Abstract
Early induction of therapeutic hypothermia (TH) is recommended in out-of-hospital cardiac arrest (CA); however, currently no reliable methods exist to initiate cooling. We investigated the effect of high flow transnasal dry air on brain and body temperatures in adult porcine animals. Adult porcine animals (n = 23) under general anesthesia were subject to high flow of transnasal dry air. Mouth was kept open to create a unidirectional airflow, in through the nostrils and out through the mouth. Brain, internal jugular, and aortic temperatures were recorded. The effect of varying airflow rate and the air humidity (0% or 100%) on the temperature profiles were recorded. The degree of brain cooling was measured as the differential temperature from baseline. A 10-minute exposure of high flow dry air caused rapid cooling of brain and gradual cooling of the jugular and the aortic temperatures in all animals. The degree of brain cooling was flow dependent and significantly higher at higher airflow rates (0.8°C ± 0.3°C, 1.03°C ± 0.6°C, and 1.3°C ± 0.7°C for 20, 40, and 80 L, respectively, p < 0.05 for all comparisons). Air temperature had minimal effect on the brain cooling over 10 minutes with similar decrease in temperature at 4°C and 30°C. At a constant flow rate (40 LPM) and temperature, the degree of cooling over 10 minutes during dry air exposure was significantly higher compared to humid air (100% saturation) (1.22°C ± 0.35°C vs. 0.21°C ± 0.12°C, p < 0.001). High flow transnasal dry air causes flow dependent cooling of the brain and the core temperatures in intubated porcine animals. The mechanism of cooling appears to be evaporation of nasal mucus as cooling is mitigated by humidifying the air. This mechanism may be exploited to initiate TH in CA.
Keywords: : therapeutic hypothermia, cardiac arrest, survival, neuroprotection, out-of-hospital cardiac arrest
Background
Cardiac arrest (CA) is a leading cause of morbidity and mortality in the developed world. Resuscitation is attempted in an estimated 260,000 patients annually in the United States and 66/100,000 population every year in Europe (Zheng et al., 2001; The Hypothermia after Cardiac Arrest Study Group, 2002). Recovery without residual neurologic damage after CA with global cerebral ischemia is rare. Of the patients with CA who survive to hospital discharge, the majority are left with permanent neurological sequelae (Neumar et al., 2008; Yu et al., 2010). After CA with no blood flow for more than 5 minutes, the generation of free radicals during reperfusion, together with other mediators, incites chemical cascades that result in cerebral injury (Lyon et al., 2010).
Several studies have shown that moderate systemic hypothermia (30°C) or mild hypothermia (∼34°C) markedly mitigates brain damage after CA in dogs (Safar et al., 2000; Behringer et al., 2003; Kuboyama et al., 2003). The mechanism is thought to be a reduction in cerebral oxygen consumption among other multifactorial chemical and physical mechanisms during and after ischemia (Lyon et al., 2010). Two randomized clinical trials demonstrated the benefit of cooling survivors of witnessed CA who had ventricular fibrillation as the presenting rhythm (Holzer et al., 1997; Bernard et al., 2002). Based on these data, the American Heart Association and the European Resuscitation Council recommend therapeutic hypothermia (TH) in the management of unconscious patients following CA (Neumar et al., 2008: Nolan et al., 2008).
Data from recent randomized trials have raised questions on the depth of hypothermia needed for clinical benefit and on the use of large volume of cold saline for rapid induction of hypothermia (Yannopoulos et al., 2009). Despite several unanswered questions, the need for TH remains; however, there is no easy way to accomplish this in an out-of-hospital setting. Current methods for induction of TH include ice-cold saline IV, ice packs, cooling blankets (Bernard et al., 2002), and cooling helmets (Wang et al., 2004). Liquid cooling helmets have been used to cool the head and act by direct conductive cooling of the scalp. Although they are useful in children and neonates, their utility in adult patients is largely unknown. Furthermore, close contact with the scalp is required for adequate heat exchange, and closed-loop temperature control is not available.
