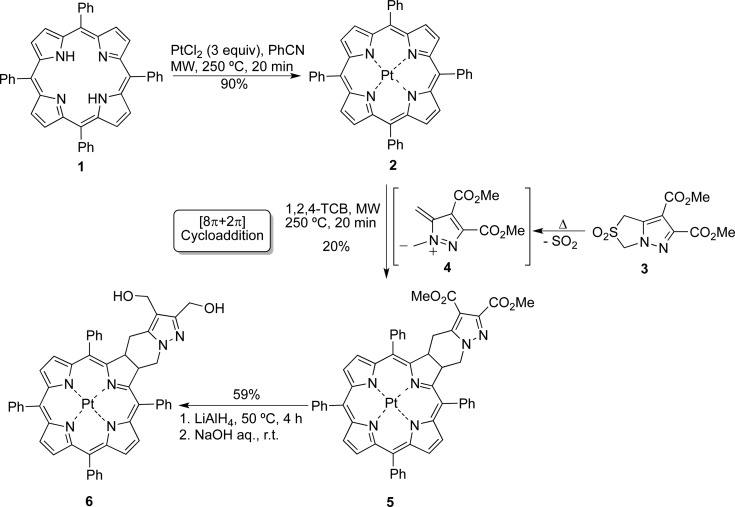
Abstract
Novel near-infrared luminescent compounds based on platinum(II) 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins are described. These compounds have high photostability and display light emission, in particular simultaneous fluorescence and phosphorescence emission in solution at room temperature, in the biologically relevant 700–850 nm red and near-infrared (NIR) spectral region, making them excellent materials for biological imaging. The simultaneous presence of fluorescence and phosphorescence emission at room temperature, with the phosphorescence strongly quenched by oxygen whereas fluorescence remains unaffected, allows these compounds to be used as ratiometric oxygen sensors in chemical and biological media. Both steady-state (fluorescence vs phosphorescence intensities) and dynamic (dependence of phosphorescence lifetimes upon oxygen concentration) luminescence approaches can be used. Photocytotoxicity studies against human melanocytic melanoma cells (A375) indicate that these compounds display potential as photosensitizers in photodynamic therapy.
Keywords: PDT, theranostics, Pt(II) chlorins, photosensitizers, oxygen sensing
Luminescence sensing and imaging provides a sensitive and low-cost approach for the in vivo study of biological systems.1 Near-infrared (NIR) emitters in the therapeutically relevant 700–850 nm region are particularly important, as their spectral features span a region where tissues are nearly transparent.2,3 Valuable applications include NIR luminescence imaging of cells for diagnosis of cancer and other abnormal conditions.4−7 Examples of the most important classes of NIR emitters are inorganic fluorophores, such as quantum dots; up-converting fluorophores containing lanthanide ions; organic dyes, such as cyanines, squaraines, phthalocyanines, porphyrins, boron dipyrromethanes (BODIPYs), and perylene dyes; and carbon nanotubes.2,6 However, although a number of materials have been reported that luminesce in this region, the only long wavelength emission compound approved by the US Food and Drug Agency (FDA) for direct usage in medical diagnostics is the cyanine dye indocyanine green.2 There is, therefore, the urgent need to develop new and efficient NIR emitting materials.
There is a major advantage if the same luminescent material that is used for imaging can also be used as fluorescent probe and sensor for both qualitative and quantitative analysis of a chemical species.8−10 One important target in cellular studies is molecular oxygen, whose concentration can provide information on the cell state. Quenching of the excited states by molecular oxygen can lead to reactive oxygen species, such as singlet oxygen or superoxide radical anions, which can be harmful for malignant cells. Combining this ability with diagnostic properties endows these systems with potential as theranostic agents.
