Abstract
Significance: Rapid recruitment and activation of macrophages may accelerate wound healing. Such accelerated healing was observed in wounds and burns of experimental animals treated with α-gal nanoparticles.
Recent Advances: α-Gal nanoparticles present multiple α-gal epitopes (Galα1-3Galβ1-4GlcNAc-R). α-Gal nanoparticles applied to wounds bind anti-Gal (the most abundant antibody in humans) and generate chemotactic complement peptides, which rapidly recruit macrophages. Fc/Fc receptor interaction between anti-Gal coating the α-gal nanoparticles and recruited macrophages activates macrophages to produce cytokines that accelerate healing. α-Gal nanoparticles applied to burns and wounds in mice and pigs producing anti-Gal, decreased healing time by 40–60%. In mice, this accelerated healing avoided scar formation. α-Gal nanoparticle-treated wounds, in diabetic mice producing anti-Gal, healed within 12 days, whereas saline-treated wounds became chronic wounds. α-Gal nanoparticles are stable for years and may be applied dried, in suspension, aerosol, ointments, or within biodegradable materials.
Critical Issues: α-Gal nanoparticle therapy can be evaluated only in mammalian models producing anti-Gal, including α1,3-galactosyltransferase knockout mice and pigs or Old World primates. Traditional experimental animal models synthesize α-gal epitopes and lack anti-Gal.
Future Directions: Since anti-Gal is naturally produced in all humans, it is of interest to determine safety and efficacy of α-gal nanoparticles in accelerating wound and burn healing in healthy individuals and in patients with impaired wound healing such as diabetic patients and elderly individuals. In addition, efficacy of α-gal nanoparticle therapy should be studied in healing and regeneration of internal injuries such as surgical incisions, ischemic myocardium following myocardial infarction, and injured nerves.
Keywords: : skin injury, wound healing, macrophage activation, α-gal glycolipids, anti-Gal antibody, myocardium regeneration, nerve regeneration, biomaterials, tissue engineering

Uri Galili, PhD
Scope and Significance
This review describes preclinical studies on acceleration of wound and burn healing by a novel method that induces rapid recruitment and activation of macrophages within injured sites. The method uses α-gal nanoparticles, which harness the natural anti-Gal antibody (the most abundant natural antibody in humans), for recruitment and activation of macrophages that orchestrate healing of injuries. Studies performed in mice and pigs producing anti-Gal demonstrate 40–60% decrease in healing time in wounds treated with α-gal nanoparticles in comparison with saline-treated wounds. α-Gal nanoparticle treatment was further found to prevent scar formation in mice.
Translational Relevance
Studies on wound therapy by α-gal nanoparticles may provide a new approach for exploiting the natural anti-Gal antibody in various clinical settings. The interaction of anti-Gal with its carbohydrate antigen—the α-gal epitope, abundantly presented on α-gal nanoparticles, enables rapid recruitment of macrophages and their activation for the secretion of a wide range of cytokines, promoting repair and regeneration of wounds, which may occur before the onset of fibrosis. The observations on accelerated healing of skin raise the possibility that such therapy may be of significance in treatment of internal injuries as well.
Clinical Relevance
The decrease in wound and burn healing time in anti-Gal-producing animals may suggest that such treatment could be effective in humans since multiple studies have shown that human anti-Gal binding to α-gal epitopes results in extensive local activation of the complement system at least as much as in the experimental animal models. In addition, the observed healing of wounds in diabetic mice by α-gal nanoparticles raises the possibility that such treatment may jump-start the healing process in chronic wounds in diabetic patients and in elderly individuals suffering from impaired wound healing.
Background
Macrophages are the pivotal cells in early stages of injury healing and tissue regeneration.1 Macrophages migrate into wounds and debride them. Subsequently, macrophages orchestrate the healing processes within the wound by secreting a variety of cytokines.1–3 Macrophages are recruited into wounds by cytokines such as MIP-1 and MCP-1 released from cells within and around injury sites.4–6 In view of the significance of macrophages in wound healing, one may hypothesize that rapid recruitment of macrophages and their activation in wounds might result in accelerated healing.
Macrophages may be recruited by the complement cleavage peptides, C5a and C3a, which are potent chemotactic factors inducing rapid extravasation and migration of monocytes and their differentiation into macrophages.7 The complement system can be activated to produce these cleavage peptides by antigen/antibody interactions. Therefore, we hypothesized that C5a and C3a may be generated within wounds by interaction between the natural anti-Gal antibody, commonly found in all humans, and the carbohydrate antigen it recognizes, the α-gal epitope.
The Natural Anti-Gal Antibody and α-Gal Nanoparticles
Anti-Gal is present in humans as ∼1% of immunoglobulins8–12 and is produced throughout life13 as a result of antigenic stimulation by gastrointestinal bacteria of the natural flora.14,15 Anti-Gal binds specifically to the α-gal epitope with the structure, Galα1-3Galβ1-4GlcNAc-R,16,17 and is produced also in Old World monkeys (monkeys of Asia and Africa) and in apes, but not in other mammals.12,18 In contrast, mammals other than Old World monkeys, apes, and humans, present on their cells multiple α-gal epitopes, which are synthesized on glycolipids and glycoproteins by α1,3-galactosyltransferase (α1,3GT), a glycosylation enzyme absent in humans.17–20 Interaction between anti-Gal and α-gal epitopes results in effective activation of the complement system.21–24 This was further demonstrated in studies on transplantation of pig cells or organs into Old World monkeys. Transplantation of pig heart or kidney into monkeys results in a rapid (hyperacute) rejection caused by binding of anti-Gal to α-gal epitopes on pig cells, activation of the complement system that bores holes in cell membranes, collapse of the vascular bed, and rejection of xenografts within 30 min to few hours.24–26 Similarly, incubation of pig cells with human serum results in activation of the complement system by this anti-Gal/α-gal epitope interaction.21–24 By-products of the complement activation cascade are small cleaved complement peptides, including C5a and C3a. Therefore, we assumed that application of nanoparticles presenting multiple α-gal epitopes (called α-gal nanoparticles) onto wounds is likely to result in binding of the anti-Gal antibody to these nanoparticles, activation of the complement cascade, and generation of C5a and C3a chemotactic gradients that may induce rapid recruitment of macrophages. The origin of wound anti-Gal and complement is serum proteins that are released into wounds from ruptured capillaries.