Recently, RhinoChill device used transnasal perfluorocarbon (PFC) spray through bilateral nasal cannula to promote heat loss by evaporation of the PFC in the nasopharynx (Baile et al., 1987; Castrén et al., 2010). This device is portable and battery operated and has demonstrated hypothermia induction in patients after CA and in neurosurgical patients. The device is designed for induction and not for prolonged use as the PFC is expensive. Two liters of ice-cold saline at 4°C is easy to implement, yet it is ineffective at consistently inducing mild TH (∼34°C) (Bernard et al., 2003). The other methods result in cutaneous vasoconstriction and shivering that counteracts the cooling effect, rendering them inefficient means of inducing mild TH. As such there is a need for a hypothermia induction method that is easy to use and effective in out-of-hospital CA (Merchant et al., 2006).
The upper respiratory tract in mammals is very efficient at conditioning inspired air (before arrival at the lungs), that is, it can humidify dry air to full saturation very quickly (Jackson et al., 1963). This humidifying process requires energy to convert water in the body to a vapor that is then mixed with the incoming dry air. We hypothesized that the physiologic process of conditioning large volumes of air through the nasal passages can be harnessed to extract heat from the body thereby causing hypothermia.
Methods
The Institutional Animal Care and Use Committee of the Johns Hopkins University approved this study.
Animal preparation
Adult female pigs (wt. ∼36 kg) were premedicated with Ketamine 22 mg/kg, Sedazine 0.9 mg/kg, and Telazol 1.1 mg/kg IM. Following induction, the animals were transferred to a procedure table and kept warm with a heating blanket. Animals were then intubated, anesthetized with isoflurane 2.0%, and mechanically ventilated with 100% oxygen. The femoral artery was cannulated and a fiber optic temperature probe (Neoptix, Canada LP) was advanced to the origin of the common carotid artery under fluoroscopic guidance. The femoral vein was then cannulated and a second temperature probe was advanced to the internal jugular vein under fluoroscopic guidance. A parietal burr hole was created, and a micropuncture catheter was advanced into the right lateral cerebral ventricle as indicated by the return of cerebrospinal fluid. Through this catheter, a third temperature probe was advanced into the lateral ventricle for measurement of the brain temperature. Blood pressure, electrocardiogram (ECG), and temperature were recorded continuously for the duration of each experiment. Respiratory rate, end tidal CO2, and ECG were monitored continuously. Blood pressure and ECG data were recorded using PowerLab® Chart™ 7.0, ADInstruments, Bella Vista, Australia. Temperature probe signals were measured using Neoptix™ Reflex™ 4-channel signal conditioner and temperature data recorded using Neoptix OptiLink™, Neoptix® Canada LP.
Experimental protocol
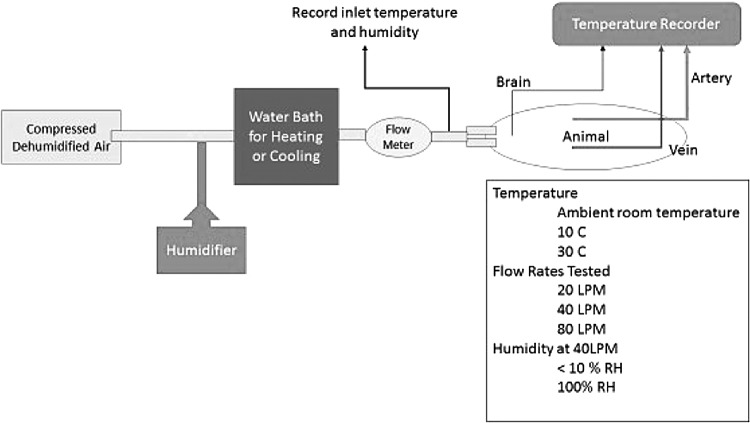
The mouth of the animal was kept open by a pair of retractors. A high flow nasal cannula was secured in the animal's nostrils for airflow delivery. Medical grade dry air was delivered to the nasal cannula at various flow rates and the change in venous, arterial, and brain temperatures was recorded. The detailed experimental setup and the protocol are shown in the Figure 1. Briefly, following the removal of the heating blanket, baseline temperatures were recorded for a 10-minute duration. Animals then received high flow air through a pair of high flow nasal cannula at a constant flow rate for a duration of 10 minutes. The animals were rewarmed with heating blankets applied to the torso for 30 minutes. Baseline temperatures were recorded following a 10-minute equilibration period, and the experiment was repeated varying the flow rates. The input temperature (carotid/aortic) and the output temperature (jugular vein) were monitored as a measure of the cooling capacity. The carotid temperature probe was placed at the origin of the carotid artery close to the aortic arch and represented the core arterial temperature. In 10 animals the airflow was continued at a flow rate of 80 LPM for 1 hour to assess the effect of prolonged flow on brain and core temperature. All animals were euthanized at the end of the protocol, and the mouth and the nasal cavity were examined for bleeding ulceration or desiccation.
