Abstract
In mammals and birds, long episodes of nondreaming sleep (“slow-wave” sleep, SW) are followed by short episodes of dreaming sleep (“rapid-eye-movement” sleep, REM). Both SW and REM sleep have been shown to be important for the consolidation of newly acquired memories, but the underlying mechanisms remain elusive. Here we review electrophysiological and molecular data suggesting that SW and REM sleep play distinct and complementary roles on memory consolidation: While postacquisition neuronal reverberation depends mainly on SW sleep episodes, transcriptional events able to promote long-lasting memory storage are only triggered during ensuing REM sleep. We also discuss evidence that the wake-sleep cycle promotes a postsynaptic propagation of memory traces away from the neural sites responsible for initial encoding. Taken together, our results suggest that basic molecular and cellular mechanisms underlie the reverberation, storage, and propagation of memory traces during sleep. We propose that these three processes alone may account for several important properties of memory consolidation over time, such as deeper memory encoding within the cerebral cortex, incremental learning several nights after memory acquisition, and progressive hippocampal disengagement.
In mammals and birds, long episodes of nondreaming sleep (“slow-wave” sleep, SW) are followed by short episodes of dreaming sleep (“rapid-eye-movement” sleep, REM) (Aserinsky and Kleitman 1953; Dement and Kleitman 1957a,b; Dement 1958; Jouvet et al. 1959; Roffwarg et al. 1962; Tradardi 1966; Jouvet 1967; Rechtschaffen and Kales 1968; Ayala-Guerrero et al. 2003). Despite early insight (Jenkins and Dallenbach 1924), it was not until the 1970s that science began to recognize the key role of sleep in memory consolidation. The main findings supporting this view are the detrimental effects of sleep deprivation on learning (Pearlman 1969, 1973; Leconte and Bloch 1970; Fishbein 1971; Pearlman and Becker 1974; Linden et al. 1975; Shiromani et al. 1979; Smith and Butler 1982; Smith and Kelly 1988; Smith and MacNeill 1993; Karni et al. 1994; Smith and Rose 1996; Stickgold et al. 2000a; Walker et al. 2002; Maquet et al. 2003; Mednick et al. 2003), the improved memory retention in rats when REM sleep is enhanced (Wetzel et al. 2003), the increase in sleep amounts following memory acquisition (Lucero 1970; Leconte and Hennevin 1971; Fishbein et al. 1974; Smith et al. 1974, 1980; Smith and Lapp 1986, 1991; Smith and Wong 1991), and the fact that theta rhythm, a learning-related (Adey et al. 1960; Elazar and Adey 1967; Landfield et al. 1972; Bennett 1973; Bennett et al. 1973; Winson 1978; Sederberg et al. 2003) hippocampal oscillation typical of high arousal (Green and Arduini 1954; Brown 1968; Sainsbury 1970; Harper 1971; Arnolds et al. 1980; Stewart and Fox 1991; Kahana et al. 1999), also characterizes REM sleep (Vanderwolf 1969; Timo-Iaria et al. 1970; Winson 1974; Cantero et al. 2003). Given the involvement of the hippocampus in memory acquisition (Scoville and Milner 1957; Mishkin 1978; Kesner and Novak 1982; Buzsaki et al. 1990; Zola-Morgan and Squire 1990; Squire 1992; Kim et al. 1995; Corkin et al. 1997; Izquierdo and Medina 1997; Bontempi et al. 1999; Lavenex and Amaral 2000; Haist et al. 2001; Winocur et al. 2001), these results indicated that sleep is a privileged off-line window for the processing of novel and ecologically relevant information (Bryson and Schacher 1969; Winson 1972, 1985, 1990, 1993).
The chase for the mechanisms underlying the mnemonic role of sleep produced two major spearheading findings: (1) neuronal firing rates observed during waking (WK) experience recur in the hippocampus during ensuing SW and REM sleep (Pavlides and Winson 1989), and (2) the blockade of protein synthesis during sleep impairs memory acquisition (Gutwein et al. 1980). The persistence of increased neuronal activity immediately after a stimulus is a widespread phenomenon that likely arises from hardwired neuronal circuit loops (Lorente de Nó 1938) but also, and more pertinent to the issue of learning, from pregenomic biochemical changes (Wang 2001) able to cause activity-dependent synaptic modification and long-lasting learning via de novo protein synthesis (Agranoff et al. 1966; Bliss and Collingridge 1993; Lamprecht and LeDoux 2004). The two pioneering studies (Gutwein et al. 1980; Pavlides and Winson 1989) suggested that sleep harbored both mechanisms postulated by Donald Hebb to be necessary and sufficient to explain memory consolidation: postacquisition neuronal reverberation, and structural synaptic plasticity (Hebb 1949).
Experience-dependent neuronal reverberation during SW sleep
Exploration of the first lead was prolific: Postacquisition neuronal reverberation during sleep or quiet WK was found to preserve the temporal firing relationships of alert, exploratory WK in the hippocampus (Wilson and McNaughton 1994; Skaggs and McNaughton 1996; Nadasdy et al. 1999; Poe et al. 2000; Hirase et al. 2001; Louie and Wilson 2001; Lee and Wilson 2002) and the cerebral cortex (Qin et al. 1997; Hoffman and McNaughton 2002), causing a correlated replay of activity patterns across two-neuron (Wilson and McNaughton 1994) or many-neuron ensembles (Louie and Wilson 2001). To this date, experience-dependent brain reactivation during sleep has been observed in rodents (Pavlides and Winson 1989; Wilson and McNaughton 1994; Skaggs and McNaughton 1996; Qin et al. 1997; Nadasdy et al. 1999; Hirase et al. 2001; Louie and Wilson 2001; Lee and Wilson 2002), nonhuman primates (Hoffman and McNaughton 2002), humans (Maquet et al. 2000), and even songbirds (Dave and Margoliash 2000), pointing to a very general biological phenomenon. Finally and most importantly, postacquisition brain reactivation during sleep has been shown to be proportional to memory acquisition in rats (Gerrard 2002) and humans (Peigneux et al. 2003), and to quantitatively predict learning (Datta 2000; Maquet et al. 2003).
In spite of the positive evidence, the neural reverberation hypothesis for memory consolidation during sleep still faces several objections. First, the neocortical reverberation detected to this date is extremely subtle and decays rapidly within <1 h of memory trace formation (Qin et al. 1997; Hoffman and McNaughton 2002). Such short-lived reverberation falls short of explaining the disruption of memory traces by sleep deprivation several hours and even days after initial acquisition (Pearlman 1969, 1973; Leconte and Bloch 1970; Fishbein 1971; Pearlman and Becker 1974; Linden et al. 1975; Shiromani et al. 1979; Smith and Butler 1982; Smith and Kelly 1988; Smith and MacNeill 1993; Karni et al. 1994; Smith and Rose 1996; Stickgold et al. 2000a; Maquet et al. 2003; Mednick et al. 2003). Second, strictu sensu neuronal reverberation during mammalian sleep has only been investigated in the hippocampo-cortical loop (Pavlides and Winson 1989; Wilson and McNaughton 1994; Skaggs and McNaughton 1996; Qin et al. 1997; Nadasdy et al. 1999; Hirase et al. 2001; Louie and Wilson 2001; Hoffman and McNaughton 2002; Lee and Wilson 2002), making it difficult to determine whether the phenomenon is particular to this neural circuit or whether it represents global experience-dependent changes in the brain. Third, neuronal reverberation has mostly been observed in highly trained animal subjects (Wilson and McNaughton 1994; Skaggs and McNaughton 1996; Qin et al. 1997; Nadasdy et al. 1999; Dave and Margoliash 2000; Louie and Wilson 2001; Hoffman and McNaughton 2002; Lee and Wilson 2002), raising skepticism about its relevance for the acquisition and consolidation of novel information (Kudrimoti et al. 1999). Finally, experience-dependent neuronal reverberation has been reported to occur in all behavioral states (Pavlides and Winson 1989; Wilson and McNaughton 1994; Skaggs and McNaughton 1996; Qin et al. 1997; Louie and Wilson 2001; Lee and Wilson 2002), including WK (Nadasdy et al. 1999; Hirase et al. 2001; Hoffman and McNaughton 2002). Although the first finding in this regard has hinted at a possible predominance of reverberation during SW sleep (Pavlides and Winson 1989), a comprehensive comparison of the relative contributions of WK and SW and REM sleep for neuronal reverberation is still missing.
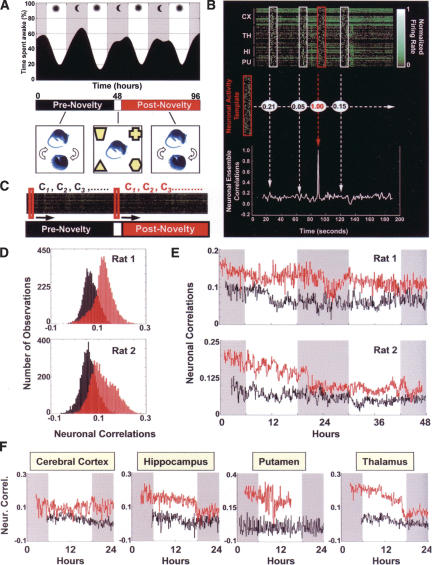
In order to address these objections, we set out to investigate the effects of a transient novel tactile experience on the long-term evolution of ongoing brain activity across the major behavioral states of the rat (Ribeiro et al. 2004). We simultaneously recorded the extracellular neuronal activity of 100-150 neurons per animal and local field potentials (LFPs) from four different forebrain regions: hippocampus (HP), primary somatosensory “barrel field” cortex (CX), ventral posteromedial thalamic nucleus (TH), and dorsal putamen (PU). Neural signals were continuously recorded across the natural sleep-wake cycle for 48-96 h, with a single 1-h exposure to four complex objects placed in the four corners of the recording box (Fig. 1A). The objects were strictly novel to the subjects and were presented half-way through the recording time around midnight (lights off), when WK reached a peak and the drive for whisker-based tactile exploration of the environment was greatest. This paradigm, designed to maximize novelty induced neuronal changes (as opposed to changes caused by behavioral overtraining), strongly increased WK relative to sleep during the exposure time and led to robust novel sensory stimulation.
