Abstract
High-dose ionising radiation is associated with circulatory disease. Risks associated with lower-dose (<0.5 Gy) exposures remain unclear, with little information on risk modification by age at exposure, years since exposure or dose-rate. Tuberculosis patients in Canada and Massachusetts received multiple diagnostic x-ray fluoroscopic exposures, over a wide range of ages, many at doses <0.5 Gy. We evaluated risks of circulatory-disease mortality associated with <0.5 Gy radiation exposure in a pooled cohort of 63,707 patients in Canada and 13,568 patients in Massachusetts. Under 0.5 Gy there are increasing trends for all circulatory disease (n = 10,209; excess relative risk/Gy = 0.246; 95% CI 0.036, 0.469; p = 0.021) and for ischaemic heart disease (n = 6410; excess relative risk/Gy = 0.267; 95% CI 0.003, 0.552; p = 0.048). All circulatory-disease and ischaemic-heart-disease risk reduces with increasing time since exposure (p < 0.005). Over the entire dose range, there are negative mortality dose trends for all circulatory disease (p = 0.014) and ischaemic heart disease (p = 0.003), possibly due to competing causes of death over this dose interval.These results confirm and extend earlier findings and strengthen the evidence for circulatory-disease mortality radiation risk at doses <0.5 Gy. The limited information on well-known lifestyle/medical risk factors for circulatory disease implies that confounding of the dose trend cannot be entirely excluded.
The well-documented effects of ionising-radiation exposure include cancer1,2, and at higher doses, various types of tissue-reaction effect, in particular circulatory disease3. Circulatory diseases have been shown to be associated with radiation treatment of malignant4,5,6,7,8,9,10,11,12 and benign disease13. There is a substantial body of radiobiological data which suggests that certain inflammatory cytokines and adhesion markers thought to be involved in circulatory disease may be differentially up and down regulated at doses above and below ~0.5 Gy14, suggesting that attention should be restricted to the moderate dose range <0.5 Gy. However, risks associated with lower-dose (<0.5 Gy) exposures remain unclear. A recent report suggested that low dose-rate space radiation exposure may increase risk of circulatory disease15, although this finding is controverisal16. A meta-analysis of groups with mean exposure <0.5 Gy indicates excess circulatory-disease risk overall for two out of four disease endpoints, but suggests that inter-cohort heterogeneity for certain endpoints limits the causal interpretability of these findings17.
Previous analyses of long-term health effects with respect to circulatory disease mortality after exposure from x-ray fluoroscopy used in the course of treatment for tuberculosis have included cohorts from Canada18 and Massachusetts19. There is excess mortality risk for ischaemic heart disease (IHD) in the Canadian cohort after adjusting for dose fractionation18. There is decreasing excess mortality risk with increasing age at exposure and time since exposure, but an unexpected inverse dose fractionation effect18. Although there is little evidence of excess risk overall in the Massachusetts study, at doses <0.5 Gy there is evidence of excess mortality risk for all circulatory disease (p = 0.074) and IHD (p = 0.068)19; there are no indications of modifying effects of age at exposure, time since exposure or dose fractionation19.
The purpose of this paper is to investigate circulatory disease risk in the Canadian and Massachusetts tuberculosis fluoroscopy cohorts by using a pooled data set, with a focus on the effects of radiation at doses <0.5 Gy, and exploring also adjustments for age at exposure, time since exposure and radiation dose rate. Combining data will boost statistical power for certain rare outcomes such as hypertensive heart disease. Interpretation of the slightly different findings in the two cohorts will benefit from a unified methodological treatment.
Results
In the pooled group exposed to <0.5 Gy, there are 58,676 persons, 48,068 from the Canadian cohort and 10,608 from the Massachusetts dataset (Table 1) and the mean cumulative lung dose is 0.18 Gy (range = 0.01, 0.50) (Supplementary Table S1). 17.4% (10,209/58,676) of patients died from circulatory disease (Table 2).
Table 1. Counts of patients for the Canadian and Massachusetts by demographic and exposure variables.
| Descriptive characteristics | Categories | Numbers with lung dose <0.5 Gy/dose unrestricted | ||
|---|---|---|---|---|
| Canada | Massachusetts | Total | ||
| Canadian province | Nova Scotia | 3431/4408 | 0/0 | 3431/4408 |
| non-Nova Scotia | 44,637/59,299 | 0/0 | 44,637/59,299 | |
| Massachusetts subcohort | Massachusetts I | 0/0 | 1101/1744 | 1101/1744 |
| Massachusetts II | 0/0 | 5327/6986 | 5327/6986 | |
| Massachusetts III | 0/0 | 4180/4838 | 4180/4838 | |
| Gender | female | 23,295/31,787 | 4934/6633 | 28,229/38,420 |
| male | 24,773/31,920 | 5674/6935 | 30,447/38,855 | |
| Smoking status | never | 2447/3456 | 2530/3390 | 4977/6846 |
| ever | 7099/10,172 | 5108/6474 | 12,207/16,646 | |
| unknown | 38,522/50,079 | 2970/3704 | 41,492/53,783 | |
| Alcohol status | never | 0/0 | 5392/7013 | 5392/7013 |
| ever | 0/0 | 2633/3281 | 2633/3281 | |
| unknown | 0/0 | 2583/3274 | 2583/3274 | |
| Tuberculosis status | minimal | 12,899/15,264 | 2187/2643 | 15,086/17,907 |
| moderate | 15,299/22,696 | 3848/5229 | 19,147/27,925 | |
| advanced | 10,609/16,253 | 3832/4932 | 14,441/21,185 | |
| unrecorded | 9261/9494 | 741/764 | 10,002/10,258 | |
| Age at entry, year | 0–19 | 10,447/14,249 | 1344/1804 | 11,791/16,053 |
| 20–39 | 26,806/37,655 | 5220/7399 | 32,026/45,054 | |
| 40–59 | 8803/9765 | 3019/3322 | 11,822/13,087 | |
| ≥60 | 2012/2038 | 1025/1043 | 3037/3081 | |
| Age at first exposure, year | not screened | 38,775/38,775 | 7229/7229 | 46,004/46,004 |
| 0–19 | 1295/4500 | 412/1098 | 1707/5598 | |
| 20–39 | 6570/17,888 | 2207/4210 | 8777/22,098 | |
| 40–59 | 1363/2448 | 712/971 | 2075/3419 | |
| ≥60 | 65/96 | 49/60 | 113/156 | |
| Age at study exit, years | 0–54 | 9222/11,696 | 2834/3562 | 12,056/15,258 |
| 55–64 | 14,025/19,117 | 1635/2014 | 15,660/21,131 | |
| 65–74 | 14,470/20,818 | 2635/3286 | 17,105/24,104 | |
| ≥75 | 10,351/12,676 | 3504/4706 | 13,855/17,382 | |
| Cumulative lung dose, Gy | 0 | 38,775/38,775 | 7754/7754 | 46,529/46,529 |
| >0–0.49 | 9293/9293 | 2854/2854 | 12,147/12,147 | |
| 0.50–0.99 | 0/5038 | 0/1123 | 0/6161 | |
| 1.00–1.99 | 0/6343 | 0/1241 | 0/7584 | |
| ≥2.00 | 0/4258 | 0/596 | 0/4854 | |
| Lung dose rate, Gy/year | 0 | 38,775/38,775 | 7754/7754 | 46,529/46,529 |
| >0.0–0.19 | 2308/3141 | 1271/1562 | 3579/4703 | |
| 0.20–0.49 | 2585/9429 | 1040/2777 | 3625/12,206 | |
| 0.50–4.99 | 4387/12,349 | 543/1475 | 4930/13,824 | |
| ≥5.00 | 13/13 | 0/0 | 13/13 | |
| Total | 48,068/63,707 | 10,608/13,568 | 58,676/77,275 | |
In each cell we provide numbers of persons with cumulative dose <0.5 Gy (to the left of the oblique dash) and without restriction on dose (to the right of the oblique dash) in the respective cohorts (Canada, Massachusetts, total (Canada + Massachusetts)).