FIG. 1.
Study protocol. Compressed dry air was delivered through a water bath for heating or cooling the air, and the flow was measured with a flow meter. A humidification circuit was connected in parallel to saturate the air to measure effect of humidification.
Delivery of airflow
Dry medical grade air was delivered to the animal's nostrils using a high flow nasal cannula. The mean temperature differential was measured as the difference between the venous, arterial, and brain probes after a 10-minute exposure from baseline. An airflow meter (TSI incorporated, MN), connected in series, measured the airflow rate with a response time of 4 minutes. The mouth of the animal was kept open with a mouth gag speculum to provide a path of least resistance through which the transnasal air could exit.
Air temperature and humidity
To determine the effect of air temperature, the air tubing was heated or cooled by passing it through a copper pipe in a water bath. The temperature of the water was controlled to provide the desired inlet temperature. A humidifier (Fisher Paykel, Auckland, New Zealand) was connected in series to the compressed airflow to humidify the air. The humidifier was used in the noninvasive mode to avoid additional heating of the air. The air temperature was kept constant at ∼30°C during dry and humid (0% or 100% humidity) air delivery by passing the air through heated or cooled copper tubing.
Statistical methods
Data are presented as mean ± SD. Mean differences in brain and body temperatures before and after cooling were assessed using analysis of variance test.
Results
A total of 68 experiments were conducted in 23 female porcine subjects. All animals were subject to the 10-minute exposure of transnasal dry airflow at varying flow rates. Each animal was subject to an average of three experiments with 30-minute rewarming and wash out period between experiments. The mean weight of the animals was 37 ± 7 kg. All animals tolerated the experimental procedures. Baseline temperatures were not statistically different between the brain, jugular, and carotid artery temperatures (36.8°C ± 1.2°C, 36.1°C ± 0.8°C, and 36.3°C ± 1.1°C, respectively, p = not significant (NS)). Mean heart rate was 96 ± 18 per minute, respiratory rate was 12 ± 2 per minute, and mean arterial pressure was 68 ± 16 mm Hg at baseline. Mean heart rate was 102 ± 12 per minute, respiratory rate was 12 ± 1 per minute, and mean arterial pressure was 60 ± 13 mm Hg at the end of the experiment. No measurable changes were observed in the heart rates, blood pressure, or oxygen saturations of the animals. Postmortem examination did not reveal any evidence of bleeding, trauma, or desiccation of the nasal or oral cavity.
Effect of airflow on brain and vascular temperatures
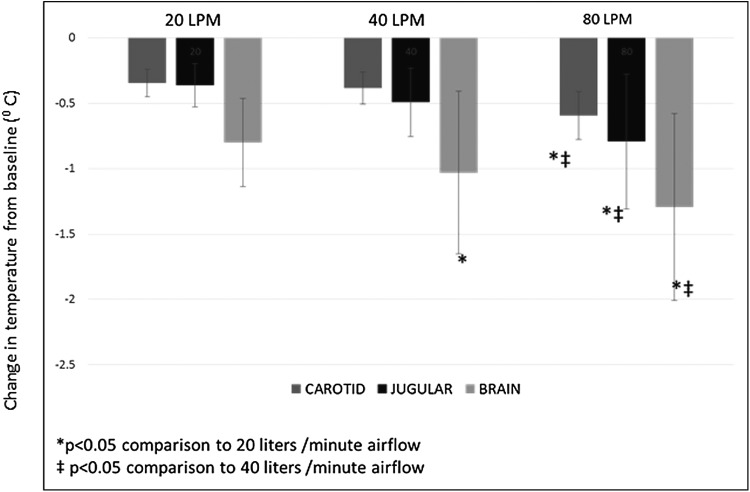
At airflow rates of 20 L/min, a 10-minute exposure to dry air caused the brain temperatures to uniformly decrease in all animals. This change was immediate and continued through the duration of airflow. The decrease in brain temperature over a 10-minute exposure was flow dependent and significantly higher at higher airflow rates (0.8°C ± 0.3°C, 1.03°C ± 0.6°C, 1.3°C ± 0.7°C for 20, 40 and 80 L respectively, p < 0.05 for all comparisons) (Fig. 2). Jugular vein cooling and systemic cooling in the carotid artery were significantly different only between 40 and 80 LPM (0.49°C ± 0.26°C vs. 0.79°C ± 0.5°C for jugular vein temperature and 0.38°C ± 0.12°C vs. 0.59°C ± 0.18°C for carotid temperature p < 0.05 for both).