Figure 1.
(A) Experimental design. (Top) A representative example of the strong circadian dynamics of the rat sleep-wake cycle. Grey bands indicate lights-off, and white bands indicate lights on; notice the fixed 12-h periods of darkness and light. (Bottom) Animals continuously recorded for up to 96 h were kept undisturbed except for a 1-h period of novel sensory stimulation (white segment) produced by the tactile exploration of four distinct novel objects placed at the corners of the recording box. Our paradigm produced marked and acute exploratory behavior without disrupting the large-scale sleep-wake structure across the many hours of recording. (B) Neuronal ensemble correlation method. Neuronal activity templates (red boxes) were compared with extensive recordings of neuronal action potentials (top, green ticks) by way of an off-line template-matching algorithm (Louie and Wilson 2001) that generalizes the notion of pair-wise correlations to neuronal ensembles of any size. Templates and targets (white boxes) were binned, firing-rate normalized, and correlated (middle). This procedure yields a time series of neuronal ensemble correlations for each template-target sliding match (bottom). (C) Templates of interest (9-sec-long, red boxes) were sampled around the origin of pre- and postnovelty periods during alert WK, and slid against their corresponding neuronal targets so as to sample neuronal correlations every 30 sec for up to 48 h. (D) Two representative examples show that postnovelty neuronal correlations (red) were significantly larger (right-shifted, P < 0.05) than prenovelty correlations (black). (E) The temporal profiles of multiple-area neuronal ensemble correlations indicate that large-scale neuronal firing patterns generated during the exploration of novel objects recur for several hours after the reference experience (postnovelty, red), while firing patterns associated with the walls of the recording box are substantially less detectable over time (prenovelty, black). Grey bands indicate lights-off, and white bands indicate lights on. (F) Long-lasting neuronal reverberation occurs in the cerebral cortex, hippocampus, putamen, and thalamus. Shown are temporal profiles of neuronal ensemble correlations calculated for single areas (all panels correspond to the same animal except PU).
To investigate the long-term effects of novel stimulation on the spatiotemporal evolution of ongoing neuronal activity, we took advantage of a neuronal ensemble correlation method previously shown to detect experience-dependent reactivation of rodent hippocampal ensembles during REM sleep (Louie and Wilson 2001). This method generalizes the concept of pairwise neuronal correlations (Wilson and McNaughton 1994; Qin et al. 1997; Hoffman and McNaughton 2002) to an arbitrarily large number of neurons, quantifying the degree of similarity between spatiotemporal patterns of neuronal activity by way of a firing-rate-normalized template-matching algorithm (Fig. 1B). Templates of alert WK neuronal ensemble activity were selected from moments when animals made whisker contact with the novel objects. Control templates were selected from epochs of alert WK 24 h (three rats) or 48 h (two rats) before novel stimulation, during which familiar tactile stimulation was produced by the contact of whiskers with the smooth walls of the recording box, to which animals were habituated. Templates were matched against the entire record of neuronal activity using the neuronal ensemble correlation method (Fig. 1C). The resulting correlation temporal profiles were averaged, aligned with reference to the light/darkness cycle to control for possible circadian effects, and compared.
First, we tested whether the neuronal ensemble correlation method could detect in our data set any trace of increased neuronal reverberation after exposure to the novel stimuli. For this we examined correlation profiles obtained for all recorded neurons (three to four brain areas pooled together) in each animal. As shown for two different animals in Figure 1D, postnovelty average correlation distributions were significantly right-shifted relative to prenovelty distributions, indicating that neuronal firing patterns concomitant with novel stimulation persisted significantly more during the ensuing time than did patterns sampled 24-48 h before novel stimulation, when animals were in the same behavioral state (alert WK) but without novel objects to explore. We then assessed whether the neuronal ensemble correlation method could detect neuronal reverberation lasting at least >1 h after exposure to novel stimulation. Figure 1E shows the temporal evolution of neuronal ensemble correlations for two different animals. Despite the marked interanimal variability in the shapes and magnitudes of these profiles, a significant and sustained increase of neuronal ensemble correlations after exposure to novel stimulation was observed in all animals. Importantly, these increases lasted well above 1 h.
In order to assess the anatomical distribution of experience-dependent neuronal reverberation, we performed the neuronal ensemble correlation analysis for each area separately. Significant changes between pre- and postnovelty correlations, indicative of neuronal reverberation, were observed in all areas studied for up to 48 h after exposure to novel stimulation (Fig. 1F). These results indicate that the tactile, gustatory, olfactory, spatial, and motor activities produced by the free exploration of novel objects engage multiple forebrain structures in widespread neuronal reverberation. Interestingly, we found that enhanced neuronal reverberation (post > precorrelations) is not the only kind of experience-dependent change possible. Antireverberation, consisting of patterns of activity that were statistically more dissimilar from novel stimulation templates than expected by chance, occurred in the HP (one of four rats), PU (two of four rats), and TH (one of five rats) but not in the CX (Ribeiro et al. 2004). In principle, the novelty-induced reverberation and antireverberation of neuronal firing patterns could play balancing roles in the delineation of new memory traces, embossing high and low relieves in the forebrain synaptic landscape where memories are encoded.
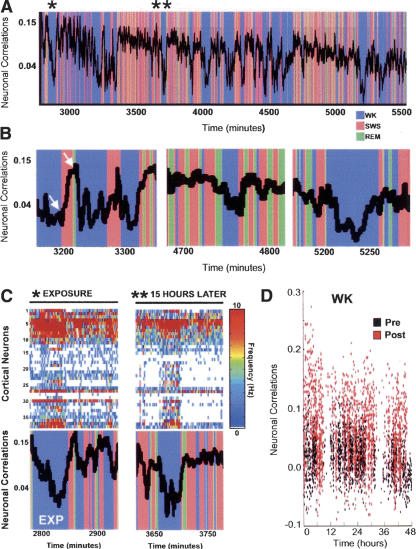
Next, we investigated how experience-dependent changes in neuronal ensemble correlations varied across the three major rat behavioral states: WK, SW sleep, or REM sleep. We found that neuronal reverberation consistently increases during SW sleep and decreases during WK, while REM sleep showed variable results across animals. A superimposition of behavioral state classification and neuronal ensemble correlations (Fig. 2A) revealed an exquisite long-term temporal match between SW episodes (red) and epochs of increased neuronal ensemble correlations in the CX. Likewise, neuronal ensemble correlation troughs show a tight correspondence with WK episodes (blue). In this example, such characteristic state-dependency persisted throughout 45 h of postnovelty recording (Fig. 2B). The fact that neuronal reverberation is sustained for long epochs during SW sleep suggests that unconsolidated synaptic changes may be not only recalled but also amplified over time during SW sleep. Indeed, a progressive increase of neuronal ensemble correlations across single SW sleep episodes was often observed (Fig. 2B, white arrows).
Figure 2.
(A) Neuronal reverberation is strongest during SW sleep. Shown is a superimposition of successive neuronal ensemble correlations and concurrent behavioral states for neocortical neurons, illustrating the state-dependency of neuronal ensemble correlations. Nearly all correlation peaks correspond to SW episodes, while almost all troughs match WK epochs. REM sleep showed SW-like increased neuronal correlations in only one out of five animals (depicted here). REM correlations in the remaining animals were either closer to WK than to SW levels, or well in between (Ribeiro et al. 2004). (B) State-dependent neuronal reverberation was sustained throughout the recording period, as shown by segments representing the beginning (3200-3300 min), middle (4700-4800 min), and end (5200-5250 min) of the experimental record. (Left) Notice the progressive increase of neuronal correlations across a single SW sleep episode (white arrows), suggesting a progressive amplification of the memory trace. (C) Blow-up of two selected data segments indicated by asterisks in A. Despite having been sampled from moments of high neuronal firing rates (*), novel stimulation templates reverberate most strongly during SW sleep when firing rates are low (* and **). The high firing rates that characterize WK correspond to decreased neuronal reverberation, probably due to sensory interference. (D) Postnovelty neuronal ensemble correlations decrease during WK but do not reach prenovelty levels. This indicates that novel experience causes sustained neuronal reverberation (Hebb 1949) rather than discrete reactivation (Wilson and McNaughton 1994; Kudrimoti et al. 1999), in the sense that traces of a given salient experience are continuously detectable during subsequent periods across all behavioral states, in a state-dependent manner.
In order to assess the contributions of different neurons to total ensemble correlations, we ran the correlation analysis on a neuron-by-neuron basis. We found that no one subset of neurons was particularly responsible for the reverberation effect, as the contribution of individual neurons was highly variable in time and showed no state-dependence (data not shown). This indicates that the neuronal changes caused by novelty were highly distributed through the neuronal populations sampled.