The incorporation of high atomic number metal ions, e.g., platinum, into porphyrins, phthalocyanines, chlorins, and bacteriochlorins can enhance triplet state formation, and, in many cases, lead to room temperature phosphorescence within the biological spectral window.11−17
Chlorins (dihydroporphyrins) show suitable spectral properties in the NIR region. However, when prepared by the classical route of porphyrin diimide reduction, they have limited chemical stability, and reoxidation to the porphyrin occurs easily. We have recently shown that their stability can be dramatically enhanced by the introduction of a fused ring. Indeed, stable 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins were obtained via an unprecedented [8π + 2π] cycloaddition of diazafulvenium methides with porphyrins.18,19 Furthermore, preliminary studies on the photodynamic activity of 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins against melanoma cells showed that they are very active photodynamic agents.20 Interestingly, a dihydroxymethylchlorin derivative was particularly active against human melanocytic melanoma A375 cells. This is of particularly importance considering the known resistance of melanoma to conventional chemotherapy and radiotherapy, and the fact that PDT of melanoma can be compromised due to the natural resistance mechanism of some melanotic melanomas.21,22 In fact, high melanin levels in such tumors can lead to optical interference via competition with the photosensitizer for light absorption.
To enhance the potential using the results already achieved with above chlorins, their Pt(II) complexes were prepared with the aim of developing NIR luminescence probes as well as new photosensitizers for photodynamic therapy (PDT) of cancer.
The platinum complex of 5,10,15,20-tetraphenylporphyrin was synthesized following a known general procedure, replacing Pt(acac)2 as the metal source with PtCl2.23 5,10,15,20-Tetraphenylporphyrin (1, TPP) reacted with PtCl2 in benzonitrile under microwave irradiation at 250 °C for 20 min, giving the Pt-complex 2 in 90% yield. The metalated porphyrin 2 (2 equiv) reacted with 2,2-dioxo-1H,3H-pyrazolo[1,5-c]thiazole 3 under microwave irradiation at 250 °C for 20 min giving the novel Pt complex of 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorin 5 in 20% yield (50% of porphyrin recovered). Thus, Pt-complex 2 participated in a [8π + 2π] cycloaddition with diazafulvenium methide 4, generated in situ from sulfone 3 through thermal extrusion of sulfur dioxide. To improve the hydrophilicity of the complex for application in biological systems, Pt(II)-chlorin 5 was converted into the corresponding dihydroxymethyl derivative 6 using LiAlH4 as reducing agent (Scheme 1).
Scheme 1. Synthesis of Pt(II) 4,5,6,7-Tetrahydropyrazolo[1,5-a]pyridine-Fused Chlorins 5 and 6.
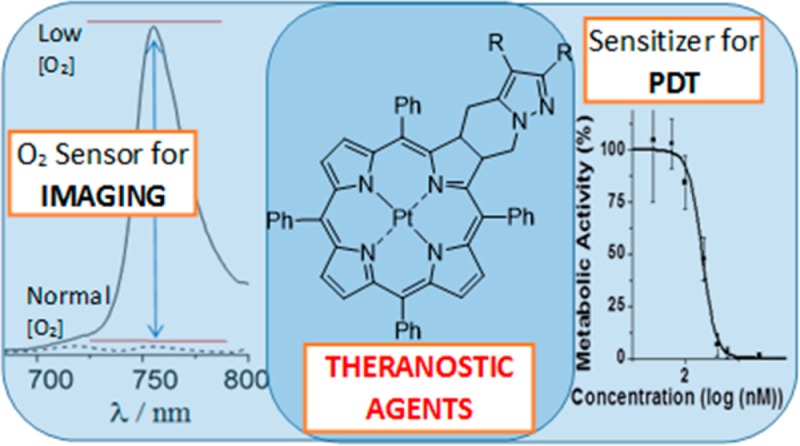
The absorption spectra of the Pt complexes 5 and 6 (in toluene solution) display wavelength maxima at ∼590 nm, together with additional absorption bands at shorter wavelengths (Figure 1 and Table 1). Note, for the compounds investigated the absorbance of the low energy band is significantly higher than those found for TPP or its Pt(II) complex (see Figure 1 and Figure S3).
