Abstract
The therapeutic treatment of negative symptoms and cognitive dysfunction associated with schizophrenia is a significant unmet medical need. Preclinical literature indicates that α7 neuronal nicotinic acetylcholine (nACh) receptor agonists may provide an effective approach to treating cognitive dysfunction in schizophrenia. We report herein the discovery and evaluation of 1c (BMS-933043), a novel and potent α7 nACh receptor partial agonist with high selectivity against other nicotinic acetylcholine receptor subtypes (>100-fold) and the 5-HT3A receptor (>300-fold). In vivo activity was demonstrated in a preclinical model of cognitive impairment, mouse novel object recognition. BMS-933043 has completed Phase I clinical trials.
Keywords: Schizophrenia, α7 neuronal nicotinic acetylcholine receptor, α7 nAChR partial agonist, quinuclidine, clinical candidate
Schizophrenia is a severe and chronic psychiatric disorder affecting approximately 1% of the general population. The clinical features of schizophrenia include hallucinations and delusions (positive symptoms), loss of motivation and social withdrawal (negative symptoms), and cognitive impairment including deficits in executive cognitive function, selective attention, and working memory.1 Cognitive impairment is inadequately treated by marketed antipsychotic drugs and contributes to the marked social and occupational dysfunction seen in patients. Improved cognition is one of the best predictors of improved functional outcome2 and represents a significant unmet medical need.3
The pathophysiology of schizophrenia has been linked to the cholinergic neurotransmission system and, in particular, to the α7 neuronal nicotinic acetylcholine receptor (α7 nAChR).3 nAChR subtypes transduce the acetylcholine signal in the limbic and cortical regions of the brain, where cholinergic receptors are highly expressed. Polymorphisms in the promoter region of the CHRNA7, a gene that encodes for the α7 nAChR, are linked to P50 sensory gating suppression in schizophrenics.4,5 Additionally, post-mortem analysis of brain tissue isolated from schizophrenia patients shows reduced expression of α7 nAChRs in the hippocampus and dentate gyrus.6
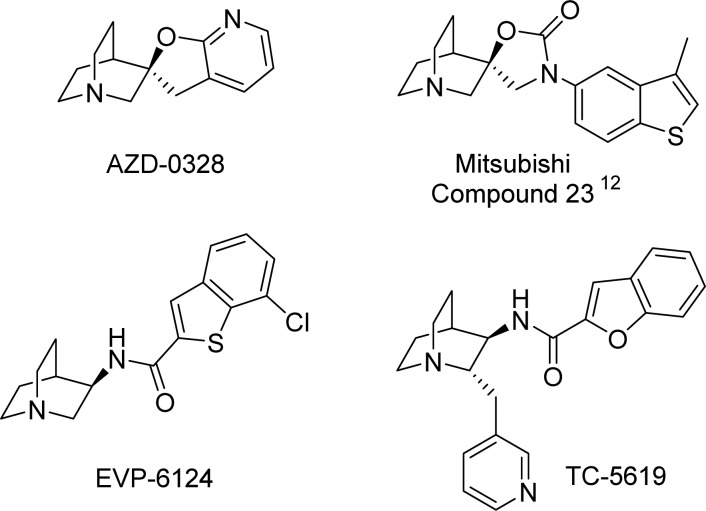
It has been noted that nicotine, a prototypical agonist of nAChRs, improves cognitive deficits and negative symptoms associated with schizophrenia.7 Nicotine also improves P50 auditory gating performance in schizophrenics.8 The high rate of tobacco smoking observed in schizophrenics is thus thought to be an indication of self-medication.9 These observations have led many groups over the past two decades to seek the development of nAChR agonists, and in particular, selective agonists of α7 nAChR to improve cognitive deficits associated with schizophrenia. Many α7 nAChR agonists have demonstrated improvement in preclinical models of memory and cognition,10−15 and several compounds have progressed to human clinical trials, including DMXB-A (GTS-21),16 EVP-6124,17,18 TC-5619,19−21 and others.22 Of particular note, clinical efficacy has been reported for both EVP-612418 and TC-561921 in Phase II trials, although positive clinical end points were not achieved in later-stage trials. Examples of α7 nAChR agonists are shown in Figure 1.
Figure 1.
Examples of α7 nAChR agonists.
In our program, we prioritized the development of compounds with potent α7 nAChR partial agonist effects, a profile expected to have reduced potential for receptor desensitization compared to agonists that fully activate the receptor.23,24 Among previously published α7 receptor agonists, many have demonstrated antagonist activity at the serotonergic 5-HT3A receptor. This is likely due to the high sequence homology between α7 receptors and 5-HT3A receptors.25 In fact, the marketed antiemetic 5-HT3A receptor antagonist tropisetron was shown to be a potent α7 receptor partial agonist.26 Since 5-HT3A receptor antagonism has been associated with off-target gastric side effects,27,28 we also prioritized the development of compounds with high selectivity relative to this target.
