Abstract
The adult cerebral cortex can adapt to environmental change. Using monocular deprivation as a paradigm, we find that rapid experience-dependent plasticity exists even in the mature primary visual cortex. However, adult cortical plasticity differs from developmental plasticity in two important ways. First, the effect of adult, but not juvenile monocular deprivation is strongly suppressed by administration of barbiturate just prior to recording visual evoked potentials, suggesting that the effect of adult experience can be inactivated acutely. Second, the effect of deprivation is less persistent over time in adults than in juveniles. This correlates with the known decline in CREB function during maturation of the visual cortex. To compensate for this decline in CREB function, we expressed persistently active VP16-CREB and find that it causes adult plasticity to become persistent. These results suggest that in development and adulthood, the regulation of a trans-synaptic signaling pathway controls the adaptive potential of cortical circuits.
A primary function of the brain is to integrate the individual into a continually changing environment. Some aspects of this integration are accomplished through developmental processes, other aspects through learning. Although learning can occur throughout life, many behaviors, from language to sexual behavior, are shaped profoundly by early life experience. In this study, we have examined how the adaptive capacity of the cerebral cortex changes with maturation.
A classical model of developmental plasticity is ocular dominance plasticity (Wiesel and Hubel 1963; Hubel and Wiesel 1998). Hubel and Wiesel showed that closing one eye of an infant cat produced a visual cortex dominated by the nondeprived eye. Closing an eye of an adult cat was ineffective. Single-unit studies in a number of mammalian systems, ranging from rodents to primates, have found that ocular dominance plasticity is restricted to a period prior to puberty (Hubel and Wiesel 1970; Blakemore et al. 1978; Olson and Freeman 1980; Gordon and Stryker 1996; Issa et al. 1999; Fagiolini and Hensch 2000). The amount of deprivation required to alter the responses of visual cortical neurons depends on the animal's age. In the cat, during the peak of the critical period (4-5 wk of age), as little as 1 d of deprivation is sufficient to cause ocular dominance changes (Olson and Freeman 1975). Near the age of puberty, weeks or months of deprivation are necessary to induce changes observable by single-unit recordings, and the changes are thought to occur only in layers 2 and 3 of the visual cortex (Daw et al. 1992).
In the clinical literature, however, there are reports suggesting that improvement of visual acuity can occur in adult patients with amblyopia, a central disorder of visual acuity, following patching of the normal eye (Selenow and Ciuffreda 1986; Saulles 1987; Rutstein and Fuhr 1992; Wick and Wingard 1992). Furthermore, a lengthy period of monocular occlusion caused by a dense cataract (Sloper and Collins 1995) can cause significant changes in visual-evoked cortical potentials (VEP). Surprisingly, even a brief period of eye patching in adulthood can result in transient changes in the VEP (Tyler and Kaitz 1977; Tyler et al. 1979). These findings suggest that the primary visual cortex of adult humans may possess the capability of significant experience-dependent plasticity that has not been observed by single-unit recordings of anesthetized animals.
Recently, Sawtell et al. (2003) and we (Guire et al. 1999; Lickey and Gordon 2002) reported that effects of monocular deprivation in rodents extend past puberty when assayed by VEPs. These results suggest that ocular dominance plasticity in animal models may continue into adulthood. Here, we ask: Can we detect ocular dominance plasticity in the mature visual cortex? If so, how might adult cortical plasticity differ from developmental plasticity? We hypothesize that adult and developmental cortical plasticity will be different at the anatomical, physiological, and molecular levels.
Some anatomical and physiological differences have been described. For example, adult somatosensory cortical plasticity appears to involve horizontal connections of neurons in layers 2 and 3, whereas developmental plasticity of the somatosensory cortex also involves thalamic inputs to layer 4 (Fox 1992; Diamond et al. 1994; Skibinska et al. 2000). Plasticity in the adult visual cortex induced by retinal lesions (Kaas et al. 1990; Chino et al. 1992) involves horizontal connections of cortical neurons, not thalamic inputs (Darian-Smith and Gilbert 1994). Also, in adults, ocular dominance plasticity might be suppressed by increased activity of inhibitory connections (Hanover et al. 1999; Huang et al. 1999; Fagiolini and Hensch 2000) and by changes in the extracellular matrix (Pizzorusso et al. 2002). In contrast to these anatomical and physiological findings, little is known regarding how adult and developmental plasticity differ at the molecular level.
Molecular studies of neural plasticity have focused on activity-dependent neuronal signaling pathways. The activity-dependent kinases protein kinase A (PKA), extracellular-regulated protein kinase (ERK), and calmodulin-dependent protein kinase II (CamKII) are required for ocular dominance plasticity (Gordon et al. 1996; Beaver et al. 2001; Di Cristo et al. 2001). Visual deprivation is also associated with changes in gene expression (Worley et al. 1991; Rossi et al. 1999). A major mediator of activity-regulated gene expression is the transcription factor CREB, a calcium- and cAMP-dependent regulator (for review, see Shaywitz and Greenberg 1999; Mayr and Montminy 2001). CREB has been implicated specifically in long-lasting plasticity (Dash et al. 1990; Bourtchuladze et al. 1994; Yin et al. 1994; Casadio et al. 1999; Barco et al. 2002; Pittenger et al. 2002). CREB-mediated gene expression is induced in the visual cortex following monocular deprivation and declines with maturation (Pham et al. 1999, 2001). These findings suggest that adult ocular dominance plasticity, if detected, may be less stable than juvenile plasticity.
To test the hypothesis that adult and juvenile forms of ocular dominance plasticity have distinct properties, we have examined ocular dominance plasticity in mice using two functional assays, VEPs and visually evoked gene expression. We find plasticity at all ages examined, even in mature mice at 1 yr of age. However, we find that adult and juvenile forms of ocular dominance plasticity differ in interesting ways. In contrast to juvenile plasticity, adult plasticity is sensitive to anesthesia and lacks persistence over time. When we enhance CREB activity by expression of persistently active VP16-CREB, we find that the effect of monocular deprivation in adults becomes persistent. These results provide a molecular mechanism underlying the enduring nature of early experience and suggest how cortical adaptation might be improved in adult humans.
Results
Effect of monocular deprivation is observed in fully mature mice using visual evoked potentials
Mice possess ocular dominance plasticity similar to cats and primates (Dräger 1978; Gordon and Stryker 1996). However, whereas cats and primates have large binocular regions spanning almost all of the visual field, in mice, the visual fields of the two eyes overlap only in the central 40-60 degrees (Wagor et al. 1980). Consequently, the visual pathway of mice is dominated by contralateral projections, which comprise about 97% of all retinal projections (Jeffery 1984; Rice et al. 1995). Correspondingly, the primary visual cortex is divided into a large monocular zone (V1m), which represents the peripheral visual field, and a smaller binocular zone (V1b), which represents the central visual field. Ocular dominance plasticity occurs in V1b.
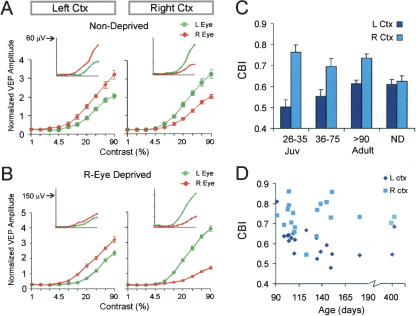
By recording VEPs in V1b in response to the presentation of a horizontal striped-pattern stimulus, we have characterized the effect of monocular deprivation in a large group of animals ranging in age from 24 d (P24) to 1 yr at the time of deprivation. Mice are sexually mature by 60 d of age, and are usually considered adult by 90 d of age. Figure 1A shows results of recordings from the left and right cortex of nondeprived mice. The inset graphs show amplitudes of the VEPs recorded from a single adult mouse, averaged over 30 stimulation trials, as a function of stimulus contrast. The amplitude of cortical potentials rises with increasing contrast of the stimulus. As expected, the responses for the left and right hemispheres were about equivalent and mirror image. In each case, the response to the contralateral eye was 1.5- to twofold greater than the response to the ipsilateral eye. To normalize for interanimal variability, we divided amplitude values by the mean amplitude recorded for the hemisphere. (We normalized with respect to the mean response, because the maximum responses showed more variability, and because, occasionally, the maximum response did not occur at the highest contrast.) The normalized amplitudes were then averaged to yield group contrast response curves. These curves reflect the relative strength of the two eyes in the entire group of nondeprived mice. They confirm that the left and right cortex have equivalent visually evoked responses.
Figure 1.