Interestingly, a comparison of the ensemble correlation temporal profile (Fig. 2C, bottom panels) with the concurrent neuronal firing record (Fig. 2C, top panels) reveals that SW correlation peaks correspond to periods of decreased firing rate, while WK correlation troughs match epochs of increased neuronal activity. Thus, although novel stimulation templates of neuronal activity were selected from WK episodes characterized by high firing rates, ensuing reverberation of these neuronal firing patterns was most pronounced during SW sleep, in a regime of lower firing rates. It should be noted, however, that neuronal ensemble reverberation decreased but did not disappear during WK (Fig. 2D), in agreement with the original findings of poststimulus changes in hippocampal firing rates (Pavlides and Winson 1989) and a more recent investigation of the same issue (Hirase et al. 2001). The inverse correlation between neuronal ensemble correlations and concurrent firing rates suggests that reverberating patterns of neuronal activity associated with past novel experience are largely—but not completely—masked during WK by incoming sensory inputs unrelated to the reference experience. By the same token, peak neuronal ensemble correlations arise during SW sleep, when sensory interference ceases. These observations corroborate the notion that the importance of sleep for memory consolidation stems from the off-line processing of memory traces, i.e., from the absence of sensory interference (Jenkins and Dallenbach 1924; Melton and Irwin 1940; Winson 1985). The consistent increase in neuronal reverberation during SW sleep, the high interanimal variability of neuronal reverberation during REM sleep, and the small contribution of REM sleep to total sleep time suggest a major role for SW sleep in the postacquisition recall of new memory traces. The function of experience-dependent brain reactivation during REM sleep (Pavlides and Winson 1989; Maquet et al. 2000; Louie and Wilson 2001; Peigneux et al. 2003) remains to be explained. One attractive possibility yet to be tested is that neuronal reverberation during REM sleep, being “noisier” than that of SW sleep, may facilitate memory trace restructuring and insight generation during sleep (Wagner et al. 2004).
In summary, our results (Ribeiro et al. 2004) establish that a poststimulus reverberation of neuronal ensemble firing patterns occurs in rats completely naive with respect to the reference stimuli, directly contradicting the notion that only the performance of highly trained behaviors is followed by neuronal reverberation (Kudrimoti et al. 1999). The new data also demonstrate that sustained experience-dependent neuronal reverberation can be detected in several forebrain areas up to 48 h after exposure to novel stimulation, suggesting that neuronal reverberation is a general property of cortical and subcortical forebrain circuits, such as the thalamus and the dorsal striatum. More recent evidence of neuronal reverberation in the ventral striatum supports this conclusion (Pennartz et al. 2004). Finally, we found strong evidence that neuronal reverberation is state-dependent and peaks during SW sleep in inverse correlation with firing rates. Taken together, the results provide evidence of reverberatory processes compatible with the memory impairment effects of sleep deprivation applied hours or days after training (Fishbein 1971; Pearlman and Becker 1974; Smith and Butler 1982; Smith and Kelly 1988; Karni et al. 1994; Stickgold et al. 2000a; Fenn et al. 2003). In conclusion, novelty-induced neuronal reverberation during SW sleep is capable of implementing the first mnemonic function postulated by Hebb (1949).
Long-term storage of synaptic changes during REM sleep
Action on the second Hebbian front, i.e., the search for a molecular link between plasticity-related protein synthesis and neural activity during sleep, was spurred by the discovery of inducible transcription factors that couple neuronal depolarization to gene regulation (Morgan and Curran 1989). The hypothesis was clear-cut: At least some of these immediate-early genes (IEGs) should be up-regulated during sleep. The first shot belonged to an Italian team, which compared forebrain IEG expression after several hours of sustained WK or sleep (SW and REM combined). Surprisingly, major IEGs were found to be strongly down-regulated during sleep (Pompeiano et al. 1994, 1995, 1997). These puzzling results were followed up and extended to other plasticity-related genes (Basheer et al. 1997; Cirelli and Tononi 2000a,b), leading some researchers to conclude that sleep plays no role in synaptic plasticity (Tononi and Cirelli 2001), being in fact related to synaptic downscaling (Tononi and Cirelli 2003). This molecular break in the logical thread that connected neuronal reverberation to the mnemonic effects of sleep provided just enough mechanistic paradox to help the late resurrection of the notion that sleep and memory are not linked (Vertes and Eastman 2000; Siegel 2001). To borrow Thomas Kuhn's terminology (Kuhn 1962), the paradigm faced an embarrassing anomaly.
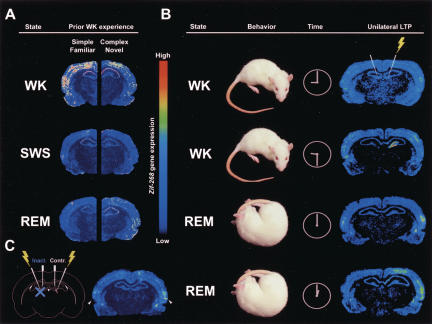
Disentangling this controversy involved the use of a curious analogy, proposed by yet another Italian group (Giuditta et al. 1995): Sleep is to information what digestion is to food. According to this view, the best way to understand how the sleeping brain facilitates learning is to contrast neural variables obtained in the presence or absence of recently acquired information. Following this advice, we assessed IEG brain expression in rats that had been exposed to a novel enriched environment for 3 h before falling asleep. Instead of lumping together SW and REM sleep, we singled out individual episodes of each phase and compared the results with those of WK. Unexposed controls showed IEG down-regulation during both sleep states (Fig. 3A, left panels), in agreement with the previous studies (Pompeiano et al. 1994, 1995, 1997; Basheer et al. 1997; Cirelli and Tononi 2000a,b). In contrast, we found the IEG zif-268 to be up-regulated to WK levels during REM—but not SW sleep—in the hippocampus and cerebral cortex of exposed animals (Fig. 3A, right panels; Ribeiro et al. 1999).
Figure 3.
(A) Experience-dependent up-regulation of zif-268 brain expression during REM sleep. Shown are autoradiograms of representative brain sections hybridized with a zif-268 radioactive riboprobe. In controls kept in their familiar home cages before the experiment (left), zif-268 expression decreased from WK to SW and REM sleep. In animals exposed to an enriched environment for 3 h before the experiment (right), zif-268 levels decreased from WK to SW sleep, but increased from the latter to REM sleep. This effect was particularly noticeable in the cerebral cortex and the hippocampus. (B) Hippocampofugal propagation of zif-268 expression during REM sleep. Unilateral LTP induction by high-frequency stimulation (yellow lightning) of the hippocampus (first-row panels) causes unilateral hippocampal up-regulation of zif-268 expression 30 min after stimulation (second-row panels). During the first REM sleep episode after LTP induction (3 h after stimulation), zif-268 expression was up-regulated in the temporal cerebral cortex and amygdala of the stimulated hemisphere, proximal to the hippocampus. Some zif-268 up-regulation was also observed in the non-stimulated hemisphere (third-row panels). During a second REM sleep episode (4 h after stimulation), this effect was even more pronounced and lateralized, reaching dorsal neocortical areas distal from the hippocampus (fourth-row panels). Our results indicate that hippocampal LTP induction during WK leads to a series of zif-268 up-regulation waves that propagate from the hippocampus to cortical areas during the course of WK and REM sleep, but that are terminated during SW sleep. (C) Effect of hippocampus inactivation during REM sleep on extrahippocampal zif-268 expression. First, LTP was bilaterally induced in the hippocampus during WK (left, yellow lightning). Three hours later, upon entering REM sleep, the left hippocampus was inactivated with tetracaine (left, blue X), while the right hippocampus received saline (control). We found a marked reduction of zif-268 expression in the temporal cerebral cortex and the amygdala of the inactivated hemisphere, as compared to the control hemisphere (right panel, arrows). This result indicates that post-LTP extrahippocampal zif-268 expression during REM sleep is under hippocampal control.
As indicated by the name “paradoxical sleep” (Jouvet et al. 1959; Jouvet 1967), REM sleep is characterized by increased neuronal activity in the forebrain, comparable to waking levels (McCarley and Hobson 1970; Destexhe et al. 1999). Such increased activity is necessary but not sufficient to induce zif-268 expression, which also requires calcium inflow via NMDA channels and phosphorilation of the cAMP response element-binding protein (CREB) (Changelian et al. 1989; Cole et al. 1989; Wisden et al. 1990; Sheng et al. 1991; Mayr and Montminy 2001; Lonze and Ginty 2002). Since NMDA channels require sustained neuronal depolarization to open (Mayer et al. 1984; Nowak et al. 1984) and REM sleep (but not SW sleep) is capable of inducing zif-268 expression (Ribeiro et al. 1999), one concludes that neuronal reverberation during REM sleep must facilitate sustained neuronal depolarization, by mechanisms yet to be determined.
The zif-268 gene (Milbrandt 1987) encodes a transcription factor (Christy and Nathans 1989) with binding sites on the promoters of hundreds of different genes (Lemaire et al. 1990). The zif-268 protein is thought to modulate synaptic plasticity by controlling the expression of genes directly involved in synaptic function, such as synapsins I and II (Thiel et al. 1994; Petersohn et al. 1995; Rosahl et al. 1995). Importantly, zif-268 expression is systematically induced by high-frequency stimulation that triggers hippocampal long-term potentiation (LTP) (Cole et al. 1989; Wisden et al. 1990; Abraham et al. 1993; Worley et al. 1993), a leading model of synaptic plasticity thought to underlie memory formation (Bliss and Lømo 1973; Bliss and Collingridge 1993). Zif-268 expression is also increased in brain regions that undergo dendritic changes after exposure to an enriched environment (Wallace et al. 1995), as well as in novelty and learning behavioral paradigms (Mello et al. 1992; Nikolaev et al. 1992; Grimm and Tischmeyer 1997). Most importantly, zif-268 expression is actually required for the long-term maintenance of hippocampal LTP, as well as spatial and nonspatial long-term memories (Jones et al. 2001; Bozon et al. 2003). In our study (Ribeiro et al. 1999), significant up-regulation of zif-268 gene expression during REM sleep was detected in the hippocampus and the cerebral cortex, two major forebrain structures intimately related to memory acquisition and long-term storage (Squire 1992; McClelland et al. 1995). Given this body of evidence, experience-dependent zif-268 expression during REM sleep provides a compelling tie between neuronal reverberation during sleep and cellular plasticity able to consolidate memories. Therefore, REM sleep fulfills—at least in principle—the second Hebbian postulate for memory consolidation (Hebb 1949).