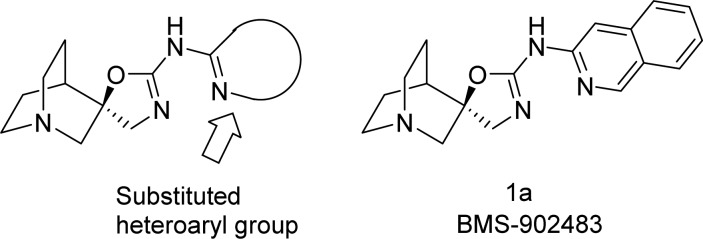
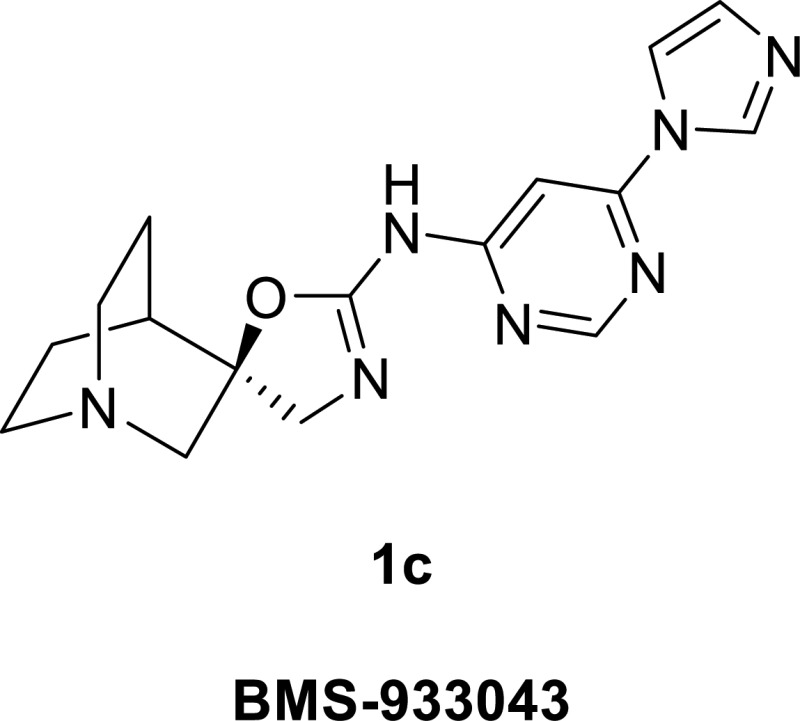
Most of the compounds in Figure 1 are characterized by a pharmacophoric model consisting of three elements: (1) a rigid bicyclic amine, which serves as a cationic center at physiological pH, (2) an exocyclic amide, carbamate, or carbonyl biosteric heterocycle serving as a central H-bond acceptor (mimicking the ester carbonyl in ACh), and (3) a lipophilic aromatic or heteroaromatic group.29−31 Our extensive SAR efforts32−34 identified a novel chemotype which conformed to this pharmacophore, with (1S,4S)-quinuclidine serving as the preferred bicyclic amine and (R)-aminooxazoline (spiroimidate) as an isostere for the central H-bond acceptor (Figure 2). In developing compounds with optimum α7 receptor partial agonism properties and high selectivity relative to the 5-HT3A receptor, we found that the choice of the heteroaryl group was an important factor. Compounds that contained 4-aminopyrimidines substituted in the 6-position with aromatic and heteroaromatic rings, and fused heteroaromatics generally provided this combination of characteristics.32−34
Figure 2.
Structures of the quinuclidine spiroimidate chemotype and compound 1a.
We identified BMS-902483 (1a) as an early example of a potent α7 nACh receptor partial agonist (Figure 2).32 This compound bound with high potency to native rat and recombinant human α7 nAChRs and demonstrated agonist activity in a Ca2+ fluorescence assay (FLIPR). In whole cell voltage clamp electrophysiology experiments, 1a showed a potent, partial agonist profile (data summarized in Table 1). Compound 1a had no agonist or antagonist activity at other nicotinic acetylcholine receptor subtypes (α1β1δε, α3β4, α4β2) and demonstrated a 50-fold margin with respect to the binding of human 5-HT3A receptors. In vivo evaluation of 1a in the mouse novel object recognition (NOR) model showed this compound to be efficacious at doses of 0.1–3 mg/kg, sc.32 As an indicator of potential cardiovascular safety, 1a was evaluated for inhibition of the hERG potassium channel in a patch clamp electrophysiological assay and was found to be a moderate inhibitor (IC50 = 3.2 μM). At the NOR minimum effective dose (0.1 mg/kg, sc), 1a was considered to possess a sufficient safety margin to advance into preclinical toxicological studies.35 Unfortunately, in a 1 month of GLP repeat dose dog study, 1a showed drug-related liver and kidney changes correlated with elevations in AST, ALT, and alkaline phosphatase. QT prolongation was also observed at high doses. Thus, further development of this compound was halted.32
Table 1. Selected in Vitro Screening Data of 1a, 1b, and 1c(42).