Visual-evoked potentials reveal an effect of monocular deprivation in fully mature mice. VEPs measured as a function of stimulus contrast in nondeprived animals show that responses of the left and right hemispheres are equivalent and mirror image (A). Following deprivation of 4 d, a difference develops between the left and right hemispheres (B). Inset graphs show the amplitude of evoked potentials as a function of contrast of the grating stimulus from a single adult animal. The amplitude (ordinate) increases with increasing contrast (abscissa) of the visual stimulus. In nondeprived animals, the amplitude from the contralateral visual input is about twofold larger than the amplitude from the ipsilateral input. Following deprivation, this difference sometimes declines in the left cortex but becomes greater in the right cortex. The large graphs show group-normalized contrast-response curves. The curves represent averages for 16 nondeprived mice and 19 right-eye deprived adults (ages P91-P415). (C) For the purpose of statistical comparison, amplitude data was transformed into contralateral bias indexes (CBI). In juveniles as well as adults, a difference between left and right hemispheres was observed following 4 d of monocular deprivation. There was no left/right difference in nondeprived (ND) mice. ANOVA (age group × hemisphere) yields a significant effect of hemispheres (P < 0.001) and a trend-level hemispheres × age interaction (P = 0.09). This trend-level interaction indicates that plasticity may weaken with age. All three deprived groups show a significant difference between left and right hemispheres (juvenile, P < 0.001; intermediate, P < 0.05; adult, P < 0.01, Bonferroni test corrected for four comparisons). n = 10 mice (juveniles), 14 (intermediate), 19 (adults). n = 16 mice for ND (four juveniles, four young, eight adults). CBIs for ND mice did not vary with age. (D) CBIs of individual hemispheres for all 19 right-eye-deprived adult animals. In almost all cases, the CBI of the right cortex was greater than the CBI of the left cortex, and this did not change with age.
Following deprivation of the right eye for 4 d, we found that the pattern of visually evoked responses changed (Fig. 1B). There was an effect of monocular deprivation at all ages examined. The inset graphs show contrast response curves for a single deprived adult mouse. In the left cortex, the right (deprived) eye responses were only slightly greater than the left (nondeprived) eye response. In contrast, in the right cortex, the responses evoked by the left eye were much greater than the right eye. This left/right difference contrasts with the mirror image pattern observed in nondeprived mice. Normalized group contrast response curves (large graphs), which reflect all monocularly deprived adults, show a clear effect of monocular deprivation; responses of the two eyes are more similar in the left cortex than in the right cortex; in the right cortex, the left eye dominates strongly. This left/right hemispheric difference is a within animal indicator of the effect of monocular deprivation.
To quantify the deprivation effect, we calculated a contralateral bias index (CBI) from the VEP amplitudes (see Materials and Methods). A contralateral bias index of 1 indicates that the hemisphere responds only to the contralateral eye, whereas a contralateral bias index of 0 indicates that the hemisphere responds only to the ipsilateral eye. In normal animals, contralateral bias indexes for both the left and right cortex are about 0.62 (Fig. 1C). Because we always deprived the right eye, a monocular deprivation effect is reflected in a greater contralateral bias index value for the right hemisphere and a lower value for the left hemisphere. Figure 1C shows that a 4-d monocular deprivation led to a large left/right asymmetry of the contralateral bias index in juveniles (see figure legend for statistics). With increasing age, there was a trend of progressive reduction in the left/right difference. In fully mature adult mice that were greater than 90 d old, the difference between the right and left hemispheres was clearly present, and in fact, was about half as large as in juveniles. In contrast, in nondeprived mice, the contralateral bias indexes of the right and left hemispheres were not different. Our sample of 19 adults, including several over 6 mo of age, showed a highly consistent effect of monocular deprivation (Fig. 1D). There were only two animals in which the contralateral bias index was lower in the right cortex than in the left cortex (P = 0.0004, binomial test).
Expression of the monocular deprivation effect in adults is sensitive to anesthesia
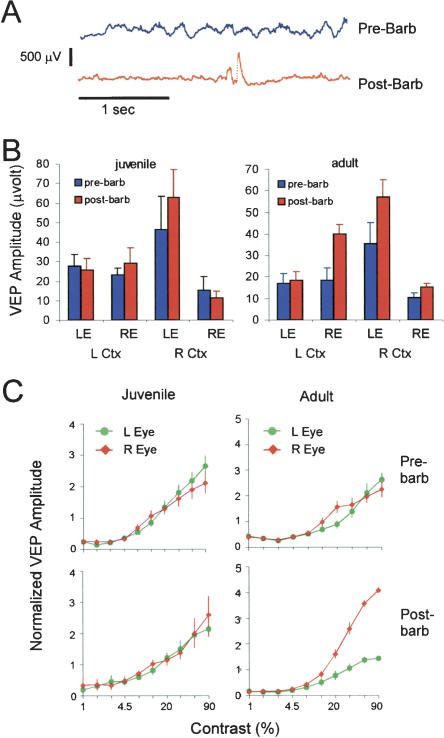
We hypothesized that the anesthetic state during recording might be another factor that permits or prevents the observation of adult plasticity. Our VEP recordings were performed under urethane anesthesia, which was also the anesthetic agent used by Sawtell et al. (2003), whereas all single-unit studies involving mice have used an anesthetic cocktail involving barbiturate. To determine whether barbiturate might affect the expression and detection of plastic changes, we performed pre/post barbiturate recordings. Mice were initially anesthetized with urethane and an initial set of VEP recordings was made. Subsequently, the animals were given a subanesthetic dose of pentobarbital (40 μg/g body weight of the mouse) by intraperitoneal injection. The effects of pentobarbital are reflected in the pattern of the electroencephalogram (EEG) (Fig. 2A). Under urethane only, the EEG showed an irregular pattern composed of a mixture of waveforms of different frequencies and amplitudes. Ten to 15 min following injection of pentobarbital, the EEG showed stereotyped intermittent high-amplitude bursts superimposed on a low amplitude background. These changes in the EEG pattern confirmed the adequacy of pentobarbital dosing.
Figure 2.
Barbiturate increases the amplitude of the contralateral deprived pathway in adults, but has no effect in juveniles. (A) Barbiturate (pentobarbital) alters the electroencephalogram pattern recorded from the visual cortex. Traces were obtained before and after intraperitoneal injection of pentobarbital, without changing the location of the recording electrode. (B) VEP amplitudes in mice right-eye-deprived for 4 d, before and after barbiturate. Amplitudes were unchanged for juveniles, but were frequently increased in adults, especially in the deprived pathways. (C) Group-normalized contrast-response curves from the left cortex of right-eye deprived mice. The curves represent averages of all cases (n = five animals for juveniles and adults). Barbiturate increased the difference between right- and left-eye responses in adult, but had no effect in juveniles. Error bars, SEM.
Surprisingly, the addition of pentobarbital did not reduce the amplitude of the VEP. Shown in Figure 2B are absolute evoked amplitudes (in microvolts). In adults right-eye deprived for 4 d, we observed increases in the amplitude of the deprived pathways, especially the crossed pathways. Pentobarbital did not affect the VEP amplitude in juveniles. The effect of barbiturate on ocular dominance is more easily appreciated from normalized contrast response curves of the left hemisphere. Both juvenile and adult groups shown in Figure 2C showed a deprivation effect in the left cortex, as the responses of the contralateral and ipsilateral eyes were similar. Following barbiturate, the adult animals showed an increase in the strength of the deprived pathway (right eye, red curve) relative to the nondeprived pathway (left eye, green curve). In contrast, the juveniles were not affected by barbiturate. In adults, as a result of barbiturate, the responses of the left hemisphere became very similar to the responses of the right hemisphere, obscuring evidence of an effect of monocular deprivation observed under urethane alone.
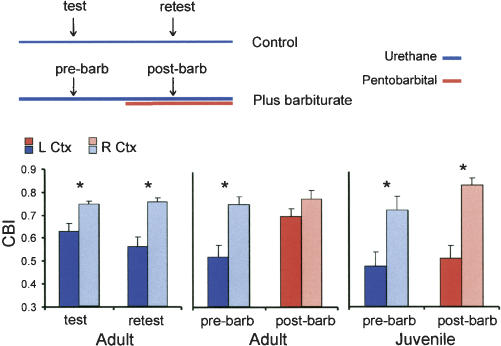
For the purpose of statistical comparison, we transformed amplitude data into contralateral bias indexes. Figure 3 shows results from animals deprived for 4 d. To rule out the possibility that the effect of monocular deprivation might be reversed by repeated visual stimulation during the first set of recordings, we performed a test/retest control. This control group was given a second set of recordings beginning about 10 min after completing the first set. The left/right hemispheric difference did not become smaller with retesting (Fig. 3). In contrast, following pentobarbital in adults, there was a large increase in the contralateral bias index of the left hemisphere. Thus, the left and right hemispheres became more similar, obscuring evidence of the effect of monocular deprivation. Note that the sample of adults used in the barbiturate test has a larger deprivation effect than average, but even this robust effect largely disappeared following barbiturate. In contrast, in juveniles, pentobarbital did not cause the difference between the left and right hemispheres to diminish. By 3-factor ANOVA, there was a significant age × drug treatment × hemisphere interaction (P < 0.01), which indicates that the barbiturate effect is different in adults compared with juveniles (see Fig. 3 legend for detailed statistics). These data show that effects of adult and juvenile experience are differentially affected by anesthetic state.