α-Gal nanoparticles are submicroscopic α-gal liposomes made of glycolipids with multiple α-gal epitopes (α-gal glycolipids), phospholipids, and cholesterol (Fig. 1).27–29 Rabbit red blood cell (RBC) membranes are used for formation of α-gal nanoparticles since they are rich in α-gal glycolipids.18,30–35 Rabbit RBC membranes are obtained by hypotonic shock of the RBC and removal of hemoglobin by repeated washes. A mixture of glycolipids, phospholipids, and cholesterol is extracted from the RBC membranes in a solution of chloroform and methanol.36 The extract is dried, then resuspended in saline in a sonication bath to generate α-gal liposomes (size of 1–10 μm) that present multiple α-gal epitopes of α-gal glycolipids.27,28 The size of these liposomes is further decreased by sonication with a sonication probe to form smaller particles with the size range of 10–300 nm, called α-gal nanoparticles, which can be sterilized by filtration through a 0.2-μm filter.28,29
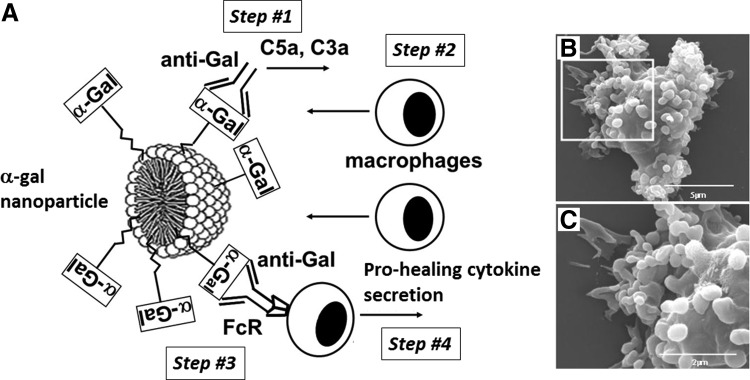
Figure 1.
Illustration of α-gal nanoparticles. (A) Suggested functions of α-gal nanoparticles in wounds. The α-gal nanoparticle comprises phospholipids and α-gal glycolipids, each capped with an α-gal epitope (α-GAL in rectangles). When α-gal nanoparticles are applied to wounds, their α-gal epitopes bind the natural anti-Gal antibody, resulting in activation of the complement system (step #1). The produced chemotactic complement cleavage peptides induce rapid recruitment of macrophages (step #2). Anti-Gal coating the α-gal nanoparticles further interacts through its Fc tail with Fcγ receptors (FcR) on macrophages (step #3). This interaction activates macrophages to produce and secrete a variety of cytokines that accelerate wound healing (step #4). (B) Binding of anti-Gal-coated α-gal nanoparticles to macrophages as a result of Fc/FcR interaction (step #3 in Fig. 1A), as evaluated by scanning electron microscopy. (C) Enlargement of the inset in (B). The surface of a representative macrophage is covered with α-gal nanoparticles. The size of the α-gal nanoparticles is ∼100–300 nm (modified from Ref.27).
α-Gal nanoparticles made of rabbit RBC membranes have phospholipid and cholesterol bilayer or monolayer, as in the micelle in Fig. 1A, in which α-gal glycolipids are anchored through their fatty acid tails. α-Gal glycolipids have 1–8 carbohydrate branches (antennae), each carrying an α-gal epitope,18,30–36 and the total number of these epitopes is 1015 per mg.28 α-Gal nanoparticles may also be prepared from synthetic α-gal glycolipids and phospholipids in a process similar to that described above.
Rapid Recruitment and Activation of Macrophages By α-Gal Nanoparticles
We hypothesized27–29 that topical application of α-gal nanoparticles to burns and wounds may enable harnessing of the natural anti-Gal antibody for recruitment and activation of macrophages, which, in turn, will accelerate the healing process in the following sequential steps (Fig. 1A): (1) Anti-Gal/α-gal nanoparticle interaction activates the complement system, which generates the chemotactic peptides, C5a and C3a. (2) These chemotactic peptides induce rapid extravasation of monocytes and their differentiation into macrophages that migrate toward the α-gal nanoparticles. (3) The recruited macrophages bind through their Fcγ receptor (FcR) the Fc portion of anti-Gal coating the α-gal nanoparticles. (4) Binding of α-gal nanoparticles to FcR of the macrophages activates these cells to secrete cytokines that promote and accelerate the healing process. Whereas steps #1–3 were predictable from previous studies on anti-Gal/α-gal epitope interaction,9 step #4 was hypothesized without previous supporting data.
The study of anti-Gal-mediated acceleration of wound healing by α-gal nanoparticles requires experimental animal models that produce the anti-Gal antibody. As indicated above, Old World monkeys, apes, and humans are the only mammals producing anti-Gal, whereas other mammals synthesize α-gal epitopes on their cells and are prevented from producing anti-Gal by immune tolerance mechanisms.9,17–19 The only two known nonprimate mammals, which lack α-gal epitopes and produce anti-Gal, are α1,3GT knockout mice37,38 (GT-KO mice) and α1,3GT knockout pigs39,40 (GT-KO pigs), in which the α1,3GT gene (GGTA1) encoding the enzyme that synthesizes α-gal epitopes was disrupted (i.e., knocked out). Whereas GT-KO pigs naturally produce anti-Gal,41–43 GT-KO mice require immunization with cells or tissue homogenates presenting α-gal epitopes (e.g., pig kidney homogenate) as antigenic stimulation for anti-Gal production. The sterile environment and food of mice do not enable the development of bacterial flora that can induce natural anti-Gal antibody production.44,45
Intradermal injection of α-gal nanoparticles into anti-Gal-producing GT-KO mice demonstrated recruitment of macrophages to injection sites within 24 h (Fig. 2A). These macrophages readily stain by the macrophage-specific anti-F4/80 antibody (Fig. 2B). Macrophages were found at the injection site for ∼2 weeks postinjection. These cells completely disappeared after 3 weeks without altering normal skin histology at the injection site and with no granulomas, chronic inflammatory responses, keloids, or fibrosis.28 Intradermal injection of α-gal nanoparticles mixed with cobra venom factor, which inhibits complement activation, resulted in no macrophage recruitment.28 Saline injection resulted in no recruitment of macrophages as well. No recruitment of macrophages was also observed following intradermal injection of α-gal nanoparticles in mice lacking anti-Gal.28 These findings suggested that steps #1 and #2 in Fig. 1A, hypothesizing recruitment of macrophages, require binding of anti-Gal to the α-gal nanoparticles to activate the complement system and generate chemotactic complement cleavage peptides that induce rapid recruitment of macrophages.28
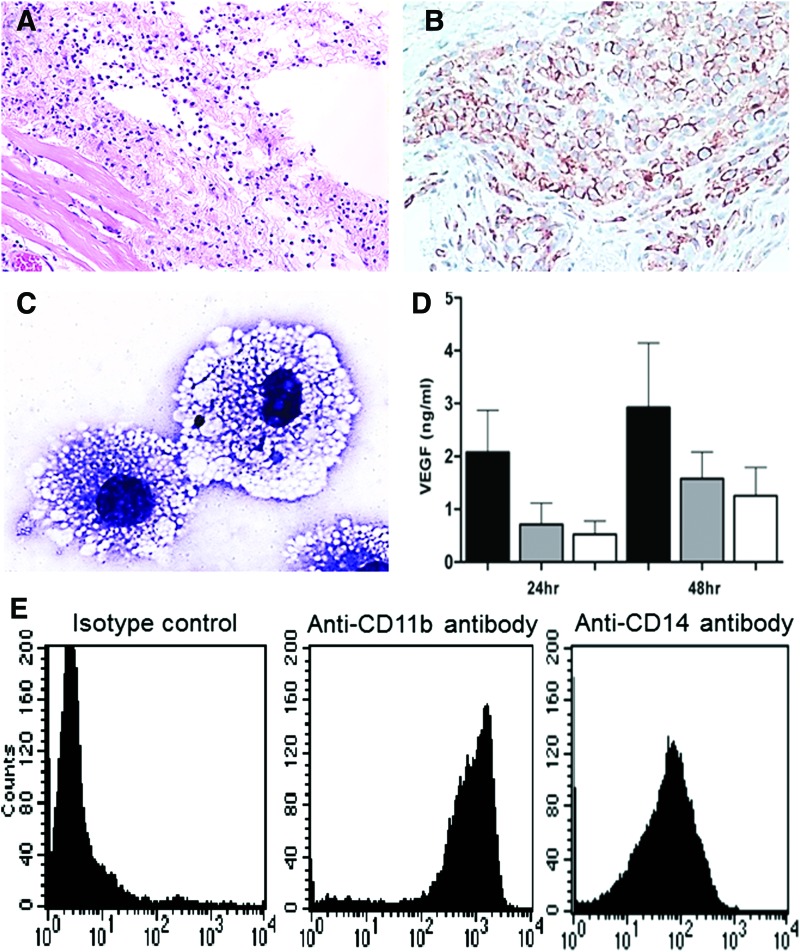
Figure 2.