Figure 1.
Normalized absorption spectra of Pt(II) chlorins 5 and 6, presented with TPP and Pt(II)-TPP (considered as references compounds) in toluene solution at T = 293 K.
Table 1. Absorption and Photophysical Dataa for the Pt(II) Chlorins and the Corresponding Free Base Chlorins in Solution at Room Temperature.
| absorption
λmax (nm) |
fluorescence
λmax (nm) |
phosphorescence λmax (nm)c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qx (0–0) | Qx (1–0) | Qy (0–0) | Qy (1–0) | B (0–0) | Q (0–0) | Q (0–1) | ϕFb,c | ϕPb,c | phosphorescence lifetime (μs) | ||
| chlorin 5 | 590 | 554 | 484 | 400 | 598 | 655 | 756 | 0.0001g | 0.0002e | 0.4c,e | |
| 0.0028e | 26.1c,f | ||||||||||
| 0.088f | |||||||||||
| chlorin 6 | 591 | 555 | 486 | 400 | 598 | 644 | 756 | 0.0001e | 0.0002g | 0.5c,e | |
| 0.0019e | 25.7c,f | ||||||||||
| 0.068f | 30.9d,f | ||||||||||
| 11.3d,e | |||||||||||
| free base chlorin of 519 | 649 | 595 | 545 | 518 | 416 | 654 | 698, 721 | 0.27 | |||
| free base chlorin of 6 | 651 | 601 | 543 | 514 | 409 | 654 | 696, 718 | 0.23 | |||
Absorption, fluorescence and phosphorescence maxima, fluorescence ϕF and phosphorescence quantum yields, ϕPh, and phosphorescence lifetimes, τPh.
Fluorescence quantum yields (λexc = 413 nm) were determined by using TPP in toluene (ΦF = 0.11)24 and tris(2,2′-bipyridyl)ruthenium(II) in water (ΦF = 0.042)25 as a standard,;
In toluene.
In aqueous micellar system (Tween80/DMSO/water (2:2:96, v/v/v)).
Presence of O2.
Deaerated solution.
O2 saturated solution; values presented in parentheses were obtained in the ns-TA setup.
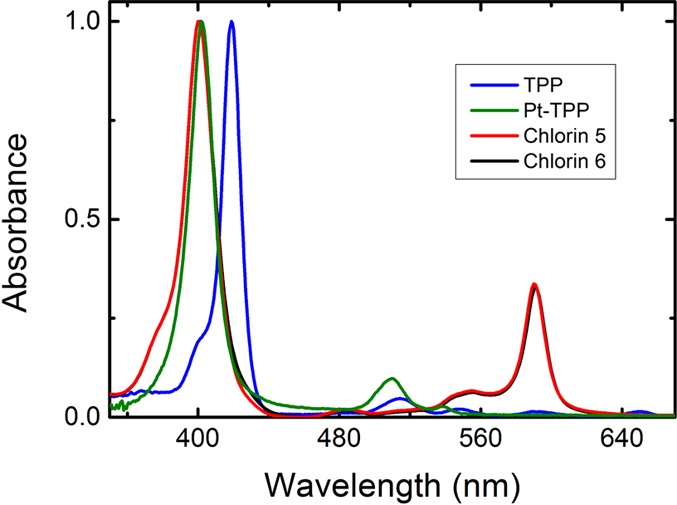
Figure 2 presents the emission spectra for chlorins 5 and 6. The fluorescence emission (in the 644–655 nm range) is structured, with a maximum at 598 nm (Table 1). However, the most interesting feature of these compounds is the additional occurrence of room temperature phosphorescence, with a maximum at 755 nm (see Figure 2). The assignment of this long wavelength band to phosphorescence was based on its similarity with the RT phosphorescence emission spectra collected with 5 μs delay after flash and the time-resolved phosphorescence spectra obtained in a laser flash photolysis setup (see Figures 2 and S4–S7 in SI). For the Pt(II) derivatives (chlorins 5 and 6) at 293 K, the RT phosphorescence spectra display an unstructured long wavelength emission band centered at ∼755 nm (Figures S4 and S7). This provides the possibilities of using these compounds for NIR imaging. There is a strong overlap between the phosphorescence and the fluorescence emission bands. However, the phosphorescence is highly sensitive to oxygen and increases when the concentration of O2 in solution is reduced (see Figure S4 in SI). This allows determination of the fluorescence quantum yields for chlorins 5 and 6 using oxygen saturated solutions, where the phosphorescence is effectively quenched, see Figure 2, while the fluorescence is little affected. The fluorescence quantum yields (ϕF) for chlorin 5 and 6 were obtained using TPP (ϕF = 0.11)24 in toluene as standard (see experimental section, SI), showing a lower ϕF = 0.0001 value, see Table 1. This is particularly relevant when compared to the free-base chlorins of 5 and 6 where the fluorescence quantum yields are more than 3 orders of magnitude higher. In addition, a blue-shift of the fluorescence was also observed upon going from the free base chlorins to the corresponding Pt(II)-chlorin derivatives (see Table 1).
