Abstract
Current evidence appoints a central role to cholinergic interneurons in modulating striatal function. Recently, a long-term potentiation (LTP) of synaptic transmission has been reported to occur in these neurons. The relationship between the pattern of cortico/thalamostriatal fibers stimulation, the consequent changes in the intracellular calcium concentration ([Ca2+]i), and the induction of synaptic plasticity was investigated in striatal cholinergic interneurons from a rat corticostriatal slice preparation by means of combined electrophysiological intracellular recordings and microfluorometric techniques. Different protocols of stimulation were considered, varying both the frequency and the duration of the train of stimuli. High-frequency stimulation (HFS) (three trains at 100 Hz for 3 sec, 20-sec interval) induced a rise in [Ca2+]i, exceeding by fivefold the resting level, and caused a LTP of synaptic transmission. Tetanic stimulation delivered at lower frequencies (5-30 Hz) failed to induce long-term changes of synaptic efficacy. The observed elevation in [Ca2+]i during HFS was primarily mediated by L-type high-voltage activated (HVA)-Ca2+ channels, as it was fully prevented by nifedipine. Conversely, blockade of NMDA and AMPA glutamate receptor did not affect either LTP or the magnitude of the [Ca2+]i rise. Interestingly, the pharmacological analysis of the post-tetanic depolarizing postsynaptic potential (DPSP) revealed that LTP was attributable, to a large extent, to the potentiation of the GABAA-mediated component. In conclusion, the expression of LTP in striatal cholinergic interneurons is a selective response to a precise stimulation pattern of induction requiring a critical rise in [Ca2+]i.
Striatal cholinergic interneurons represent a small population of large aspiny neurons that provide the striatum with one of the highest levels of acetylcholine in the brain (Lehman and Langer 1983; Graybiel 1990; Mesulam et al. 1992; Contant et al. 1996). In physiological conditions, cholinergic interneurons discharge tonically and, hence, they are believed to correspond to the tonically active neurons (TANs) identified during in vivo recordings from the striatum of behaving animals (Kimura et al. 1984; Wilson et al. 1990; Bennett and Wilson 1999). TANs develop a transient response, a pause in tonic firing, to conditioning stimuli during behavioral training in classical conditioning tasks, that requires dopaminergic and glutamatergic inputs to the striatum from the substantia nigra and the thalamus, respectively (Kimura et al. 1984; Apicella et al. 1991; Lapper and Bolam 1992; Aosaki et al. 1994, 1995; Matsumoto et al. 2001; Apicella 2002). In the past years, different mechanisms have been proposed for the TAN pause-response generation (Yan and Surmeier 1997; Bennett and Wilson 1998, 1999; Watanabe and Kimura 1998; Bennett et al. 2000). Recently, both an increase in spontaneous GABAA-dependent synaptic activity and plastic changes occurring at cortico/thalamostriatal synapses have been suggested to play a role in the generation of this phenomenon (Suzuki et al. 2001; Bonsi et al. 2003a).
It is currently accepted that an essential requirement for the induction of synaptic plasticity is the increase in [Ca2+]i in the postsynaptic neuron (Lynch et al. 1983; Malenka et al. 1988; Calabresi et al. 2000; Kemp and Bashir 2001; Lisman 2001; Suzuki et al. 2001; Bonsi et al. 2003b). A variety of experimental models have been tested in the past years to investigate the correlation between the pattern of [Ca2+]i increase and the expression of bidirectional synaptic plasticity (Hansel et al. 1997; Yang et al. 1999; Cho et al. 2001; Cormier et al. 2001). Although these studies have clearly demonstrated that the [Ca2+]i rise is a crucial checkpoint in the successful induction of either LTD or LTP, the correlation between afferent fiber activation, postsynaptic [Ca2+]i level and synaptic plasticity occurrence has not been extensively investigated.
In this study, we combined microfluorometric techniques with intracellular electrophysiological recordings in order to further characterize the molecular mechanisms of LTP induction in cholinergic interneurons from corticostriatal slices.