| compound | 1a | 1b | 1c | |
|---|---|---|---|---|
| cLogP | 1.4 | 2.8 | 0.27 | |
| FLIPR rat α7 (EC50, nM)a | 9.3 ± 5.3 | 11 ± 6 | 23 ± 10 | |
| rat α7 | BTXb binding (Ki, nM) | 4.8 | 3.3 | |
| human α7 | BTXb binding (Ki, nM) | 1.3 | 8.1 | |
| rat α7 nAChR electrophysiology | ||||
| peak Ymax, area Ymax (%) | 40, 54 | 13, 49 | 27, 67 | |
| area EC50 (nM) | 140 | 0.49 | 100 | |
| human α7 nAChR electrophysiology | ||||
| peak Ymax, area Ymax (%) | 26, 62 | 24, 78 | ||
| area EC50 (nM) | 240 | 300 | ||
| nicotinic ACh-related receptors (EC50, μM)c | >100 | >100 | ||
| HEK293 human 5-HT3A (IC50, nM)a | 480 ± 160 | 9200 ± 1400 | 8100 ± 2300 | |
| metabolic stability, % remaining (human, rat, mouse, dog, monkey) | 96, 1, 89, 74, 78 | 91, 95, 93, 98, 100 | ||
| CYP inhibition, IC50 (μM)d | >40 | >30 | ||
| hERG, patch clamp assay (IC50, μM) | 3.2 | 4.0 | >30e | |
| plasma free fraction, % free (human, rat, mouse, dog, monkey) | 25, 27, 23, 44, 35 | 87, 87, 84, 93, 87 | ||
| Caco-2, efflux ratio | 1.1 | 2.5 | ||
n ≥ 4.
[125I]-bungarotoxin binding.
Panel of nicotinic acetylcholine receptors α1β1δε, α3β4, and α4β2
Panel of human CYP isozymes: 3A4-BFC, 3A4-BZR, 1A2, 2B6, 2C8, 2C9, 2C19, 2D6.
12% inhibition at 10 μM and 38% inhibition at 30 μM concentrations
In developing an alternative to 1a, we required a candidate with reduced potential for cardiovascular liability, as measured in our hERG patch clamp assay, while maintaining target efficacy at low exposures. Since compound lipophilicity is a contributing factor to binding at the hERG channel,36 we felt that compounds with lower lipophilicity would have the best potential to be weaker inhibitors of the hERG channel. Therefore, we used cLogP values37 as an estimate of lipophilicity to help guide our selection process. cLogP values less than that of 1a (1.4) were targeted.
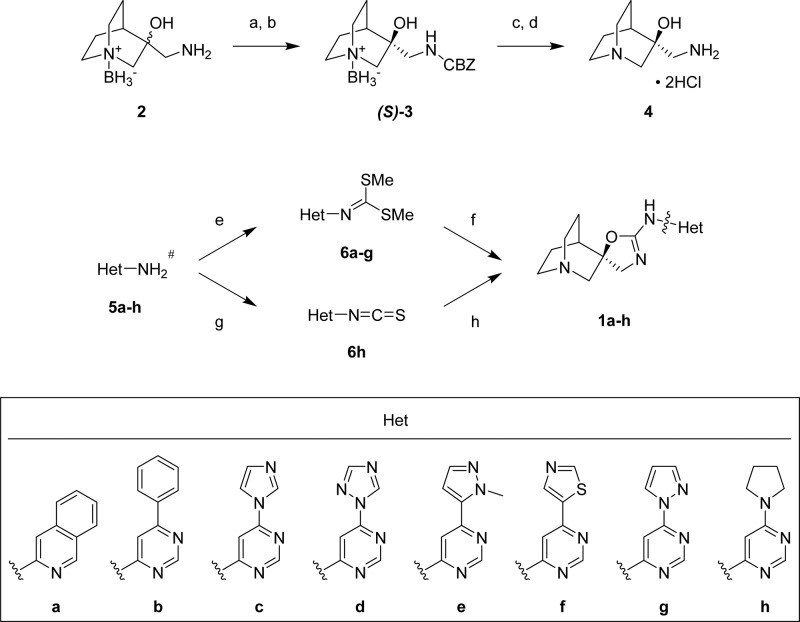
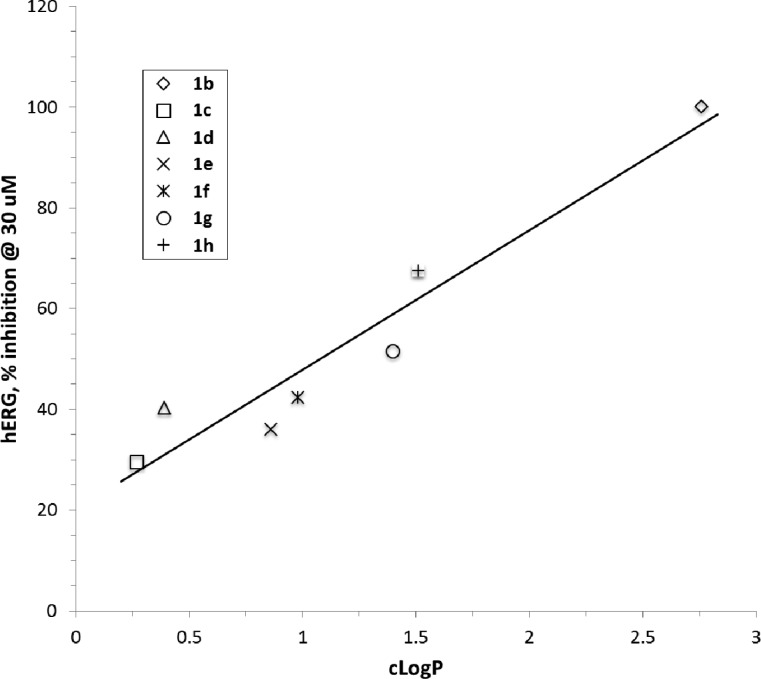
A group of compounds that initially attracted our attention was a series of deannulated analogues of 1a, the 6-aryl substituted 4-aminopyrimidines.34 The prototype of this series, 1b, exhibited a potent and selective α7 partial agonist profile (rat EP EC50 = 0.49 nM, peak Ymax 13%, area Ymax 49%; >800-fold selective versus the 5-HT3A receptor; see Table 1).38 However, this compound was a moderate hERG inhibitor (IC50 = 4.0 μM) with a cLogP value of 2.8 and would not meet our cardiovascular risk criteria. We next considered compounds 1c–h, a group of 4-aminopyrimidines substituted in the 6-position with a five-membered heteroaryl or heterocyclic group, as shown in Scheme 1. These compounds had significantly lower cLogP values than either 1a or 1b (Figure 3). Compounds 1c–h were surveyed in a single-point hERG patch clamp assay in order to quickly assess this liability. It was observed that decreasing hERG potency correlated well with the lower cLogP values (Figure 3). Among this group, 1c (BMS-933043) had the lowest cLogP value (0.27) and hERG channel inhibition (38% at 30 μM test concentration) and was chosen for extensive in vitro and in vivo profiling.