Postsynaptic propagation of gene expression during REM sleep
It is currently believed that memories initially stored in the hippocampus are relocated over time to the cerebral cortex, by way of mechanisms long-sought but still unknown (Scoville and Milner 1957; Mishkin 1978; Kesner and Novak 1982; Buzsaki et al. 1990; Zola-Morgan and Squire 1990; Squire 1992; Kim et al. 1995; Corkin et al. 1997; Izquierdo and Medina 1997; Bontempi et al. 1999; Lavenex and Amaral 2000; Haist et al. 2001; Winocur et al. 2001). For this reason, the fact that zif-268 up-regulation during REM sleep occurred in the hippocampo-cortical circuit was of utmost interest. To further investigate the relationship between hippocampo-cortical processing and gene expression during REM sleep, we assessed zif-268 expression in several WK, SW, and REM groups in which the induction of hippocampal LTP substituted for exposure to an enriched environment as presleep stimulus. A comprehensive analysis of these results revealed a sequence of three spatiotemporally distinct waves of zif-268 up-regulation after the induction of hippocampal LTP (Ribeiro et al. 2002): The first gene expression wave began locally at the stimulation site ∼30 min after stimulation, reached proximal brain areas relative to the stimulation site after 3 h of sustained wakefulness, and was terminated during SW sleep. A second wave began during ensuing REM sleep in brain regions proximal to the stimulus site, propagated to distal brain regions during subsequent WK, and was terminated during another SW sleep episode. Finally, a third wave of zif-268 up-regulation began during a second bout of REM sleep in all proximal and distal extrahippocampal regions studied. Altogether, hippocampal regions showed a gradual decrease of zif-268 expression from the first to the second and third waves. Conversely, the most distal extrahippocampal regions studied (somatosensory and motor cortices), several synapses away from the site of LTP induction, showed an opposite gene expression profile: a gradual increase of zif-268 expression from the first to the second and third waves. These results indicate that zif-268 up-regulation after hippocampal LTP induction gradually propagates from the hippocampus to distal neocortical regions, as REM sleep recurs (Fig. 3B).
To test the possibility that neocortical zif-268 up-regulation during REM sleep is under hippocampal control, we used intra-cerebral microinjections of a sodium-channel blocker to transiently inactivate the hippocampus during post-LTP REM sleep. Hippocampal inactivation during REM sleep blocked zif-268 up-regulation in the cerebral cortex (Fig. 3C), indicating that the hippocampus is able to instruct cortical gene expression during REM sleep (Ribeiro et al. 2002). In contrast, diffusion controls injected during WK showed elevated zif-268 expression levels in both hemispheres (Ribeiro et al. 2002). This shows that post-LTP cortical zif-268 expression during WK is hippocampus independent, presumably owing to the intense thalamocortical processing that characterizes WK. Taken together, these findings indicate that REM sleep constitutes a privileged window for hippocampus-driven cortical activation, free from waking interference and, in principle, capable of playing an instructive role in the communication of memory traces from the hippocampus to the cortex. To our knowledge, these results provide the first experimental evidence that REM sleep may play a key role in the exodus of memory associations from the hippocampus to the cerebral cortex, uncovering hippocampo-cortical interactions that have been postulated for decades (Scoville and Milner 1957; Marr 1971; McClelland et al. 1995; Izquierdo and Medina 1997; Eichenbaum 2000).
A model for the complementary roles of SW and REM sleep in memory consolidation
It has been recently proposed that the neuronal reverberation of newly acquired synaptic changes during SW sleep may lead to the recall and storage of new memories by way of “calcium-mediated intracellular cascades” capable of opening the “molecular gates to plasticity” (Sejnowski and Destexhe 2000; Destexhe and Sejnowski 2003). This hypothesis is partially contradicted by evidence that calcium-dependent gene expression related to synaptic plasticity is shut down during SW sleep (Pompeiano et al. 1994; Ribeiro et al. 1999, 2002). Based on our results and the current literature, we have proposed instead that SW and REM sleep play distinct and complementary roles on memory consolidation, with memory recall (neuronal reverberation) occurring mainly during SW sleep and memory storage (plasticity-related gene expression) taking place during REM sleep (Ribeiro et al. 2004).
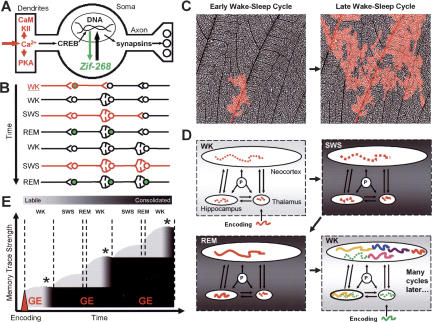
Our model proposes that intrinsic pontine activation during SW sleep, being free of sensory interference, would be biased toward previously potentiated synapses, causing neuronal firing patterns originally produced during novel WK experience to reverberate significantly above chance levels during SW sleep (Pavlides and Winson 1989; Wilson and McNaughton 1994; Ribeiro et al. 2004). At the same time, the large-amplitude slow oscillations typical of SW sleep (Steriade and McCarley 1990; Steriade et al. 1993) would promote marked periodic fluctuations of calcium levels in activated synapses, as suggested by extracellular calcium measurements (Massimini and Amzica 2001). As a consequence, SW sleep would be concomitant with the activation of multiple calcium-dependent kinases with a role in memory formation, such as Ca2+-calmodulin-dependent protein kinase II (CaMKII) (Deisseroth and Tsien 2002; Lisman et al. 2002) and protein kinase A (PKA) (Fig. 4A; Abel et al. 1997). This would result in a pretranscriptional amplification of synaptic changes encoding novel memory traces during SW sleep, as suggested by recent data (Fig. 2B, arrows in first panel). Finally, such changes would be transcriptionally stored during REM sleep by way of CREB-dependent gene expression (Ribeiro et al. 1999, 2002), able to trigger plasticity-related protein synthesis (Gutwein et al. 1980; Thiel et al. 1994; Petersohn et al. 1995) and hence consolidate newly acquired memory traces in the following hours (Fig. 4A). Experience-dependent plasticity-related gene expression during REM sleep is compatible with the notion of sleep-dependent synaptic downscaling (Tononi and Cirelli 2003), as long as the latter happens in neuronal circuits that were not activated by WK experience. Indeed, we predict that the combination of synaptic upscaling in activated circuits and synaptic downscaling in non-activated circuits should markedly increase the signal-to-noise ratio of memory consolidation during sleep, carving high-relief memory traces in a background of low synaptic plasticity.
Figure 4.
(A) SW and REM sleep trigger different steps of the calcium-dependent metabolic cascade that leads to synaptic plasticity. Neuronal reverberation during SW sleep is likely associated with enhanced calcium inflow and the phosphorilation of multiple signaling kinases at the dendritic level, such as PKA and CaMKII (red). However, CREB-dependent induction of zif-268 gene expression in the cell nucleus only occurs during REM sleep (green). The zif-268 gene encodes a transcription factor that modulates synaptic plasticity by controlling the expression of genes directly involved in synaptic function, such as synapsins. (B) Our model proposes that the recursive reinduction of zif-268 during REM sleep causes a postsynaptic propagation of newly acquired synaptic changes. According to this model, a WK event capable of encoding a new memory triggers calcium-dependent pretranscriptional signaling cascades (represented in red) and plasticity-related gene regulation (represented in green), leading to delayed synaptic plasticity during ensuing WK (schematically represented by the addition of synapses). Next, neuronal reverberation during SW sleep promotes calcium entry in the neuron downstream of the one originally activated during encoding, and ensuing REM sleep then triggers plasticity-related gene regulation. The repetition of this process leads to the further establishment of new synapses downstream the activated circuit, effectively propagating the memory trace at every WK-SW-REM cycle. (C) The postsynaptic propagation of newly acquired synaptic changes caused by the succession of wake-sleep states should result in the progressive ingraining of memory traces (red) within the cortical neuronal matrix (black). Figure adapted from a leaf micrograph, with permission from Marcelo Magnasco. (D) According to our model, new memories are encoded as labile traces during WK in several forebrain sites (dashed lines, top left), amplified during SW sleep (thicker dashed lines, top right), and consolidated during REM by way of plasticity-related gene expression (solid lines, bottom left). Notice that in this model the thalamus does not harbor a consolidated trace, due to the very low expression of CREB-dependent genes in most of the thalamus (Mello et al. 1992; Beckmann et al. 1997; Ribeiro et al. 1999). Due to postsynaptic propagation, the reiteration of WK-SW-REM cycles should promote a tidal migration of memory traces within the forebrain. In particular, due to the much larger coding capacity (i.e., number of available synapses) of the cerebral cortex in comparison with the hippocampus, such migration should generate a net hippocampofugal flow of information, progressively flushing memory traces away from the hippocampus and into the cerebral cortex (bottom right). According to this view, the hippocampus would only keep a record of the most recent memory traces (green and yellow lines), while the cerebral cortex would store a much larger repertoire of traces (lines of various colors). P denotes pontine (intrinsic) activation. (E) We have proposed (Ribeiro et al. 2004) that SW and REM sleep play distinct and complementary roles in the processing of new memory traces, with memory recall (reverberation) occurring during SW sleep and memory storage (plasticity-related gene expression, GE) taking place during REM sleep. Such functional dissociation implies that memory processing progresses in cycles of pretranscriptional amplification of labile traces during SW sleep and transcriptional trace consolidation triggered by REM sleep. According to this scheme, the combined action of SW and REM sleep would cause a progressive increase in the strength and consolidation level of memories, produced over several hours via plasticity-related protein synthesis. The model also predicts that after some time in WK such effects would be completed, and memory strength would then start to decrease due to sensory interference (indicated by asterisks). The recurrence of sleep would therefore prevent sensory interference from further degrading the strength of recently acquired memory traces.