Figure 2.
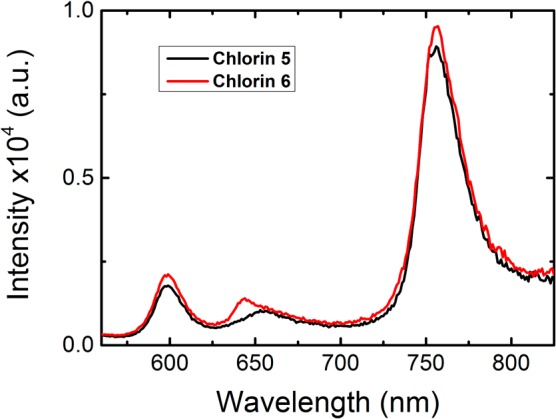
Room temperature luminescence spectra (λexc= 400 nm) for chlorins 5 and 6 in oxygen saturated toluene solutions prepared by bubbling oxygen for 3 h.
Degassed solutions of the Pt(II) chlorins in toluene show a much stronger phosphorescence at 756 nm compared with aerated ones, while the fluorescence emission intensity at 600 nm remains unaltered, as can be seen in Figure S4. The phosphorescence quantum yields (ϕPh) for chlorins 5 and 6 were obtained by using tris(2,2′-bipyridyl)ruthenium(II) in water (ϕF = 0.042)25 as standard. For further details please see the experimental section in SI. The values of the phosphorescence quantum yields (Table 1) depend on the dissolved oxygen concentration in the solution, varying from 0.0002 in oxygen saturated toluene (3 h of bubbling with oxygen); through 0.0028 with an air equilibrated solution ([O2] = 1.8 × 10–3 M), finally to 0.088 with a nitrogen saturated solution. The strong quenching of the phosphorescence band of chlorins 6 and 5 by molecular oxygen and the increase in its intensity by over 2 orders of magnitude on going from oxygen saturated to degassed solutions (see Table 1Figure S4) opens the possibility of sensing oxygen in chemical and biological media by studying the ratio of the intensity of phosphorescence to fluorescence bands (at ∼600 nm).26 One particularly important application of such ratiometric sensing is in cancer diagnosis since it could, in principle, enable the determination of intracellular oxygen concentration, thereby allowing distinction between normoxic and hypoxic cells. This also opens the way for in vivo oxygen tumor imaging by phosphorescence quenching.15
To mimic biological conditions, a micellar system of the surfactant polysorbate 80 (Tween 80) in aqueous DMSO solution (Tween80/DMSO/water (2/2/96, v/v/v)) was used. In this medium similar spectroscopic properties (absorption and emission wavelength maxima) were observed as those in toluene solution spectra (Figure 3).
Figure 3.