FIG. 2.
Effect of airflow rate on brain, jugular vein, and carotid artery temperature. Shown in the figure is the decrease in brain, jugular, and carotid temperatures from baseline values over a 10-minute exposure to 20, 40, and 80 LPM. The decrease in brain temperature was airflow dependent with greater cooling at higher airflow rates. Rates of jugular and carotid cooling were also higher between flow rates of 40 and 80 LPM.
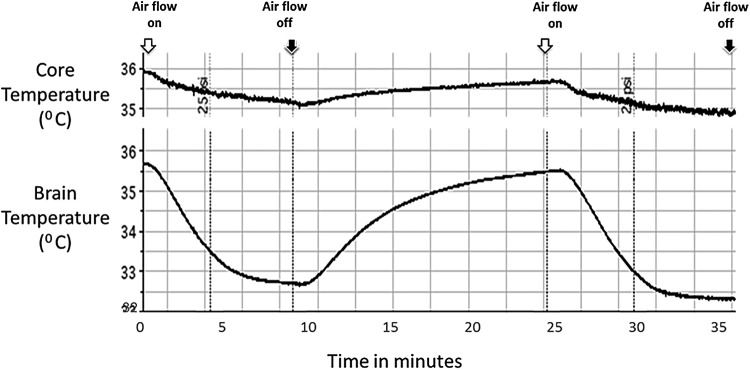
Figure 2 shows the effect of 10-minute exposure of 80 LPM dry air on brain and body temperature of a pig. Brain temperature decreased from 35.7°C to 32.6°C in 10 minutes and recovered rapidly following cessation of flow to 35.5°C within 10 minutes. During the same time, the core body temperature (right atrial temperature) decreased from 36°C to 35.1°C and showed a similar pattern of recovery on cessation of airflow. Reinstating the airflow as shown in Figure 3 consistently reproduced the same phenomenon. The overall change in brain temperature with 80 LPM of transnasal airflow was maximal in the first 10 minutes of airflow and was 0.3°C ± 0.1°C per minute for the first 10 minutes.
FIG. 3.
Shown in this figure is a representative example of induction and recovery of brain and core arterial temperature during 10 minutes of high flow dry gas at a flow rate of 80 LPM. White arrows denote the time the airflow was turned on and the black arrows denote turning off of airflow. Rapid decline in brain temperature from baseline is noted immediately after airflow is turned on, which recovers promptly once the flow is off. A more gradual response is noted in the core arterial temperature during the same time. The results were reproducible as shown in the figure.
Effect of prolonged exposure to high flow transnasal dry air
In 10 animals following completion of the 10-minute protocol, the animals were rewarmed and subject to high flow dry air for 1 hour. Shown in Figure 2 is the effect of longer durations of airflow on brain and body temperature. As shown in the figure, maximal decrease in brain temperature occurs in the first few minutes of the transnasal airflow followed by a plateau response. However, the animal continues to lose heat as evidenced by a gradual decline in the core body temperature overtime.
Effect of air temperature on rate of cooling
Effect of air temperature was assessed in five animals. Dry air (40 LPM, 0.3% relative humidity) at temperature of 4°C had a similar decrease in brain temperature compared with dry air at 30°C (1.3°C ± 0.10°C vs. 1.2°C ± 0.15°C, p = NS) over a 10-minute exposure.