Results
Characterization of striatal cholinergic interneurons
Data were collected from 97 striatal neurons, identified as cholinergic interneurons by means of both morphological and electrophysiological criteria. The cells were visualized up to ∼100 μm beneath the surface of the slice by IR-DIC videomicroscopy. Only neurons showing a large and polygonal soma and two/four dendritic branches, morphological features of striatal cholinergic interneurons, were considered in this study (Kawaguchi 1992,1993; Kawaguchi et al. 1995). The recorded neurons were characterized by the well-established electrophysiological properties attributed to cholinergic interneurons, that is, depolarized resting membrane potential (-60.1 ± 1.4 mV); long-duration action potential (>1 msec at half amplitude); strong spike accommodation and pronounced afterhyperpolarization upon injection of supra-threshold depolarizing current pulses; prominent hyperpolarization-activated cation current, Ih, evoked by hyperpolarizing current pulses (n = 97; data not shown) (Jiang and North 1991; Kawaguchi 1993).
Intrastriatal synaptic stimulation evoked DPSPs in the recorded neuron (n = 92). As previously described (Pisani et al. 2000, 2002), these DPSPs were characterized both by a NMDA and AMPA receptor-dependent excitatory component, and by a GABAA-mediated component.
[Ca2+]i elevations and induction of synaptic plasticity
Test DPSPs were evoked by 0.1 Hz synaptic stimulation delivered intrastriatally with a bipolar electrode. Stable control responses were acquired for 10-20 min, then a conditioning train of supra-threshold stimuli was delivered. After tetanus, the stimulation was reverted to the test parameters and the DPSPs recorded for 30-60 min. Measurement of [Ca2+]i started 10 min before delivery of the tetanus, to determine the resting level (0.443 ± 0.009 340/380 ratio (R); n = 66; P < 0.01), continued throughout the conditioning protocol and terminated upon return of [Ca2+]i to baseline.
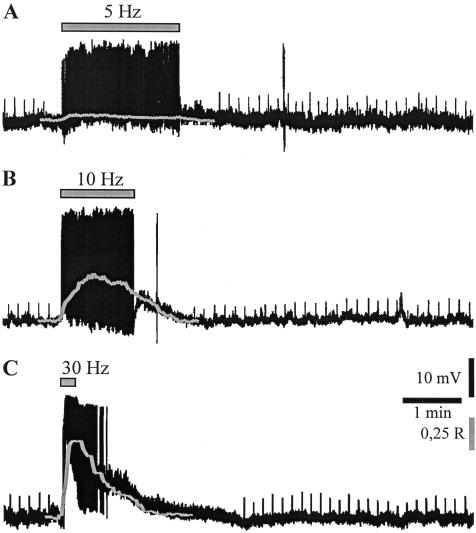
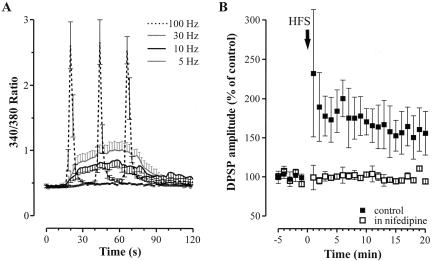
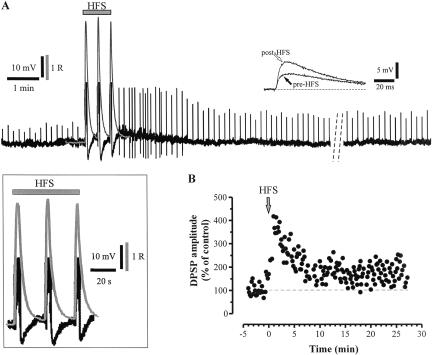
A conditioning train delivered at 5 Hz for 2 min slightly depolarized the recorded neurons (Fig. 1A, 3.0 ± 0.6 mV; n = 9; P < 0.01) and caused a modest simultaneous increase in [Ca2+]i (Fig.1A and Fig. 3A, below; 0.488 ± 0.02 R, 109.0 ± 0.5% of resting level; n = 8; P < 0.01). Accordingly, no significant modifications of the DPSP amplitude were observed (Fig. 1A, 98.3 ± 1.6; n = 9; P > 0.05). By increasing the stimulation frequency to 10 Hz for 1 min, larger depolarizations (7.9 ± 1.0 mV; n = 11; P < 0.01) and concomitant [Ca2+]i increases (0.794 ± 0.034 R, 174.0 ± 7.4% of resting level; n = 11; P < 0.01) were observed (Fig. 1B and Fig. 3A, below). However, the amplitude of DPSPs, measured after tetanic stimulation, was not significantly modified with respect to test DPSPs (103.1 ± 2.1%; n = 11; P > 0.05). Expectedly, when the frequency of synaptic stimulation was further increased to 