Abstract
Background
Anaplastic thyroid carcinoma has an extremely poor prognosis, and no known drugs have exhibited acceptable efficacy. In recent years, novel anticancer tyrosine kinase inhibitors have been developed. We encountered a case of tracheal stenosis due to mediastinal and tracheal infiltration of anaplastic carcinoma for which lenvatinib exhibited remarkable effects; owing to this, airway management could be performed, even though the patient's condition was considered critical.
Case Report
A 55-year-old man presented with locally advanced anaplastic thyroid carcinoma that was observed to have mediastinal infiltration. Tracheal stenosis due to infiltration of the trachea occurred, and the condition of the patient rapidly deteriorated. Radiation and chemotherapy consisting of cetuximab, cisplatin, and fluorouracil were ineffective, but his tracheal stenosis was relieved 2 weeks after initiation of lenvatinib, after which the patient could be discharged. However, the lenvatinib was ineffective for his liver, bone, and brain metastatic lesions, and the patient remained in a critical condition.
Conclusion
We encountered a case in which lenvatinib was effective for locally advanced anaplastic thyroid carcinoma, leading to an improvement in quality of life and a prolonged life. The drug was effective for the primary lesion, but mixed efficacy was noted for distant metastatic lesions.
Keywords: Anaplastic thyroid cancer, Lenvatinib, Tyrosine kinase inhibitor, Advanced thyroid cancer, Tracheal infiltration
Introduction
Anaplastic thyroid carcinoma is one of the carcinomas with the poorest prognosis. The frequency of its occurrence is low but it is highly malignant, and local expansion and distant metastasis progress rapidly; to date, no effective treatment has been established [1, 2]. Multimodal therapy has been performed, but there are no known effective anticancer drugs [3, 4, 5, 6, 7, 8].
In recent years, many molecular targeted therapies have been developed, and therapies targeting the thyroid are no exception [9, 10]. In Japan, the tyrosine kinase inhibitor lenvatinib was approved in March 2015 for the treatment of all types of thyroid cancer, including anaplastic carcinoma. Because there is little clinical experience in using lenvatinib for anaplastic carcinoma, its efficacy is unknown.
We administered lenvatinib to a patient who rapidly developed tracheal stenosis due to tracheal infiltration of advanced anaplastic thyroid carcinoma, and remarkable efficacy was observed. Here, we report the clinical course of a patient in whom tracheal stenosis was relieved and who could be discharged following lenvatinib administration.
Case Report
A 55-year-old man presented with the chief complaint of dysphagia. A suspected right level IV lymph node metastasis and a neoplastic lesion located in the inferior pole of the thyroid to the mediastinum were observed. Because the boundary between the inferior pole of the thyroid gland and the tumor was unclear, and a calcified lesion was observed in the tumor, thyroid cancer was suspected.
We performed fine needle biopsy on the caudal thyroid tumor and right level IV lymph node, created cell blocks at the time of cytodiagnosis, and performed thyroglobulin, thyroid transcription factor-1 (TTF-1) and Hecter Battifora mesothelial epitope-1 (HBME-1) immunostaining. However, the results of immunostaining were negative, and based on the results of the cytodiagnosis, poorly differentiated squamous cell carcinoma was suspected. A generalized examination consisting of contrast-enhanced computed tomography (CT), FDG-positron emission tomography (PET), and endoscopy did not reveal a clear primary site in the head and neck region, and in addition to the mediastinal tumor, a right level IV cervical lymph node metastasis, pulmonary metastases, and bone metastases were observed (Fig. 1a).
Fig. 1.
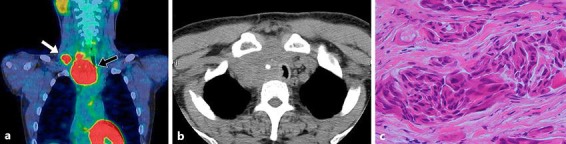
a The PET/CT image shows an increase in FDG uptake in the mediastinum (black arrow) and the cervical metastatic lymph node (white arrow). b The chest CT shows the mediastinal tumor infiltration of the large vessels and the trachea. c The histopathological finding shows anaplastic carcinoma. Hematoxylin and eosin staining. ×60.