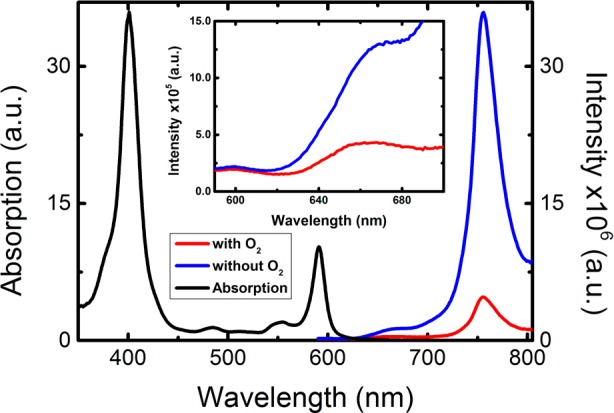
Room temperature luminescence spectra (λexc = 410 nm) together with the normalized absorption spectra for Pt(II) chlorin 6 in aqueous micellar system (Tween80/DMSO/water (2:2:96, v/v/v)), collected in the presence (air saturated solution) and absence of O2 (after deoxygenation by bubbling N2 for 20 min). Inset: Magnified view of the fluorescence emission bands present in the 590–700 nm range.
The potential for oxygen sensing of chlorin 6 was analyzed quantitatively using phosphorescence intensities at different oxygen concentrations and the Stern–Volmer equation (eq 1),24
| 1 |
where KSV is the Stern–Volmer constant, pO2 is the oxygen partial pressure, I0 = (luminescence intensity at 755 nm/luminescence intensity at 600 nm), and I = (luminescence intensity at 755 nm/luminescence intensity at 600 nm) at several pO2 from 0 to 21%. A good linear fit was observed up to 21% oxygen, with a KSV value of 0.18 (Figure 4). The fluorescence intensity monitored at 600 nm was practically unchanged, showing that chlorin 6 can be used as a ratiometric phosphorescence probe for measuring oxygen pressures and, consequently, solution concentrations.
Figure 4.
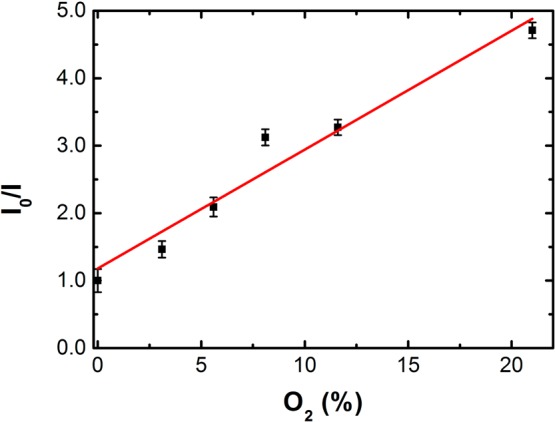
Plot of I0(755 nm/600 nm)/I(755 nm/600 nm) as a function of O2 concentration, from 0 to 21% (gas phase), for chlorin 6 in aqueous micellar system (Tween80/DMSO/water (2:2:96, v/v/v)).
The phosphorescence lifetime of chlorin 6 was also determined in the presence and absence of oxygen in Tween80/DMSO/H2O (2:2:96) (Table 1) and changed from 11.3 μs (aerated solution with O2) to 30.9 μs (degassed solution), see Figure S6A,B in SI. The observed decrease in phosphorescence lifetime makes this class of compounds a good candidate for applications in phosphorescence lifetime imaging microscopy,27 in addition to its use as steady-state ratiometric O2 sensor probes.
The photostability of chlorin 6 was determined by monitoring the phosphorescence emission of a deaerated solution in water at 756 nm. The phosphorescence intensity at 756 nm remained unchanged over ∼4.5 h irradiation when excited with a broad band Xe lamp. Finally, the thermal stability of chlorins 5 and 6 was evaluated, using isothermal thermogravimetric analysis of the samples at 60 °C during 3 h. Negligible mass loss (less than 1%) was observed for the two compounds studied (SI, Figure S8). These results demonstrate that chlorins 5 and 6 have high thermal stability and photostability.