30 Hz, the amplitude of membrane depolarization and [Ca2+]i elevation were even larger, despite the duration of the stimulation (15-60 sec) (Fig. 1C and Fig. 3A, below; 14.4 ± 2.1 mV; n = 13; P < 0.01, and 1.017 ± 0.043 R, 238.9 ± 7.2% of resting level; n = 11; P < 0.01). However, long-term modifications of the DPSP amplitude were not observed (101.9 ± 2.3%; n = 13; P > 0.05). When the HFS (100 Hz frequency, 3-sec duration, 20-sec interval) was delivered, each of the three trains strongly depolarized the cell (Fig. 2A; 26.4 ± 0.5 mV; n = 37; P < 0.01) and large, concomitant [Ca2+]i transients were recorded (Figs. 2A, 3A; 2.674 ± 0.253 R, 588.9 ± 55.7% of resting level; n = 21; P < 0.01). Noteworthy, at the end of each train of the HFS, [Ca2+]i returned to basal levels. In accordance with the previous observation by Suzuki et al. (2001), HFS stimulation induced a sustained potentiation of the DPSP amplitude up to 30-60 min (Figs. 2B, 3B; 156.4 ± 28.0% of control; n = 37; P < 0.01). Independently from the frequency pattern, each tetanus was followed by a slow depolarizing hump not paralleled, however, by significant changes in [Ca2+]i (Figs. 1, 4A). It has been reported that intrastriatal repetitive stimulation induces the appearance of a slow, substance P-mediated DPSP (Aosaki and Kawaguchi 1996).
Figure 1.
Relationship between frequency of synaptic stimulation, [Ca2+]i elevations, and induction of synaptic plasticity. (A) A conditioning train of synaptic stimulation delivered intrastriatally at 5 Hz for 2 min (gray bar) slightly depolarized the recorded cholinergic interneuron and caused a modest simultaneous increase in [Ca2+]i (gray line). The amplitude of the DPSPs (upward deflections) elicited by the test stimuli after the tetanus was not significantly modified as compared with control responses. R.M.P., 62 mV. (R) Ratio values calculated from pairs of 340 and 380 nm images corrected for background fluorescence. (B) In another neuron, tetanic stimulation at 10 Hz (1 min) depolarized the cell and induced a concomitant [Ca2+]i increase (gray line), without affecting the DPSPs amplitude. R.M.P., 59 mV. (C) In another recording, the protocol of synaptic stimulation at 30 Hz for 15 sec induced a large membrane depolarization and a significant [Ca2+]i elevation (gray line). Long-term modifications of the DPSP amplitude were not observed. R.M.P., 65 mV. Spikes induced by tetanic stimulation are truncated.
Figure 3.
Involvement of L-type HVA calcium channels in the transient postsynaptic [Ca2+]i rise during LTP induction. (A) Comparison between the amplitude of [Ca2+]i transients induced by 5-100 Hz synaptic stimulation. The ratio values obtained from different experiments were averaged and plotted at selected timepoints (every 2 sec). Only experiments in which the 30-Hz stimulation was delivered for 1 min were considered in this graph. (B) Summary plot showing the effect of 20 min pretreatment with 10 μM of nifedipine (empty squares) on LTP induction.
Figure 2.