The functional dissociation of the two main sleep phases with regard to memory consolidation implies that they separately satisfy the two Hebbian learning postulates (Hebb 1949). Accordingly, the deleterious effects of sleep deprivation on memory consolidation would be a consequence of the disruption of the underlying neuronal reverberation and gene expression during SW and REM sleep, respectively. Such a mechanism fulfills earlier conceptual notions of a two-step process for memory consolidation during sleep (Giuditta 1985; Giuditta et al. 1995; Stickgold 1998) and is in line with evidence that SW and REM sleep have synergistic effects on human procedural learning (Stickgold et al. 2000b; Mednick et al. 2003) and developmental plasticity (Marks et al. 1995; Shaffery et al. 1998; Frank et al. 2001).
Our model has far-reaching implications. The postsynaptic nature of CREB-dependent gene expression (Lemaire et al. 1990; Thiel et al. 1994; Lonze and Ginty 2002), its putative consequences on synaptic strengthening (Jones et al. 2001; Bozon et al. 2003), and in particular the hippocampofugal waves of zif-268 gene up-regulation during REM sleep (Ribeiro et al. 2002) led us to propose (Pavlides and Ribeiro 2003; Ribeiro et al. 2004) that the cyclical reiteration of memory recall during SW sleep and memory storage during REM sleep promotes a postsynaptic propagation of synaptic changes downstream the neuronal circuits first activated by a novel experience (Fig. 4B). One interesting corollary of this propagation is that neuronal reverberation during SW sleep should be most critical for memory consolidation shortly after memory acquisition, because failure to do so would provoke an irreversible loss of the recently acquired and therefore still labile memory traces. By the same token, the memory-magnifying effects of REM sleep should become more relevant as the wake-sleep cycle recurs, due to the progressive recruitment of larger neuronal networks over time. This interpretation is in agreement with the fact that early posttraining SW sleep is more important for learning than is late SW sleep (Stickgold et al. 2000b), while the exact opposite is verified for REM sleep (Smith and Rose 1996; Stickgold et al. 2000b). Further support for this interpretation derives from the finding that zif-268 up-regulation is anatomically more extensive in late than in early REM sleep (Ribeiro et al. 2002).
Within a given neuronal network such as the cerebral cortex, sleep-dependent postsynaptic propagation of synaptic changes would cause memory traces to gradually reach farther and farther away from the original neuronal circuits initially involved in memory encoding, becoming progressively more ingrained in the neuronal matrix at every WK-SW-REM cycle (Fig. 4C). Thus, postsynaptic propagation during sleep may fully account for some important findings of psychology, such as deeper memory encoding over time (Hebb 1942; Craik and Lockhart 1972; Cermak and Craik 1979), incremental learning for multiple nights after memory trace acquisition (Stickgold et al. 2000b; Walker et al. 2003), and the gradual change of dream reports—from literal simulations of WK experience into highly abstract metaphors of the same experience—as human subjects go from early to late REM sleep in a single night (Emberger 2001; Stickgold 2003).
The same rationale exposed above, when applied to memory processing across multiple neuronal networks, implies that the repetition of the WK-SW-REM cycle promotes a tidal migration of memory traces within the forebrain. For instance, due to the much larger coding capacity (i.e., number of available synapses) of the cerebral cortex in comparison with the hippocampus, this migration would generate a net hippocampofugal flow of information as sleep recurs, progressively flushing memory traces away from the hippocampus to the cerebral cortex (Fig. 4D). In principle, this mechanism would be able to explain the increased segregation of memory traces in the cerebral cortex over time, because regions not easily accessible at the moment of initial memory encoding would be eventually reached, diminishing the cortical overlap of explicit memories (McClelland et al. 1995). If our hypotheses are correct, the spatiotemporal dynamics of gene regulation during REM sleep (Ribeiro et al. 2002) will prove crucial for the progressive hippocampal disengagement after explicit memory acquisition (Scoville and Milner 1957; Mishkin 1978; Kesner and Novak 1982; Buzsaki et al. 1990; Zola-Morgan and Squire 1990; Squire 1992; Kim et al. 1995; Corkin et al. 1997; Izquierdo and Medina 1997; Bontempi et al. 1999; Lavenex and Amaral 2000; Haist et al. 2001; Winocur et al. 2001).
Our model begins with molecular and cellular considerations (Fig. 4A) and generates consequences at the level of local, regional, and global (systemic) neuronal circuitry (Fig. 4B-D, respectively). It predicts that the combined action of SW and REM sleep should determine a gradual increase in the strength and consolidation level of memories, produced over several hours via plasticity-related protein synthesis (Fig. 4E). This increase should develop in a saltatory manner, reflecting the boosting effects of sleep cycles. We expect the consolidation dynamics of both explicit and implicit memories to be overall similar; for although they rely on different anatomical substrates (Thompson and Kim 1996), they likely depend on the same cellular mechanisms. However, the consolidation speeds of explicit and implicit memories differ substantially. Explicit memories most often involve the simple association of pre-existing representations, requiring the modification and/or addition of few synapses. As a consequence, the consolidation of explicit memories is usually very fast. In contrast, the acquisition of implicit memories involves a very large number of synaptic modifications, reflected in their typical slow consolidation. This difference alone may explain why sleep deprivation is much more detrimental to implicit than to explicit memory consolidation (Fowler et al. 1973; Karni et al. 1994; Smith 1995, 2001; Stickgold et al. 2000a; Laureys et al. 2002; Walker et al. 2002; Maquet et al. 2003; Mednick et al. 2003). According to this reasoning, more taxing explicit memory tasks, involving the association of novel representations rather than pre-existing ones, should be sensitive to postacquisition sleep deprivation. In fact, support for this hypothesis comes from the now classical experiments of the sleep and learning field, which showed that lack of sleep strongly impairs the retention of newly learned nonsense syllables (Jenkins and Dallenbach 1924).
In conclusion, our results suggest that basic molecular and cellular mechanisms underlie the reverberation, storage, and propagation of memory traces during sleep. We propose that these three sleep processes alone may be sufficient to account for several major hallmarks of memory consolidation over time, such as deeper memory encoding within the cerebral cortex, incremental learning several nights after memory acquisition, and progressive hippocampal disengagement. If corroborated by further experimentation, this model will vindicate the early insight of those that postulated an intimate link between sleep and learning (Jenkins and Dallenbach 1924; Bryson and Schacher 1969; Winson 1972, 1985, 1990, 1993).
Acknowledgments
We thank Jonathan Winson, Robert Stickgold, Carlyle Smith, David Bryson, Ivan de Araújo, and David Schwartz for fruitful discussions of the views expressed here; Jonathan Ross for help with data analysis; and Susan Halkiotis for secretarial assistance. This work was supported by a fellowship from the Pew Latin American Program in Biomedical Sciences (S.R.) and by NIH 5 R01 DE11451 and 5 R01 DE13810 grants (M.A.L.N.).
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.75604.
References
- Abel, T., Nguyen, P.V., Barad, M., Deuel, T.A., Kandel, E.R., and Bourtchouladze, R. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615-626. [DOI] [PubMed] [Google Scholar]
- Abraham, W.C., Mason, S.E., Demmer, J., Williams, J.M., Richardson, C.L., Tate, W.P., Lawlor, P.A., and Dragunow, M. 1993. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience 56: 717-727. [DOI] [PubMed] [Google Scholar]
- Adey, W.R., Dunlop, C.W., and Hendrix, C.E. 1960. Hippocampal slow waves: Distribution and phase relationship in the course of approach learning. Arch. Neurol. 3: 74-90. [DOI] [PubMed] [Google Scholar]
- Agranoff, B.W., Davis, R.E., and Brink, J.J. 1966. Chemical studies on memory fixation in goldfish. Brain Res. 1: 303-309. [DOI] [PubMed] [Google Scholar]
- Arnolds, D.E., Lopes da Silva, F.H., Aitink, J.W., Kamp, A., and Boeijinga, P. 1980. The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalogr. Clin. Neurophysiol. 50: 324-328. [DOI] [PubMed] [Google Scholar]
- Aserinsky, E. and Kleitman, N. 1953. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118: 273-274. [DOI] [PubMed] [Google Scholar]
- Ayala-Guerrero, F., Mexicano, G., and Ramos, J.I. 2003. Sleep characteristics in the turkey Meleagris gallopavo. Physiol. Behav. 78: 435-440. [DOI] [PubMed] [Google Scholar]
- Basheer, R., Sherin, J.E., Saper, C.B., Morgan, J.I., McCarley, R.W., and Shiromani, P.J. 1997. Effects of sleep on wake-induced c-fos expression. J. Neurosci. 17: 9746-9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, A.M., Davidson, M.S., Goodenough, S., and Wilce, P.A. 1997. Differential expression of Egr-1-like DNA-binding activities in the naive rat brain and after excitatory stimulation. J. Neurochem. 69: 2227-2237. [DOI] [PubMed] [Google Scholar]
- Bennett, T.L. 1973. The effects of centrally blocking hippocampal theta activity on learning and retention. Behav. Biol. 9: 541-552. [DOI] [PubMed] [Google Scholar]
- Bennett, T.L., Nunn Hebert, P., and Moss, D.E. 1973. Hippocampal theta activity and the attention component of discrimination learning. Behav. Biol. 8: 173-181. [DOI] [PubMed] [Google Scholar]
- Bliss, T.V. and Collingridge, G.L. 1993. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31-39. [DOI] [PubMed] [Google Scholar]
- Bliss, T.V.P. and Lømo, T. 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232: 331-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi, B., Laurent-Demir, C., Destrade, C., and Jaffard, R. 1999. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400: 671-675. [DOI] [PubMed] [Google Scholar]
- Bozon, B., Kelly, A., Josselyn, S.A., Silva, A.J., Davis, S., and Laroche, S. 2003. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos. Trans. R. Soc. Lond. B 358: 805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B.B. 1968. Frequency and phase of hippocampal theta activity in the spontaneously behaving cat. Electroencephalogr. Clin. Neurophysiol. 24: 53-62. [DOI] [PubMed] [Google Scholar]
- Bryson, D. and Schacher, S. 1969. Behavioral analysis of mammalian sleep and learning. Perspect. Biol. Med. 13: 71-79. [DOI] [PubMed] [Google Scholar]
- Buzsaki, G., Chen, L.S., and Gage, F.H. 1990. Spatial organization of physiological activity in the hippocampal region: Relevance to memory formation. Prog. Brain Res. 83: 257-268. [DOI] [PubMed] [Google Scholar]
- Cantero, J.L., Atienza, M., Stickgold, R., Kahana, M.J., Madsen, J.R., and Kocsis, B. 2003. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J. Neurosci. 23: 10897-10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak, L. and Craik, F. 1979. Levels of processing in human memory. John Wiley & Sons, Indianapolis, IN.