Scheme 1. Synthesis of Quinuclidine Spiroimidates 1a–h.
Reagents: (a) benzyl chloroformate, Na2CO3, CH2Cl2/H2O (21%); (b) chiral supercritical fluid chromatography purification; (c) 3 M aqueous HCl/acetone; (d) H2, Pd–C (69% for steps c, d); (e) NaH, CS2, CH3I, THF or NaOH, CS2, CH3I, DMF (8–77%); (f) 4, Cs2CO3 (43–96%); (g) 1,1′-thiocarbonyldipyridin-2(1H)-one (33%); (h) 4, Cs2CO3, N,N-diisopropylcarbodiimide (11%).
Synthesis of 5a–h is described in the Supporting Information.
Figure 3.
Relationship of hERG inhibition to cLogP values for 1b–h. cLogP values were calculated using the LogP calculator available in the ACD/Laboratories ChemSketch software package.37
Quinuclidine spiroimidates 1a–h were prepared according to the methods described by Cook and co-workers32 and are shown in Scheme 1. Briefly, treatment of borane-protected quinuclidine 2(39−41) with benzyl chloroformate (CBZ-Cl) gave the corresponding racemic CBZ- and borane-protected amino alcohol, which was then separated into its individual enantiomers by chiral chromatography. The CBZ- and borane-protecting groups were removed from the preferred isomer (S)-3(32) in a one-pot procedure by treatment with aqueous HCl, followed by hydrogenolysis in the presence of catalytic palladium to afford the chiral amino alcohol 4 as the dihydrochloride salt. Final compounds 1a–h were then obtained by one of two methods. Heterocyclic amines 5a–g were converted to the corresponding intermediate dimethylimidodithioates 6a–g by treatment under basic conditions with carbon disulfide and methyl iodide. Condensation of 4 and 6a–g in the presence of Cs2CO3 then provided 1a–g. Heterocyclic amine 5h was alternatively converted to the isothiocyanate 6h, which was reacted with 4 followed by ring closure with diisopropylcarbodiimide to yield 1h.
In the α7 FLIPR assay, 1c exhibited an EC50 = 23 nM (Table 1). Like 1a, this compound was devoid of agonist (EC50 > 100 μM) activity at HEK293 cells expressing related rat nicotinic acetylcholine receptors (α1β1δε, α3β4, α4β2). In whole cell voltage clamp electrophysiology experiments, 1c exhibited a potent, partial agonist profile (rat EC50 = 100 nM, peak Ymax 27%, area Ymax 67%; human EC50 = 300 nM, peak Ymax 24%, area Ymax 78%). In in vitro competition binding studies, 1c potently displaced antagonist [125I]-bungarotoxin (BTX) binding from recombinant rat α7 (Ki = 3.3 nM) and human α7 receptors (Ki = 8.1 nM). Compound 1c demonstrated functional antagonism at 5-HT3A receptors with an IC50 = 8.1 μM, corresponding to >300-fold selectivity versus α7 receptor agonism. Additionally, 1c exhibited no significant pharmacological activities in our internal screening panel of 30 other receptor and enzyme targets, which included muscarinic receptor subtypes (hM1, hM3, hM4, hM5).42