Quenching of the phosphorescence may be expected to produce singlet oxygen (1Δg). Its formation was confirmed by direct measurement of the characteristic singlet oxygen phosphorescence emission centered at 1270 nm following irradiation of aerated solutions of chlorin 6. The singlet oxygen quantum yield (ϕΔ) was obtained by comparing the sensitized phosphorescence emission spectra from singlet oxygen (Figure S9 in SI), obtained with optically matched solutions of the samples and that of the reference phenazine28 (for further details see the experimental section in SI).
A value of ϕΔ = 0.58 was obtained for chlorin 6. This strongly suggests that the platinum(II) ring-fused chlorins can also be potentially interesting photosensitizers for photodynamic therapy.
In this context, the photocytotoxicity of chlorins 5 and 6 against human melanocytic melanoma cells (A375) was evaluated. It was observed that the di(hydroxymethyl)-substituted chlorin 6 shows high photodynamic activity with an IC50 of 144 nM (confidence interval at 95%: [121.8;171.0]) (Figure 5), comparable to the IC50 value of 156 nM obtained for Photofrin under the same conditions. Additionally, this chlorin 6 was significantly more active than the diester-substituted chlorin 5 (IC50 > 5 μM), in agreement to what was previously observed for the corresponding metal-free chlorins.20 Experiments with A375 cells in the dark confirmed that the cytotoxicity is light-dependent. In the case of chlorin 6, some cytotoxicity was observed in the absence of light, but with an IC50 value of 22.29 μM. Thus, the potential of platinum(II) 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins as photosensitizers for PDT of melanocytic melanoma cells has been demonstrated.
Figure 5.
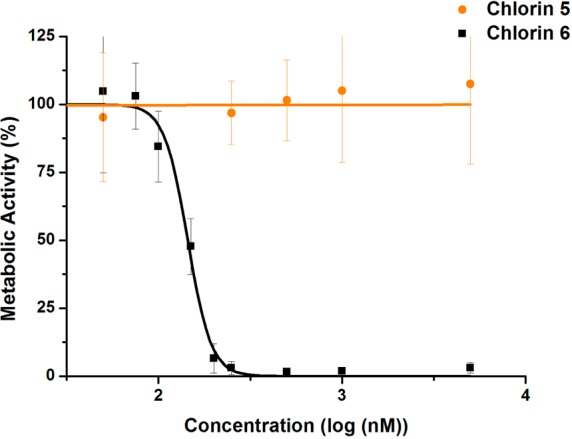
Metabolic activity of A375 human melanoma cells submitted to photodynamic treatment using the photosensitizer chlorins 5 and 6. The values represent the average and standard deviation for each concentration.
In conclusion, new near-infrared luminescent oxygen sensors based on platinum(II) 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine-fused chlorins are reported. Their high thermal and photochemical stability and attractive photophysical features, including oxygen-dependent room temperature phosphorescence and potential in ratiometric emission measurements, make them excellent compounds to be used as probes for molecular oxygen, biological imaging, and photodynamic therapy. This implies that the described compounds are true, stable Pt-chlorin-type theranostic agents.
Acknowledgments
This work was funded by FEDER–Fundo Europeu de Desenvolvimento Regional through the COMPETE 2020–Operational Programme for Competitiveness and Internationalisation (POCI), and by Portuguese funds through FCT–Fundação para a Ciência e a Tecnologia in the framework of the project POCI-01-0145-FEDER-PTDC/QEQ-MED/0262/2014, Portuguese Agency for Scientific Research. The Coimbra Chemistry Centre (CQC) is supported by FCT through project no. 007630 UID/QUI/00313/2013, cofunded by COMPETE2020-UE. IBILI is supported by FCT through project UID/NEU/04539/2013, cofunded by COMPETE2020-UE (POCI-01-0145-FEDER-007440). The FCT is also acknowledged for a postdoctoral grant to J.P. (ref SFRH/BPD/108469/2015). We acknowledge the UC-NMR facility for obtaining the NMR data (www.nmrccc.uc.pt) and Professor Mário N. Berberan-Santos (Instituto Superior Técnico) for valuable discussions. The authors also gratefully acknowledge Perkin-Helmer, Inc., and Intrumentos de Laboratório Científico, Lda (ILC) for providing the EnSpire Multimode Plate Reader and Dr. Hans-Peter Steffens (Perkin-Helmer, Inc.) for technical support. M.O.S. acknowledges grant SFI IvP 13/1894.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00476.