Large [Ca2+]i transients are associated with LTP induction in cholinergic interneurons. (A) Each train of the HFS (100 Hz frequency, 3-sec duration, 20-sec interval) induced a large [Ca2+]i transient (gray line), closely paralleling the membrane depolarization of the recorded neuron, as shown at higher magnitude in the inset. Note that spikes induced by HFS stimulation are truncated. After HFS delivery, the amplitude of the DPSPs (upward deflections) was significantly potentiated (see also the two representative traces indicated by the arrows, shown at higher sweep speed). The potentiation lasted up to 60 min (dotted lines in A indicate 20 min interval). R.M.P., 61 mV. (R) Ratio values calculated from pairs of 340 and 380 nm images corrected for background fluorescence. (B) Amplitude of the DPSPs recorded from another neuron, plotted as a function of time.
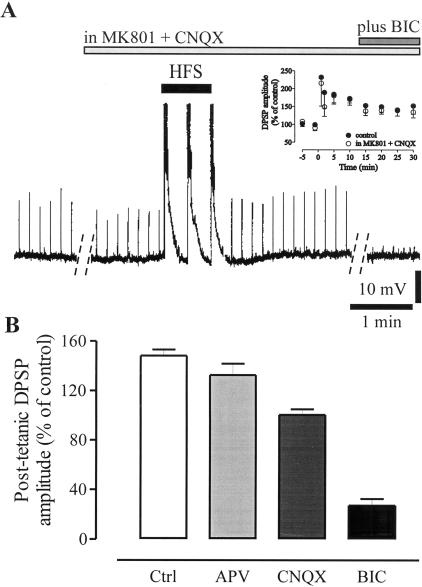
Figure 4.
The main component of the potentiated DPSP is GABAergic. (A) Delivery of HFS in the presence of 30 μM MK801 plus 10 μM CNQX was able to induce a LTP of the DPSP amplitude (upward deflections and empty circles in the summary plot shown in the inset) similar to the LTP recorded in control condition (•). Further addition of 30 μM BIC to the perfusion solution containing MK801 plus CNQX nearly abolished the DPSP. Dotted lines represent a 20-min interval. Spikes induced by HFS are truncated. R.M.P., 65 mV. (B) The graph shows the relative contribution of NMDA-, AMPA-, and GABA-mediated components to the potentiated DPSP measured, following 20 min of stable expression of LTP, after a 10-20-min incubation in the respective antagonists (10 μM APV, 10 μM CNQX, or 30 μM BIC). Values are expressed as percentage of the potentiated DPSP amplitude.
Several sources of Ca2+ entry from the extracellular space could be involved in the HFS-induced [Ca2+]i elevations. Blockade of L-type Ca2+ channels has been reported to prevent the induction of synaptic plasticity in striatal medium spiny neurons (Calabresi et al. 1994). Preincubation of the corticostriatal slices with nifedipine (10 μM, 20 min), a selective blocker of L-type Ca2+ channels, did not affect, per se, the synaptic potential; however, it significantly inhibited the induction of LTP (Fig. 3B; 98.0 ± 1.7%; n = 10; P < 0.01). Accordingly, in nifedipine, [Ca2+]i rise during HFS was negligible (data not shown; 0.476 ± 0.011 R, 106.9 ± 2.5%; n = 7; P < 0.05). Another possible source of Ca2+ contributing to LTP induction could be represented by both NMDA and AMPA receptor subtypes. Cholinergic interneurons have been reported to express Ca2+-permeable AMPA receptors (Bernard et al. 1997). Slices were therefore incubated with the NMDA and AMPA receptor antagonists, MK801 (30 μM) and CNQX (10 μM), and a 30% reduction in the amplitude of the synaptic potential was observed. In the presence of MK801 and CNQX, HFS still induced an LTP, comparable to LTP obtained in control condition (Fig. 4A; in MK801 plus CNQX, 138.1 ± 1.2%; n = 12; P > 0.05 with respect to control LTP). Accordingly, in MK801 plus CNQX, [Ca2+]i transients reached levels that did not significantly differ from those recorded in controls (data not shown; in MK801 plus CNQX, 2.447 ± 0.183 R, 549.2 ± 33.6% of resting level; n = 8; P > 0.05 with respect to control LTP values). After recording a stable LTP for at least 30 min, the GABAA receptor antagonist BIC (30 μM) was added to the perfusion solution containing MK801 plus CNQX, and a nearly complete inhibition of the DPSPs was observed (Fig. 4A; 8.8 ± 2.6%; n = 12; P < 0.01), suggesting that this neuronal subtype expresses a LTP of GABAergic synaptic transmission whose induction does not require NMDA and AMPA receptor activation.