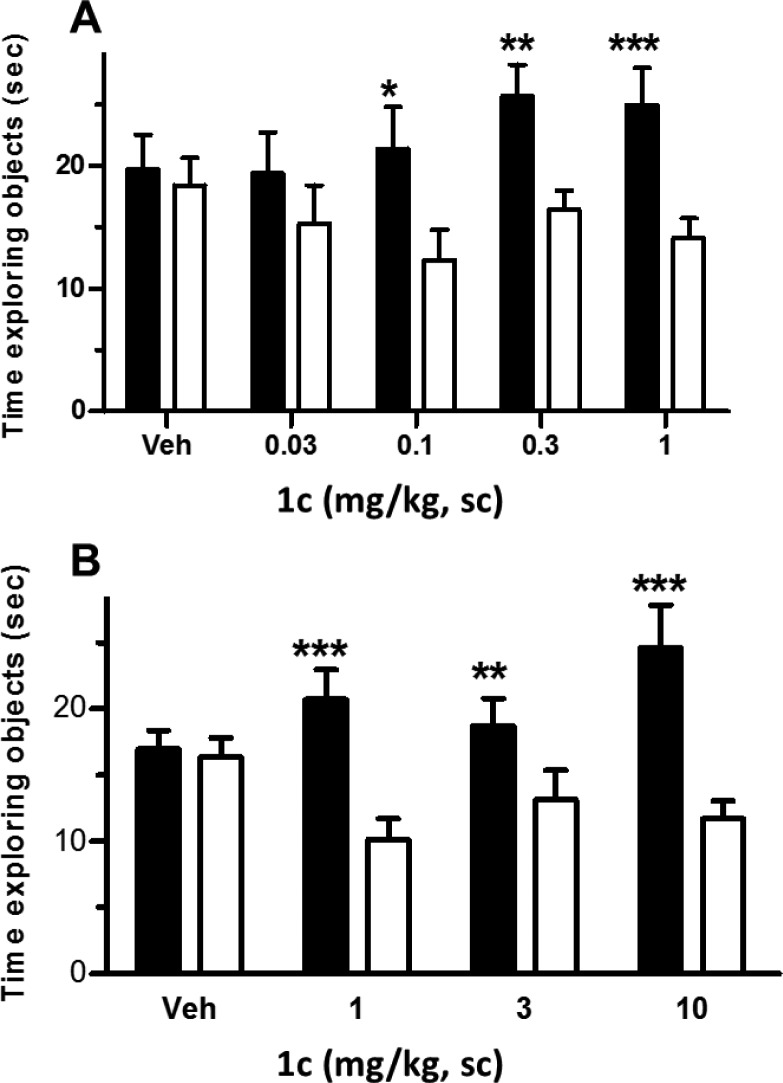
The effect of 1c on episodic memory was evaluated in the mouse NOR model, our primary measure of cognitive improvement (Figure 4). This model utilizes the natural tendency of mice to spend more time exploring novel, unfamiliar objects relative to familiar objects encountered previously during the training (drug) phase of the task. Mice were treated subcutaneously (0.03–10 mg/kg, sc) with 1c 30 min prior to training. Object recognition memory retention was examined 24 h later. A robust increase in novel object exploration was demonstrated at doses of 0.1–10 mg/kg, sc, indicative of improved object recognition memory (Figure 4). The associated average plasma exposure for 1c, determined 30 min after dosing in satellite groups of mice, was 52 nM at the minimum efficacious dose (MED), 0.1 mg/kg, sc.
Figure 4.
Effects of 1c in mouse novel object recognition experiments. (A) Low dose experiment. (B) High dose experiment. Filled bars correspond to time of exploration of novel objects. Open bars correspond to time of exploration of familiar objects. Paired t tests were used to compare the statistical difference between time exploring novel and familiar objects; *p < 0.05, **p < 0.01, ***p < 0.001.
Compound 1c was evaluated in the hERG patch clamp assay and shown to inhibit the hERG channel 12% at 10 μM and 38% at 30 μM concentrations. Thus, the hERG IC50 was determined to be >30 μM, a potency at least 10-fold weaker than that of 1a (Table 1). In order to place this parameter in the context of plasma drug levels at the MED in the NOR model, we used uncorrected plasma exposures since mouse plasma free fraction levels were very high (84%). At the MED of 1c, 0.1 mg/kg, sc, the plasma exposure was >600-fold less than the hERG IC50, representing a significant improvement in hERG-related cardiovascular risk compared to 1a.
Table 2 outlines the pharmacokinetic parameters of 1c in preclinical species. Compound 1c demonstrated high clearance and a short T1/2 in mouse and rat (1.1 and 0.7 h, respectively), moderate clearance and a t1/2 of 4.4 h in cynomolgous monkeys, and moderate clearance and a t1/2 of 5.5 h in dog. Bioavailability was good to excellent across species (45–100%). In mice, the brain-to-plasma ratio was 0.21 at 30 min postdose (1 mg/kg). The major metabolite of 1c was the corresponding quinuclidine N-oxide 7,42 which had greatly reduced α7 activity (EC50 in FLIPR assay >50 μM). Screening in ADME profiling and against a panel of 30 receptor and enzyme targets did not reveal pharmacological liabilities for this metabolite.42
Table 2. Single-Dose Pharmacokinetic Parameters of 1c(42).