Macrophage recruitment by α-gal nanoparticles in GT-KO mice. (A) One milligram of α-gal nanoparticle suspension in saline was injected intradermally (indicated as the empty area). Inspection after 24 h demonstrates multiple macrophages recruited around the injection site (H&E staining 100 × ). (B) The injection site after 4 days immunostained with the macrophage-specific anti-F4/80 antibody, demonstrating that most of the recruited cells are macrophages (peroxidase immunostaining 200 × ). (C) Macrophages recruited into PVA sponge discs implanted subcutaneously and containing 1 mg α-gal nanoparticles, studied after 6 days. Most of the recruited cells display ample vacuolated cytoplasm due to the uptake of anti-Gal-coated α-gal nanoparticles and activation of the macrophages, with size of ∼30 μm (Wright staining 1,000 × ). (D) Secretion of VEGF by GT-KO mouse peritoneal macrophages cocultured with anti-Gal-coated α-gal nanoparticles (black columns), α-gal nanoparticles without anti-Gal (gray columns), or macrophages alone (white columns). VEGF was quantified in culture media after 24 or 48 h (mean + SD n = 4 mice/group). p < 0.05 between the group with anti-Gal-coated α-gal nanoparticles and the other two groups. (E) Flow cytometry analysis of cells recruited by α-gal nanoparticles into implanted PVA sponge discs indicates that >90% of the recruited cells are macrophages as they are CD11b+ and CD14+ cells. (Representative results from five mice with similar results, modified from Refs.27,28) PVA, polyvinyl alcohol. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Cells recruited by α-gal nanoparticles could be further studied by subcutaneous implantation of biologically inert sponge discs (made of polyvinyl alcohol) that contained α-gal nanoparticles. The sponge discs were explanted after 6 days and the recruited cells retrieved after squeezing these sponges in phosphate-buffered saline. The recruited cells displayed a morphology of large macrophages filled with vacuoles representing internalized α-gal nanoparticles (Fig. 2C). Flow cytometry analysis indicated that >90% of the cells were stained with macrophage-specific anti-CD11b and anti-CD14 antibodies (Fig. 2E), whereas no B cells or T cells were found among the recruited cells.27 Recent studies have suggested that these macrophages are primarily M2 macrophages as they are producing arginase and IL12, but lack IL10 (in preparation).
Once macrophages reach the α-gal nanoparticles, they bind them through Fc/FcR interaction with the Fc portion of anti-Gal coating these nanoparticles (step #3 in Fig. 1A). This binding is visualized in Fig. 1B, C with macrophages of GT-KO pig coincubated with anti-Gal-coated α-gal nanoparticles. No such binding is observed with α-gal nanoparticles that lack the coating anti-Gal antibody (not shown). This Fc/FcR interaction was assumed to generate transmembrane signal(s) that activates these macrophages to produce various cytokines known to promote wound healing, as described in step #4 in Fig. 1A. Cytokines displaying increased production in the activated macrophages included VEGF, FGF, IL1, PDGF, and CSF.28 An example for elevated cytokine production is that of VEGF secreted by macrophages and measured in the culture medium (Fig. 2D). Macrophages coincubated with α-gal nanoparticles that were not coated with anti-Gal secreted VEGF at a level similar to that of macrophages incubated in the absence of these nanoparticles. However, macrophages incubated with anti-Gal-coated α-gal nanoparticles displayed a much higher secretion of VEGF already within 24 h of incubation.
α-Gal Nanoparticle Effects on Wound Healing in Gt-Ko Mice
The ability of α-gal nanoparticle-recruited macrophages to accelerate wound healing was studied in anti-Gal-producing GT-KO mice. Oval, full-thickness skin wounds (∼6 × 9 mm) were formed under anesthesia in the dorsal region of the mouse flank. Wounds were covered with spot bandage dressing coated with 10 mg α-gal nanoparticles, nanoparticles lacking α-gal epitopes (from GT-KO pig RBC), or with saline. Inspection of wounds on day 6 revealed 95–100% healing (i.e., regeneration of epidermis),28 as shown in the representative example in Fig. 3B, D, whereas wounds treated with nanoparticles lacking α-gal epitopes or with saline displayed <20% of surface healing at that time (Fig. 3A, C). Healing of saline-treated wounds was observed in these mice only after 12–14 days.28 Overall, there was 60–70% decrease in healing time in comparison with saline-treated wounds.28 Histological evaluation of wounds further indicated that the processes of vascularization, fibroblast migration, and collagen deposition in the dermis also are accelerated in wounds treated with α-gal nanoparticles in comparison with saline-treated wounds.28 The studies on α-gal nanoparticle-treated wounds raise the possibility that the observed accelerated vascularization and migration of fibroblasts, as well as the faster regrowth of the epidermis over the wound, are all associated with increased concentration of cytokines produced by α-gal nanoparticle interaction with the recruited macrophages.28
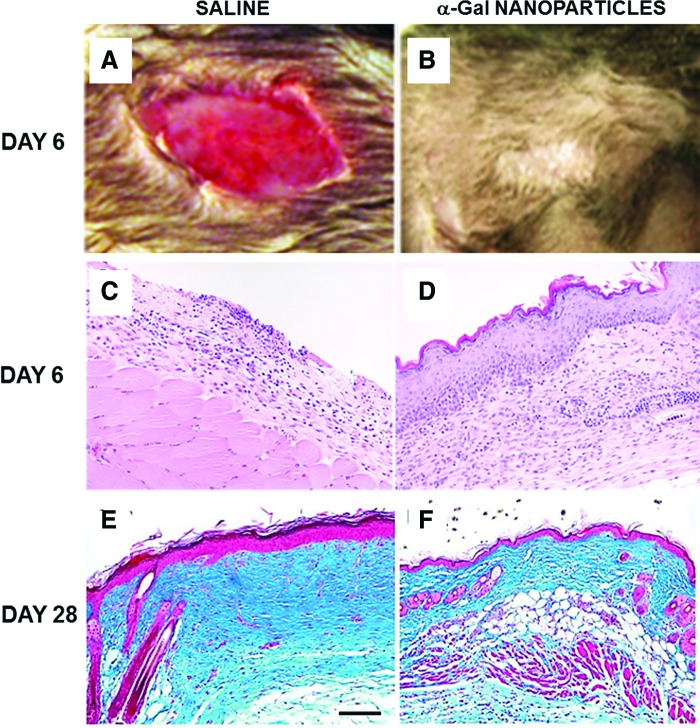
Figure 3.