Experimental section, 1H NMR, 13C NMR, absorption, and fluorescence emission spectra for TPP and Pt(II) complex, luminescence spectra of chlorins 5 and 6 (in the absence and presence of oxygen), thermal stability, testing of photodynamic activity of chlorins 5 and 6, and singlet oxygen formation on photolysis of chlorin 6 (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Massoud T. F.; Gambhir S. S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- Pansare V.; Hejazi S.; Faenza W.; Prud’homme R. K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. 10.1021/cm2028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.; Chopra A.; Shan L.; Eckelman W. C.; Menkens A. E. Essential parameters to consider for the characterization of optical imaging probes. Nanomedicine 2012, 7, 1101–1107. 10.2217/nnm.12.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevick-Muraca E. M.; Houston J. P.; Gurfinkel M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr. Opin. Chem. Biol. 2002, 6, 642–650. 10.1016/S1367-5931(02)00356-3. [DOI] [PubMed] [Google Scholar]
- Frangioni J. V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Luo S.; Zhang E.; Su Y.; Cheng T.; Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Hötzer B.; Medintz I. L.; Hildebrandt N. Fluorescence in Nanobiotechnology: Sophisticated Fluorophores for Novel Applications. Small 2012, 8, 2297–2326. 10.1002/smll.201200109. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Lin W.; Zheng K.; He L.; Huang W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. 10.1039/C2CS35313J. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Cheng J.; Zhou L.; Zhou X.; Xiang H. Ratiometric optical oxygen sensing: a review in respect of material design. Analyst 2012, 137, 4885–4901. 10.1039/c2an35907c. [DOI] [PubMed] [Google Scholar]
- Wang X.-d.; Wolfbeis O. S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. 10.1039/C4CS00039K. [DOI] [PubMed] [Google Scholar]
- Eastwood D.; Gouterman M. Porphyrins. J. Mol. Spectrosc. 1970, 35, 359–375. 10.1016/0022-2852(70)90179-7. [DOI] [Google Scholar]
- Papkovsky D. B.; O’Riordan T. C. Emerging Applications of Phosphorescent Metalloporphyrins. J. Fluoresc. 2005, 15, 569–584. 10.1007/s10895-005-2830-x. [DOI] [PubMed] [Google Scholar]
- Zems Y.; Moiseev A. G.; Perepichka D. F. Convenient Synthesis of a Highly Soluble and Stable Phosphorescent Platinum Porphyrin Dye. Org. Lett. 2013, 15, 5330–5333. 10.1021/ol402590c. [DOI] [PubMed] [Google Scholar]
- Borisov S. M.; Papkovsky D. B.; Ponomarev G. V.; DeToma A. S.; Saf R.; Klimant I. Photophysical properties of the new phosphorescent platinum(II) and palladium(II) complexes of benzoporphyrins and chlorins. J. Photochem. Photobiol., A 2009, 206, 87–92. 10.1016/j.jphotochem.2009.05.018. [DOI] [Google Scholar]
- Esipova T. V.; Karagodov A.; Miller J.; Wilson D. F.; Busch T. M.; Vinogradov S. A. Two New “Protected” Oxyphors for Biological Oximetry: Properties and Application in Tumor Imaging. Anal. Chem. 2011, 83, 8756–8765. 10.1021/ac2022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata M.; Hirohara S.; Tanaka R.; Kinoshita I.; Ohkubo K.; Fukuzumi S.; Tanihara M.; Yano S. In vitro heavy-atom effect of palladium(II) and platinum(II) complexes of pyrrolidine-fused chlorin in photodynamic therapy. J. Med. Chem. 2009, 52, 2747–2753. 10.1021/jm8015427. [DOI] [PubMed] [Google Scholar]