| mouse |
rat |
dog |
monkey |
|||||
|---|---|---|---|---|---|---|---|---|
| iv | po | iv | po | iv | po | iv | po | |
| dose (mg/kg) | 1 | 10 | 1 | 10 | 1 | 5 | 1 | 5 |
| Vss (L/kg) | 7.0 | 2.9 | 5.5 | 5.7 | ||||
| CLTp (mL/min/kg) | 96 | 70 | 15 | 17 | ||||
| Cmax (μM) | 0.55 | 1.6 | 3.2 | 1.3 | ||||
| t1/2 (h) | 1.1 | 0.7 | 5.5 | 4.4 | ||||
| AUC (μM·h) | 0.53 | 3.2 | 0.75 | 7.2 | 3.4 | 19 | 3.0 | 11 |
| %F | 45 | 97 | 100 | 70 | ||||
In summary, 1c (BMS-933043) is a potent and selective α7 nACh receptor partial agonist, which was active in a preclinical model of cognitive improvement in mice. Compound 1c had reduced cardiovascular liability compared to earlier analogues based on reduced interaction with the hERG channel. A full description of the preclinical phamacology of compound 1c was recently reported.43 Based on the profile described in these reports, 1c was advanced into Phase I clinical studies.44
Acknowledgments
We thank Michael Sinz for his assistance in interpreting pharmacological screening data and pharmacokinetic studies.
Glossary
ABBREVIATIONS
- α7 nAChR
α7 neuronal nicotinic acetylcholine receptor
- 5-HT3A
5-hydroxytryptamine 3A
- ACh
acetylcholine
- BTX
bungarotoxin
- EP
electrophysiology
- NOR
novel object recognition
- FLIPR
fluorescence imaging plate reader
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00032.
Experimental details for synthetic procedures and associated chemical data for compounds 1–7, pharmacological screening data, and biological methods (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Jones C. K.; Byun N.; Bubser M. Muscarinic and Nicotinic Acetylcholine Receptor Agonists and Allosteric Modulators for the Treatment of Schizophrenia. Neuropsychopharmacology 2012, 37, 16–42. 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. F.; Kern R. S.; Heaton R. K. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004, 72, 41–51. 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Young J. W.; Geyer M. A. Evaluating the role of the alpha-7nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem. Pharmacol. 2013, 86, 1122–1132. 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin R. J.; Turetsky B. I.; Moberg P.; Gur R. C.; Gur R. E. P50 abnormalities in schizophrenia: relationship to clinical and neuropsychological indices of attention. Schizophr. Res. 1998, 33, 157–167. 10.1016/S0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- Freedman R.; Leonard S.; Gault J. M.; Hopkins J.; Cloninger C. R.; Kaufmann C. A.; et al. Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7). J. Med. Genet. 2001, 105, 20–22. . [DOI] [PubMed] [Google Scholar]
- Freedman R.; Hall M.; Adler L. E.; Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry 1995, 38, 22–33. 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Sacco K. A.; Termine A.; Seyal A.; Dudas M. M.; Vessicchio J. C.; Krishnan-Sarin S.; et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch. Gen. Psychiatry 2005, 62, 649–659. 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Adler L. E.; Hoffer L. J.; Griffith J.; Waldo M. C.; Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol. Psychiatry 1992, 32, 607–616. 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Lohr J. B.; Flynn K. Smoking and schizophrenia. Schizophr. Res. 1992, 8 (2), 93–102. 10.1016/0920-9964(92)90024-Y. [DOI] [PubMed] [Google Scholar]
- Sydserff S.; Sutton E. J.; Song D.; Quirk M. C.; Maciag C.; Li C.; Jonak G.; Gurley D.; Gordon J. C.; Christian E. P.; et al. Selective α7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem. Pharmacol. 2009, 78, 880–888. 10.1016/j.bcp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Castner S. A.; Smagin G. N.; Piser T. M.; Wang Y.; Smith J. S.; Christian E. P.; Mrzljak L.; Williams G. V. Immediate and sustained improvements in working memory after selective stimulation of α7 nicotinic acetylcholine receptors. Biol. Psychiatry 2011, 69, 12–18. 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Tatsumi R.; Fujio M.; Takanashi S.; Numata A.; Katayama J.; Satoh H.; Katayama J.; et al. (R)-3′-(3-methylbenzo[b]-thiophen-5-yl)spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin]-2′-one, A Novel and Potent Alpha 7 Nicotinic Acetylcholine Receptor Partial Agonist Displays Cognitive Enhancing Properties. J. Med. Chem. 2006, 49, 4374–83. 10.1021/jm060249c. [DOI] [PubMed] [Google Scholar]