Healing of representative excisional wounds in anti-Gal-producing GT-KO mice treated with α-gal nanoparticles. (A, B) Gross appearance of a day 6 wound (9 × 6 mm) treated with saline (A) or with 10 mg saline α-gal nanoparticles (B). Whereas the saline-treated wounds do not display healing, α-gal nanoparticle-treated wounds are covered with regenerating epidermis (representative mice of n = 20/group). (C, D) Histology of the wounds in (A, B). Saline-treated wounds display no healing, whereas α-gal nanoparticle-treated wounds are covered with regenerating epidermis (representative mice of n = 20/group, H&E 100 × ). (E, F) Healed wounds 28 days post-treatment. Saline-treated wounds (E) display distinct fibrosis and scar formation, characterized by multiple fibroblasts, extensive deposits of collagen (stained blue), few blood vessels, no skin appendages, and hypertrophic epidermis. Bar represents 100 μm. Wounds treated with α-gal nanoparticles (F) display restoration of normal skin structure with loose connective tissue, skin appendages such as hair shafts and sebaceous glands, and thin epidermis (Trichrome staining, representative mice of n = 5/group, 200 × ) (modified from Ref.28). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
α-Gal nanoparticles also have a long-term effect on healed wound. Physiologic healing in saline-treated wounds is characterized by distinct fibrosis and scar formation, as the default healing process in large injuries. Trichrome staining of such wounds on day 28 demonstrates the formation of dense fibrotic tissue in the wound dermis, no skin appendage regrowth, relatively poor vascularization, and hyperplasia of the epidermis (Fig. 3E). In contrast, wounds treated with α-gal nanoparticles displayed restoration of the normal histology in most of the healed wounds, including loose connective tissue in the dermis, regrowth of skin appendages such as hair shafts and sebaceous glands, fat tissue and smooth muscle in the hypodermis, and normal thin epidermis (Fig. 3F).28 These differences suggest that healing in α-gal nanoparticle-treated wounds restores normal structure of the injured skin before onset of the fibrosis process and scar formation. Fibrosis and scar formation are also the causes for irreversible damage in the course of healing of internal injuries, such as surgical incisions, ischemic heart muscle following myocardial infarction, and severed nerves. It would be of interest to determine whether such damage may be avoided by administration of α-gal nanoparticles to these injuries to induce accelerated repair and regeneration of the normal tissue before initiation of the fibrosis default process.
Healing of Burns in Gt-Ko Mice
Studies on anti-Gal-mediated accelerated healing of burns in GT-KO mice preceded those of wound healing and were performed with α-gal liposomes, which have the same structure as α-gal nanoparticles, but are ∼5–10-fold larger.27 α-Gal liposomes are made of one or several lipid bilayers studded with multiple α-gal glycolipids presenting α-gal epitopes. Subsequent conversion into smaller α-gal nanoparticles by additional sonication was performed in wound healing studies because the nanoparticle preparations can be sterilized by filtration through 0.2-μm filters. In addition, α-gal nanoparticles were found to be somewhat more effective in wound healing than α-gal liposomes, possibly because of better dispersion of the multiple small nanoparticles in wounds.28
Thermal injuries (∼2 × 3 mm) in the shaven skin were performed under anesthesia by a brief touch with the heated end of a metal spatula. This resulted in skin damage comparable to a second-degree burn in humans, in which the epidermis and approximately half of the upper region of the dermis are injured. Burn injuries in anti-Gal-producing GT-KO mice were treated with spot bandages coated with 10 mg α-gal liposomes or with saline. The healing of α-gal liposome-treated burns was twice as fast as that of saline-treated burns.27 Accelerated migration of macrophages and neutrophils was observed already by day 3 in burns treated with α-gal liposomes (Fig. 4B) in comparison with saline-treated burns (Fig. 4A). Wounds treated with α-gal liposomes displayed complete regeneration of the epidermis, including formation of stratum corneum by day 6, as shown in the representative example in Fig. 4D, whereas no significant regeneration of epidermis was observed in saline-treated wounds (Fig. 4C). Similar studies in wild-type mice synthesizing autologous α-gal epitopes and lacking anti-Gal antibody demonstrated no acceleration in healing following α-gal liposome treatment,27 suggesting that the observed acceleration in the healing process is dependent on anti-Gal interaction with α-gal epitopes. These studies on burn healing further suggest that the mechanism described in Fig. 1A for accelerated healing of wounds treated with α-gal nanoparticles is likely to mediate accelerated healing of burns as well.
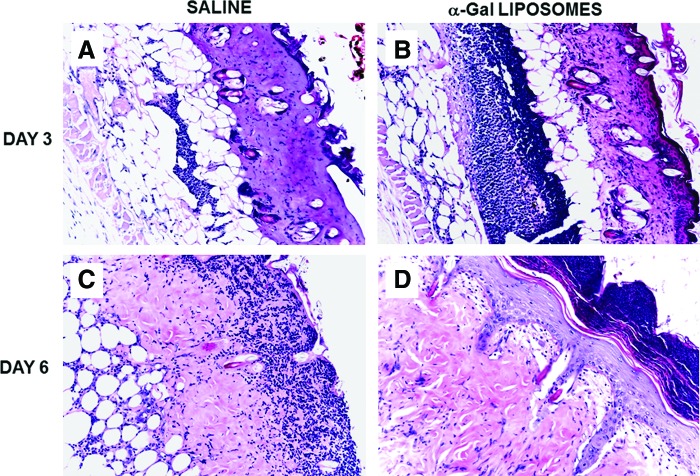
Figure 4.