- Pershukevich P. P.; Galievsky V. A.; Stasheuski A. S.; Makarova E. A.; Luk’yanets E. A.; Solovyov K. N. Phosphorescence of palladium and platinium complexes of benzo-fused hydroporphyrazines. J. Appl. Spectrosc. 2011, 77, 790–801. 10.1007/s10812-011-9404-2. [DOI] [Google Scholar]
- Pereira N. A. M.; Serra A. C.; Pinho e Melo T. M. V. D. Novel Approach to Chlorins and Bacteriochlorins: [8π+2π] Cycloaddition of Diazafulvenium Methides with Porphyrins. Eur. J. Org. Chem. 2010, 2010, 6539–6543. 10.1002/ejoc.201001157. [DOI] [Google Scholar]
- Pereira N. A. M.; Fonseca S. M.; Serra A. C.; Pinho e Melo T. M. V. D.; Burrows H. D. [8π+2π] Cycloaddition of meso-Tetra- and 5,15-Diarylporphyrins: Synthesis and Photophysical Characterization of Stable Chlorins and Bacteriochlorins. Eur. J. Org. Chem. 2011, 2011, 3970–3979. 10.1002/ejoc.201100465. [DOI] [Google Scholar]
- Pereira N. A. M.; Laranjo M.; Pineiro M.; Serra A. C.; Santos K.; Teixo R.; Abrantes A. M.; Gonçalves A. C.; Sarmento Ribeiro A. B.; Casalta-Lopes J.; Botelho M. F.; Pinho e Melo T. M. V. D. Novel 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine fused chlorins as very active photodynamic agents for melanoma cells. Eur. J. Med. Chem. 2015, 103, 374–380. 10.1016/j.ejmech.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Huang Y.-Y.; Vecchio D.; Avci P.; Yin R.; Garcia-Diaz M.; Hamblin M. R. Melanoma resistance to photodynamic therapy: new insights. Biol. Chem. 2013, 394, 239–250. 10.1515/bchm-2012-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawczyk-Krupka A.; Bugaj A. M.; Latos W.; Zaremba K.; Sieron A. Photodynamic therapy in treatment of cutaneous and choroidal melanoma. Photodiagn. Photodyn. Ther. 2013, 10, 503–509. 10.1016/j.pdpdt.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Dean M. L.; Schmink J. R.; Leadbeater N. E.; Brückner C. Microwave-promoted insertion of Group 10 metals into free base porphyrins and chlorins: scope and limitations. Dalton Trans. 2008, 1341–1345. 10.1039/b716181f. [DOI] [PubMed] [Google Scholar]
- Turro N. J.; Ramamurthy V.; Scaiano J. C.. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, 2010. [Google Scholar]
- Van Houten J.; Watts R. J. Temperature dependence of the photophysical and photochemical properties of the tris(2,2′-bipyridyl)ruthenium(II) ion in aqueous solution. J. Am. Chem. Soc. 1976, 98, 4853–4858. 10.1021/ja00432a028. [DOI] [Google Scholar]
- Valeur B.; Berberan-Santos M. N.. Chemical Sensing via Fluorescence. In Molecular Fluorescence; Valeur B., Berberan-Santos M. N., Eds.; Wiley-VCH: Weinheim, 2012; pp 409–478. [Google Scholar]
- Chen Y.-C.; Clegg R. M. Fluorescence lifetime-resolved imaging. Photosynth. Res. 2009, 102, 143–155. 10.1007/s11120-009-9458-7. [DOI] [PubMed] [Google Scholar]
- Redmond R. W.; Gamlin J. N. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.