Figure 3.
The effect of monocular deprivation is obscured by anesthesia in adults, but not in juveniles. The schematic depicts the experimental design. All mice were right-eye deprived for 4 d prior to testing. In the control group (test/retest), recording was simply repeated beginning about 10 min after the first set of recordings. In the experimental group, barbiturate was given after the first recording, and then recording was repeated 10 min later. The bar graphs show the contralateral bias index (CBI) of the left (dark color) and right (light color) cortex for each treatment group. An effect of a 4-d monocular deprivation, as reflected in the difference of the CBIs of left and right hemispheres, was observed in all groups except the adult post-barbiturate group. Asterisks represent significant paired comparisons (P < 0.01, 3-factor ANOVA followed by Bonferroni test, n = five animals in all groups). When the adult and juvenile barbiturate treatment groups were compared directly, there was a significant age × treatment × hemisphere interaction (P < 0.01, 3-factor ANOVA). This indicates that the barbiturate effect is different in adults compared with juveniles.
An assay based on visually-evoked gene expression detects rapid and robust adult visual plasticity
Because the VEP measures synaptic potentials rather than action potentials, it is possible that the plasticity we found is “subthreshold”, and does not translate into changes in cellular function. Therefore, we wanted an assay that reflects integrative cellular function. To design such an assay, we took advantage of the fact that visual experience after a period of binocular deprivation leads to expression of Fos protein (c-fos and its variants) in the visual cortex (Rosen et al. 1992). In our assay, we subject mice to a period of right-eye deprivation, followed by suture of the left eyelid to produce binocular deprivation (Fig. 4A). The plasticity effect occurs during monocular deprivation, when the inputs coming from the left and right eyes are unmatched. Binocular deprivation serves to equalize activity to a low level throughout the visual cortex. Fos expression is then induced during a 2-h test period of normal vision that follows the reopening of the deprived (right) or nondeprived (left) eye. The mouse is not anesthetized during this test period.
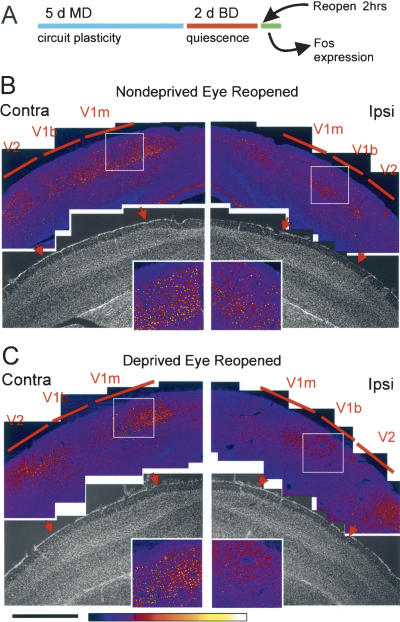
Figure 4.
A method for measuring ocular dominance plasticity using visually evoked gene expression. (A) Schematic of the Fos assay. Following monocular (MD) and binocular (BD) deprivation, either the deprived (right) or nondeprived (left) eye was reopened. Composite confocal images show cells labeled with Fos following reopening of the nondeprived eye (B) and deprived eye (C). Intensity of the Fos signal is shown in a color scale. The areas of interest are shown magnified in insets. Note that in V1b (right insets) the density of cells labeled with Fos is much lower when the deprived eye is opened than when the nondeprived eye is opened, whereas in V1m (left insets) it is not different. These samples received identical immunohistochemical reaction and imaging parameters. Bisbenzimide staining (gray images) of the same sections provided a reference for the location of V1. Red arrows indicate the boundaries of V1 as shown by cytoarchitectonics. “Contra” and “ipsi” refer to the reopened eye. Scale bar, 1 mm.
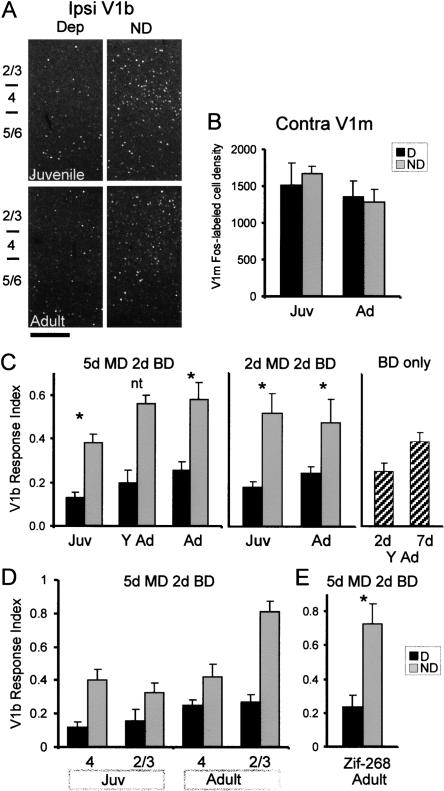
If monocular deprivation induces a plastic change, then the pattern of Fos expression might be different when the animal sees through the deprived eye as compared with the nondeprived eye. This was, in fact, what we observed in juvenile mice subjected to monocular deprivation for 5 d. When the nondeprived (left) eye was reopened, many labeled cells were visible in the binocular zone of V1 (V1b) (Fig. 4B). In contrast, reopening the deprived (right) eye produced very few Fos-labeled cells in V1b, especially the ipsilateral V1b (Fig. 4C). There was little effect of deprivation on responses in the monocular zone (V1m) (cf. boxed areas shown magnified in insets).
When we examined fully mature mice, we found an effect of monocular deprivation comparable to that observed in juvenile mice. Reopening the nondeprived eye produced markedly greater Fos expression in ipsilateral V1b than reopening the deprived eye (Fig. 5A). As we expected, there was no significant effect of deprivation in V1m (Fig. 5B).
Figure 5.
Visually evoked gene expression detects rapid plasticity in the binocular zone of V1 (V1b) in juvenile and adult mice. The age groups shown are juvenile (Juv, P26-P32), young adult (Y Ad, P45-P70), and adult (Ad, P90-P110). (A) Representative confocal images of juvenile and adult V1b following reopening of the nondeprived (ND) or deprived (Dep) eye. Scale bar, 200 microns. (B) Fos-labeled cell density in V1m contralateral to the reopened eye. There was no effect of deprivation (P > 0.2, 2-factor ANOVA). n = four to five animals in each group. (C) The V1b response index, defined as the ratio of ipsilateral V1b Fos density to contralateral V1m Fos density (see text), shows robust effects of MD. In the 5-d MD group, differences between deprived and nondeprived responses were significant for both juveniles and adults (P < 0.01, ANOVA with Bonferroni post-hoc test, see legend to Fig. 6; [asterisk] significant comparison; [nt] paired test not performed). In the 2-d MD group, there was a significant effect of deprivation both in juveniles and adults (P < 0.01, 2-factor ANOVA and Bonferroni post-hoc test). In young adult mice subjected to binocular deprivation (BD) of 2 or 7 d, followed by reopening of one eye, the longer duration of binocular deprivation did not lead to a lower V1b response index. Number of animals, left to right, 5-d MD, 4, 4, 3, 6, 4, 5; 2-d MD, 4, 3, 4, 4; BD-only, 4, 4. (D) Reanalysis of the 5-d monocular deprivation effect by layers. In juveniles, the deprivation effect was greater in layer 4, whereas in adults, it was greater in layer 2/3. (E) The effect of monocular deprivation in adults can be demonstrated using zif-268. There was a statistically significant difference between D and ND (n = four deprived, five nondeprived mice, P < 0.01, Student's t-test). This analysis used sections from the same animals as in C (adults, 5 da MD). Error bars, SEM. (Asterisks) Significant paired tests.