The results of the biopsy performed on the level IV lymph node metastasis to determine the tissue type revealed a metastasis of a squamous cell carcinoma. Based on this, we diagnosed occult primary squamous cell carcinoma and initiated treatment. Chemotherapy consisting of cetuximab, cisplatin, and fluorouracil was initiated 1 month following the initial presentation. The result was stable disease. We administered an additional course of chemotherapy consisting of cetuximab, cisplatin, and fluorouracil, but the result was progressive disease, and edema of his cervical and facial regions and tracheal stenosis worsened.
Endotracheal fiber examination showed tracheal invasion of the tumor, and CT revealed mediastinal tumor infiltration of the large vessels of the chest (left common carotid artery, brachiocephalic artery, and brachiocephalic vein) and thrombosis of the bilateral internal jugular veins (Fig. 1b).
Because the airway was becoming blocked, we performed a tracheotomy and placed a long intubation tube just above the tracheal bifurcation. A mediastinal tumor biopsy was also simultaneously performed. After performing airway management, we administered palliative irradiation (16 Gy/2 Fr) but no efficacy was noted. Biopsy results revealed papillary thyroid carcinoma and partially anaplastic transformation (Fig. 1c).
The anaplastic thyroid carcinoma was stage IVC, and the tracheal stenosis progressed to the region just above the bifurcation of the trachea because of the infiltrated tumor. Therefore, we thought that suffocation would be unavoidable in the near future (Fig. 2).
Fig. 2.
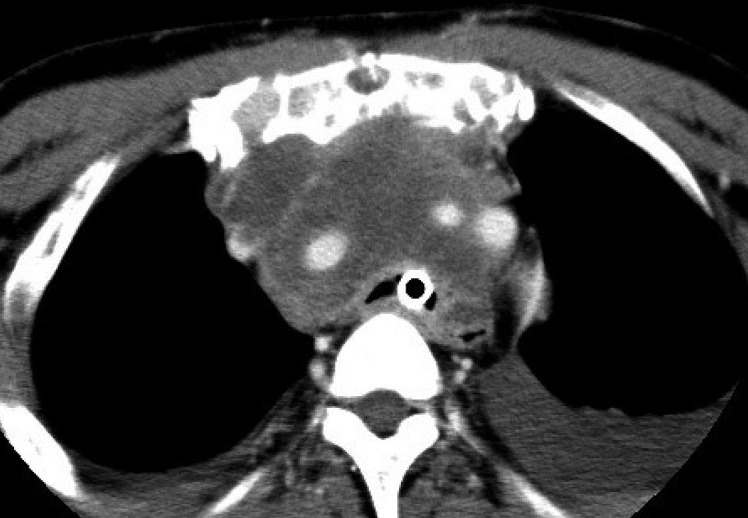
CT shows severe tracheal stenosis as a result of tumor invasion.
Because there are no conventional treatments that are believed to be effective for anaplastic carcinoma, we initiated the administration of lenvatinib (full dose, 24 mg) after having obtained consent from the patient and his family members and having performed a heart evaluation and screening for brain metastases.
Effects of Lenvatinib
On the fourth day after lenvatinib administration, his facial edema began to be ameliorated, followed by amelioration of the edema of his upper limbs. The tumor that had infiltrated the trachea shrank starting on day 10 of administration, and a space between the intubation tube and tracheal wall was observed. On day 14 of administration, the tumor in the trachea disappeared, and because there was no need to maintain a cavity with the intubation tube, the tube was changed to a common short cannula.
CT showed a rapid prominent reduction of the mediastinal tumor, reduction of the pulmonary lesion, and a decrease in pleural effusion. Necrosis of the mediastinal tumor developed, and a necrotic cavity formed without development of granulomas or scarring; as a result, a dead space developed in the anterior region of the trachea (Fig. 3a). In contrast, an increase in the size and number of bone and liver metastases was noted (Fig. 3b).