- Malysz J.; Anderson D. J.; Gronlien J. H.; Ji J.; Bunnelle W. H.; Hakerud M.; Thorin-Hagene K.; Ween H.; Helfrich R.; Hu M.; et al. In vitro pharmacological characterization of a novel selective α7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J. Pharmacol. Exp. Ther. 2010, 334, 863–874. 10.1124/jpet.110.167072. [DOI] [PubMed] [Google Scholar]
- Othman A. A.; Lenz R. A.; Zhang J.; Li J.; Awni W. M.; Dutta S. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective α7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J. Clin. Pharmacol. 2011, 51, 512–526. 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- Pichat P.; Bergis O. E.; Terranova J.-P.; Urani A.; Duarte C.; Santucci V.; Gueudet C.; Voltz C.; Steinberg R.; Stemmelin J.; et al. SSR180711, a Novel Selective α7 Nicotinic Receptor Partial Agonist: (II) Efficacy in Experimental Models Predictive of Activity Against Cognitive Symptoms of Schizophrenia. Neuropsychopharmacology 2007, 32, 17–34. 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- Kitagawa H.; Takenouchi T.; Azuma R.; Wesnes K. A.; Kramer W. G.; Clody D. E.; Burnett A. L. Neuropsychopharmacology 2003, 28, 542. 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- Prickaerts J.; van Goethem N. P.; Chesworth R.; Shapiro G.; Boess F. G.; Methfessel C.; Reneerkens O. A. H.; Flood D. G.; Hilt D.; Gawryl M.; et al. EVP-6124, a novel and selective α7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of α7 nicotinic acetylcholine receptors. Neuropharmacology 2012, 62, 1099–1110. 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Barbier A. J.; Hilhorst M.; Vliet A. V.; Snyder P.; Palfreyman M. G.; Gawryl M.; Dgetluck N.; Massaro M.; Tiessen R.; Timmerman W.; et al. Pharmacodynamics, Pharmacokinetics, Safety, and Tolerability of Encenicline, a Selective α7 Nicotinic Receptor Partial Agonist, in Single Ascending-Dose and Bioavailability Studies. Clin. Ther. 2015, 37, 311–324. 10.1016/j.clinthera.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Hauser T. A.; Kucinski A.; Jordan K. G.; Gatto G. J.; Wersinger S. R.; Hesse R. A.; Stachowiak E. K.; Stachowiak M. K.; Papke R. L.; Lippiello P. M.; et al. TC-5619: An alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem. Pharmacol. 2009, 78, 803–812. 10.1016/j.bcp.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurov A. A.; Kombo D. C.; Hauser T. A.; Miao L.; Dull G.; Genus J. F.; Fedorov N. B.; Benson L.; Sidach S.; Xiao Y.; et al. Discovery of (2S,3R)-N-[2-(Pyridin-3-ylmethyl)-1-azabicyclo[2.2.2]oct-3-yl]benzo[b]furan-2-carboxamide (TC-5619), a Selective α7 Nicotinic Acetylcholine Receptor Agonist, for the Treatment of Cognitive Disorders. J. Med. Chem. 2012, 55, 9793–9809. 10.1021/jm301048a. [DOI] [PubMed] [Google Scholar]
- Lieberman J. A.; Dunbar G.; Segreti A. C.; Girgis R. R.; Seoane F.; Beaver J. S.; Duan N.; Hosford D. A. A Randomized Exploratory Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) for Cognitive Enhancement in Schizophrenia. Neuropsychopharmacology 2013, 38, 968–975. 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace T. L.; Bertrand D. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin. Ther. Targets 2013, 17, 139–155. 10.1517/14728222.2013.736498. [DOI] [PubMed] [Google Scholar]
- O’Neill J.; Broad L.; Sher E.; Astles P.; Zwart R. Selective α7 Nicotinic Acetylcholine Receptor Ligands for the Treatment of Neuropsychiatric Diseases. Drugs Future 2007, 32, 161–170. 10.1358/dof.2007.032.02.1064020. [DOI] [Google Scholar]
- Dinklo T.; Lesage A. S.; Grantham C. G. Desensitization characteristics of the human α7nAChR/5-HT3a chimera receptor. J. Mol. Neurosci. 2006, 30, 109–110. 10.1385/JMN:30:1:109. [DOI] [PubMed] [Google Scholar]
- Gurley D.; Lanthorn T. Nicotinic Agonists Competitively Antagonize Serotonin at Mouse 5-HT3 Receptors expressed in Xenopus Oocytes. Neurosci. Lett. 1998, 247, 107–110. 10.1016/S0304-3940(98)00306-1. [DOI] [PubMed] [Google Scholar]
- Macor J. E.; Gurley D.; Lanthorn T.; Loch J.; Mullen G.; Mack R. A.; Tran O.; Wright N.; Gordon J. C. The 5-HT3 Antagonist Tropisetron (ICS 205–930) is a Potent, Partial Agonist at α7 Nicotinic Receptors. Bioorg. Med. Chem. Lett. 2001, 11, 319–321. 10.1016/S0960-894X(00)00670-3. [DOI] [PubMed] [Google Scholar]