Healing of burns in GT-KO mice treated with α-gal liposomes. (A, B) Representative second-degree burns (∼2 × 3 mm) in an anti-Gal-producing GT-KO mouse treated with a spot bandage covered with saline (A) or with 10 mg α-gal liposomes (B) and studied after 3 days. Note that the number of macrophages and neutrophils recruited in the injured dermis of the α-gal liposome-treated burn is >5-fold higher than that in the saline-treated burn. (C, D) Wounds, as in (A, B), studied after 6 days. The burn treated with α-gal liposomes (D) is covered with the regenerating epidermis, including stratum corneum, and the debris of recruited macrophages and neutrophils is found above the intact epidermis. The saline-treated burn (C) lacks regenerating epidermis and displays macrophages and neutrophils within the dermis migrating toward the surface of the wound. Each burn pair is from the same representative mouse of five mice/time point (modified from Ref.27, H&E staining, 200 × ). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Healing of Wounds in Gt-Ko Pigs
As indicated above, GT-KO mice and GT-KO pigs are the only mammals that can serve as nonprimate experimental models capable of producing anti-Gal. Thus, the study of wound healing following α-gal nanoparticle treatment was also performed in GT-KO pigs. Studies in pigs were performed to determine whether the observations described above in GT-KO mice can be validated in wounds of a large animal model in which the skin has a histological structure similar to that in humans.29 Analyses of natural anti-Gal antibody activity in GT-KO pig serum samples indicated that this antibody displays characteristics similar to those of human anti-Gal, in that it readily binds to α-gal nanoparticles and activates the pig complement cascade.43
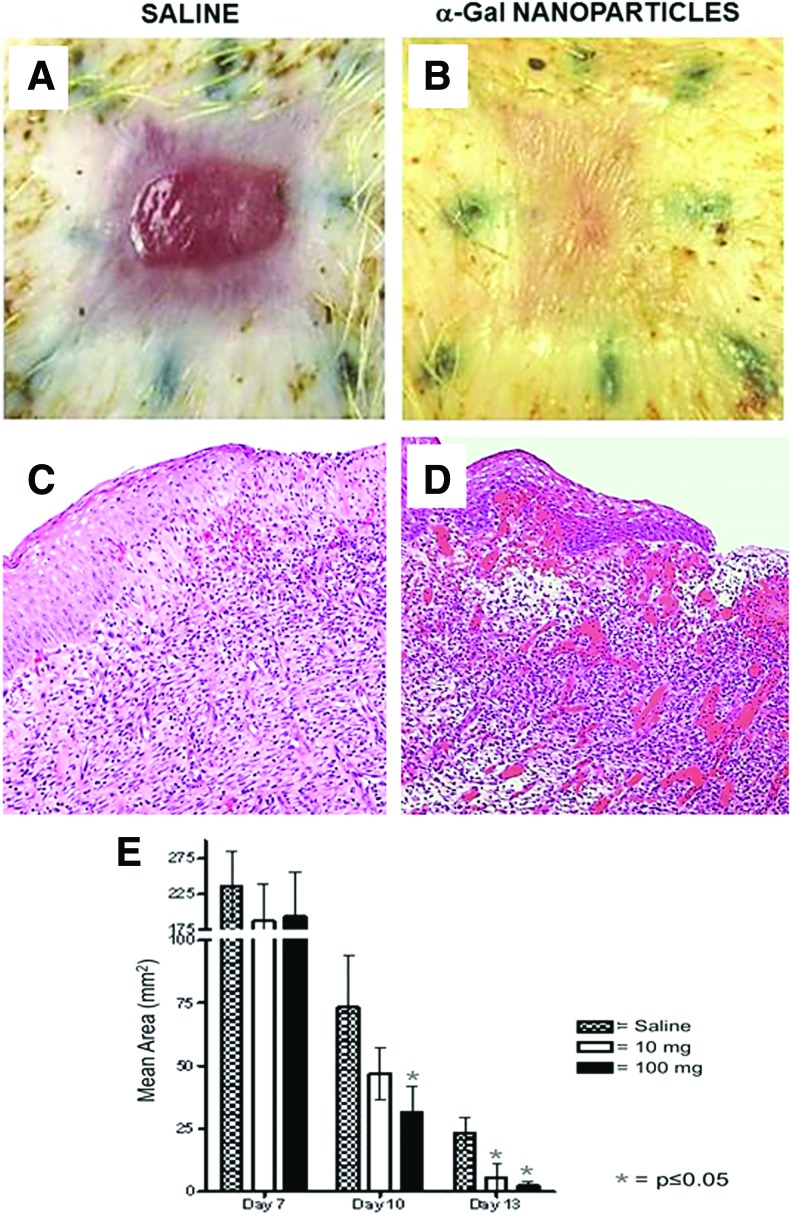
Excisional 20 × 20 mm square wounds (∼3 mm deep) were formed on the back of 3-month-old GT-KO pigs under anesthesia. Borders of the wounds were marked by tattooed dots before wounding. Wounds were covered with dressing coated with 100 mg α-gal nanoparticles, 10 mg α-gal nanoparticles, or saline.29 Treated pigs were euthanized and wound healing was evaluated by gross appearance and histology. No granulation tissue was observed on day 3; however, on day 7, all wounds were filled with granulation tissue. Although wound size (defined as area of the open wound, not covered by regenerating epidermis) was not significantly different on day 7 (Fig. 5E), wounds treated with α-gal nanoparticles contained many more macrophages than saline-treated wounds and deposits of collagen could be observed in the α-gal nanoparticle-treated wounds, but not in saline-treated wounds.29 Measurements on day 10 indicated that wounds treated with 100 and 10 mg α-gal nanoparticles were ∼60% (p < 0.05) and ∼35% smaller than saline-treated wounds, respectively (Fig. 5E).
Figure 5.