Fig. 3.
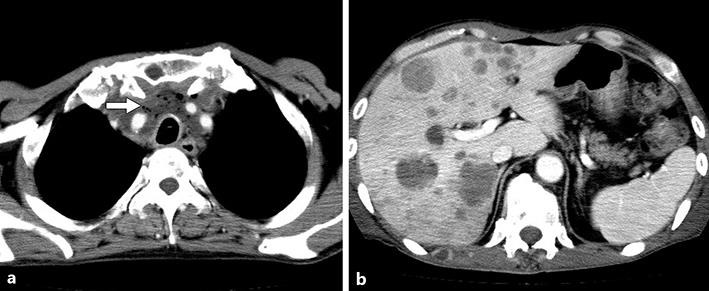
a A rapid, prominent reduction of the mediastinal tumor led to the dead space in the mediastinum. The air within the mediastinal tumor represents the dead space caused by tumor necrosis after lenvatinib administration (arrow). b Abdominal CT shows progression of the liver metastases.
Complications of Lenvatinib
The patient's blood pressure increased to 168/96 mm Hg 3 days after initiation of lenvatinib, but it improved after he had been receiving nifedipine for several days. Five days after initiating lenvatinib, his thyroid function began to decrease, and the decrease in function reached the lowest point 2 weeks after initiation. For this reason, we finally decided to administer levothyroxine (100 μg). No other side effects were noted. On day 19, the patient began to make utterances using a speech valve, and he was discharged 4 weeks following the initiation of lenvatinib.
Prognosis
The tracheal stenosis was relieved, and the patient's life was temporarily prolonged, but the drug was completely ineffective for his liver and bone metastatic lesions, which increased in size and number. Because the tumor necrosis and dead space following necrosis of the tumor surrounding the trachea were not filled after discharge, the dose of the drug was decreased to 14 mg at 6 weeks after initiation.
Because the effects of anticancer drugs greatly differ between mediastinal primary site and liver field foci, we performed tissue biopsy of the suspected liver metastasis to rule out metastases from another site or primary liver cancer. However, histological findings similar to those of the primary focus were noted.
A large dead space remained in the mediastinum, and because mediastinitis due to the influx of saliva from the trachea hole was also a concern, and further brain metastases were revealed, administration of lenvatinib was discontinued 7 weeks and 3 days after initiation. The brain and liver metastases advanced, and the patient died 3 months after initiation of lenvatinib.
Discussion
Lenvatinib is a tyrosine kinase inhibitor-type anticancer drug with a novel mechanism that is not seen in conventional anticancer drugs. It exhibits selective inhibitory activity for receptor tyrosine kinases (RTK) that contribute to tumor angiogenesis or tumor malignancy such as vascular endothelial growth factor receptors (VEGFR) VEGFR1, VEGFR2, VEGFR3; fibroblast growth factors (FGFR) FGFR1, FGFR2, FGFR3, FGFR4; and platelet-derived growth factors (PDGFR) PDGFRα, KIT, and RET [11, 12, 13]. Currently, the drug has been approved for the treatment of differentiated thyroid cancer in Western countries, but the indication for anaplastic cancer was approved in March 2015 in Japan ahead of other countries. Because not much time has passed since the drug was approved in Japan, there are almost no reports concerning the use of lenvatinib for anaplastic cancer.
We encountered a patient in whom tracheal stenosis developed due to infiltration of locally advanced anaplastic thyroid carcinoma, making airway management difficult. Because a reduction was observed and tracheal stenosis was relieved 2 weeks after lenvatinib administration, the patient could be discharged. In this case, lenvatinib exhibited remarkable effects on the locally advanced anaplastic cancer, and the avoidance of tracheal occlusion contributed to the dramatic improvement of the patient's quality of life and prolonged his life. The patient could return home, and he and his family had time to accept the situation.