Effect of air humidity on brain cooling
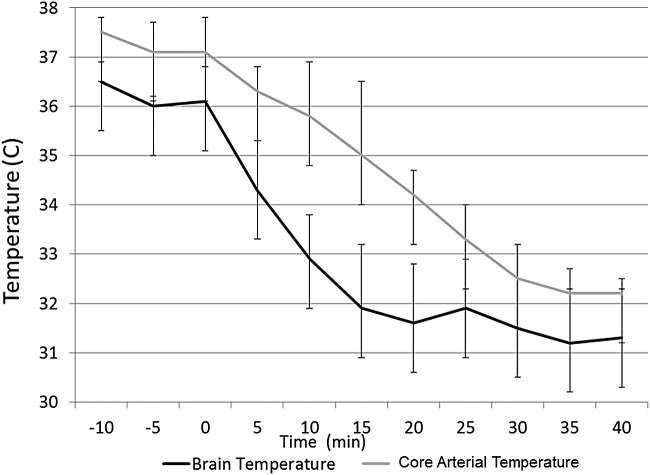
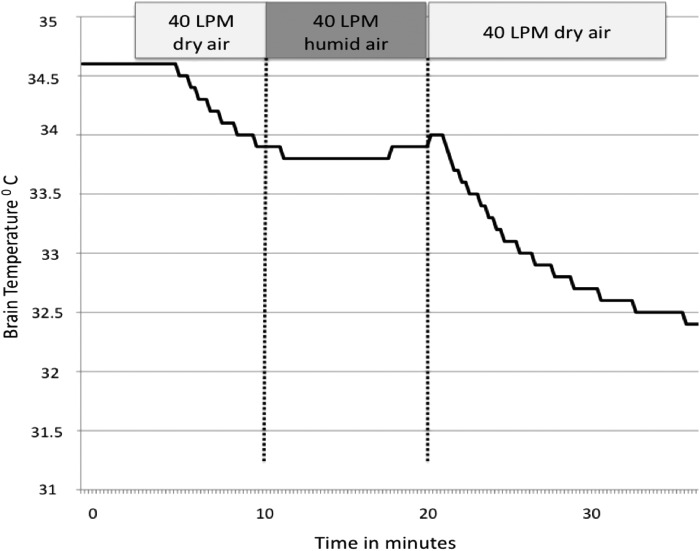
To test the hypothesis that dry air causes evaporative cooling, we tested the effect of high flow transnasal humid air (relative humidity ∼100%, air temperature 21°C) on brain temperature. In five animals dry air was initiated and switched to humid air after 10 minutes of cooling. Figure 4 shows the effect of humidifying the air. At a constant flow rate (40 LPM), the rate of cooling over 10 minutes during dry air exposure was significantly higher compared to humid air (1.22°C ± 0.35°C vs. 0.21°C ± 0.12°C, p < 0.001). Initiation of humid air abruptly slowed the decline in temperature, which resumed after reinstating dry air (Fig. 5).
FIG. 4.
Brain and body temperature during transnasal airflow of dry air at 80 L/min. Initial preferential decline in brain temperature is followed by more gradual decline in core body temperature.
FIG. 5.
Effect of humidity of inflow air on brain temperature. Ambient air at room temperature and a flow of 40 LPM was administered at 0% humidity and 100% humidity in sequence. Brain temperature decreased from 34.7°C to 33.8°C during exposure to dry air. The cooling effect was completely mitigated by humidifying the inflowing air at the same flow rate of 40 LPM (34.8°C to 34.9°C during 10-minute exposure to humid air). Cooling resumes once the dry air is turned back on in support of evaporation as the mechanism behind the cooling process.
Discussion
Our study reveals that transnasal dry air causes a flow dependent decrease in brain and body temperature of porcine subjects. This effect appears to be independent of the temperature of the inflowing air and humidifying the air can mitigate the effect. Furthermore, our results suggest evaporation of nasal mucosal water to be the possible mechanism behind the heat loss. Humidification of the inflowing air results in mitigation of cooling in support of this hypothesis. The effect of core temperature loss at prolonged flows also suggests that the cooling is systemic and probably mediated by nasal vascular cooling.