Healing of wounds in GT-KO pigs treated with α-gal nanoparticles. (A, B) Excisional wounds (20 × 20 mm and ∼3 mm deep) in a representative GT-KO pig of eight pigs treated with wound dressing covered with saline or with 100 mg α-gal nanoparticles. The figure shows a representative pair of wounds from the same pig and inspected on day 13 post-treatment. Saline-treated wound (A) displays partial regrowth of the epidermis, whereas α-gal nanoparticle-treated wound (B) is covered with regenerating epidermis. No differences are observed in wound contraction indicated by the elongated shape of the tattooed dots around the wounds. (C, D) Histology of the granulation tissue under the leading front of the regenerating epidermis in saline- (C) and α-gal nanoparticle-treated wounds (D), in which the wound is almost completely covered with regenerating epidermis. There are many more blood vessels and recruited cells in α-gal nanoparticle-treated wounds than in saline-treated wounds (representative pigs of n = 8, H&E 100 × ). (E) Size of wounds measured as area not covered by regenerating epidermis in GT-KO pig wounds treated with 100 or 10 mg α-gal nanoparticles or with saline at 7, 10, or 13 days. Mean ± SD from nine pigs on day 7 and eight pigs on days 10 and 13 (modified from Ref.29). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The greatest differences between α-gal nanoparticles and saline-treated wounds were observed on day 13. Regeneration of epidermis was observed also in saline-treated wounds, in which the open wound area was ∼25 mm2 (i.e., 0.5 × 0.5 cm) (Fig. 5). However, many of the wounds treated with 100 mg α-gal nanoparticles were completely covered by regenerating epidermis (Fig. 5B). On average, wounds treated with 100 and 10 mg α-gal nanoparticles had on day 13 ∼ 90% and 80% smaller noncovered areas, respectively, than saline-treated wounds (p < 0.05) (Fig. 5E). The data in Fig. 5E further suggest that the treatment of wounds displayed some extent of dose dependence, in that wounds treated with 10 mg α-gal nanoparticles healed faster than saline-treated wounds, but slower than wounds treated with 100 mg of these nanoparticles. However, the extent of wound contraction (marked by stretching of tattooed dots) in α-gal nanoparticle-treated wounds was similar to that in saline-treated wounds (Fig. 5A, B).29 Full healing of saline-treated wounds was observed on days 18–22 (not shown). Thus, healing time of wounds treated with 100 mg α-gal nanoparticles was shortened by ∼40%. No local or systemic toxicity responses were observed in any of the treated pigs, including histologic specimens of the pig kidneys and myocardium.29 Monitoring the wounds for 60 days revealed no keloid formation in both α-gal nanoparticle- and saline-treated wounds. Interestingly, the regenerating dermis in α-gal nanoparticle-treated wounds displayed the presence of skin appendages such as hair.29
Histological analysis of day 13 wounds, in comparable areas of the epidermis regenerating front, demonstrated many more blood vessels and cells with macrophage morphology in α-gal nanoparticle-treated wounds than in saline-treated wounds (Fig. 5D, C respectively). The increased vascularization of wounds treated with α-gal nanoparticles may be associated with elevated concentration of VEGF within the granulation tissue produced by the many activated macrophages that bound the anti-Gal-coated α-gal nanoparticles, as observed in GT-KO mice (Fig. 2D).28 Overall, these observations suggest that accelerated healing observed in GT-KO mouse wounds treated with α-gal nanoparticles also can be demonstrated in GT-KO pig wounds. Therefore, it may be of interest to determine the effects of α-gal nanoparticle treatment also on healing of wounds and burns in humans.
A possible limitation, as yet unproven, for α-gal nanoparticle treatment of injuries in humans is associated with the production of anti-Gal IgE in a very small proportion of the population.46–48 Individuals producing anti-Gal IgE are usually those who are allergic to beef, pork, and lamb meat, all containing very high concentrations of α-gal epitopes.19 It may be possible that topical application of α-gal nanoparticles on wounds and burns could result in an allergic skin reaction. A skin prick test with α-gal nanoparticles before treatment may enable identification of individuals allergic to α-gal epitopes.
Wound Healing in Diabetic Gt-Ko Mice
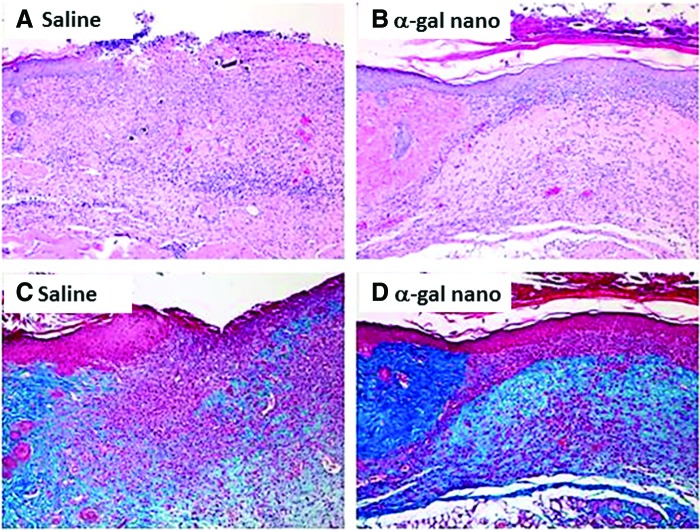
A relatively common disease in which wound healing is impaired is diabetes. It is well established that impairment in wound healing in diabetes is associated with deficient recruitment and activation of macrophages in wounds and in lower concentrations of cytokines, which normally are required for healing and regeneration of wounds.49,50 Thus, it was of interest to determine whether α-gal nanoparticles can affect wound healing in diabetic mice. This was addressed in preliminary studies in GT-KO mice that became diabetic following five daily intraperitoneal injections of streptozotocin (50 mg/kg). Mice that were hyperglycemic for >1 month were subjected to wounding, as in Fig. 3A, and then treated with α-gal nanoparticles or with saline on wound dressing. No indication of epidermis regeneration was observed in saline-treated wounds after 12 days (Fig. 6A, C) or even after 1 month (not shown). In contrast, α-gal nanoparticle-treated wounds displayed complete regeneration of the epidermis within 12 days post-treatment (Fig. 6B, D), suggesting that the recruitment and activation of macrophages by α-gal nanoparticles may overcome the obstacles in macrophage migration and activation in diabetes. These preliminary observations further suggest that it may be of interest to study α-gal nanoparticle therapy as a possible approach for inducing wound healing in patients with impaired wound healing such as diabetic and elderly individuals.
Figure 6.
Wound healing in streptozotocin-induced diabetic mice treated with α-gal nanoparticles. (A) Control wound treated for 12 days with saline. No significant regenerating epidermis (H&E). (B) A wound treated for 12 days with 10 mg α-gal nanoparticles. The border of the wound (wound bed) is indicated by the intact dermis in the left area. The wound is completely covered with regenerating epidermis (H&E). (C, D) Trichrome staining of sections (A) and (B), respectively. The collagen in the wound bed is stained in the left area as deep blue. Representative mice of five diabetic mice/group (100 × ). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Summary and Future Directions
Wound and burn healing may be accelerated by the use of α-gal nanoparticles, which harness the immunologic potential of the natural anti-Gal antibody, the most abundant antibody in humans constituting ∼1% of immunoglobulins. Binding of anti-Gal to the multiple α-gal epitopes on α-gal nanoparticles applied to injuries activates the complement cascade that generates complement cleavage chemotactic peptides, which induce rapid migration of macrophages into the injury. Studies in knockout mice and pigs lacking α-gal epitopes and producing anti-Gal demonstrated binding of anti-Gal-coated α-gal nanoparticles to macrophages through Fc/Fc receptor interaction and activation of such macrophages to secrete various cytokines. Topical application of α-gal nanoparticles to wounds in these experimental animal models resulted in accelerated healing of wounds and burns and decrease of healing time by ∼40–60%. This healing of wounds in anti-Gal-producing mice treated with α-gal nanoparticles occurs before the onset of fibrosis, thus a scar is avoided. Physiologic repair and regeneration mechanisms in wounds also function in internal injuries. Thus, it may be of interest to determine whether application of α-gal nanoparticles to internal injured tissues, such as surgical incisions, ischemic heart muscle, and severed nerves, can induce tissue regeneration and prevent fibrosis of the injured tissue. Preliminary studies in mice with streptozotocin-induced diabetes further suggest that α-gal nanoparticle wound therapy may induce healing of chronic wounds, which fail to heal under physiologic conditions. The α-gal nanoparticles are highly stable for years in suspension or in a dried state on wound dressing. Thus, application of these nanoparticles to injuries may be feasible in the form of dried nanoparticles on wound dressings and as suspensions, aerosols, hydrogels, water-based ointment, or incorporated into sheets of biodegradable scaffold materials such as collagen.