- Changelian, P.S., Feng, P., King, T.C., and Milbrandt, J. 1989. Structure of the NGFI-A gene and detection of upstream sequences responsible for its transcriptional induction by nerve growth factor. Proc. Natl. Acad. Sci. 86: 377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy, B. and Nathans, D. 1989. DNA binding site of the growth factor-inducible protein Zif268. Proc. Natl. Acad. Sci. 86: 8737-8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli, C. and Tononi, G. 2000a. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J. Neurosci. 20: 9187-9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 2000b. Gene expression in the brain across the sleep-waking cycle. Brain Res. 885: 303-321. [DOI] [PubMed] [Google Scholar]
- Cole, A.J., Saffen, D.W., Baraban, J.M., and Worley, P.F. 1989. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340: 474-476. [DOI] [PubMed] [Google Scholar]
- Corkin, S., Amaral, D.G., Gonzalez, R.G., Johnson, K.A., and Hyman, B.T. 1997. H.M.'s medial temporal lobe lesion: Findings from magnetic resonance imaging. J. Neurosci. 17: 3964-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik, F. and Lockhart, R. 1972. Levels of processing: A framework for memory research. J. Verb. Learn. Verb. Behav. 11: 671-684. [Google Scholar]
- Datta, S. 2000. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J. Neurosci. 20: 8607-8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave, A.S. and Margoliash, D. 2000. Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290: 812-816. [DOI] [PubMed] [Google Scholar]
- Deisseroth, K. and Tsien, R.W. 2002. Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron 34: 179-182. [DOI] [PubMed] [Google Scholar]
- Dement, W.C. 1958. The occurrence of low voltage, fast, electroencephalogram patterns during behavioral sleep in the cat. Electroenceph. Clin. Neurophysiol. 10: 291-296. [DOI] [PubMed] [Google Scholar]
- Dement, W. and Kleitman, N. 1957a. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr. Clin. Neurophysiol. Suppl 9: 673-690. [DOI] [PubMed] [Google Scholar]
- ____. 1957b. The relation of eye movements during sleep to dream activity: An objective method for the study of dreaming. J. Exp. Psychol. 53: 339-346. [DOI] [PubMed] [Google Scholar]
- Destexhe, A. and Sejnowski, T.J. 2003. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol. Rev. 83: 1401-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe, A., Contreras, D., and Steriade, M. 1999. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci. 19: 4595-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum, H. 2000. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 1: 41-50. [DOI] [PubMed] [Google Scholar]
- Elazar, Z. and Adey, W.R. 1967. Electroencephalographic correlates of learning in subcortical and cortical structures. Electroencephalogr. Clin. Neurophysiol. 23: 306-319. [DOI] [PubMed] [Google Scholar]
- Emberger, K.M. 2001. To sleep perchance to ski: The involuntary appearance of visual and kinesthetic imagery at sleep onset following play on the Alpine Racer II. Ph.D. thesis, Harvard University, Cambridge, MA.
- Fenn, K.M., Nusbaum, H.C., and Margoliash, D. 2003. Consolidation during sleep of perceptual learning of spoken language. Nature 425: 614-616. [DOI] [PubMed] [Google Scholar]
- Fishbein, W. 1971. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav. 6: 279-282. [DOI] [PubMed] [Google Scholar]
- Fishbein, W., Kastaniotis, C., and Chattman, D. 1974. Paradoxical sleep: Prolonged augmentation following learning. Brain Res. 79: 61-75. [DOI] [PubMed] [Google Scholar]
- Fowler, M.J., Sullivan, M.J., and Ekstrand, B.R. 1973. Sleep and memory. Science 179: 302-304. [DOI] [PubMed] [Google Scholar]
- Frank, M.G., Issa, N.P., and Stryker, M.P. 2001. Sleep enhances plasticity in the developing visual cortex. Neuron 30: 275-287. [DOI] [PubMed] [Google Scholar]
- Gerrard, J.L. 2002. “Reactivation of hippocampal ensemble activity patterns in the aging rat: Insights into memory consolidation within the aged brain” Ph.D. thesis, University of Arizona, Tucson.
- Giuditta, A., ed. 1985. A sequential hypothesis for the function of sleep. Fisher-Verlag, Stuttgart, Germany.
- Giuditta, A., Ambrosini, M.V., Montagnese, P., Mandile, P., Cotugno, M., Zucconi, G.G., and Vescia, S. 1995. The sequential hypothesis of the function of sleep. Behav. Brain Res. 69: 157-166. [DOI] [PubMed] [Google Scholar]
- Green, J.D. and Arduini, A.A. 1954. Hippocampal electrical activity in arousal. J. Neurophysiol. 17: 533-557. [DOI] [PubMed] [Google Scholar]
- Grimm, R. and Tischmeyer, W. 1997. Complex patterns of immediate early gene induction in rat brain following brightness discrimination training and pseudotraining. Behav. Brain Res. 84: 109-116. [DOI] [PubMed] [Google Scholar]
- Gutwein, B.M., Shiromani, P.J., and Fishbein, W. 1980. Paradoxical sleep and memory: long-term disruptive effects of Anisomycin. Pharmacol. Biochem. Behav. 12: 377-384. [DOI] [PubMed] [Google Scholar]
- Haist, F., Bowden Gore, J., and Mao, H. 2001. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat. Neurosci. 4: 1139-1145. [DOI] [PubMed] [Google Scholar]
- Harper, R.M. 1971. Frequency changes in hippocampal electrical activity during movement and tonic immobility. Physiol. Behav. 7: 55-58. [DOI] [PubMed] [Google Scholar]
- Hebb, D.O. 1942. The effects of early and late brain injury upon test scores, and the nature of normal adult intelligence. Proc. Am. Philos. Soc. 85: 275-292. [Google Scholar]
- ____. 1949. The organization of behavior: A neuropsychological theory. John Wiley & Sons, New York.
- Hirase, H., Leinekugel, X., Czurko, A., Csicsvari, J., and Buzsaki, G. 2001. Firing rates of hippocampal neurons are preserved during subsequent sleep episodes and modified by novel awake experience. Proc. Natl. Acad. Sci. 98: 9386-9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, K.L. and McNaughton, B. 2002. Coordinated reactivation of distributed memory traces in primate neocortex. Science 297: 2070-2073. [DOI] [PubMed] [Google Scholar]
- Izquierdo, I. and Medina, J.H. 1997. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 68: 285-316. [DOI] [PubMed] [Google Scholar]
- Jenkins, J.B. and Dallenbach, K.M. 1924. Oblivescence during sleep and waking. Am. J. Psychol. 35: 605-612. [Google Scholar]
- Jones, M.W., Errington, M.L., French, P.J., Fine, A., Bliss, T.V., Garel, S., Charnay, P., Bozon, B., Laroche, S., and Davis, S. 2001. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 4: 289-296. [DOI] [PubMed] [Google Scholar]
- Jouvet, M. 1967. The states of sleep. Sci. Am. 216: 62-68. [DOI] [PubMed] [Google Scholar]
- Jouvet, M., Michel, F., and Courjon, J. 1959. Sur un stade d'activité électrique cérébrale rapide au cours du sommeil physiologique. C.R. Soc. Biol. (Paris) 153: 1024-1028. [Google Scholar]
- Kahana, M.J., Sekuler, R., Caplan, J.B., Kirschen, M., and Madsen, J.R. 1999. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399: 781-784. [DOI] [PubMed] [Google Scholar]
- Karni, A., Tanne, D., Rubenstein, B.S., Askenasy, J.J., and Sagi, D. 1994. Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265: 679-682. [DOI] [PubMed] [Google Scholar]
- Kesner, R.P. and Novak, J.M. 1982. Serial position curve in rats: Role of the dorsal hippocampus. Science 218: 173-175. [DOI] [PubMed] [Google Scholar]
- Kim, J.J., Clark, R.E., and Thompson, R.F. 1995. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav. Neurosci. 109: 195-203. [DOI] [PubMed] [Google Scholar]
- Kudrimoti, H.S., Barnes, C.A., and McNaughton, B.L. 1999. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19: 4090-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, T.S. 1962. The structure of scientific revolutions. University of Chicago Press, Chicago.
