Also, some authors carefully observed the clinical features and the titers of autoantibodies in the BP patient, and they found that circulating levels of IgE autoantibodies correlate well with urticarial erythema and that those of IgG autoantibodies are instead associated with blister formation [10]. Until a larger, controlled trial is performed, it seems most prudent to use omalizumab in patients with recalcitrant disease who have elevated IgE levels, eosinophilia, or both [3]. The ideal dosing of omalizumab in BP has not yet been decided. For now, the recommended dosing of omalizumab has been based on its use in asthma. In asthma studies, the only statistically significant adverse reaction compared with placebo was hypersensitivity reactions to the medication including anaphylaxis (0.1%) [11].
We first thought that omalizumab was a reasonable treatment because of our patient's high IgE serum level and recent literature about good response. But while IgE levels gradually decreased in our patient, BP180 antibody titers stayed at the same level (>200 IU) without any clinical improvement during follow-up after omalizumab treatment. So this observation made us think that IgG BP180 antibodies probably played the major role in the pathogenesis of BP instead of IgE BP180 antibodies. We also speculated that blister formation without urticarial erythema could be the reason in our patient that omalizumab provided no benefit.
Rituximab, which is a monoclonal antibody directed against the CD20 antigen on B lymphocytes, has been reported to be effective in various autoimmune diseases, including autoimmune bullous dermatoses [12]. A specific protocol for the use of rituximab to treat BP patients is not yet available. There are only small case series and case reports about the efficacy and safety of rituximab in BP [13]. The first report using rituximab as first-line treatment in BP, which demonstrated a significantly better rate of complete remission compared to conventional treatment, was published in 2014 [14]. Since infection and mortality rates are the major concerns, careful and close monitoring seems necessary [13]. But the study of Cho et al. [14] provided a comparison and showed first-line combination therapy of rituximab has similar or even lower rates of infection and mortality than conventional treatment. They suggested that this might have resulted from rapid tapering and withdrawing systemic steroids [14]. These data suggest that rituximab is a useful option for BP patients who are recalcitrant to conventional therapy. Clinical improvement in patients with pemphigus has been associated with decreased levels of circulating autoantibodies [15]. In our case we also observed a decline in BP180 antibody titers with rituximab treatment.
Conclusions
Although further prospective studies are needed to determine the most appropriate dosing schedule, our case supports the knowledge about the effectiveness and safety of rituximab not only in pemphigus but also in BP. On the other hand, although it did not work in our case, omalizumab may be a potentially effective agent in some carefully selected patients with certain subtypes of BP.
Statement of Ethics
Consent for using involved drugs (such as omalizumab and rituximab) has been taken from the Ministry of Health and a written consent has been taken from the patient for publication.
Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Messingham KA, Holahan HM, Fairley JA. Unraveling the significance of IgE autoantibodies in organ-specific autoimmunity: lessons learned from bullous pemphigoid. Immunol Res. 2014;59:273–278. doi: 10.1007/s12026-014-8547-7. [DOI] [PubMed] [Google Scholar]
- 2.Messingham KN, Pietras TA, Fairley JA. Role of IgE in bullous pemphigoid: a review and rationale for IgE directed therapies. G Ital Dermatol Venereol. 2012;147:251–257. [PubMed] [Google Scholar]
- 3.Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- 4.Joly P, Baricault S, Sparsa A, Bernard P, Bédane C, Duvert-Lehembre S, Courville P, Bravard P, Rémond B, Doffoel-Hantz V, Bénichou J. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132:1998–2004. doi: 10.1038/jid.2012.35. [DOI] [PubMed] [Google Scholar]
- 5.Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71:468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London VA, Kim GH, Fairley JA, Woodley DT. Successful treatment of bullous pemphigoid with omalizumab. Arch Dermatol. 2012;148:1241–1243. doi: 10.1001/archdermatol.2012.1604. [DOI] [PubMed] [Google Scholar]
- 7.Dufour C, Souillet AL, Chaneliere C, Jouen F, Bodemer C, Jullien D, Cambazard F, Joly P, Reix P. Successful management of severe infant bullous pemphigoid with omalizumab. Br J Dermatol. 2012;166:1140–1142. doi: 10.1111/j.1365-2133.2011.10748.x. [DOI] [PubMed] [Google Scholar]
- 8.Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, Fairley JA. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. 2003;120:784–788. doi: 10.1046/j.1523-1747.2003.12146.x. [DOI] [PubMed] [Google Scholar]
- 9.Delaporte E, Dubost-Brama A, Ghohestani R, Nicolas JF, Neyrinck JL, Bergoend H, Janin A, Capron M. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642–3647. [PubMed] [Google Scholar]
- 10.Kamiya K, Aoyama Y, Noda K, Yamaguchi M, Hamada T, Tokura Y, Iwatsuki K. Possible correlation of IgE autoantibody to BP180 with disease activity in bullous pemphigoid. J Dermatol Sci. 2015;78:77–79. doi: 10.1016/j.jdermsci.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Milgrom H, Fick RB, Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, Metzger WJ. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 12.Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9:10–25. doi: 10.1016/j.intimp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Shetty S, Ahmed AR. Treatment of bullous pemphigoid with rituximab: critical analysis of the current literature. J Drugs Dermatol. 2013;12:672–677. [PubMed] [Google Scholar]
- 14.Cho YT, Chu CY, Wang LF. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302–304. doi: 10.1111/bjd.13633. [DOI] [PubMed] [Google Scholar]
- 15.Joly P, Mouquet H, Roujeau JC, D'Incan M, Gilbert D, Jacquot S, Gougeon ML, Bedane C, Muller R, Dreno B, Doutre MS, Delaporte E, Pauwels C, Franck N, Caux F, Picard C, Tancrede-Bohin E, Bernard P, Tron F, Hertl M, Musette P. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357:545–552. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]