Take-Home Messages.
• The interaction between anti-Gal, which is the most abundant natural antibody in humans, and its carbohydrate antigen, the α-gal epitope, may be harnessed for promoting wound and burn healing.
• Studies in mice and pigs producing the anti-Gal antibody demonstrated that application of nanoparticles presenting multiple α-gal epitopes (α-gal nanoparticles) to wounds and burns results in acceleration of the healing process to the extent that healing time decreases by ∼40–60%.
• α-Gal nanoparticles applied to injuries bind anti-Gal and activate the complement system, which generates chemotactic factors that induce rapid recruitment of macrophages. In mice, the accelerated healing following α-gal nanoparticle treatment occurs before the onset of fibrosis, thereby scar formation may be avoided.
• Recruited macrophages, binding anti-Gal-coated α-gal nanoparticles through Fc/Fc receptor interaction, are further activated to secrete cytokines that accelerate the healing process as well as induce healing of wounds in diabetic mice.
• In view of the abundance of the natural anti-Gal antibody in humans, it would be of interest to evaluate safety and efficacy of α-gal nanoparticle therapy in clinical trials on healing of wounds and burns in healthy individuals and in patients with impaired wound healing mechanisms such as diabetic patients and elderly individuals.
Abbreviations and Acronyms
- α-gal epitope
Galα1-3Galβ1-4GlcNAc-R carbohydrate epitope
- α1,3GT
α1,3-galactosyltransferase
- α-gal nanoparticles
submicroscopic liposomes presenting multiple α-gal epitopes
- GT-KO mice or pigs
knockout mice or pigs for the α1,3-galactosyltransferase gene that lack α-gal epitopes and can produce the anti-Gal antibody
- PVA
polyvinyl alcohol
- RBC
red blood cell
Acknowledgments and Funding Sources
The studies described in this review were partially funded by an intramural fund at University of Massachusetts Medical School. No extramural funding was used. Animal experimentation: all studies with mice and pigs were approved by the IACUC committee at UMass Medical School.
Author Disclosure and Ghostwriting
The author has no conflict of interests in the studies described in this review. The article was written by the author with no ghostwriting.
About the Author
Uri Galili, PhD, received his PhD degree in Immunology in 1977 at the Hebrew University School of Medicine, Jerusalem, Israel. He did his postdoctoral research in the Department of Tumor Biology at the Karolinska Institute, Stockholm, Sweden (1977). Subsequently, he performed his research at Hadassah Medical Center, Jerusalem, Israel (1979–1984), where he discovered the natural anti-Gal antibody as the most abundant natural antibody in humans. He continued in 1984 his research in the United States as professor at the University of California Medical Center, San Francisco, CA, where he identified the α-gal epitope as the antigen recognized by anti-Gal and studied the molecular basis for the evolution of the natural anti-Gal antibody and the α-gal epitope in mammals. At MCP Hahnemann School of Medicine, Philadelphia, PA (1991), he studied the significance of anti-Gal/α-gal epitope interaction in mediating hyperacute rejection of pig xenografts and initiated studies on harnessing the immunologic potential of anti-Gal in cancer immunotherapy and in amplification of immune response to microbial vaccines. Subsequently, at Rush Medical School, Chicago, IL (1999), he developed methods for preventing anti-Gal production by inducing immune tolerance to the α-gal epitope. After moving to UMass Medical School, Worcester, MA (2004), he developed a method for in situ conversion of tumors into autologous vaccines targeted to antigen-presenting cells by intratumoral injection of α-gal glycolipids and performed clinical trials with this novel immunotherapy. At UMass Medical School, he further developed the α-gal nanoparticles, which enable harnessing of anti-Gal for induction of accelerated wound and burn healing and for induction of tissue repair and regeneration in internal injuries. Dr. Galili retired in 2013 and presently volunteers as scientific researcher at Rush Medical School, Chicago, IL.
References
- 1.DiPietro LA, Koh TJ. Macrophages and wound healing. Adv Wound Care 2016;2:71–75 [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 4.Piccolo MT, Wang Y, Sannomiya P, et al. Chemotactic mediator requirements in lung injury following skin burns in rats. Exp Mol Pathol 1999;66:220–226 [DOI] [PubMed] [Google Scholar]
- 5.Low QE, Drugea IA, Duffner LA, et al. Wound healing in MIP-1α(-/-) and MCP-1(-/-) mice. Am J Pathol 2001;159:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich SA, Messingham KA, Gregory MS, et al. Elevated monocyte chemoattractant protein-1 levels following thermal injury precede monocyte recruitment to the wound site and are controlled, in part, by tumor necrosis factor-α. Wound Repair Regen 2003;11:110–119 [DOI] [PubMed] [Google Scholar]
- 7.Snyderman R, Pike M. 1984. Chemoattractant receptors on phagocytic cells. Annu Rev Immunol 2003;2:257–281 [DOI] [PubMed] [Google Scholar]
- 8.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J Exp Med 1984;160:1519–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013;140:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu PB, Holzknecht ZE, Bruno D, Parker W, Platt JL. Modulation of natural IgM binding and complement activation by natural IgG antibodies. J Immunol 1996;157:5163–5168 [PubMed] [Google Scholar]
- 11.Parker W, Lin SS, Yu PB, et al. Naturally occurring anti-α-galactosyl antibodies: relationship to xenoreactive anti-α-galactosyl antibodies. Glycobiology 1999;9:865–873 [DOI] [PubMed] [Google Scholar]
- 12.Teranishi K, Manez R, Awwad M, Cooper DK. Anti-Gal α1-3Gal IgM and IgG antibody levels in sera of humans and old world non-human primates. Xenotransplantation 2002;9:148–154 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Anaraki F, Henion TR, Galili U. Variations in activity of the human natural anti-Gal antibody in young and elderly populations. J Gerontol (Med Sci). 1995;50A:M227–M233 [DOI] [PubMed] [Google Scholar]
- 14.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffis JM. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun 1988;56:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posekany KJ, Pittman HK, Bradfield JF, Haisch CE, Verbanac KM. Induction of cytolytic anti-Gal antibodies in α-1,3-galactosyltransferase gene knockout mice by oral inoculation with Escherichia coli O86:B7 bacteria. Infect Immun 2002;70:6215–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galili U, Macher BA, Buehler J, Shohet SB. Human natural anti-α-galactosyl IgG. II. The specific recognition of α(1–3)-linked galactose residues. J Exp Med 1985;162:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macher BA, Galili U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochem Biophys Acta 2008;1780:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the anti-Gal antibody and the Galα1 → 3Gal epitope in primates. Proc Natl Acad Sci USA 1987;84:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galili U, Shohet SB, Kobrin E, Stults CLM, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J Biol Chem 1988;263:17755–17762 [PubMed] [Google Scholar]