To control for interanimal variability in Fos expression, we defined the V1b response index as the density of labeled cells in ipsilateral V1b divided by the density of labeled cells in contralateral V1m of the same section. This analysis revealed plasticity in adults as well as juveniles after monocular deprivation of 5 d (Fig. 5C). The V1b response index was much greater when Fos expression was evoked by the nondeprived than by the deprived eye (P < 0.01, ANOVA followed by Bonferroni paired test, see legends to Figs. 5 and 6 for details). Furthermore, monocular deprivation for as little as 2 d is sufficient to cause changes in both juveniles and adults (Fig. 5C). Because our plasticity assay involves depriving the right-eye pathway longer than the left-eye pathway, it is possible that the changes we observed resulted from the longer period of inactivity of one pathway compared with the other. To determine whether this is the case, we subjected animals only to binocular deprivation for 2 or 7 d (Fig. 5C, right graph). We then induced Fos by reopening one eye for 2 h. We found that the longer period of deprivation did not reduce the V1b response index compared with the shorter period of deprivation.
Figure 6.
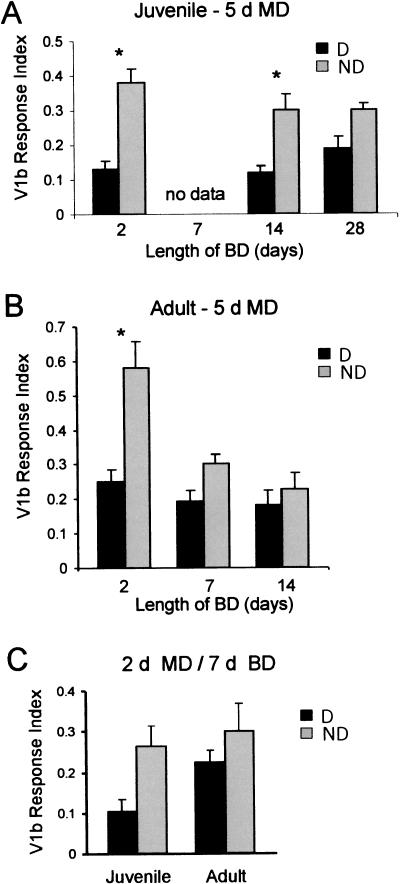
The effect of monocular deprivation is more persistent in juveniles than adults. (A) Juvenile (P24-P28) and (B) adult (>P90) mice were right-eye deprived (MD) for 5 d, followed by suture of the left eyelid (BD) for 2, 7 (adults only), 14, or 28 (juveniles only) d. Then, either the right eye (deprived, D) or left eye (nondeprived, ND) was reopened. Visually evoked Fos expression was analyzed. Three-factor ANOVA (factors: deprived or nondeprived eye reopened, length of BD, and age) showed significant effects of deprivation (P < 0.001), BD (P = 0.001), and age (P < 0.05). There was also a significant age × deprivation × BD interaction (P < 0.01). n = four to six mice for all groups. These results suggest that the effect of monocular deprivation in adults is less persistent than in juveniles. Asterisks show P < 0.01 (Bonferroni post-hoc test corrected for six comparisons). (C) Juvenile and adult mice were subjected to MD for 2 d, followed by BD of 7 d. Two-factor ANOVA revealed an overall significant effect of deprivation (P < 0.05, n = four to six animals in both groups). A Bonferroni test corrected for two comparisons shows that the deprivation effect in juveniles was near significance level (P = 0.07), whereas the effect in adults was not (P > 0.25).
Cortical plasticity may occur in different cortical layers in adults versus juveniles. In the somatosensory system, plasticity in adults has been observed primarily in layer 2/3 and not in layer 4, the thalamic input layer (Diamond et al. 1994; Fox 1994). To determine whether monocular deprivation affects layer 4 and layer 2/3 differently, we reanalyzed the 5-d monocular deprivation data (Fig. 5D). In this analysis, the density of Fos-expressing cells in a specific layer of V1b was divided by the density of Fos-expressing cells in the corresponding layer of V1m. We found that the effect of monocular deprivation in juveniles was similar for layers 4 and 2/3, but the plastic effect in adults was much greater in layer 2/3 than in layer 4. Therefore, adult ocular dominance plasticity differs from developmental plasticity in cortical location.
To determine whether a different gene marker would also reveal an effect of monocular deprivation in adults, we repeated the analysis on adults monocularly deprived for 5 d using zif-268, a protein known to be induced by vision (Worley et al. 1991). We found a robust effect of monocular deprivation in V1b similar to that obtained with Fos (P < 0.01, Student's t-test, see Fig. 5E). Taken together, these data indicate that visually evoked gene expression can be used as a population index of activity in an assay of cortical plasticity.
The effect of monocular deprivation is more persistent in juveniles compared with adults
To determine persistence of the monocular deprivation effect in juveniles versus adults, we interposed a temporal delay between the deprivation and the reopening of an eye. We subjected animals to binocular deprivation during the temporal delay. We chose binocular deprivation because changes during binocular deprivation are likely to reflect passive decay of plastic effects over time. An alternative strategy to test for persistence is to simply reopen both eyes, but binocular vision is known to engage activity-dependent recovery mechanisms (Mitchell et al. 2001; Kind et al. 2002).
We subjected mice to monocular deprivation for 5 d, followed by binocular deprivation of varying durations. The effect of monocular deprivation was assayed by using visually evoked Fos expression. In juveniles, we found that a 5-d monocular deprivation produces effects that survive at least 14 d of binocular deprivation (Fig. 6A). In contrast, in the adult, the effect of monocular deprivation was marginal after 7 d of binocular deprivation and disappears entirely after 14 d of binocular deprivation (Fig. 6B). A similar result was obtained using a 2-d monocular deprivation protocol (Fig. 6C). Compared with adults, juveniles show greater effect of monocular deprivation after 7 d of binocular deprivation. These data suggest that adult mice have decreased capacity for retention of plastic change in the visual cortex.
Enhancement of CREB activity causes the effect of monocular deprivation in adults to become persistent
CREB-mediated gene expression is induced following monocular deprivation of juveniles and declines with maturation of the visual cortex (Pham et al. 1999). We hypothesized that decline of CREB function with age might underlie the lack of stability of adult visual plasticity. To test this hypothesis, we used transgenic mice that express VP16-CREB, a chimeric construct consisting of CREB linked to the potent transcriptional activation domain of VP16 (Barco et al. 2002). The VP16 domain is not regulated by calcium or cAMP, and therefore, expression of VP16-CREB leads to constitutive up-regulation of CREB target genes (Barco et al. 2002).
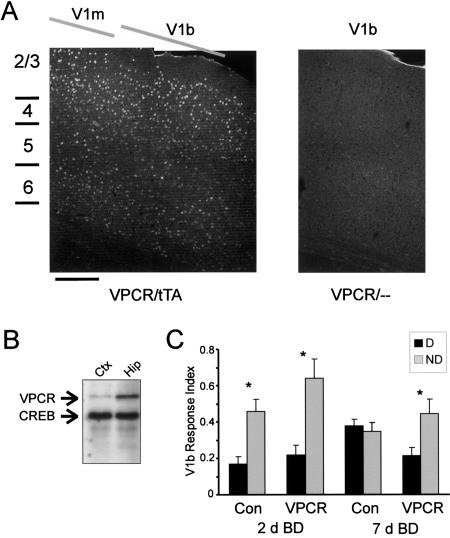
Expression of VP16-CREB was controlled by a doxycycline-regulated promoter (Barco et al. 2002). This system requires both VP16-CREB and doxycycline-responsive transactivator (tTA) transgenes. Two weeks prior to experimentation, we removed doxycycline from the diet in order to induce expression of VP16-CREB. Appropriate expression was confirmed by immunofluorescence and Western blotting (Fig. 7A,B). Using an antibody to the VP16 domain, expression of VP16-CREB was observed in mice doubly transgenic for VP16-CREB and tTA, and not in mice carrying VP16-CREB only. We found expression throughout V1 and in all layers of the visual cortex, although the highest expression was in layers 2 and 3 (Fig. 7A). Western blotting using a CREB antibody showed that the amount of VP16-CREB expression was low relative to CREB expression, with higher expression in the hippocampus (Fig. 7B). This matches the pattern observed with immunofluorescence (Barco et al. 2002).
Figure 7.