- Briggs C.; McKenna D. Activation and Inhibition of the Human α7 Nicotinic Acetylcholine Receptor by Agonists. Neuropharmacology 1998, 37, 1095–1102. 10.1016/S0028-3908(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Gallo-Torres H.; Brinker A.; Avigan M. Alosetron: Ischemic Colitis and Serious Complications of Constipation. Am. J. Gastroenterol. 2006, 101, 1080–1083. 10.1111/j.1572-0241.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- Beers W. H.; Reich E. Structure and activity of acetylcholine. Nature 1970, 228, 917–22. 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Dukat M. Central nicotinic receptor ligands and pharmacophores. Pharm. Acta Helv. 2000, 74, 103–14. 10.1016/S0031-6865(99)00022-9. [DOI] [PubMed] [Google Scholar]
- Toyohara J.; Hashimoto K. α7 Nicotinic Receptor Agonists; Potential Therapeutic Drugs for Treatment of Cognitive Impairments in Schizophrenia and Alzheimer’s Disease. Open Med. Chem. J. 2010, 4, 37–56. 10.2174/1874104501004020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.; Zusi F. C.; McDonald I. M.; King D.; Hill M. D.; Iwuagwu C.; Mate R. A.; Fang H.; Zhao R.; Wang B.; Cutrone J.; Ma B.; Gao Q.; Knox R.; Gallagher L.; Ferrante M.; Post-Munson D.; Molski T.; Easton A.; Lidge R.; Jones K.; Digavalli S.; Healy F.; Lentz K.; Benitex Y.; Clarke W.; Natale J.; Siuciak J.; Lodge N.; Zaczek R.; Denton R.; Morgan D.; Bristow L.; Macor J. E.; Olson R. E. Design and Synthesis of a New Series of 4-Heteroarylamino-1′-azaspiro[oxazole-5, 3′-bicyclo[2.2.2]octanes as α7 Nicotinic Receptor Agonists. 1. Development of Pharmacophore and Early Structure Activity Relationship. J. Med. Chem. 2016, 59, 11171–11181. 10.1021/acs.jmedchem.6b01506. [DOI] [PubMed] [Google Scholar]
- Hill M. D.; Fang H.; King H. D.; Iwuagwu C. I.; McDonald I. M.; Cook J.; Zusi F. C.; Mate R. A.; Knox R. J.; Post-Munson D.; Easton A.; Miller R.; Lentz K.; Clarke W.; Benitex Y.; Lodge N.; Zaczek R.; Denton R.; Morgan D.; Bristow L.; Macor J. E.; Olson R. Development of 4-Heteroarylamino-1′-azaspiro[oxazole-5,3′-bicyclo[2.2.2]octanes] as α7 Nicotinic Receptor Agonists. ACS Med. Chem. Lett. 2017, 8, 133–137. 10.1021/acsmedchemlett.6b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwuagwu C.; King D.; McDonald I. M.; Cook J.; Zusi F. C.; Hill M. D.; Mate R. A.; Fang H.; Knox R.; Gallagher L.; Post-Munson D.; Easton A.; Lidge R.; Benitex Y.; Siuciak J.; Lodge N.; Zaczek R.; Morgan D.; Bristow L. J.; Macor J. E.; Olson R. E. Design and Synthesis of a Novel Series of 4-Heteroarylamino-1′-azaspiro[oxazole-5,3′-bicyclo[2.2.2]octanes as α7 Nicotinic Receptor Agonists 2. Development of 4-Heteroaryl SAR. Bioorg. Med. Chem. Lett. 2017, 10.1016/j.bmcl.2017.01.058. [DOI] [PubMed] [Google Scholar]
- Because of the high free fraction observed for 1a, the uncorrected total plasma exposure was used for this assessment.
- Diller D. J. In silico hERG modeling: challenges and progress. Curr. Comput.-Aided Drug Des. 2009, 5, 106–121. 10.2174/157340909788451928. [DOI] [Google Scholar]
- cLogP values were calculated using the LogP calculator available in the ACD/Labs ChemSketch software package, v12.
- Papke R. L.; Porter Papke J. K. Comparative pharmacology of rat and human α7 nAChR conducted with net charge analysis. Br. J. Pharmacol. 2002, 137, 49–61. 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain C.; Kneen C.; Baker R. Synthesis of Indole Oxazolines, Potent 5-HT3 Antagonists. Tetrahedron Lett. 1990, 31, 2445–2448. 10.1016/S0040-4039(00)97385-4. [DOI] [Google Scholar]
- Swain C.; Baker R.; Kneen C.; Herbert R.; Moseley J.; Saunders J.; Seward E. M.; Stevenson G. I.; Beer M.; et al. Novel 5-HT3 Antagonists: Indol-3-ylspiro(azabicycloalkane-3,5′(4′H)oxazoles). J. Med. Chem. 1992, 35, 1019–1031. 10.1021/jm00084a007. [DOI] [PubMed] [Google Scholar]
- Swain C.; Kneen C.; Baker R. Synthesis of Indole Oxazolines, Potent 5-HT3 Antagonists. J. Chem. Soc., Perkin Trans. 1 1990, 3183–3186. 10.1039/p19900003183. [DOI] [Google Scholar]
- The structure and synthesis of 7, along with all pharmacological methods and screening data, is presented in the Supporting Information.
- Bristow L. J.; Easton A. E.; Li Y.; Sivarao D. V.; Lidge R.; Jones K. M.; Post-Munson D.; Daly C.; Lodge N. J.; Molski T.; Pieschl R.; Chen P.; Westphal R.; Zaczek R.; Gallagher L.; Hendricson A.; Cook J.; Iwuagwu C.; King D.; Macor J. E.; Olson R.; Morgan D.; Benitex Y. The Novel, Nicotinic Alpha7 Receptor Partial Agonist, BMS-933043, Improves Cognition and Sensory Processing in Preclinical Models of Schizophrenia. PLoS One 2016, 11, e0159996. 10.1371/journal.pone.0159996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01605994. https://clinicaltrials.gov/ct2/show/NCT01605994.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.