- Lamprecht, R. and LeDoux, J. 2004. Structural plasticity and memory. Nat. Rev. Neurosci. 5: 45-54. [DOI] [PubMed] [Google Scholar]
- Landfield, P.W., McGaugh, J.L., and Tusa, R.J. 1972. Theta rhythm: A temporal correlate of memory storage processes in the rat. Science 7: 87-89. [DOI] [PubMed] [Google Scholar]
- Laureys, S., Peigneux, P., Perrin, F., and Maquet, P. 2002. Sleep and motor skill learning. Neuron 35: 5-7. [DOI] [PubMed] [Google Scholar]
- Lavenex, P. and Amaral, D.G. 2000. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus 10: 420-430. [DOI] [PubMed] [Google Scholar]
- Leconte, P. and Bloch, V. 1970. Déficit de la rétention d'un conditionnement après privation de sommeil paradoxal chez le rat. Comptes Rendus de l' Académie des Sciences (Paris) 271D: 226-229. [PubMed] [Google Scholar]
- Leconte, P. and Hennevin, E. 1971. Augmentation de la durée de sommeil paradoxal consécutive à un apprentissage chez le rat. C.R. Acad. Sci. (Paris) 273: 86-88. [PubMed] [Google Scholar]
- Lee, A.K. and Wilson, M.A. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36: 1183-1194. [DOI] [PubMed] [Google Scholar]
- Lemaire, P., Vesque, C., Schmitt, J., Stunnenberg, H., Frank, R., and Charnay, P. 1990. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol. Cell. Biol. 10: 3456-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, E.R., Bern, D., and Fishbein, W. 1975. Retrograde amnesia: Prolonging the fixation phase of memory consolidation by paradoxical sleep deprivation. Physiol. Behav. 14: 409-412. [DOI] [PubMed] [Google Scholar]
- Lisman, J., Schulman, H., and Cline, H. 2002. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3: 175-190. [DOI] [PubMed] [Google Scholar]
- Lonze, B.E. and Ginty, D.D. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605-623. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó, R. 1938. Analysis of the activity of the chains of internuncial neurons. J. Neurophysiol. 1: 207-244. [Google Scholar]
- Louie, K. and Wilson, M.A. 2001. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29: 145-156. [DOI] [PubMed] [Google Scholar]
- Lucero, M.A. 1970. Lengthening of REM sleep duration consecutive to learning in the rat. Brain Res. 20: 319-322. [DOI] [PubMed] [Google Scholar]
- Maquet, P., Laureys, S., Peigneux, P., Fuchs, S., Petiau, C., Phillips, C., Aerts, J., Del Fiore, G., Degueldre, C., Meulemans, T., et al. 2000. Experience-dependent changes in cerebral activation during human REM sleep. Nat. Neurosci. 3: 831-836. [DOI] [PubMed] [Google Scholar]
- Maquet, P., Schwartz, S., Passingham, R., and Frith, C. 2003. Sleep-related consolidation of a visuomotor skill: Brain mechanisms as assessed by functional magnetic resonance imaging. J. Neurosci. 23: 1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, G.A., Shaffery, J.P., Oksenberg, A., Speciale, S.G., and Roffwarg, H.P. 1995. A functional role for REM sleep in brain maturation. Behav. Brain Res. 69: 1-11. [DOI] [PubMed] [Google Scholar]
- Marr, D. 1971. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. B262: 23-81. [DOI] [PubMed] [Google Scholar]
- Massimini, M. and Amzica, F. 2001. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J. Neurophysiol. 85: 1346-1350. [DOI] [PubMed] [Google Scholar]
- Mayer, M.L., Westbrook, G.L., and Guthrie, P.B. 1984. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309: 261-263. [DOI] [PubMed] [Google Scholar]
- Mayr, B. and Montminy, M. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2: 599-609. [DOI] [PubMed] [Google Scholar]
- McCarley, R.W. and Hobson, J.A. 1970. Cortical unit activity in desynchronized sleep. Science 167: 901-903. [DOI] [PubMed] [Google Scholar]
- McClelland, J.L., McNaughton, B.L., and O'Reilly, R.C. 1995. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102: 419-457. [DOI] [PubMed] [Google Scholar]
- Mednick, S.C., Nakayama, K., and Stickgold, R. 2003. Sleep-dependent learning: A nap is as good as a night. Nat. Neurosci. 6: 697-698. [DOI] [PubMed] [Google Scholar]
- Mello, C.V., Vicario, D.S., and Clayton, D.F. 1992. Song presentation induces gene expression in the songbird forebrain. Proc. Natl. Acad. Sci. 89: 6818-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton, A.W. and Irwin, J.M. 1940. The influence of degree of interpolated learning on retroactive inhibition and the overt transfer of specific responses. Am. J. Psychol. 53: 173-203. [PubMed] [Google Scholar]
- Milbrandt, J. 1987. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science 238: 797-799. [DOI] [PubMed] [Google Scholar]
- Mishkin, M. 1978. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 273: 297-298. [DOI] [PubMed] [Google Scholar]
- Morgan, J.I. and Curran, T. 1989. Stimulus-transcription coupling in neurons: Role of cellular immediate-early genes. Trends Neurosci. 12: 459-462. [DOI] [PubMed] [Google Scholar]
- Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J., and Buzsaki, G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19: 9497-9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev, E., Kaminska, B., Tischmeyer, W., Matthies, H., and Kaczmarek, L. 1992. Induction of expression of genes encoding transcription factors in the rat brain elicited by behavioral training. Brain Res. Bull. 28: 479-484. [DOI] [PubMed] [Google Scholar]
- Nowak, L., Bregestovski, P., Ascher, P., Herbet, A., and Prochiantz, A. 1984. Magnesium gates glutamate-activated channels in mouse central neurons. Nature (Lond) 307: 462-465. [DOI] [PubMed] [Google Scholar]
- Pavlides, C. and Ribeiro, S. 2003. Recent evidence of memory processing in sleep. In Sleep and brain plasticity (eds. P. Maquet et al.), pp. 327-362. Oxford University Press, Oxford, UK.
- Pavlides, C. and Winson, J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 9: 2907-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman, C.A. 1969. Effect of rapid eye movement (dreaming) sleep deprivation on retention of avoidance learning in rats. Rep. U.S. Naval Submarine Med. Center 22: 1-4. [PubMed] [Google Scholar]
- ____. 1973. REM sleep deprivation impairs latent extinction in rats. Physiol. Behav. 11: 233-237. [DOI] [PubMed] [Google Scholar]
- Pearlman, C. and Becker, M. 1974. REM sleep deprivation impairs bar-press acquisition in rats. Physiol. Behav. 13: 813-817. [DOI] [PubMed] [Google Scholar]
- Peigneux, P., Laureys, S., Fuchs, S., Destrebecqz, A., Collette, F., Delbeuck, X., Phillips, C., Aerts, J., Del Fiore, G., Degueldre, C., et al. 2003. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage 20: 125-134. [DOI] [PubMed] [Google Scholar]
- Pennartz, C.M., Lee, E., Verheul, J., Lipa, P., Barnes, C.A., and McNaughton, B.L. 2004. The ventral striatum in off-line processing: Ensemble reactivation during sleep and modulation by hippocampal ripples. J. Neurosci. 24: 6446-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersohn, D., Schoch, S., Brinkmann, D.R., and Thiel, G. 1995. The human synapsin II gene promoter: Possible role for the transcription factor zif268/egr-1, polyoma enhancer activator 3, and AP2. J. Biol. Chem. 270: 24361-24369. [DOI] [PubMed] [Google Scholar]
- Poe, G.R., Nitz, D.A., McNaughton, B.L., and Barnes, C.A. 2000. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855: 176-180. [DOI] [PubMed] [Google Scholar]
- Pompeiano, M., Cirelli, C., and Tononi, G. 1994. Immediate-early genes in spontaneous wakefulness and sleep: Expression of c-fos and NGIF-A mRNA protein. J. Sleep Res. 3: 80-96. [DOI] [PubMed] [Google Scholar]
- Pompeiano, M., Cirelli, C., Arrighi, P., and Tononi, G. 1995. c-Fos expression during wakefulness and sleep. Neurophysiol. Clin. 25: 329-341. [DOI] [PubMed] [Google Scholar]
- Pompeiano, M., Cirelli, C., Ronca-Testoni, S., and Tononi, G. 1997. NGFI-A expression in the rat brain after sleep deprivation. Brain Res. Mol. Brain Res. 46: 143-153. [DOI] [PubMed] [Google Scholar]
- Qin, Y.L., McNaughton, B.L., Skaggs, W.E., and Barnes, C.A. 1997. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos. Trans. R. Soc. Lond. B 352: 1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen, A. and Kales, A. 1968. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. National Institutes of Health, Washington, DC.