One of the important functions of the upper respiratory tract is to condition the inspired air before its arrival at the lungs (Liese et al., 1973, 1974; Cain et al., 1990). This conditioning involves heating the air to body temperature and humidifying the air to full saturation at that temperature. The air conditioning capacity of the nose protects the lower airways by warming and humidifying the air from ambient conditions ranging from temperatures of −42°C to 48°C, from 0% to 100% relative humidity (Mercke et al., 1974), and from 5 L/min to sustained flow rates of 20 to 30 L/min (Cole, 1953). As a consequence of conduction and evaporation, heat is lost from the nasal surfaces.
To accomplish the function of conditioning the air, the nose in mammals has highly convoluted turbinates with extensive vascularization with a distensible vascular network, seromucous glands, and goblet cells (Cole, 1953; Cole, 1998). The turbinates increase enormously the surface area and are richly vascular and capable of engorgement in response to environmental stimuli, especially cold and dry air stimulation (Baile et al., 1987; Hayes et al., 1995). Unidirectional flow of ambient air through the nose results in a 65% increase in nasal mucosal blood flow, which is mitigated by humidification and warming of the inspired air (Togias et al., 1985; Togias et al., 1988; Hayes et al., 1995). Moreover, warm dry air results in significantly increased upper airway blood flow compared with cold dry air hyperventilation. Respiratory water loss also follows a similar paradigm with a water loss of 0.66 ± 0.17 g H2O/min for cold dry air ventilation and 0.90 ± 0.16 g H2O/min for warm dry air hyperventilation (Baile et al., 1987). This water loss by the body is in fact heat loss due to the high latent heat of evaporation of water (2.27 kJ/g), which is provided by the nasal blood flow. Thus the rate of water loss by this process is directly associated with heat loss by the body, making humidification an excellent way to extract heat (Fig. 5).
The human nasal capacity for humidification is remarkable. In normal volunteers exposed to nasal dry air inhalation at 40 L/min, the expired air was found to be 100% saturated (Rouadi et al., 1999). Warm dry air inhalation is associated with a higher water gradient between the inspired and expired air compared to cold dry air due to the higher water carrying capacity of the warm air. Thus, this unique property of the nasal countercurrent heat exchanger can theoretically be harnessed to enhance heat loss from the upper airways in humans and as similar as in swines.
Although other investigators have tried cooling the body through the nasal passages using air/oxygen and observed the hypothermic effect, the mechanism behind the effect was not well appreciated (Boller et al., 2010; Busch et al., 2010). Einer-Jensen et al. flushed the nostrils of pigs with 20 L/min of oxygen and observed a modest decrease in brain temperature (1.5°C) (Einer-Jensen and Khorooshi, 2000; Einer-Jensen et al., 2001). They attributed the effect to specialized vasculature called “rete mirabile” and concluded that the brain temperature in pigs is partly independent of the body temperature. Similar observations have been made in intubated humans with low airflow of 17 L/min with brain temperature changes of ∼0.15°C. Recently, nasal PFC spray using the RhinoChill device has been shown to be effective in induction of TH in the out-of-hospital setting (Abou-Chebl et al., 2011). Evaporation of the PFC in the nose results in convective cooling of the brain and core body temperature. Interestingly, the PFC is evaporated by high flow of dry oxygen at similar flow rates used in our study. This device, however, carries a risk of aspiration of the liquid PFC and absorption in the blood stream (Harris et al., 2016). The study, however, provides proof of principle that whole body and brain cooling are possible using a transnasal approach.
Our study reveals the mechanism behind the cooling effect of high flow dry air. Air temperature had no appreciable effect, and the effect was mitigated by humidification of the inflowing air. Higher airflow rates were associated with higher rates of cooling, and the relative humidity of the outflowing air was close to 100% suggesting significant water extraction, in support of our hypothesis that the cooling is mediated by water evaporation.
Finally, at least in the porcine model, transnasal cooling appears to directly influence brain temperature due to the proximity of the brain to the nasal turbinates. This process in the pigs appears to selectively cool the brain before cooling the body and, thus, also provides a unique model to study the effects of selective brain cooling compared to whole-body cooling using this noninvasive approach. However, the additional core cooling during the transnasal dry air process suggests that this local cooling is being transmitted to the remainder of the body by convective mechanisms through the circulating blood thereby suggesting a role for this method in mammals lacking specialized vascular structures for selective brain cooling.