Enhancement of CREB activity by expression of VP16-CREB leads to increased stability of cortical plasticity in adults. (A) VP16-CREB is expressed in the visual cortex of VP16-CREB/tTA double-transgenic mice, but not in VP16-CREB/- single transgenic mice. The confocal composites shown are of VP16 immunofluorescence. VP16-CREB expression was strongest in layers 2 and 3, and weaker in other layers of the cortex; there was similar expression in V1m and V1b (location of V1m and V1b determined by bisbenzimide cytoarchitectonics). Scale bar, ∼200 μm. (VPCR) VP16-CREB; (tTA) tetracycline-regulated transactivator. (B) Western blotting using visual cortex and hippocampus samples from VP16-CREB/tTA mice. The antibody detected much less VP16-CREB than CREB; there was much less expression of VP16-CREB in the cortex than in the hippocampus. (C) VP16-CREB expressing mice show greater stability of ocular dominance plasticity. Mice were subjected to 2 d of monocular deprivation, followed by 2 or 7 d of binocular deprivation. We then opened the deprived (D) or nondeprived (ND) eye. After 2 d of binocular deprivation, there was a highly significant effect of deprivation (P < 0.001, ANOVA), but no effect of transgene and no significant deprivation × transgene interaction. This indicates that both VP16-CREB/tTA mice and controls exhibit ocular dominance shift after 2 d of binocular deprivation. After 7 d of binocular deprivation, there was a deprivation effect in VP16-CREB mice, but not in control mice. ANOVA shows a significant deprivation × transgene interaction (P = 0.02). (Asterisks) Significant paired comparisons using the Bonferroni correction (P < 0.01 for 2 d BD; P < 0.05 for 7 d BD). n = five to eight mice for all groups.
To determine whether VP16-CREB mice might show enhanced persistence of adult plasticity, we subjected mice (>P90) to 2 d of monocular deprivation, followed by either 2 or 7 d of binocular deprivation (Fig. 7C). To quantify the effect of monocular deprivation, we used zif-268 instead of Fos as an activity marker. The reason for our use of zif-268 here is that Fos is up-regulated constitutively in VP16-CREB/tTA mice (Barco et al. 2002). This constitutive expression of Fos was observed in the visual cortex even in the absence of visual input (data not shown). In contrast, zif-268 is not up-regulated in VP16-CREB mice (A. Barco and E. Kandel, unpubl.). We found that after 2 d of binocular deprivation, both controls and VP16-CREB mice showed robust plastic changes. In both groups, cortical responses to opening of the nondeprived eye were greater than responses to the deprived eye. After 7 d of binocular deprivation, the controls showed no effect of deprivation; opening of the nondeprived or deprived eye produced identical responses. However, in VP16-CREB mice, the nondeprived eye produced a twofold greater response than the deprived eye. These results suggest that enhancement of CREB activity increases the stability of adult ocular dominance plasticity.
Discussion
There is clear evidence for plasticity in the adult brain, and yet studies of sensory systems development suggest the special importance of childhood experience. To study how childhood experience differs from adult experience, we have reexamined the effects of monocular deprivation. We have found that there is robust ocular dominance plasticity in the adult primary visual cortex by using two assays, VEPs and visually evoked gene expression, but this adult plasticity in the visual cortex differs from juvenile visual plasticity. First, adult plasticity can be inactivated by anesthesia. Second, adult plasticity lacks persistence over time. Remarkably, up-regulation of CREB activity causes adult ocular dominance plasticity to become persistent. Thus, the well-documented decline of CREB activity may underlie the loss of stability of cortical plasticity with maturation.
Assay method is critical in the observation of adult cortical plasticity
Although recordings of single units in anesthetized animals have not revealed evidence of ocular dominance plasticity in post-pubertal animals, we have used population indexes of neural activity and have found rapid plasticity in the adult primary visual cortex. Using visually evoked gene expression, we have found that as little as 2 d of monocular deprivation is sufficient to induce a robust plastic effect in fully mature mice older than 3 mo. Using VEPs, we have found an effect of monocular deprivation even in mice 1 yr of age.
A recent study by Sawtell et al. (2003) also reported changes in the VEP after monocular deprivation. In comparing our VEP results with Sawtell et al. (2003), it is important to note that this previous study considered 60-day-old mice as adults, and did not record from mice older than 90 d, whereas, here, we define as adults animals older than 90 d. Our VEP data show plasticity of the hemisphere ipsilateral to the nondeprived eye in mice younger than 90 d, but do not always show plasticity in this hemisphere in mice older than 90 d. This is consistent with data on 60-90-day-old adults reported by Sawtell et al. (2003). In contrast, we found that plasticity of the hemisphere contralateral to the nondeprived eye continues past 90 d of age. Sawtell et al. (2003) did not measure ocular dominance in the contralateral hemisphere.
The observation of plasticity in adults is likely to be dependent on procedural details of the assay. There seem to be three important factors: anesthesia, hemispheric differences, and sampling of neural activity. (1) Anesthesia is clearly important. An anesthetic cocktail containing barbiturate has been used in nearly all single-unit studies involving mice (for examples, see Gordon and Stryker 1996; Hensch et al. 1998; Hanover et al. 1999; Fagiolini and Hensch 2000) and in many studies involving other mammals (Wiesel and Hubel 1963; Issa et al. 2000; Mower et al. 2002); yet, we have found that barbiturate obscures the effect of monocular deprivation in adults. (2) Almost all prior studies of ocular dominance plasticity in mice have recorded only from the hemisphere ipsilateral to the nondeprived eye (for examples, see Gordon and Stryker 1996; Gordon et al. 1996; Hensch et al. 1998; Huang et al. 1999). However, our results show (Fig. 1) that in adults over 90 d of age, the hemisphere ipsilateral to the nondeprived eye (left hemisphere, in our case) is not distinguishable from controls. The effect of monocular deprivation is detected most readily when the right and left hemispheres are compared. In rodents, >97% of retinal axons project to the contralateral hemisphere (Jeffery 1984; Rice et al. 1995), and this pre-existing bias may drive plasticity more efficiently in the hemisphere contralateral to the nondeprived eye. (3) Recordings of single units and VEPs detect different types of electrical activity. The VEP reflects summated synaptic potentials produced by synchronous activity of many cells, and therefore, may yield a different sampling of activity compared with recordings of single cells.
To verify that changes observed by VEPs reflect changes in cellular function, we developed a gene expression assay that does not involve anesthesia and that reflects a natural visually evoked activity. This assay is relatively straightforward and could be applied easily to a large group of mice. The gene expression assay has obvious limitations compared with single-unit recording, which has yielded spectacular insights into receptive fields and single neuron response properties. But here, we are not concerned with plasticity of individual response properties or receptive fields. The gene transcriptional response measured by our technique is an integration of activity-dependent signaling over minutes to hours, as opposed to the millisecond time-frame of electrophysiology. Thus, a gene-expression assay may reveal effects of deprivation not detected by an assay of single-cell electrical activity. The results of the gene expression assay are in agreement with data derived from VEPs. This is significant, because sensitivity thresholds derived from VEPs closely agree with results derived from psychophysics (Lombroso et al. 1969; Tyler et al. 1979; Allen et al. 1986).
Effect of adult ocular dominance plasticity can be inactivated
The effect of barbiturate on detection of adult ocular dominance plasticity shows that some plastic changes resulting from adult experience can be inactivated acutely. In contrast, the effect of developmental plasticity appears to be resistant to inactivation by anesthesia. These results suggest that cortical plasticity in juveniles and adults involve different processes. A major effect of barbiturate is enhancement of GABA receptor function (Olsen 1987; Saunders and Ho 1990), which mediates inhibition in the cortex. Barbiturate also interferes with glutamate (Hobbs et al. 1996) and nicotinic acetylcholine receptors (Tonner and Miller 1995; Violet et al. 1997; Downie et al. 2000), which have net excitatory effects in the cortex. The result of these effects should be depression of neural activity. However, we found that in adults, the predominant effect of barbiturate on the VEP is enhancement of the deprived crossed pathway. This observation suggests that at the doses used, the primary effect is disruption of inhibitory circuitry in the visual cortex. Another possibility is that barbiturate causes some visually evoked responses to become more synchronized, increasing the amplitude of summated responses.
We think that the effect of barbiturate reveals a mechanistic difference between adult and juvenile plasticity, rather than a maturational difference in sensitivity to the drug. Specifically, the differential effect of barbiturate on juveniles and adults does not reflect gross differences in the maturation of GABA synapses, because the GABA system in the visual cortex is mature by P30 (Luhmann and Prince 1991). Differences in metabolism of barbiturate or penetration of the drug into the cortex are unlikely to confound our results, because in each case, we confirmed that the animal received an adequate dose by observing evidence of barbiturate narcosis on the EEG. Finally, the effect of barbiturate is unlikely to have been caused by toxic effects on visual cortical neurons, as visual responses were enhanced.