Pharmacological characterization of LTP
To investigate whether LTP of the glutamate-mediated component could be induced by HFS delivery, slices were incubated with the GABAA receptor antagonist. Pretreatment with BIC (30 μM, 10 min) significantly reduced the amplitude of the DPSP recorded from cholinergic interneurons (26 ± 5% of control; n = 4; P < 0.01). Delivery of HFS in the presence of BIC did not cause a significant modification of the amplitude of the glutamatergic potential (data not shown; 124.6 ± 7.2%; n = 4; P > 0.05).
In another set of experiments, we addressed the relative contribution of glutamate and GABA receptors to the DPSP after induction of LTP. Receptor antagonists were applied after obtaining a stable LTP of DPSP amplitude (∼20 min post-HFS). NMDA receptor blockade by MK801 or APV (10 μM) did not significantly modify the DPSP amplitude (Fig. 4B; 89.5 ± 6.2%; n = 10; P > 0.05), whereas bath application of CNQX reduced the DPSP amplitude to 67.7 ± 3.1% (Fig. 4B; n = 9; P < 0.01). Notably, once LTP was steadily expressed, perfusion of the slice with 30 μM of BIC largely inhibited the synaptic potential, thus suggesting that the potentiated DPSP is mainly mediated by GABAA receptor activation (Fig. 4B; 28.2 ± 5.5%; n = 14; P < 0.01). The proportional reduction in the amplitude of the DPSP by NMDA, AMPA, or GABAA receptor antagonists measured before and after LTP induction was not significantly different.
In cholinergic interneurons, the induction of LTP has been reported to be dopamine dependent (Suzuki et al. 2001). Blockade of the D1-like dopamine receptor by preincubation of the slice with the selective antagonist SCH 23390 (10 μM, 10 min) fully prevented the HFS-induced LTP (data not shown; 99.9 ± 6.1%; n = 5; P > 0.05).
Discussion
The rise in postsynaptic [Ca2+]i is an essential requirement for the induction of long-term changes in synaptic efficacy in different brain areas (Kemp and Bashir 2001; Lisman 2001). A close relationship between the magnitude of postsynaptic [Ca2+]i rise and the induction of long-term modifications of synaptic efficacy has been demonstrated in striatal medium spiny neurons (Bonsi et al. 2003b). In the present work, we show that (1) LTP induction was observed only after HFS stimulation, and was closely dependent on a transient postsynaptic increase in [Ca2+]i to fivefold the resting level; (2) L-type HVA Ca2+ channels represent a major source of [Ca2+]i rise, whereas Ca2+ influx through both NMDA and Ca2+-permeable AMPA receptors appears negligible; (3) a main component of the DPSP, after LTP induction, is GABAA receptor mediated. A GABAergic LTP is induced in the presence of both NMDA and AMPA glutamate receptor antagonists.
In behaving monkeys, the TANs, which are considered to correspond to these interneurons, respond to the presentation of sensory stimuli associated to reward with a 100-200-msec pause of their discharge that is dependent on the dopaminergic input from nigrostriatal neurons (Kimura et al. 1984; Aosaki et al. 1994, 1995; Apicella 2002). Although different cellular and synaptic mechanisms have been proposed, the origin of this response is still debated. Recently, a long-lasting synaptic potentiation of GABAergic responses has been suggested as the basis for expression of this pause response (Suzuki et al. 2001). In agreement with an involvement of GABA-mediated transmission, is the evidence that cholinergic interneurons recorded in corticostriatal slices from behaviorally conditioned animals showed an increase in spontaneous GABAA-dependent synaptic activity (Bonsi et al. 2003a). Notably, our pharmacological analysis of the relative contribution of GABA and glutamate to the DPSPs recorded after induction of LTP is in support of a major involvement of GABAergic transmission in the plastic changes occurring at cholinergic interneuron synapses after HFS.