However, multiple distant metastases were noted, and the lenvatinib was not found to be effective for the patient's distant metastases except for those in the lungs. A histopathological comparison of the primary tumor site for which efficacy was noted and the liver metastatic lesion for which no efficacy was noted revealed no differences. Because in cases of lung lesions, there are lesions for which efficacy is noted and lesions for which efficacy is not observed, it is still unclear whether the difference of the efficacy for the lesions was due to differences in drug concentration or to differences in tissue type and hemodynamics, but the possibility of mutations affecting the metastasis sites was also considered.
In this patient, lenvatinib exhibited a rapid shrinking effect on the tumors without forming scar tissue or granulation tissue in the tumor necrosis site, creating a large dead space. The tyrosine kinase inhibitor lenvatinib does not appear to cause the scarring and granulation generally seen following tumor necrosis when other anticancer drugs are used. Based on these findings, when using lenvatinib, it is necessary to be aware from the start that tissue defects and dead space formation may occur after tumor necrosis in addition to the possibility of fistula formation of the trachea and esophagus and rupture-induced hemorrhage of large blood vessels. In fact, the rupture of a major artery has been previously reported in Japan [14].
To date, no efficacy of anticancer drugs for anaplastic thyroid carcinoma has been reported. However, the present case shows the possibility that lenvatinib can be a first-line anticancer drug for the treatment of anaplastic cancer.
Conclusion
We treated a case in which lenvatinib was effective for locally advanced anaplastic thyroid carcinoma, leading to improved quality of life and a prolonged life span. A remarkable effect on the primary lesion was noted, but the efficacy for distant metastatic lesions was mixed. In the site in which the tumor rapidly shrank, a delay in granuloma formation was observed only in the tumor necrotic tissue.
Statement of Ethics
The authors declare that the subject has given informed consent and that the study was performed in accordance with the ethical guidelines of the Helsinki Declaration.
Disclosure Statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg. 2015;36:1247–1254. doi: 10.1007/s00268-012-1437-z. [DOI] [PubMed] [Google Scholar]
- 2.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shaha AR, Tuttle RM. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb JA, Hill CS., Jr Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med. 1974;290:193–197. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 4.Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155–2160. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Biganzoli L, Gebbia V, Maiorino L, Caraci P, Iirillo A. Thyroid cancer: different outcomes to chemotherapy according to tumour histology. Eur J Cancer. 1995;31:2423–2424. doi: 10.1016/0959-8049(95)00415-7. [DOI] [PubMed] [Google Scholar]
- 6.Santini F, Bottici V, Elisei R, Montanelli L, Mazzeo S, Basolo F, Pinchera A, Pacini F. Cytotoxic effects of carboplatinum and epirubicin in the setting of an elevated serum tyrotropin for advanced poorly differentiated thyroid cancer. J Clin Endocrinol Metab. 2002;87:4160–4165. doi: 10.1210/jc.2001-011151. [DOI] [PubMed] [Google Scholar]
- 7.Matuszczyk A, Petersenn S, Voigt W, Kegel T, Dralle H, Schmoll HJ, Bockisch A, Mann K. Chemotherapy with paclitaxel and gemcitabine in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm Metab Res. 2010;42:61–64. doi: 10.1055/s-0029-1238294. [DOI] [PubMed] [Google Scholar]
- 8.Ain KB, Lee C, Williams KD. Phase 2 trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid. 2007;17:663–670. doi: 10.1089/thy.2006.0289. [DOI] [PubMed] [Google Scholar]
- 9.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refactory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 10.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 12.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto K, Ikemori-Kawada M, Jestel A, von Konig K, Funahashi Y, Matsushima T, Tsuruoka A, Inoue A, Matsui J. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2014;6:89–94. doi: 10.1021/ml500394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onoda N, Tokumoto M, Noda S. A case of recurrent anaplastic thyroid cancer treated by lenbatinib after successful long-term multimodal therapy (in Japanese). J Japan Surg Assoc. 2016;77:291–295. [Google Scholar]