We hypothesize that barbiturate interferes with a specific aspect of neuronal function that is essential for expression of adult, but not juvenile plasticity. Because a major effect of barbiturate is potentiation of GABAA receptors, we speculate that the effects of monocular deprivation on GABA neurotransmission might be different in adults versus juveniles. Monocular deprivation decreases GABA synthesis in the visual cortex to a greater extent in juveniles than adults (Hendry and Jones 1988). This suggests that barbiturate potentiation of GABA receptor function might mask the effect of monocular deprivation in adults more than in juveniles. In slices of adult visual cortex, electrical stimulation of layer 4 produces potentiation of field potential responses in layers 2 and 3. This effect appears to be caused by depression of inhibitory inputs onto pyramidal cells and not by potentiation of excitatory inputs; in juveniles, there is both potentiation of excitatory inputs and depression of inhibitory inputs (Yoshimura et al. 2003). Therefore, potentiation of GABA receptors by barbiturates might obscure the effect of monocular deprivation in adults, but not in juveniles.
Molecular mechanisms that regulate plasticity during maturation of the visual cortex
Whereas plasticity does not disappear in the adult visual cortex, properties of plasticity change with maturation. An important property of plasticity is its persistence. Some types of sensory plasticity are transient (Pettet and Gilbert 1992), lasting only minutes, whereas effects of prolonged juvenile deprivation may be life-long (Mitchell 1988). Yet, there have not been systematic studies of changes in persistence of sensory plasticity with maturation. Using a visually evoked gene expression assay, we have found that, compared with juveniles, the effect of monocular deprivation in adults is not as persistent over time. Whereas Sawtell et al. (2003) concluded that adult ocular dominance plasticity is stable, they used a long period of monocular deprivation (12 d) and also did not compare directly the persistence of plasticity in adults versus juveniles. Our results suggest that CREB activity is a crucial factor that regulates the persistence of visual cortical plasticity with maturation.
CREB is a calcium- and cAMP-regulated transcription factor critical for some forms of neuronal plasticity (Dash et al. 1990; Yin et al. 1994; Silva et al. 1998; Mayr and Montminy 2001; Barco et al. 2002; Mower et al. 2002; Pittenger et al. 2002). CREB protein expression does not appear to change significantly with age, but CREB function as assayed by a CRE-lacZ transgene reporter declines with cortical maturation (Pham et al. 1999). In vitro studies of long-term potentiation (LTP) have led to the hypothesis that CREB enhances the consolidation phase of synaptic plasticity, producing a long-lasting enhancement of synaptic efficacy (Casadio et al. 1999; Barco et al. 2002).
In VP16-CREB mice, CREB target genes are expressed in the absence of high-frequency stimulation. In hippocampal slices from these mice, a single stimulus train produces long-lasting LTP, presumably because CREB-regulated proteins are already present for insertion into active synapses (Barco et al. 2002). This is consistent with our finding that, in the visual cortex of adults, monocular deprivation causes plastic changes, but these changes are not very stable, because CREB activity is low in adults. The expression of VP16-CREB rescues this deficit, enabling stable maintenance of plastic change.
Adult ocular dominance plasticity and visual perception
The relationship between the adult form of ocular dominance plasticity that we have found and visual perception is not yet clear. In humans, monocular eye patching in adulthood for treatment of amblyopia appears to be partially effective in enhancing visual acuity in the amblyopic eye (see Introduction). However, ocular dominance plasticity in adults may have more impact on stereopsis, which relies on the integration of information from the two eyes. In fact, patients with prolonged and dense monocular cataracts have been reported to suffer from a disorder of central fusion (Pratt-Johnson and Tillson 1989; Sharkey and Sellar 1994). Because visual perception in rodents is difficult to measure, we know little concerning the effect of visual deprivation in these animals. A recent study found that a 2-wk visual deprivation in juvenile mice (P19-P32) results in impairment of visual acuity, whereas a similar deprivation in older animals (P32-P51) may not (Prusky and Douglas 2003). However, the design of this study did not allow the detection of nonpersistent changes in visual acuity, because all animals were given a 20-d binocular recovery between the end of deprivation and testing. During this period of binocular vision, the mice were trained in the visual task. Because we have found that adult plasticity is not persistent over time, any change of acuity in adults should be lost during the prolonged period of binocular visual experience.
Taken altogether, our results show potential for rapid change in the adult cortex, but also reveal its limitations. These data may have relevance for rehabilitation of adult patients with neuropsychiatric disorders, which frequently rely on techniques based on learning. Whereas repetition can enhance retention of learned behavior, our results suggest that the mobilization of CREB signaling might make effects of therapies more permanent.
Materials and Methods
Antibodies and mice
C-Fos, Zif-268, and VP16 antibodies were obtained from Santa Cruz Biotechnology. CREB antibody was obtained from Cell Signaling Technologies. VP16-CREB and tTA mice were maintained as separate lines on C57BL/6 background. Genotyping was by PCR (Barco et al. 2002). The mice were mated in the presence of doxycycline (200 μg/kg regular mouse diet, Bio-Serv, Inc.) and the progeny were maintained on doxycycline until 2 wk prior to experimentation. Wild-type mice (pigmented C57BL/6) were purchased from Charles River or Simonsen.
Surgeries
Eyelid suture was performed as described previously (Gordon and Stryker 1996). If openings developed during the course of deprivation, the mice were not used. Following reopening, the cornea was inspected for clarity. Mice were not used if their corneas were not clear.
VEP recordings
Mice were anesthetized with an IP injection of 20% urethane solution (7-7.5 μL per gram body weight of the mouse). Cerebral edema was controlled with dexamethasone, and vital signs were monitored throughout. After positioning the animal in a stereotaxic instrument, craniotomies were made over the primary visual cortex of both hemispheres. Pipette electrodes, containing 0.5% pontamine blue in 0.9% saline were positioned 550 microns vertically below the dura in V1b (or about 400 microns deep relative to the plane tangential to the cortical surface). The deprived eye was reopened, and the swept contrast VEP was recorded as described previously (Allen et al. 1986; Guire et al. 1999). The recording position was mapped and verified to represent the central 40 degrees (or binocular portion) of the visual field. The recording location was also verified by iontophoretic deposition of pontamine blue and histological examination after completion of the experiment.
PowerDiva software from Anthony Norcia (Smith Kettlewell Institute of Visual Sciences, San Francisco) was used for data acquisition and analysis. The visual stimulus was a horizontal sinusoidal contrast grating (0.04 cycles/degree, mean luminance 7.5 ed/m2) covering the central 90 degrees of the visual field. The grating reversed phase 6.2 times per sec. One stimulus presentation (1 trial) lasted 9.7 sec and consisted of a contrast sweep from 1% to 90% contrast in 9 log steps (i.e., the stimulus increases in contrast in a “staircase” fashion as follows: 1%, 1.65%, 2.7%, 4.5%, 7.4%, 12.2%, 20%, 33%, 55%, 90%). The response was extracted from noise using digital filtration (Tang and Norcia 1995). The visually evoked response has frequencies that are even multiples (or harmonics) of the stimulus frequency. We defined as the response the sum of the amplitudes of the first three even harmonics. An example of response as a function of contrast is shown in Figure 1. Responses evoked over the range of from 4.5% to 90% contrast were averaged, yielding a mean value that we define as “Amplitude” of response. Averaging the responses evoked by contrasts of from 4.5% to 90% yields more consistent results than averaging all amplitudes, because the perceptual threshold for mice is about 4%; below 4% contrast, there is only noise. The contralateral bias index (CBI) was defined as (Ac)/(Ac+Ai), where Ac and Ai are response amplitudes from the contralateral eye and ipsilateral eye, respectively.
In experiments involving barbiturate, sodium pentobarbital (6.5 mg/mL in saline) was given by intraperitoneal injection at a dose of 40 μg/g body weight of the mouse. After waiting 10 min and observing evidence of barbiturate narcosis in the EEG, recording was resumed as above.
Gene expression assay of plasticity
Following deprivation, one eyelid was opened and the mouse was allowed 2 h of monocular visual experience. The animal was then deeply anesthetized with pentobarbital and immediately perfused with 4% paraformaldehyde. The brain was postfixed overnight and sectioned coronally on a vibrating microtome at 40 microns. Sections through the middle of V1 in the rostrocaudal axis were selected for analysis. Immunofluorescence staining for Fos or zif-268 was performed on free-floating sections using standard procedures. To minimize artifacts arising from variability in immunofluorescence staining, we always reacted samples from comparison groups at the same time. Contralateral V1m and ipsilateral V1b sections were always reacted in the same well of a multi-well plate.