Limitations: While the change in the degree of cooling was dependent on the humidity of the air, we were only able to test the extremes (0% relative humidity and 100% relative humidity) as it was very difficult to precisely control partial humidification (50% relative humidity) using our humidifier. Cooling steps lasted for 10-minute intervals only as maximal cooling effect on the brain was reached at this time point. We did not attempt to maintain this cooling response or the temperature differential in these animals. Longer duration of cooling was performed in 10 animals, which resulted in profound core hypothermia over 1 hour period, and it was very difficult to rewarm the animals back to normothermia and this is responsible for differences in brain and arterial temperatures at baseline.
Conclusion
In conclusion, we report a novel, noninvasive hypothermia induction method that utilizes the physiologic process of nasal conditioning to extract heat from the body without any adverse consequences. This method appears to cause preferential brain hypothermia, which might be desirable as whole-body hypothermia is associated with a host of side effects. Further proof of concept experiments is warranted to investigate the plausibility of better brain cooling control using this technique.
Author Disclosure Statement
No competing financial interests exist.
References
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004;109:2786–2791 [DOI] [PubMed] [Google Scholar]
- Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the Rhino Chill device: preliminary safety data in brain-injured patients. Stroke 2011;42:2164–2169 [DOI] [PubMed] [Google Scholar]
- Baile EM, Dahlby RW, Wiggs BR, Parsons GH, Pare PD. Effect of cold and warm dry air hyperventilation on canine airway blood flow. J Appl Physiol 1987;62:526–532 [DOI] [PubMed] [Google Scholar]
- Behringer W, Safar P, Wu X, Kentner R, Radovsky A, Kochanek PM, Dixon CE, Tisherman SA. Survival without brain damage after clinical death of 60–120 mins in dogs using suspended animation by profound hypothermia. Crit Care Med 2003;31:1523–1531 [DOI] [PubMed] [Google Scholar]
- Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 2003;56:9–13 [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Boller M, Lampe JW, Katz JM, Barbut D, Becker LB. Feasibility of intra-arrest hypothermia induction: a novel nasopharyngeal approach achieves preferential brain cooling. Resuscitation 2010;81:1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch HJ, Eichwede F, Fodisch M, Taccone FS, Wobker G, Schwab T, Hopf HB, Tonner P, Hachimi-Idrissi S, Martens P, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation 2010;81:943–949 [DOI] [PubMed] [Google Scholar]
- Cain JB, Livingstone SD, Nolan RW, Keefe AA. Respiratory heat loss during work at various ambient temperatures. Respir Physiol 1990;79:145–150 [DOI] [PubMed] [Google Scholar]
- Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, Eichwede F, Mols P, Schwab T, Vergnion M, Storm C, Pesenti A, Pachl J, Guérisse F, Elste T, Roessler M, Fritz H, Durnez P, Busch HJ, Inderbitzen B, Barbut D. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC Intranasal Cooling Effectiveness). Circulation 2010;122:729–736 [DOI] [PubMed] [Google Scholar]
- Cole P. Further observations on the conditioning of respiratory air. J Laryngol Otol 1953;67:669–681 [DOI] [PubMed] [Google Scholar]
- Cole P. Physiology of the nose and paranasal sinuses. Clin Rev Allergy Immunol 1998;16:25–54 [DOI] [PubMed] [Google Scholar]
- Einer-Jensen N, Khorooshi MH. Cooling of the brain through oxygen flushing of the nasal cavities in intubated rats: an alternative model for treatment of brain injury. Exp Brain Res 2000;130:244–247 [DOI] [PubMed] [Google Scholar]
- Einer-Jensen N, Khorooshi MH, Petersen MB, Svendsen P. Rapid brain cooling in intubated pigs through nasal flushing with oxygen: prevention of brain hyperthermia. Acta Vet Scand 2001;42:459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Bansbach J, Dietrich I, Kalbhenn J, Schmutz A. RhinoChill®-more than an ice-cream headache (1) serious adverse event transnasal evaporative cooling. Resuscitation 2016;103:e5–e6 [DOI] [PubMed] [Google Scholar]