Table 2. Mortality counts by disease endpoint in the Canadian and Massachusetts cohorts, in relation to dose range, attained age range, lag period from start of follow-up to entry into analysis cohort.
| Endpoint/type of circulatory disease | ICD9 codes | Number of deaths/person years |
||
|---|---|---|---|---|
| Canada | Massachusetts | Total | ||
| Lung dose < 0.5 Gy, age < 100, lag 5 years | ||||
| Cerebrovascular disease | 430–438 | 1192 | 369 | 1561 |
| Ischaemic heart disease | 410–414 | 4876 | 1534 | 6410 |
| Hypertensive heart disease | 401–405 | 181 | 63 | 244 |
| Heart disease apart from IHD + hypertensive | 390–400, 406–409, 415–429 | 926 | 383 | 1309 |
| All other circulatory disease apart from heart + cerebrovascular | 439–459 | 518 | 167 | 685 |
| All circulatory disease | 390–459 | 7693 | 2516 | 10,209 |
| Person years follow-up | 1,179,270 | 247,711 | 1,426,981 | |
| Lung dose unrestricted, age < 100, lag 5 years | ||||
| Cerebrovascular disease | 430–438 | 1481 | 472 | 1953 |
| Ischaemic heart disease | 410–414 | 6211 | 1947 | 8158 |
| Hypertensive heart disease | 401–405 | 234 | 89 | 323 |
| Heart disease apart from IHD + hypertensive | 390–400, 406–409, 415–429 | 1182 | 497 | 1679 |
| All other circulatory disease apart from heart + cerebrovascular | 439–459 | 659 | 211 | 870 |
| All circulatory disease | 390–459 | 9767 | 3216 | 12,983 |
| Person years follow-up | 1,599,120 | 345,921 | 1,945,041 | |
| Lung dose unrestricted, age unrestricted, lag 0 years | ||||
| Cerebrovascular disease | 430–438 | 1585 | 493 | 2078 |
| Ischaemic heart disease | 410–414 | 6516 | 2086 | 8602 |
| Hypertensive heart disease | 401–405 | 286 | 102 | 388 |
| Heart disease apart from IHD + hypertensive | 390–400, 406–409, 415–429 | 1330 | 543 | 1873 |
| All other circulatory disease apart from heart + cerebrovascular | 439–459 | 701 | 222 | 923 |
| All circulatory disease | 390–459 | 10,418 | 3446 | 13,864 |
| Person years follow-up | 1,904,580 | 405,193 | 2,309,773 | |
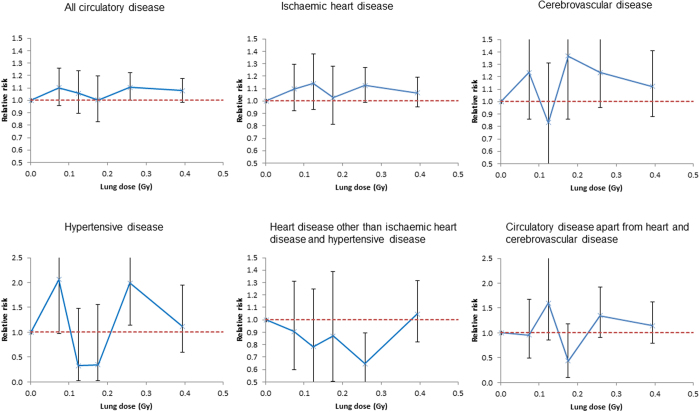
Under 0.5 Gy circulatory-disease mortality increases with dose (excess relative risk/Gy = 0.246; 95% CI 0.036, 0.469; p = 0.021) as also does IHD (excess relative risk/Gy = 0.267; 95% CI 0.003, 0.552; p = 0.048) (Table 3, Fig. 1). The Kaplan-Meier plots of Supplementary Fig. S1 demonstrate that the survival probabilities are very similar in the various dose groups until the age of 60 years, after which point they increasingly diverge. For no disease endpoint is there any significant modifying effect of age at first exposure, or radiation dose rate (p > 0.2). However, there is a pronounced (p < 0.005) reduction of relative risk for all circulatory disease and IHD with increasing time since last exposure (Table 4, Fig. 2); there are similar findings over the full dose range (Supplementary Table S3). There is no modifying effect of cohort on excess relative risk for any endpoint (p > 0.1) (results not shown).