Immunofluorescence data were collected by confocal microscopy (Bio-Rad Radiance 2000) using a 10× objective. We used identical confocal settings for images from the same animal (i.e., data that go into the numerator and denominator to calculate the V1b response index were obtained in an identical manner). Bisbenzimide staining allowed visualization of cytoarchitectonics. V1 was located easily by the prominence of layer 4 compared with surrounding V2, as demonstrated by Antonini et al. (1999). The binocular zone (V1b) is the lateral portion of V1, and can be distinguished from V1m by the slightly higher density of cells in layer 4 and by the presence of cells responding to the ipsilateral eye. V1b is at least 500 microns wide (medial to lateral) in the sections that we sampled.
Quantification of 512 by 512 pixels confocal images was performed blind using ImageJ software. Images were thresholded to remove background and quantified by using the particle counter function. The threshold was determined on the basis of the intensity of fluorescence staining of cells in V1m. The intensity of the 10 brightest cells was measured by ImageJ and averaged. A value 0.4-0.5 times the average cell intensity was used as a threshold, which was kept constant for all images from the same animal. A portion of V1b was selected for counting; this area was 300 microns wide, extending from the V1/V2 border medially, and spanned layers 2/3 and 4. The counting area was kept constant throughout the experiment. Because a typical Fos-labeled nucleus is about 20 pixels in area, we counted particles that were >10 pixels in area. An adjustment was made for large particles (>40 pixels in area) that invariably resulted from closely spaced cells. The V1b response index was defined as the V1b cell density of the ipsilateral hemisphere, divided by V1m cell density of the contralateral hemisphere (ipsi and contra defined with respect to the reopened eye). In experiments where zif-268 was used as the activity marker, there was a significant level of background zif-268 expression independent of vision. This background expression (in V1m contralateral to the non-reopened eye) was subtracted from cell density values used to calculate the V1b response index.
Western blotting
Preparation of tissue extracts and Western blotting were performed as described previously (Obrietan et al. 1999).
Statistical analysis
Systat statistical software was used for analysis. For statistical comparisons involving only two groups, we used Student's t-test. For comparisons involving multiple groups, we used analysis of variance (ANOVA), followed by paired tests using Bonferroni corrections. The Bonferroni correction adjusts for the increased chance that a spurious positive result may be obtained when multiple tests are performed.
Acknowledgments
We thank Aundrea Graves, Jason Puracal, and Kim Chen for excellent technical assistance. This work was supported by grants from the National Institutes of Health, the Whitehall Foundation, and the Medical Research Foundation of Oregon. E.R.K. is an investigator with the Howard Hughes Medical Institute.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.75304.
References
- Allen, D., Norcia, A.M., and Tyler, C.W. 1986. Comparative study of electrophysiological and psychophysical measurement of the contrast sensitivity function in humans. Am. J. Optom. Physiol. Opt. 63: 442-449. [DOI] [PubMed] [Google Scholar]
- Antonini, A., Fagiolini, M., and Stryker, M.P. 1999. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19: 4388-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco, A., Alarcon, J.M., and Kandel, E.R. 2002. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108: 689-703. [DOI] [PubMed] [Google Scholar]
- Beaver, C.J., Ji, Q., Fischer, Q.S., and Daw, N.W. 2001. Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat. Neurosci. 4: 159-163. [DOI] [PubMed] [Google Scholar]
- Blakemore, C., Garey, L.J., and Vital-Durand, F. 1978. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J. Physiol. 283: 223-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze, R., Frenguelli, B., Blendy, J., Cioffi, D., Schutz, G., and Silva, A.J. 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59-68. [DOI] [PubMed] [Google Scholar]
- Casadio, A., Martin, K.C., Giustetto, M., Zhu, H., Chen, M., Bartsch, D., Bailey, C.H., and Kandel, E.R. 1999. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221-237. [DOI] [PubMed] [Google Scholar]
- Chino, Y.M., Kaas, J.H., Smith III, E.L., Langston, A.L., and Cheng, H. 1992. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Res. 32: 789-796. [DOI] [PubMed] [Google Scholar]
- Darian-Smith, C. and Gilbert, C.D. 1994. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 368: 737-740. [DOI] [PubMed] [Google Scholar]
- Dash, P.K., Hochner, B., and Kandel, E.R. 1990. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345: 718-721. [DOI] [PubMed] [Google Scholar]
- Daw, N.W., Fox, K., Sato, H., and Czepita, D. 1992. Critical period for monocular deprivation in the cat visual cortex. J. Neurophysiol. 67: 197-202. [DOI] [PubMed] [Google Scholar]
- Di Cristo, G., Berardi, N., Cancedda, L., Pizzorusso, T., Putignano, E., Ratto, G.M., and Maffei, L. 2001. Requirement of ERK activation for visual cortical plasticity. Science 292: 2337-2340. [DOI] [PubMed] [Google Scholar]
- Diamond, M.E., Huang, W., and Ebner, F.F. 1994. Laminar comparison of somatosensory cortical plasticity. Science 265: 1885-1888. [DOI] [PubMed] [Google Scholar]
- Downie, D.L., Franks, N.P., and Lieb, W.R. 2000. Effects of thiopental and its optical isomers on nicotinic acetylcholine receptors. Anesthesiology 93: 774-783. [DOI] [PubMed] [Google Scholar]
- Dräger, U.C. 1978. Observations on monocular deprivation in mice. J. Neurophysiol. 41: 28-41. [DOI] [PubMed] [Google Scholar]
- Fagiolini, M. and Hensch, T.K. 2000. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404: 183-186. [DOI] [PubMed] [Google Scholar]
- Fox, K. 1992. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. 12: 1826-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1994. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J. Neurosci. 14: 7665-7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J.A. and Stryker, M.P. 1996. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 16: 3274-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J.A., Cioffi, D., Silva, A.J., and Stryker, M.P. 1996. Deficient plasticity in the primary visual cortex of α-calcium/calmodulin-dependent protein kinase II mutant mice. Neuron 17: 491-499. [DOI] [PubMed] [Google Scholar]
- Guire, E.S., Lickey, M.E., and Gordon, B. 1999. Critical period for the monocular deprivation effect in rats: Assessment with sweep visually evoked potentials. J. Neurophysiol. 81: 121-128. [DOI] [PubMed] [Google Scholar]
- Hanover, J.L., Huang, Z.J., Tonegawa, S., and Stryker, M.P. 1999. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci. 19: RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry, S.H. and Jones, E.G. 1988. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron 1: 701-712. [DOI] [PubMed] [Google Scholar]
- Hensch, T.K., Fagiolini, M., Mataga, N., Stryker, M.P., Baekkeskov, S., and Kash, S.F. 1998. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, W.R., Rall, T.W., and Verdoorn, T.A. 1996. Hypnotics and sedatives; ethanol. In The pharmacological basis of therapeutics, Ninth Edition (ed. A.G. Gilman), pp. 361-396. McGraw-Hill, New York.