- Ribeiro, S., Goyal, V., Mello, C.V., and Pavlides, C. 1999. Brain gene expression during REM sleep depends on prior waking experience. Learn. Mem. 6: 500-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, S., Mello, C.V., Velho, T., Gardner, T.J., Jarvis, E.D., and Pavlides, C. 2002. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci. 22: 10914-10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, S., Gervasoni, D., Soares, E.S., Zhou, Y., Lin, S.C., Pantoja, P., Lavine, M., and Nicolelis, M. 2004. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2: 126-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg, H.P., Dement, W.C., Muzio, J.N., and Fisher, C. 1962. Dream imagery: Relationship to rapid eye movements of sleep. Arch. Gen. Psychiatry 7: 235-258. [DOI] [PubMed] [Google Scholar]
- Rosahl, T.W., Spillane, D., Missler, M., Herz, J., Selig, D.K., Wolff, J.R., Hammer, R.E., Malenka, R.C., and Sudhof, T.C. 1995. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375: 488-493. [DOI] [PubMed] [Google Scholar]
- Sainsbury, R.S. 1970. Hippocampal activity during natural behavior in the guinea pig. Physiol. Behav. 5: 317-324. [DOI] [PubMed] [Google Scholar]
- Scoville, W.B. and Milner, B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psych. 20: 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg, P.B., Kahana, M.J., Howard, M.W., Donner, E.J., and Madsen, J.R. 2003. Theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci. 23: 10809-10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski, T.J. and Destexhe, A. 2000. Why do we sleep? Brain Res. 886: 208-223. [DOI] [PubMed] [Google Scholar]
- Shaffery, J.P., Oksenberg, A., Marks, G.A., Speciale, S.G., Mihailoff, G., and Roffwarg, H.P. 1998. REM sleep deprivation in monocularly occluded kittens reduces the size of cells in LGN monocular segment. Sleep 21: 837-845. [DOI] [PubMed] [Google Scholar]
- Sheng, M., Thompson, M.A., and Greenberg, M.E. 1991. CREB: A Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427-1430. [DOI] [PubMed] [Google Scholar]
- Shiromani, P., Gutwein, B.M., and Fishbein, W. 1979. Development of learning and memory in mice after brief paradoxical sleep deprivation. Physiol. Behav. 22: 971-978. [DOI] [PubMed] [Google Scholar]
- Siegel, J.M. 2001. The REM sleep-memory consolidation hypothesis. Science 294: 1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs, W.E. and McNaughton, B.L. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271: 1870-1873. [DOI] [PubMed] [Google Scholar]
- Smith, C. 1995. Sleep states and memory processes. Behav. Brain Res. 69: 137-145. [DOI] [PubMed] [Google Scholar]
- ____. 2001. Sleep states and memory processes in humans: Procedural versus declarative memory systems. Sleep Med. Rev. 5: 491-506. [DOI] [PubMed] [Google Scholar]
- Smith, C. and Butler, S. 1982. Paradoxical sleep at selective times following training is necessary for learning. Physiol. Behav. 29: 469-473. [DOI] [PubMed] [Google Scholar]
- Smith, C. and Kelly, G. 1988. Paradoxical sleep deprivation applied 2 days after end of training retards learning. Physiol. Behav. 43: 213-216. [DOI] [PubMed] [Google Scholar]
- Smith, C. and Lapp, L. 1986. Prolonged increases in both PS and number of REMS following a shuttle avoidance task. Physiol. Behav. 36: 1053-1057. [DOI] [PubMed] [Google Scholar]
- ____. 1991. Increases in number of REMS and REM density in humans following an intensive learning period. Sleep 14: 325-330. [DOI] [PubMed] [Google Scholar]
- Smith, C. and MacNeill, C. 1993. A paradoxical sleep-dependent window for memory 53-56-H after the end of avoidance training. Psychobiology 21: 109-112. [Google Scholar]
- Smith, C. and Rose, G.M. 1996. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol. Behav. 59: 93-97. [DOI] [PubMed] [Google Scholar]
- Smith, C. and Wong, P.T.P. 1991. Paradoxical sleep increases predict successful learning in a complex operant task. Behav. Neurosci. 105: 282-288. [DOI] [PubMed] [Google Scholar]
- Smith, C., Kitahama, K., Valatx, J.L., and Jouvet, M. 1974. Increased paradoxical sleep in mice during acquisition of a shock avoidance task. Brain Res. 77: 221-230. [DOI] [PubMed] [Google Scholar]
- Smith, C., Young, J., and Young, W. 1980. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep 3: 67-68. [PubMed] [Google Scholar]
- Squire, L.R. 1992. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99: 195-231. [DOI] [PubMed] [Google Scholar]
- Steriade, M. and McCarley, R.W. 1990. Brainstem control of wakefulness and sleep. Plenum Press, New York.
- Steriade, M., McCormick, D.A., and Sejnowski, T.J. 1993. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679-685. [DOI] [PubMed] [Google Scholar]
- Stewart, M. and Fox, S.E. 1991. Hippocampal theta activity in monkeys. Brain Res. 538: 59-63. [DOI] [PubMed] [Google Scholar]
- Stickgold, R. 1998. Sleep: Off-line memory reprocessing. Trends Cogn. Sci. 2: 484-492. [DOI] [PubMed] [Google Scholar]
- ____. 2003. Memory, cognition, and dreams. In Sleep and brain plasticity (eds. P. Maquet et al.), pp. 17-39. Oxford University Press, Oxford, UK.
- Stickgold, R., James, L., and Hobson, J.A. 2000a. Visual discrimination learning requires sleep after training. Nat. Neurosci. 3: 1237-1238. [DOI] [PubMed] [Google Scholar]
- Stickgold, R., Whidbee, D., Schirmer, B., Patel, V., and Hobson, J.A. 2000b. Visual discrimination task improvement: A multi-step process occurring during sleep. J. Cogn. Neurosci. 12: 246-254. [DOI] [PubMed] [Google Scholar]
- Thiel, G., Schoch, S., and Petersohn, D. 1994. Regulation of synapsin I gene expression by the zinc finger transcription factor zif268/egr-1. J. Biol. Chem. 269: 15294-15301. [PubMed] [Google Scholar]
- Thompson, R.F. and Kim, J.J. 1996. Memory systems in the brain and localization of a memory. Proc. Natl. Acad. Sci. 93: 13438-13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timo-Iaria, C., Negrao, N., Schmidek, W.R., Hoshino, K., Lobato de Menezes, C.E., and Leme da Rocha, T. 1970. Phases and states of sleep in the rat. Physiol. Behav. 5: 1057-1062. [DOI] [PubMed] [Google Scholar]
- Tononi, G. and Cirelli, C. 2001. Some considerations on sleep and neural plasticity. Arch. Ital. Biol. 139: 221-241. [PubMed] [Google Scholar]
- ____. 2003. Sleep and synaptic homeostasis: A hypothesis. Brain Res. Bull. 62: 143-150. [DOI] [PubMed] [Google Scholar]
- Tradardi, V. 1966. Sleep in the pigeon. Arch. Ital. Biol. 104: 516-521. [PubMed] [Google Scholar]
- Vanderwolf, C.H. 1969. Hippocampal electrical activity and voluntary movement in the rat. Electroenceph. Clin. Neurophysiol. 26: 407-418. [DOI] [PubMed] [Google Scholar]
- Vertes, R.P. and Eastman, K.E. 2000. The case against memory consolidation in REM sleep. Behav. Brain Sci. 23: 867-876. [DOI] [PubMed] [Google Scholar]
- Wagner, U., Gais, S., Haider, H., Verleger, R., and Born, J. 2004. Sleep inspires insight. Nature 427: 352-355. [DOI] [PubMed] [Google Scholar]
- Walker, M.P., Brakefield, T., Morgan, A., Hobson, J.A., and Stickgold, R. 2002. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron 35: 205-211. [DOI] [PubMed] [Google Scholar]
- Walker, M.P., Brakefield, T., Seidman, J., Morgan, A., Hobson, J.A., and Stickgold, R. 2003. Sleep and the time course of motor skill learning. Learn. Mem. 10: 275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, C.S., Withers, G.S., Weiler, I.J., George, J.M., Clayton, D.F., and Greenough, W.T. 1995. Correspondence between sites of NGFI-A induction and sites of morphological plasticity following exposure to environmental complexity. Brain Res. Mol. Brain Res. 32: 211-220. [DOI] [PubMed] [Google Scholar]
- Wang, X.J. 2001. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 24: 455-463. [DOI] [PubMed] [Google Scholar]
- Wetzel, W., Wagner, T., and Balschun, D. 2003. REM sleep enhancement induced by different procedures improves memory retention in rats. Eur. J. Neurol. 18: 2611-2617. [DOI] [PubMed] [Google Scholar]
- Wilson, M.A. and McNaughton, B.L. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676-679. [DOI] [PubMed] [Google Scholar]
- Winocur, G., McDonald, R.M., and Moscovitch, M. 2001. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus 11: 18-26. [DOI] [PubMed] [Google Scholar]
- Winson, J. 1972. Interspecies differences in the occurrence of theta. Behav. Biol. 7: 479-487. [DOI] [PubMed] [Google Scholar]
- ____. 1974. Patterns of hippocampal theta rhythm in the freely moving rat. Electroenceph. Clin. Neurophysiol. 36: 291-301. [DOI] [PubMed] [Google Scholar]
- ____. 1978. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201: 160-163. [DOI] [PubMed] [Google Scholar]
- ____. 1985. Brain and psyche. Anchor Press, New York.
- ____. 1990. The meaning of dreams. Sci. Am. 263: 86-96. [DOI] [PubMed] [Google Scholar]
- ____. 1993. The biology and function of rapid eye movement sleep. Curr. Opin. Neurobiol. 3: 243-248. [DOI] [PubMed] [Google Scholar]
- Wisden, W., Errington, M.L., Williams, S., Dunnett, S.B., Waters, C., Hitchcock, D., Evan, G., Bliss, T.V., and Hunt, S.P. 1990. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron 4: 603-614. [DOI] [PubMed] [Google Scholar]
- Worley, P.F., Bhat, R.V., Baraban, J.M., Erickson, C.A., McNaughton, B.L., and Barnes, C.A. 1993. Thresholds for synaptic activation of transcription factors in hippocampus: Correlation with long-term enhancement. J. Neurosci. 13: 4776-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan, S.M. and Squire, L.R. 1990. The primate hippocampal formation: Evidence for a time-limited role in memory storage. Science 250: 288-290. [DOI] [PubMed] [Google Scholar]