Table 3. Excess relative risk estimates for circulatory disease mortality endpoints by dose range using 5 year dose lag and 5 years from study entry to start of follow-up.
| Dose range, Gy | Excess relative risk/Gy (+95% confidence intervals (CI)) | |||||
|---|---|---|---|---|---|---|
| All circulatory disease | IHD | Cerebrovascular | Hypertensive | Heart disease apart from IHD and hypertensive | All other circulatory diseases apart from heart and cerebrovascular | |
| 0–0.10 | 1.135 (−0.494, 2.900) | 1.371 (−0.708, 3.664) | 1.998 (−2.102, 7.027) | 15.340 (2.298, 35.100) | −1.586 (−4.990, 2.759) | −1.724 (−6.643, 5.339) |
| p-valuea | 0.177 | 0.204 | 0.367 | 0.015 | 0.443 | 0.589 |
| 0–0.20 | 0.322 (−0.444, 1.151) | 0.618 (−0.353, 1.687) | 0.979 (−1.043, 3.453) | −0.448 (−4.791, 7.003) | −1.121 (−2.726, 0.914) | −0.564 (−3.212, 3.143) |
| p-valuea | 0.421 | 0.221 | 0.370 | 0.882 | 0.257 | 0.733 |
| 0–0.30 | 0.371 (−0.006, 0.772) | 0.490 (0.013, 1.006) | 0.915 (−0.101, 2.109) | 3.033 (0.169, 7.216) | −1.292 (−1.998, −0.417) | 0.856 (−0.622, 2.749) |
| p-valuea | 0.054 | 0.044 | 0.080 | 0.035 | 0.006 | 0.283 |
| 0–0.40 | 0.268 (0.002, 0.551) | 0.337 (0.001, 0.699) | 0.661 (−0.059, 1.499) | 1.065 (−0.746, 3.711) | −0.588 (−1.131, 0.064) | 0.540 (−0.531, 1.892) |
| p-valuea | 0.049 | 0.049 | 0.074 | 0.290 | 0.075 | 0.352 |
| 0–0.50 | 0.246 (0.036, 0.469) | 0.268 (0.003, 0.552) | 0.441 (−0.119, 1.090) | 1.121 (−0.351, 3.228) | −0.226 (−0.679, 0.307) | 0.507 (−0.322, 1.541) |
| p-valuea | 0.021 | 0.047 | 0.129 | 0.155 | 0.385 | 0.253 |
ap-value for departure of trend from null. All models adjust for cohort/sub-cohort, gender, smoking status, tuberculosis status, attained age, calendar year at risk by stratification. All CI are profile-likelihood based.
Figure 1. Relative risk estimates (and their 95% confidence intervals) against cumulative lagged dose (lagged by 5 years) for the restricted dose range [0, 0.5] Gy.
We show results for the dose categories 0–0.049, 0.050–0.099, 0.100–0.149, 0.150–0.199, 0.200–0.299, 0.300–0.499 Gy. The dashed red line corresponds to relative risk =1.
Table 4. Excess relative risk (ERR) estimates (and 95% confidence intervals (CI)) and adjustment factors for circulatory disease mortality endpoints (and 95% CI) for 0–0.5 Gy dose range in models that adjust for (a) age at first exposure, (b) years since last exposure or (c) radiation dose rate.
| Type of adjustment to ERR/Gy | Excess relative risk/Gy (+95% CI)/% adjustment (+95% CI)/p-value | |||||
|---|---|---|---|---|---|---|
| All circulatory disease | IHD | Cerebrovascular | Hypertensive | Heart disease apart from IHD and hypertensive | All other circulatory diseases apart from heart and cerebrovascular | |
| Linear ERR/Gy adjusted for age at first exposure | 0.165 (0.013, 0.478) | 0.214 (−0.088a, 0.544) | 0.510 (−0.159a, 1.242) | 1.166 (−0.688a, 3.456) | −0.236 (−0.664, 0.309a) | 0.461 (−0.621a, 1.688) |
| Age at first exposure adjustment (% change in ERR/Gy per year of age at first exposure) | −13.0 (−25.8, 8.5) | 3.2 (−32.5, 25.9) | −4.3 (−18.3a, 12.1a) | −0.8 (−14.2a, 14.6a) | 3.6 (−7.9a, 16.7a) | 1.2 (−13.2a, 17.9a) |
| p-valueb for modification of ERR | 0.251 | 0.552 | 0.593 | 0.890 | 0.353 | 0.899 |
| Linear ERR/Gy adjusted for time since last exposure | 0.272 (−0.024a, 0.582) | 0.215 (−0.109a, 0.659) | 0.540 (−0.203a, 1.338) | 1.047 (−0.824a, 3.179) | −0.215 (−0.701, 0.408) | 0.007 (−0.059a, 1.387) |
| Years since last exposure adjustment (% change in ERR/Gy per year since last exposure) | −10.5 (−49.2, −3.9) | −14.7 (−42.6, −6.4) | −6.1 (−17.3a, 6.5a) | 1.6 (−8.5a, 13.0a) | 0.6 (−14.2a, 17.9a) | 21.6 (−7.9a, 60.6a) |
| p-valueb for modification of ERR | 0.002 | < 0.001 | 0.402 | 0.693 | 0.899 | 0.244 |
| Linear ERR/Gy adjusted for dose rate | 0.247 (0.036, 0.470) | 0.268 (−0.014a, 0.551) | 0.467 (−0.101, 1.117) | 1.108 (−0.633a, 3.220) | −0.228 (−0.678, 0.379a) | 0.500 (−0.467a, 1.535) |
| Dose rate adjustment (% change in ERR/Gy per Gy/year) | 3.11 (−95.85, 67.50) | −19.32 (−99.28, 60.32) | 32.51 (−30.36a, > 100a) | −20.05 (−98.62a, > 100a) | −59.51 (−99.98a, > 100a) | −22.65 (−99.63a, > 100a) |
| p-valueb for modification of ERR | 0.950 | 0.684 | 0.565 | 0.877 | 0.570 | 0.847 |
aWald-based CI.
b2-sided p-value for departure of trend from null.
All models adjust for cohort/sub-cohort, gender, smoking status, tuberculosis status, attained age, calendar year at risk by stratification. All CI are profile-likelihood based. The adjustments for age at first exposure, years since last exposure and dose rate are centered at the person-year-weighted mean values for the <0.5 Gy data, namely 27.98 years, 25.09 years and 0.61 Gy/year, respectively. 5 year dose lag and period from entry to start of follow-up. Unless otherwise stated all CI are based on the profile likelihood.
Figure 2. Variation of excess relative risk (+95% CI) with years since last exposure for all circulatory disease and ischaemic heart disease.