- Huang, Z.J., Kirkwood, A., Pizzorusso, T., Porciatti, V., Morales, B., Bear, M.F., Maffei, L., and Tonegawa, S. 1999. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739-755. [DOI] [PubMed] [Google Scholar]
- Hubel, D.H. and Wiesel, T.N. 1970. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 206: 419-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1998. Early exploration of the visual cortex. Neuron 20: 401-412. [DOI] [PubMed] [Google Scholar]
- Issa, N.P., Trachtenberg, J.T., Chapman, B., Zahs, K.R., and Stryker, M.P. 1999. The critical period for ocular dominance plasticity in the Ferret's visual cortex. J. Neurosci. 19: 6965-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa, N.P., Trepel, C., and Stryker, M.P. 2000. Spatial frequency maps in cat visual cortex. J. Neurosci. 20: 8504-8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, G. 1984. Retinal ganglion cell death and terminal field retraction in the developing rodent visual system. Brain Res. 315: 81-96. [DOI] [PubMed] [Google Scholar]
- Kaas, J.H., Krubitzer, L.A., Chino, Y.M., Langston, A.L., Polley, E.H., and Blair, N. 1990. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 248: 229-231. [DOI] [PubMed] [Google Scholar]
- Kind, P.C., Mitchell, D.E., Ahmed, B., Blakemore, C., Bonhoeffer, T., and Sengpiel, F. 2002. Correlated binocular activity guides recovery from monocular deprivation. Nature 416: 430-433. [DOI] [PubMed] [Google Scholar]
- Lickey, M.E. and Gordon, B. 2002. Monocular deprivation effect in the primary visual cortex of adult mice. Soc. Neurosci. Abs. 28: 75.21. [Google Scholar]
- Lombroso, C.T., Duffy, F.H., and Robb, R.M. 1969. Selective suppression of cerebral evoked potentials to patterned light in amblyopia ex anopsia. Electroencephalogr. Clin. Neurophysiol. 27: 238-247. [DOI] [PubMed] [Google Scholar]
- Luhmann, H.J. and Prince, D.A. 1991. Postnatal maturation of the GABAergic system in rat neocortex. J. Neurophysiol. 65: 247-263. [DOI] [PubMed] [Google Scholar]
- Mayr, B. and Montminy, M. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2: 599-609. [DOI] [PubMed] [Google Scholar]
- Mitchell, D.E. 1988. The extent of visual recovery from early monocular or binocular visual deprivation in kittens. J. Physiol. 395: 639-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D.E., Gingras, G., and Kind, P.C. 2001. Initial recovery of vision after early monocular deprivation in kittens is faster when both eyes are open. Proc. Natl. Acad. Sci. 98: 11662-11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower, A.F., Liao, D.S., Nestler, E.J., Neve, R.L., and Ramoa, A.S. 2002. cAMP/Ca2+ response element-binding protein function is essential for ocular dominance plasticity. J. Neurosci. 22: 2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan, K., Impey, S., Smith, D., Athos, J., and Storm, D.R. 1999. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J. Biol. Chem. 274: 17748-17756. [DOI] [PubMed] [Google Scholar]
- Olsen, R.W. 1987. GABA-drug interactions. Prog. Drug Res. 31: 223-241. [DOI] [PubMed] [Google Scholar]
- Olson, C.R. and Freeman, R.D. 1975. Progressive changes in kitten striate cortex during monocular vision. J. Neurophysiol. 38: 26-32. [DOI] [PubMed] [Google Scholar]
- ____. 1980. Profile of the sensitive period for monocular deprivation in kittens. Exp. Brain Res. 39: 17-21. [DOI] [PubMed] [Google Scholar]
- Pettet, M.W. and Gilbert, C.D. 1992. Dynamic changes in receptive-field size in cat primary visual cortex. Proc. Natl. Acad. Sci. 89: 8366-8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, T.A., Impey, S., Storm, D.R., and Stryker, M.P. 1999. CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron 22: 63-72. [DOI] [PubMed] [Google Scholar]
- Pham, T.A., Rubenstein, J.L., Silva, A.J., Storm, D.R., and Stryker, M.P. 2001. The cre/creb pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron 31: 409-420. [DOI] [PubMed] [Google Scholar]
- Pittenger, C., Huang, Y.Y., Paletzki, R.F., Bourtchouladze, R., Scanlin, H., Vronskaya, S., and Kandel, E.R. 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34: 447-462. [DOI] [PubMed] [Google Scholar]
- Pizzorusso, T., Medini, P., Berardi, N., Chierzi, S., Fawcett, J.W., and Maffei, L. 2002. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248-1251. [DOI] [PubMed] [Google Scholar]
- Pratt-Johnson, J.A. and Tillson, G. 1989. Intractable diplopia after vision restoration in unilateral cataract. Am. J. Ophthalmol. 107: 23-26. [DOI] [PubMed] [Google Scholar]
- Prusky, G.T. and Douglas, R.M. 2003. Developmental plasticity of mouse visual acuity. Eur. J. Neurosci. 17: 167-173. [DOI] [PubMed] [Google Scholar]
- Rice, D.S., Williams, R.W., and Goldowitz, D. 1995. Genetic control of retinal projections in inbred strains of albino mice. J. Comp. Neurol. 354: 459-469. [DOI] [PubMed] [Google Scholar]
- Rosen, K.M., McCormack, M.A., Villa-Komaroff, L., and Mower, G.D. 1992. Brief visual experience induces immediate early gene expression in the cat visual cortex. Proc. Natl. Acad. Sci. 89: 5437-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, F.M., Bozzi, Y., Pizzorusso, T., and Maffei, L. 1999. Monocular deprivation decreases brain-derived neurotrophic factor immunoreactivity in the rat visual cortex. Neuroscience 90: 363-368. [DOI] [PubMed] [Google Scholar]
- Rutstein, R.P. and Fuhr, P.S. 1992. Efficacy and stability of amblyopia therapy. Opt. Vision Sci. 69: 747-754. [DOI] [PubMed] [Google Scholar]
- Saulles, H. 1987. Treatment of refractive amblyopia in adults. J. Am. Opt. Assoc. 58: 959-960. [PubMed] [Google Scholar]
- Saunders, P.A. and Ho, I.K. 1990. Barbiturates and the GABAA receptor complex. Prog. Drug Res. 34: 261-286. [DOI] [PubMed] [Google Scholar]
- Sawtell, N.B., Frenkel, M.Y., Philpot, B.D., Nakazawa, K., Tonegawa, S., and Bear, M.F. 2003. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 38: 977-985. [DOI] [PubMed] [Google Scholar]
- Selenow, A. and Ciuffreda, K.J. 1986. Vision function recovery during orthoptic therapy in an adult esotropic amblyope. J. Am. Opt. Assoc. 57: 132-140. [PubMed] [Google Scholar]
- Sharkey, J.A. and Sellar, P.W. 1994. Acquired central fusion disruption following cataract extraction. J. Pediat. Ophthalmol. Strabismus 31: 391-393. [DOI] [PubMed] [Google Scholar]
- Shaywitz, A.J. and Greenberg, M.E. 1999. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68: 821-861. [DOI] [PubMed] [Google Scholar]
- Silva, A.J., Kogan, J.H., Frankland, P.W., and Kida, S. 1998. CREB and memory. Annu. Rev. Neurosci. 21: 127-48. [DOI] [PubMed] [Google Scholar]
- Skibinska, A., Glazewski, S., Fox, K., and Kossut, M. 2000. Age-dependent response of the mouse barrel cortex to sensory deprivation: A 2-deoxyglucose study. Exp. Brain Res. 132: 134-138. [DOI] [PubMed] [Google Scholar]
- Sloper, J.J. and Collins, A.D. 1995. Delayed visual evoked potentials in adults after monocular visual deprivation by a dense cataract. Invest. Ophthalmol. Vis. Sci. 36: 2663-2671. [PubMed] [Google Scholar]
- Tang, Y. and Norcia, A.M. 1995. An adaptive filter for steady-state evoked responses. Electroencephalogr. Clin. Neurophysiol. 96: 268-277. [DOI] [PubMed] [Google Scholar]
- Tonner, P.H. and Miller, K.W. 1995. Molecular sites of general anaesthetic action on acetylcholine receptors. Eur. J. Anaesthesiol. 12: 21-30. [PubMed] [Google Scholar]
- Tyler, C.W. and Kaitz, M.F. 1977. Binocular interactions in the human visual evoked potential after short-term occlusion and anisometropia. Invest. Ophthalmol. Vis. Sci. 16: 1070-1076. [PubMed] [Google Scholar]
- Tyler, C.W., Apkarian, P., Levi, D.M., and Nakayama, K. 1979. Rapid assessment of visual function: An electronic sweep technique for the pattern visual evoked potential. Invest. Ophthalmol. Vis. Sci. 18: 703-713. [PubMed] [Google Scholar]
- Violet, J.M., Downie, D.L., Nakisa, R.C., Lieb, W.R., and Franks, N.P. 1997. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology 86: 866-874. [DOI] [PubMed] [Google Scholar]
- Wagor, E., Mangini, N.J., and Pearlman, A.L. 1980. Retinotopic organization of striate and extrastriate visual cortex in the mouse. J. Comp. Neurol. 193: 187-202. [DOI] [PubMed] [Google Scholar]
- Wick, B. and Wingard, M. 1992. Anisometropic amblyopia: Is the patient ever too old to treat? Opt. Vision Sci. 69: 866-878. [DOI] [PubMed] [Google Scholar]
- Wiesel, T.N. and Hubel, D.H. 1963. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26: 1003-1017. [DOI] [PubMed] [Google Scholar]
- Worley, P.F., Christy, B.A., Nakabeppu, Y., Bhat, R.V., Cole, A.J., and Baraban, J.M. 1991. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc. Natl. Acad. Sci. 88: 5106-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, J.C., Wallach, J.S., Del Vecchio, M., Wilder, E.L., Zhou, H., Quinn, W.G., and Tully, T. 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49-58. [DOI] [PubMed] [Google Scholar]
- Yoshimura, Y., Ohmura, T., and Komatsu, Y. 2003. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J. Neurosci. 23: 6557-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]