- 20.Oriol R, Candelier JJ, Taniguchi S, et al. Major carbohydrate epitopes in tissues of domestic and African wild animals of potential interest for xenotransplantation research. Xenotransplantation 1999;6:79–89 [DOI] [PubMed] [Google Scholar]
- 21.Good AH, Cooper DK, Malcolm AJ, et al. Identification of carbohydrate structures which bind human anti-porcine antibodies: implication for discordant xenografting in man. Transplant Proc 1992;24:559–562 [PubMed] [Google Scholar]
- 22.Galili U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: a major obstacle for xenotranplantation in humans. Immunol Today 1993;14:480–482 [DOI] [PubMed] [Google Scholar]
- 23.Sandrin MS, McKenzie IF. Galα(1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunol Rev 1994;141:169–190 [DOI] [PubMed] [Google Scholar]
- 24.Collins BH, Cotterell AH, McCurry KR, et al. Cardiac xenografts between primate species provide evidence of the α-galactosyl determinant in hyperacute rejection. J Immunol 1994;154:5500–5510 [PubMed] [Google Scholar]
- 25.Simon PM, Neethling FA, Taniguchi S, et al. Intravenous infusion of Galα1-3Gal oligosaccharides in baboon delays hyperacute rejection of porcine heart xenografts. Transplantation 1998;56:346–353 [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Lorf T, Sablinski T, et al. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galα1-3Galβ14Glc-R immunoaffinity column. Transplantation 1998;65:172–179 [DOI] [PubMed] [Google Scholar]
- 27.Galili U, Wigglesworth K, Abdel-Motal UM. Accelerated healing of skin burns by anti-Gal/α-gal liposomes interaction. Burns 2010;36:239–251 [DOI] [PubMed] [Google Scholar]
- 28.Wigglesworth K, Racki WJ, Mishra R, Szomolanyi-Tsuda E, Greiner DL, Galili U. Rapid recruitment and activation of macrophages by anti-Gal/α-gal liposome interaction accelerates wound healing. J Immunol 2011;186:4422–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz Z, Ignotz R, Lalikos J, Galili U. Accelerated porcine wound healing with α-Gal nanoparticles. Plast Reconst Surg 2012;129:242–251 [DOI] [PubMed] [Google Scholar]
- 30.Eto T, Iichikawa Y, Nishimura K, Ando S, Yamakawa T. Chemistry of lipids of the posthemolytic residue or stroma of erythrocytes. XVI. Occurance of ceramide pentasaccharide in the membrane of erythrocytes and reticulocytes in rabbit. J Biochem (Tokyo) 1968;64:205–213 [DOI] [PubMed] [Google Scholar]
- 31.Stellner K, Saito H, Hakomori S. Determination of aminosugar linkage in glycolipids by methylation. Aminosugar linkage of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys 1973;133:464–472 [DOI] [PubMed] [Google Scholar]
- 32.Dabrowski U, Hanfland P, Egge H, Kuhn S, Dabrowski J. Immunochemistry of I/i-active oligo- and polyglycosylceramides from rabbit erythrocyte membranes. Determination of branching patterns of a ceramide pentadecasaccharide by 1H nuclear magnetic resonance. J Biol Chem 1984;259:7648–7651 [PubMed] [Google Scholar]
- 33.Egge H, Kordowicz M, Peter-Katalinic J, Hanfland P. Immunochemistry of I/i-active oligo- and polyglycosylceramides from rabbit erythrocyte membranes. J Biol Chem 1985;260:4927–4935 [PubMed] [Google Scholar]
- 34.Hanfland P, Kordowicz M, Peter-Katalinić J, Egge H, Dabrowski J, Dabrowski U. Structure elucidation of blood group B-like and I-active ceramide eicosa- and pentacosasaccharides from rabbit erythrocyte membranes by combined gas chromatography-mass spectrometry; electron-impact and fast-atom-bombardment mass spectrometry; and two-dimensional correlated, relayed-coherence transfer, and nuclear Overhauser effect 500-MHz 1H-n.m.r. spectroscopy. Carbohydr Res 1988;178:1–21 [DOI] [PubMed] [Google Scholar]
- 35.Ogawa H, and Galili U. Profiling terminal N-acetyllactoamines of glycans on mammalian cells by an immuno-enzymatic assay. Glycoconjugate J 2006;23:663–674 [DOI] [PubMed] [Google Scholar]
- 36.Galili U, Wigglesworth K, Abdel-Motal UM. Intratumoral injection of α-gal glycolipids induces xenograft-like destruction and conversion of lesions into endogenous vaccines. J Immunol 2007;178:4676–4687 [DOI] [PubMed] [Google Scholar]
- 37.Thall AD, Maly P, Lowe JB. Oocyte Gal α1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem 1995;270:21437–21440 [DOI] [PubMed] [Google Scholar]
- 38.Tearle RG, Tange MJ, Zannettino ZL, et al. The α-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation 1996;61:13–19 [DOI] [PubMed] [Google Scholar]
- 39.Lai L, Kolber-Simonds D, Park KW. et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002;295:1089–1092 [DOI] [PubMed] [Google Scholar]
- 40.Phelps CJ, Koike C, Vaught TD, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science 2003;299:411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dor FJ, Tseng YL, Cheng J, et al. α1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation 2004;78:15–20 [DOI] [PubMed] [Google Scholar]
- 42.Fang J, Walters A, Hara H, et al. Anti-gal antibodies in α1,3-galactosyltransferase gene-knockout pigs. Xenotransplantation 2012;19:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galili U. α1,3Galactosyltransferase knockout pigs produce the natural anti-Gal antibody and simulate the evolutionary appearance of this antibody in primates. Xenotransplantation 2013;20:267–276 [DOI] [PubMed] [Google Scholar]
- 44.LaTemple DC, Galili U. Adult and neonatal anti-Gal response in knock-out mice for α-galactosyltransferase. Xenotransplantation 1998;5:191–196 [DOI] [PubMed] [Google Scholar]
- 45.Tanemura M, Yin D, Chong AS, Galili U. Differential immune response to α-gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation. J Clin Invest 2000;105:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1,3-galactose. J Allergy Clin Immunol 2009;123:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morisset M, Richard C, Astier C, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-α-1,3-galactose. Allergy 2012;67:699–704 [DOI] [PubMed] [Google Scholar]
- 48.Nunez R, Carballada F, Gonzalez-Quintela A, Gomez-Rial J, Boquete M, Vidal C. Delayed mammalian meat-induced anaphylaxis due to galactose-α-1,3-galactose in 5 European patients. J Allergy Clin Immunol 2011;128:1122–1124 [DOI] [PubMed] [Google Scholar]
- 49.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 50.Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular 2007;15:350–355 [DOI] [PubMed] [Google Scholar]