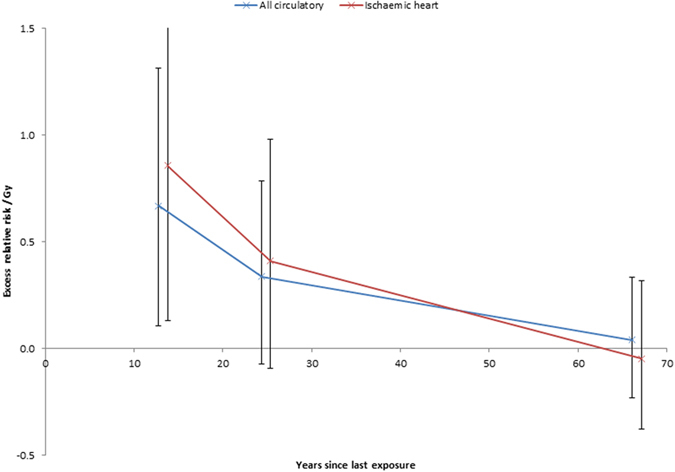
We show results for the categories 0–19, 20–29 and ≥30 years since last exposure.
Sensitivity analysis using 10-year (rather than 5-year) lag shows that there are positive dose trends for all circulatory disease (p = 0.018) and hypertensive heart disease (p = 0.027), as also to a lesser extent for IHD (p = 0.077) (Supplementary Table S4). Over the full dose range, the sensitivity analysis in Supplementary Table S4 and Supplementary Fig. S2 demonstrate that there is a decreasing trend in mortality risk with dose for all circulatory disease (p = 0.014) and IHD (p = 0.003). Removing the upper age limit (of 100 years) makes no difference to any results (results not shown). There is limited information on antibiotic use (Isoniazid, Streptomycin, Poly-aminosalicylic acid), and diabetes, all available only for a small part (1394/13,568) of the Massachusetts dataset (Table 1, Supplementary Table S2); information on alcohol consumption is available for the full Massachusetts cohort. These variables do not confound or otherwise modify the radiation dose response, but rather act as independent risk factors. When these variables are included in the model they have (as a group) highly significant independent effect, apart from radiation exposure, on all circulatory disease, IHD and heart disease apart from IHD and hypertensive disease (p < 0.01, results not shown); the adjusted trends with dose are very similar to those of the main analysis (Table 3, Supplementary Table S4). The significance of the ensemble of indicators is largely driven by the effects of alcohol consumption, diabetes and the indicator of the informative (for antibiotics and diabetes) subcohort of the Massachusetts dataset, which at least for all circulatory disease and IHD are conventionally statistically significant (p < 0.05) (Supplementary Table S4).
To assess the possibility of competing risks from causes of death other than circulatory disease, analyses of all circulatory-disease mortality using the subdistribution hazard (see Statistical Methods) yielded a risk estimate for dose <0.5 Gy that is consistent with the main analysis (excess relative risk/Gy = 0.339; p = 0.002 vs main analysis excess relative risk/Gy = 0.246; p = 0.021) (Supplementary Table S5). However, there is more discrepancy in the subdistribution hazard estimate for the full dose range (excess relative risk/Gy = −0.001; p = 0.933 vs main analysis excess relative risk/Gy = −0.024; p = 0.014) (Supplementary Table S5).
Discussion
We found increased radiation dose-related excess mortality risk for all circulatory disease, IHD, and hypertensive heart disease in a pooled analysis of 58,676 tuberculosis patients from Canada and Massachusetts exposed to repeated x-ray fluoroscopies and with cumulative dose <0.5 Gy. This contrasts with analysis over the full dose range, when a negative trend in excess mortality risk with dose was observed for all circulatory disease, IHD, all deaths, and all deaths excluding circulatory disease. There is a strong reduction in radiation risk with increasing time since last exposure, but age at first exposure and radiation dose rate do not modify risk. Our findings <0.5 Gy are robust to a variant formulation using the subdistribution hazard, suggesting that competing risks from other causes of death are operating independently from circulatory disease. However, this is not the case over the full dose range.
Our results are similar to, but somewhat stronger than those of the previous analysis of the Massachusetts cohort19, which found no dose trends for any circulatory disease endpoint over the full dose range, and indications of increasing trends with dose for all circulatory disease and IHD <0.5 Gy (Supplementary Table S6). The previous analysis of the Canadian cohort18 reported weaker indications of excess IHD mortality risk <0.5 Gy (Supplementary Table S6). The Canadian study also reported an increasing trend for IHD mortality with dose in the entire dose range with an inverse risk modification by dose fractionation using 10-year lag, the evidence for which became much weaker when dose was restricted to less than 0.5 Gy, or when 5-year lag was used18. We did not have individual annual doses for the Massachusetts cohort, but analyses using average dose-rates for both cohorts did not find any modification by dose-rate for dose <0.5 Gy, or over the full dose range (Table 4); it may be that this somewhat different definition of radiation dose rate may account for the discrepancies in the dose-fractionation-modification findings from the previous analysis of the Canadian data18.
Our findings in relation to time-since-exposure modifications to relative risk (Table 4, Fig. 2, Supplementary Table S3) are similar to those in the Canadian TB cohort18 and in the Massachusetts data19 over the full dose range. The absence of any strong modification of risk by age at exposure (Table 4, Supplementary Table S3) contrasts with the pronounced inverse modification, with excess relative risk decreasing with increasing age at exposure in the Canadian TB cohort18 and the modification in the opposite direction, in the Massachusetts data over the full dose range19, the combination of which doubtless explains our null finding overall. In the Japanese atomic bomb survivor Life Span Study (LSS) cohort modifications to excess relative risk/Gy for age at exposure are consistent with those observed here for all endpoints17; the magnitude of the time since exposure adjustment in the LSS is inconsistent with the modification observed here for IHD (Table 4, Fig. 2), although consistent with the adjustment for other endpoints. The modification to excess relative risk/Gy with time after exposure for IHD and cerebrovascular disease (CeVD) in a US cohort of persons who received X-radiation treatment for peptic ulcer13 are consistent with those observed here. It may be significant that the type of radiation used in this study, moderate energy X-rays, is quite similar to the type of low energy, and largely unfiltered, fluoroscopy X-rays used here20, albeit for therapeutic rather than diagnostic purposes, and contrasts with the rather higher energy radiation to which nuclear workers and the LSS21,22 were exposed (Supplementary Table S6); it is well known that higher-energy gamma rays are less biologically effective per unit dose than X-rays in relation to a number of experimental endpoints, in particular chromosome translocations, dicentrics, cell transformation, cell killing, specific locus mutations and various others23. Also, a typical chest fluoroscopy in the period 1930–1950, when most of the dose in the cohort was incurred, would last about 15 s and patients would receive 0.01–0.10 Gy, and thus should not be considered a low dose-rate exposure24; in this respect it is similar to the peptic ulcer study13 and to the LSS21,22, and contrasts with the generally low dose-rate exposure in most of the other moderate/low dose cohorts listed in Supplementary Table S6.