- Hayes MJ, McGregor FB, Roberts DN, Schroter RC, Pride NB. Continuous nasal positive airway pressure with a mouth leak: effect on nasal mucosal blood flux and nasal geometry. Thorax 1995;50:1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer M, Behringer W, Schorkhuber W, Zeiner A, Sterz F, Laggner AN, Frass M, Siostrozonek P, Ratheiser K, Kaff A. Mild hypothermia and outcome after CPR. Hypothermia for Cardiac Arrest (HACA) Study Group. Acta Anaesthesiol Scand Suppl 1997;111:55–58 [PubMed] [Google Scholar]
- Jackson DC, Schmidt-Nielsen K. Countercurrent heat exchange in the respiratory passages. Proc Natl Acad Sci U S A 1964;51:1192–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993;21:1348–1358 [DOI] [PubMed] [Google Scholar]
- Liese W, Joshi R, Cumming G. Humidification of respired gas by nasal mucosa. Ann Otol Rhinol Laryngol 1973;82:330–332 [DOI] [PubMed] [Google Scholar]
- Liese W, Warwick WJ, Cumming G. Water vapour pressure in expired air. Respiration 1974;31:252–261 [DOI] [PubMed] [Google Scholar]
- Lyon RM, Robertson CE, Clegg GR. Therapeutic hypothermia in the emergency department following out-of-hospital cardiac arrest. Emerg Med J 2010;27:418–423 [DOI] [PubMed] [Google Scholar]
- Merchant RM, Soar J, Skrifvars MB, Silfvast T, Edelson DP, Ahmad F, Huang KN, Khan M, Vanden Hoek TL, Becker LB, et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med 2006;34:1935–1940 [DOI] [PubMed] [Google Scholar]
- Mercke U, Hakansson CH, Toremalm NG. The influence of temperature on mucociliary activity. Temperature range 20 degrees C-40 degrees C. Acta Otolaryngol 1974;78:444–450 [DOI] [PubMed] [Google Scholar]
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–2483 [DOI] [PubMed] [Google Scholar]
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008;79:350–379 [DOI] [PubMed] [Google Scholar]
- Rouadi P, Baroody FM, Abbott D, Naureckas E, Solway J, Naclerio RM. A technique to measure the ability of the human nose to warm and humidify air. J Appl Physiol 1999;87:400–406 [DOI] [PubMed] [Google Scholar]
- Safar P, Tisherman SA, Behringer W, Capone A, Prueckner S, Radovsky A, Stezoski WS, Woods RJ. Suspended animation for delayed resuscitation from prolonged cardiac arrest that is unresuscitable by standard cardiopulmonary-cerebral resuscitation. Crit Care Med 2000;28:N214–N218 [DOI] [PubMed] [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Togias AG, Naclerio RM, Proud D, Fish JE, Adkinson NF, Jr., Kagey-Sobotka A, Norman PS, Lichtenstein LM. Nasal challenge with cold, dry air results in release of inflammatory mediators. Possible mast cell involvement. J Clin Invest 1985;76:1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togias AG, Proud D, Lichtenstein LM, Adams GK, 3rd, Norman PS, Kagey-Sobotka A, Naclerio RM. The osmolality of nasal secretions increases when inflammatory mediators are released in response to inhalation of cold, dry air. Am Rev Respir Dis 1988;137:625–629 [DOI] [PubMed] [Google Scholar]
- Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honnings D, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurgery 2004;100:272–277 [DOI] [PubMed] [Google Scholar]
- Yannopoulos D, Zviman M, Castro V, Kolandaivelu A, Ranjan R, Wilson RF, Halperin HR. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation 2009;120:1426–1435 [DOI] [PubMed] [Google Scholar]
- Yu T, Barbut D, Ristagno G, Cho JH, Sun S, Li Y, Weil MH, Tang W. Survival and neurological outcomes after nasopharyngeal cooling or peripheral vein cold saline infusion initiated during cardiopulmonary resuscitation in a porcine model of prolonged cardiac arrest. Crit Care Med 2010;38:916–921 [DOI] [PubMed] [Google Scholar]
- Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158–2163 [DOI] [PubMed] [Google Scholar]