A previous meta-analysis of groups exposed at low to moderate doses (mean whole-body dose <0.5 Sv) observed excess risk for IHD and stroke, and somewhat weaker evidence of excess risk for all circulatory disease excluding heart disease and stroke17. In particular, there is excess mortality and morbidity risk in the LSS21,22 and in various groups of nuclear workers25,26,27, which are consistent with our risk estimates for all circulatory disease and IHD <0.5 Gy (Supplementary Table S6). The findings over the full dose range are somewhat inconsistent, but as above, there are indications of interference from other causes of death over this full dose range in our data. Recent reviews have proposed biological mechanisms for the effects of radiation on circulatory disease14,28,29. At high therapeutic doses >5 Gy, damage to endothelial cells and capillaries may explain the adverse effects on the circulatory system29. At lower doses, 0.5–5 Gy, pro-inflammatory effects have been observed experimentally in vivo and in vitro, contrasting with anti-inflammatory effects at doses <0.5 Gy14,28,30. These different biological processes corresponding to different dose ranges suggest that at low and moderate doses, in particular <0.5 Gy, should be analysed separately from moderate and high doses. On the other hand, risk estimates in studies of medically exposed groups, which typically have organ doses much greater than 0.5 Gy4,11,12,13,31, are comparable to groups exposed at lower doses (Supplementary Table S6), suggesting that biological mechanism operating at high doses and dose rates may be similar to low and moderate doses and dose rates.
The present pooled analysis is the first such pooled analysis for any disease endpoints from the Canadian and Massachusetts tuberculosis fluoroscopy groups. Major strengths of the analysis are that it includes a large cohort that contains both sexes and various ages at exposure, and that has been followed through most of the 20th century. Lung dose is evaluated, which should be a reasonable surrogate of dose to the heart19. The outcome and exposure information are both register-based, so most biases (e.g., due to misclassification of exposure or outcome) are unlikely. Although the combined dataset has information on smoking status and tuberculosis disease severity, both of which can modify circulatory disease risk, it lacks information on many other lifestyle factors, socio-economic status, medical risk factors for circulatory disease such as diabetes, obesity, and hypertension, also treatment-related factors for circulatory disease. Pooling data has resulted in the exclusion of variables such as alcohol consumption that is available in only one cohort19. There is limited information on alcohol consumption, antibiotic use (Isoniazid, Streptomycin, Poly-aminosalicylic acid), and diabetes, in general available only for a small part (n = 1394) of the Massachusetts dataset (Table 1, Supplementary Table S2), and all derived by questionnaire to the study subjects. Analysis adjusting for these variables suggested that for certain endpoints they are highly statistically significant; nevertheless the trends with dose were very similar to those of the main analysis (Table 3, Supplementary Table S4), implying that they do not confound the dose response. Although there is information on Isoniazid in the Canadian dataset32 the data is unfortunately unavailable for the present analysis. The significance of the effect of alcohol consumption is unsurprising in view of the similar findings in the previous analysis of the Massachusetts data19. The excess risk associated with diabetes is also unsurprising, as this has been consistently identified as a risk factor for circulatory disease33,34. However, in radiation-exposed groups that have such lifestyle or medical information, there is no evidence that lifestyle factors interact with radiation risk of circulatory disease4,21,22,26,27. It is not expected that, conditional on calendar period, treatment for circulatory disease would be associated with fluoroscopy dose, so that it is improbable that such factors would confound the dose response.
The previous meta-analysis suggested that if the association between low-level exposure to radiation and the risk of circulatory disease reflects an underlying causal relationship, linear in dose, then the overall excess risk of mortality after exposure to low doses or low dose-rates of radiation may therefore be about twice that currently assumed17. Since the excess relative risks that are derived here are consistent with those estimated previously, the implications for low dose radiation risk are unaltered.
In conclusion, our analysis of the combined Canadian and Massachusetts tuberculosis fluoroscopy cohorts corroborate certain key findings of previous analyses of the separate cohorts. For doses under 0.5 Gy, there are increasing trends with dose for IHD, hypertensive heart disease, and all circulatory disease. Although there is no positive dose trend for circulatory disease mortality risk in the full dose range, there are indications of interference from other causes of death over this range. Fluoroscopy is still a widely used method of diagnostic imaging35, in particular for interventional procedures, where doses can be considerable36, so these findings have considerable significance for the long term risks that may be associated with currently used methods of radiological diagnosis.
Materials and Methods
Cohort characteristics and follow-up
Medical records of patients treated for tuberculosis in all 46 Canadian institutions from 1930 to 1952 and in 12 Massachusetts hospitals from 1915 to 1968 were combined for this analysis. In the Canadian cohort multiple admissions to different institutions were identified by computerised record linkage of the patient records37, resulting in a cohort of 92,707 patients. Deaths in the cohort were ascertained via computerised record linkage with the Canadian Mortality Database. Because information on cause of death is available only since 1950, we included in the cohort only those n = 68,608 known to be alive at the beginning of 1950. Exclusions were made for those with incorrect age (n = 1653), invalid last contact status or year (n = 850), age of more than 100 years at the end of follow-up (n = 2392), and other record irregularities (n = 6), leaving a cohort of 63,707 patients for analysis in the Canadian cohort. Deaths in the Massachusetts cohort were retrospectively ascertained from the Vital Statistics Offices in the state of last known residence by linking to the mortality files of the Social Security Administration and the National Death Index and by contacting relatives and friends38. Vital status was also confirmed through records from the post office, motor vehicle departments, credit bureaus, and other sources39. Of the 13,716 members of the full Massachusetts cohort, exclusions were made for lack of adequate follow-up information (n = 144), and missing last exposure date (n = 4), leaving an analysis dataset of 13,568 persons. The combined cohort therefore contains 77,275 patients, 63,707 (82%) from the Canadian data and 13,568 (18%) from the Massachusetts study. More details about the methods used to assemble the separate cohorts can be found in earlier publications39,40,41. Details of individual dates of entry and exit from treatment, smoking status (unknown smoking status/ever smoker/never smoker), and most advanced stage of TB recorded (unknown/minimal/moderate/advanced) were abstracted from medical treatment records, and for some lifestyle data available for both cohorts (e.g., smoking) via interviews and questionnaires. For a group of 1502 members of the Massachusetts cohort (1472 with lung dose <0.5 Gy) with more than a single fluoroscopy the start and end of exposure dates are only known to be within a given calendar year; for these individuals the initial and final exposures were assumed to be separated by 4 months (~third of a year), the theoretically-expected separation for dates constrained to lie within a year. 404/13,568 cohort members with vital status not known after a certain date had follow-up censored then.
The study entry date is defined as the later of the entry date into the sanatoria for treatment beginning in 1915, and in the Canadian cohort, January 1, 1950. Follow-up ended on the earlier of the loss to follow-up, date of death, or December 31, 1987 for the Canadian cohort, or December 31, 2002 for the Massachusetts cohort.
The causes of death were recoded to the International Classification of Diseases, Ninth Revision (ICD-9)42. Our study focuses on deaths from all circulatory diseases and individual analysis of IHD, CeVD, hypertensive heart disease, heart disease apart from IHD and hypertensive heart disease, and other circulatory diseases, with associated ICD-9 codes given in Table 2. All information is for underlying cause of death. There is no information available to the investigators on contributing causes of death in either cohort.
Dosimetry
Dosimetry methods for each cohort are detailed elsewhere18,19,43. In both cohorts, dose estimation accounted for the number of fluoroscopic screenings, data of typical fluoroscopic procedures during the period of exposure, and phantom studies43. Radiation doses to the lungs during fluoroscopic screenings were treated as surrogate doses to the heart and circulatory system. The fluoroscopy fields would encompass the heart more completely (i.e., the heart generally would be in the direct beam and also receive additional scattered radiation from the rest of the field); the fluoroscopy fields would not always encompass both lungs, so sometimes the lungs would be partially irradiated. Lung dose would therefore generally be expected to be slightly lower than heart dose, possibly by as much as a factor of 219,44.
Statistical methods
Person-years at risk were calculated for each stratum defined by cohort (Canada or Massachusetts), sub-cohort (Nova Scotia/non-Nova Scotia Canadian, Massachusetts I/II/III39), gender, tuberculosis stage, smoking status, attained age, calendar year at risk, cumulative lagged dose and dose rate categories; missing data were separately coded and incorporated into the person-year table. Sensitivity analyses were also conducted in which the upper age limit (of 100 years) was removed. In contrast to previous analyses of the Canadian data which used individual annual doses and actual duration of fluoroscopic procedures18, the current analyses assumed that dose was uniformly distributed over the exposure duration (=date discharge − date entry to sanatorium), so as to be comparable with the previous analysis of the Massachusetts cohort19 for which annual doses are not available. Dose rate was defined as the ratio: cumulative dose/exposure duration. For most analyses, the dose lag (and time from start of follow-up to entry into the analysis dataset) was 5 years, but a 10 year dose lag (and entry lag) was also assessed.
We modelled the relative risk (RR) for circulatory disease mortality using Poisson regression45, so that the expected number of deaths in stratum i (defined by the above non-dose variables) and dose group j with mean dose Dij and mean person year PYij of follow-up is:
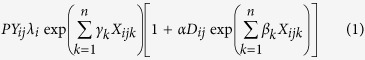 |
for some auxiliary modifying variables 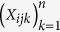 , which included age at first exposure, time since last exposure and doserate. The model is linear in radiation dose, analogous to models previously used to assess circulatory disease in radiation exposed populations13,17,22. The excess relative risk per Gy α, the baseline mortality-rate modifying parameters γk, the excess relative risk modifying parameters
, which included age at first exposure, time since last exposure and doserate. The model is linear in radiation dose, analogous to models previously used to assess circulatory disease in radiation exposed populations13,17,22. The excess relative risk per Gy α, the baseline mortality-rate modifying parameters γk, the excess relative risk modifying parameters 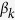 , and the semi-parametric background rate λi were estimated from the model fit. In analysis adjusting for antibiotic use (Isoniazid, Streptomycin, Poly-aminosalicylic acid), and diabetes, we employ an indicator of the informative part of the Massachusetts cohort, and indicators for the presence of each of these exposures or medical conditions, adjusting the background risk via the parameters γk; the results of the analysis adjusting for these variables and alcohol consumption (again adjusting the background risk via the parameters γk) are provided in Supplementary Table S4. Sensitivity to the effects of competing risks from other types of mortality was assessed by fitting a Poisson model analogous to the subdistribution hazard of Fine and Gray46, which assumes that patients that died from causes other than circulatory disease were censored at the last day of follow-up in each cohort. Parameter estimation was by likelihood maximisation45 and was conducted in EPICURE47. All hypothesis tests were 2-sided. When possible, confidence intervals were estimated from the profile likelihood45, otherwise by Wald test inversion. Supplementary Table S6 cites results from a number of studies, some not already referenced in the main text48,49,50,51,52,53,54,55,56,57,58,59,60,61,62.
, and the semi-parametric background rate λi were estimated from the model fit. In analysis adjusting for antibiotic use (Isoniazid, Streptomycin, Poly-aminosalicylic acid), and diabetes, we employ an indicator of the informative part of the Massachusetts cohort, and indicators for the presence of each of these exposures or medical conditions, adjusting the background risk via the parameters γk; the results of the analysis adjusting for these variables and alcohol consumption (again adjusting the background risk via the parameters γk) are provided in Supplementary Table S4. Sensitivity to the effects of competing risks from other types of mortality was assessed by fitting a Poisson model analogous to the subdistribution hazard of Fine and Gray46, which assumes that patients that died from causes other than circulatory disease were censored at the last day of follow-up in each cohort. Parameter estimation was by likelihood maximisation45 and was conducted in EPICURE47. All hypothesis tests were 2-sided. When possible, confidence intervals were estimated from the profile likelihood45, otherwise by Wald test inversion. Supplementary Table S6 cites results from a number of studies, some not already referenced in the main text48,49,50,51,52,53,54,55,56,57,58,59,60,61,62.
Ethics approval
Ethics approval was previously obtained for the individual data sets, and extensions to this approval are not required for this study.
Additional Information
How to cite this article: Tran, V. et al. Radiation-associated circulatory disease mortality in a pooled analysis of 77,275 patients from the Massachusetts and Canadian tuberculosis fluoroscopy cohorts. Sci. Rep. 7, 44147; doi: 10.1038/srep44147 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
V.T., M.P.L. and A.V.B. were supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics. L.B.Z. was supported by the National Institutes of Health, the National Cancer Institute (grants R03CA188614 and R01CA197422). The authors are grateful for the detailed and helpful comments of the two referees.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.P.L., L.B.Z. and A.V.B. designed the analysis. V.T. and M.P.L. performed the statistical analysis. All authors participated in writing the paper. All authors reviewed the manuscript.
References
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. 13–322 (United Nations, New York, 2008).
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII - Phase 2. (National Academy Press, 2006). [PubMed]
- International Commission on Radiological Protection. ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs - threshold doses for tissue reactions in a radiation protection context. ICRP publication 118. Ann. ICRP 41 (1-2), 1–322, doi: 10.1016/j.icrp.2007.10.003 (2012). [DOI] [PubMed] [Google Scholar]
- Darby S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368, 987–998, doi: 10.1056/NEJMoa1209825 (2013). [DOI] [PubMed] [Google Scholar]
- Gyenes G., Rutqvist L. E., Liedberg A. & Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother. Oncol. 48, 185–190, doi: S0167-8140(98)00062-0 (1998). [DOI] [PubMed] [Google Scholar]
- Cuzick J. et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J. Clin. Oncol. 12, 447–453 (1994). [DOI] [PubMed] [Google Scholar]
- Darby S. C., McGale P., Taylor C. W. & Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 6, 557–565, doi: 10.1016/S1470-2045(05)70251-5 (2005). [DOI] [PubMed] [Google Scholar]
- Glanzmann C., Kaufmann P., Jenni R., Hess O. M. & Huguenin P. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother.Oncol. 46, 51–62, doi: S0167814097001254 (1998). [DOI] [PubMed] [Google Scholar]
- Castellino S. M. et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 117, 1806–1816, doi: 10.1182/blood-2010-04-278796 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow A. J. et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J. Natl. Cancer Inst. 99, 206–214, doi: 10.1093/jnci/djk029 (2007). [DOI] [PubMed] [Google Scholar]
- Mulrooney D. A. et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606, doi: 10.1136/bmj.b4606 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukenova M. et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J. Clin. Oncol. 28, 1308–1315, doi: 10.1200/JCO.2008.20.2267 (2010). [DOI] [PubMed] [Google Scholar]
- Little M. P., Kleinerman R. A., Stovall M., Smith S. A. & Mabuchi K. Analysis of dose response for circulatory disease after radiotherapy for benign disease. Int. J. Radiat. Oncol. Biol. Phys. 84, 1101–1109, doi: 10.1016/j.ijrobp.2012.01.053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan T. J. et al. Circulatory disease risk. Report of the independent Advisory Group on Ionising Radiation. Vol.RCE-16 1–116 (Health Protection Agency, Holborn Gate, 330 High Holborn, London, 2010). [Google Scholar]
- Delp M. D., Charvat J. M., Limoli C. L., Globus R. K. & Ghosh P. Apollo Lunar Astronauts Show Higher Cardiovascular Disease Mortality: Possible Deep Space Radiation Effects on the Vascular Endothelium. Sci. Rep. 6, 29901, doi: 10.1038/srep29901 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta F. A., Hamada N. & Little M. P. No evidence for an increase in circulatory disease mortality in astronauts following space radiation exposures. Life Sci. Space Res. (Amst.) 10, 53–56, doi: 10.1016/j.lssr.2016.08.002 (2016). [DOI] [PubMed] [Google Scholar]
- Little M. P. et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ. Health Perspect. 120, 1503–1511, doi: 10.1289/ehp.1204982 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotska L. B., Little M. P. & Cornett R. J. Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian fluoroscopy cohort study. Am. J. Epidemiol. 179, 120–131, doi: 10.1093/aje/kwt244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. P., Zablotska L. B., Brenner A. V. & Lipshultz S. E. Circulatory disease mortality in the Massachusetts tuberculosis fluoroscopy cohort study. Eur. J. Epidemiol. 31, 287–309, doi: 10.1007/s10654-015-0075-9 (2016). [DOI] [PubMed] [Google Scholar]
- Brenner D. J. Does fractionation decrease the risk of breast cancer induced by low-LET radiation? Radiat. Res. 151, 225–229 (1999). [PubMed] [Google Scholar]
- Yamada M., Wong F. L., Fujiwara S., Akahoshi M. & Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat. Res. 161, 622–632, doi: 10.1667/RR3183 (2004). [DOI] [PubMed] [Google Scholar]
- Shimizu Y. et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ 340, b5349, doi: 10.1136/bmj.b5349 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Report No. 104. The relative biological effectiveness of radiations of different quality. 1–218 (NCRP, Bethesda, MD, USA, 1990).
- Wakeford R. & Tawn E. J. The meaning of low dose and low dose-rate. J. Radiol. Prot. 30, 1–3, doi: 10.1088/0952-4746/30/1/E02 (2010). [DOI] [PubMed] [Google Scholar]
- McGeoghegan D., Binks K., Gillies M., Jones S. & Whaley S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int. J. Epidemiol. 37, 506–518, doi: 10.1093/ije/dyn018 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizova T. V. et al. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat. Res. 174, 155–168, doi: 10.1667/RR1789.1 (2010). [DOI] [PubMed] [Google Scholar]
- Azizova T. V., Haylock R. G. E., Moseeva M. B., Bannikova M. V. & Grigoryeva E. S. Cerebrovascular diseases incidence and mortality in an extended Mayak Worker Cohort 1948-1982. Radiat. Res. 182, 529–544, doi: 10.1667/RR13680.1 (2014). [DOI] [PubMed] [Google Scholar]
- Little M. P. et al. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat. Res. 169, 99–109, doi: 10.1667/RR1070.1 (2008). [DOI] [PubMed] [Google Scholar]
- Schultz-Hector S. & Trott K. R. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int. J. Radiat. Oncol. Biol. Phys. 67, 10–18, doi: 10.1016/j.ijrobp.2006.08.071 (2007). [DOI] [PubMed] [Google Scholar]
- Mitchel R. E. J. et al. Low-dose radiation exposure and atherosclerosis in ApoE-/- mice. Radiat. Res. 175, 665–676, doi: 10.1667/RR2176.1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. P., Zablotska L. B. & Lipshultz S. E. Ischemic heart disease after breast cancer radiotherapy. N. Engl. J. Med. 368, 2523–2524, doi: 10.1056/NEJMc1304601#SA1 (2013). [DOI] [PubMed] [Google Scholar]
- Howe G. R., Lindsay J., Coppock E. & Miller A. B. Isoniazid exposure in relation to cancer incidence and mortality in a cohort of tuberculosis patients. Int. J. Epidemiol. 8, 305–312 (1979). [DOI] [PubMed] [Google Scholar]
- Wilson P. W. F. et al. Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847, doi: 10.1161/01.CIR.97.18.1837 (1998). [DOI] [PubMed] [Google Scholar]
- Stamler J., Neaton J. D., Garside D. B. & Daviglus M. L. In Coronary heart disease epidemiology: from aetiology to public health, 2nd edition(eds Marmot M. & Elliott P.) 32–70 (Oxford University Press, 2005). [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and effects of ionizing radiation. UNSCEAR 2008 Report to the General Assembly with scientific annexes. Annex A. Medical radiation exposures. 1–220 (United Nations, New York, 2010). [Google Scholar]
- International Commission on Radiological Protection. Avoidance of radiation injuries from medical interventional procedures. Ann. ICRP 30 (2), 7–67, doi: S0146645301000045 (2000). [DOI] [PubMed] [Google Scholar]
- Howe G. R. & Lindsay J. A generalized iterative record linkage computer system for use in medical follow-up studies. Comput. Biomed. Res. 14, 327–340 (1981). [DOI] [PubMed] [Google Scholar]
- Boice J. D. Jr. Follow-up methods to trace women treated for pulmonary tuberculosis, 1930-1954. Am. J. Epidemiol. 107, 127–139 (1978). [DOI] [PubMed] [Google Scholar]
- Davis F. G., Boice J. D. Jr., Hrubec Z. & Monson R. R. Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res. 49, 6130–6136 (1989). [PubMed] [Google Scholar]
- Boice J. D. Jr., Preston D., Davis F. G. & Monson R. R. Frequent chest X-ray fluoroscopy and breast cancer incidence among tuberculosis patients in Massachusetts. Radiat. Res. 125, 214–222 (1991). [PubMed] [Google Scholar]
- Howe G. R. Lung cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with lung cancer mortality in the atomic bomb survivors study. Radiat. Res. 142, 295–304 (1995). [PubMed] [Google Scholar]
- World Health Organization (WHO). International Classification of Diseases, Ninth Revision (ICD-9). (WHO, Geneva, 1998).
- Sherman G. J., Howe G. R., Miller A. B. & Rosenstein M. Organ dose per unit exposure resulting from fluoroscopy for artificial pneumothorax. Health Phys. 35, 259–269 (1978). [DOI] [PubMed] [Google Scholar]
- Rosenstein M. Personal communication to Mark Little (2014).
- McCullagh P. & Nelder J. A. Generalized linear models. 2nd edition. In Monographs on statistics and applied probability 37, 1–526 (Chapman and Hall/CRC, Boca Raton, FL, 1989). [Google Scholar]
- Fine J. P. & Gray R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Statist. Assoc. 94, 496–509, doi: 10.2307/2670170 (1999). [DOI] [Google Scholar]
- Epicure version 2.0.1.0. (Risk Sciences International, 55 Metcalfe, K1P 6L5, Canada, 2015).
- Little M. P. Radiation and circulatory disease. Mutat. Res./Rev. Mutat. Res. 770(B), 299–318, doi: 10.1016/j.mrrev.2016.07.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa Y. et al. Cardiovascular disease risk among atomic bomb survivors exposed in utero, 1978–2003. Radiat. Res. 170, 269–274, doi: 10.1667/RR1434.1 (2008). [DOI] [PubMed] [Google Scholar]
- Moseeva M. B., Azizova T. V., Grigoryeva E. S. & Haylock R. Risks of circulatory diseases among Mayak PA workers with radiation doses estimated using the improved Mayak Worker Dosimetry System 2008. Radiat. Environ. Biophys. 53, 469–477, doi: 10.1007/s00411-014-0517-x (2014). [DOI] [PubMed] [Google Scholar]
- Azizova T. V., Grigoryeva E. S., Haylock R. G. E., Pikulina M. V. & Moseeva M. B. Ischaemic heart disease incidence and mortality in an extended cohort of Mayak workers first employed in 1948–1982. Br. J. Radiol. 88, 20150169, doi: 10.1259/bjr.20150169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V. K. et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 90, 199–207, doi: 10.1097/01.HP.0000175835.31663.ea (2006). [DOI] [PubMed] [Google Scholar]
- Kashcheev V. V. et al. Radiation-epidemiological Study of Cerebrovascular Diseases in the Cohort of Russian Recovery Operation Workers of the Chernobyl Accident. Health Phys. 111, 192–197, doi: 10.1097/HP.0000000000000523 (2016). [DOI] [PubMed] [Google Scholar]
- Kreuzer M., Dufey F., Sogl M., Schnelzer M. & Walsh L. External gamma radiation and mortality from cardiovascular diseases in the German WISMUT uranium miners cohort study, 1946–2008. Radiat. Environ. Biophys. 52, 37–46, doi: 10.1007/s00411-012-0446-5 (2013). [DOI] [PubMed] [Google Scholar]
- Laurent O. et al. Relationship between occupational exposure to ionizing radiation and mortality at the French electricity company, period 1961-2003. Int. Arch. Occup. Environ. Health 83, 935–944, doi: 10.1007/s00420-010-0509-3 (2010). [DOI] [PubMed] [Google Scholar]
- Lane R. S. D., Frost S. E., Howe G. R. & Zablotska L. B. Mortality (1950–1999) and cancer incidence (1969–1999) in the cohort of Eldorado uranium workers. Radiat. Res. 174, 773–785, doi: 10.1667/RR2237.1 (2010). [DOI] [PubMed] [Google Scholar]
- Muirhead C. R. et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br. J. Cancer 100, 206–212, doi: 10.1038/sj.bjc.6604825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. B. & Wing S. Radiation and mortality of workers at Oak Ridge National Laboratory: positive associations for doses received at older ages. Environ. Health Perspect. 107, 649–656, doi: sc271_5_1835 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M. et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int. J. Epidemiol. 36, 1126–1135, doi: 10.1093/ije/dym138 (2007). [DOI] [PubMed] [Google Scholar]
- Talbott E. O., Youk A. O., McHugh-Pemu K. P. & Zborowski J. V. Long-term follow-up of the residents of the Three Mile Island accident area: 1979-1998. Environ. Health Perspect. 111, 341–348, doi: 10.1289/ehp.5662 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestinina L. Y. et al. Chronic low-dose exposure in the Techa River Cohort: risk of mortality from circulatory diseases. Radiat. Environ. Biophys. 52, 47–57, doi: 10.1007/s00411-012-0438-5 (2013). [DOI] [PubMed] [Google Scholar]
- Grosche B. et al. Mortality from cardiovascular diseases in the Semipalatinsk historical cohort, 1960–1999, and its relationship to radiation exposure. Radiat. Res. 176, 660–669, doi: 10.1667/RR2211.1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.