In our experimental conditions, a prominent GABAergic component of the synaptic potential was observed that seemed to be selectively potentiated after HFS. The short delay and the persistence of the GABAergic potential in the presence of the glutamate receptor antagonists suggested a monosynaptic nature of this synaptic potential. On the contrary, Suzuki et al. (2001) reported a significant potentiation of both glutamate- and GABAA-mediated synaptic potential, and observed that the latter was disynaptic. These authors suggested the hypothesis, supported by the observation that the HFS induced an increase in the frequency of the GABAergic IPSCs, that LTP was expressed presynaptically. In our experimental condition, the involvement of a presynaptic component in LTP expression cannot be ruled out. The apparent discrepancies with the data reported by Suzuki et al. (2001) could be due to the different experimental procedures used. These authors utilized whole-cell patch clamp recordings from sagittal corticostriatal slices of the mouse (P14-P21) and delivered the HFS (100 Hz, 1 sec) by means of a stimulating electrode placed subcortically. The different site of synaptic stimulation appears of crucial importance, as we had previously reported that the GABAergic component of the DPSP is significantly higher when the synaptic stimulation is delivered intrastriatally rather than cortically (Pisani et al. 2000). To correlate different patterns of synaptic stimulation with [Ca2+]i modifications and induction of synaptic plasticity, a systematic analysis in a wide range of frequencies was performed in the present study, and interestingly, each pattern of stimulation was paralleled by a defined rise in [Ca2+]i. When tetanus was delivered at frequencies between 5 and 30 Hz, we measured elevations in [Ca2+]i that did not exceed by more than twofold the resting level. Accordingly, no significant change in synaptic efficacy could be detected. Conversely, the HFS protocol induced [Ca2+]i increases that consistently exceeded this level and was able to cause long-lasting changes of synaptic efficacy in striatal cholinergic interneurons. These data are in accordance with previous results obtained in medium spiny striatal neurons (Bonsi et al. 2003b) and match with the hypothesis of [Ca2+]i acting as a checkpoint in the control of the induction of long-lasting changes in synaptic efficacy, possibly by finely modulating kinase and phosphatase activities (Kemp and Bashir 2001; Lisman 2001).
One of the main sources of Ca2+ entry in the intracellular environment is represented by HVA-Ca2+ channels. Cholinergic interneurons express several types of HVA-Ca2+ channels (Yan and Surmeier 1996). Nifedipine was able to block the induction of LTP in striatal cholinergic interneurons, thus providing evidence for L-type Ca2+ channels as a principal source of [Ca2+]i increase during HFS. Moreover, this observation is in accordance with the results of Suzuki et al. (2001) who reported that either intracellular BAPTA or nimodipine prevented LTP induction in these neurons. NMDA receptor activation plays a central role in the induction of some forms of long-term synaptic plasticity by contributing to the postsynaptic [Ca2+]i increase (Kemp and Bashir 2001; Lisman 2001). Likewise, an additional source of Ca2+ entry during HFS can be represented by Ca2+-permeable AMPA receptors. However, in our experimental condition, when HFS was delivered in the presence of both MK801 and CNQX, the observed [Ca2+]i elevations were not significantly different from those recorded in control condition. Accordingly, a potentiation of the synaptic efficacy similar in amplitude and time-course to control LTP was recorded.
Thus, sensory stimuli of behavioral significance might determine plastic changes at GABAergic synapses impinging onto cholinergic interneurons. However, these synapses would undergo long-lasting changes only under a specific high-frequency pattern of stimulation, determining a sustained rise in [Ca2+]i.
Materials and Methods
Preparation and maintenance of the corticostriatal slices
Male Wistar rats (20-30 postnatal day) were used for the experiments. Preparation and maintenance of the slices have been described in detail previously (Pisani et al. 2000, 2002; Bonsi et al. 2003a,b). Animals were sacrificed under ether anesthesia by cervical dislocation, the brain was quickly removed, and corticostriatal coronal slices (200-μm thick) were cut with a vibratome in Krebs' solution (see composition below). A slice was then transferred into a recording chamber mounted on the stage of an upright microscope (BX51WI, Olympus), equipped with a 20 ±, 0.95 n.a. water immersion objective (XLUMPLFL20XW, Olympus), and fully submerged in a continuously flowing Krebs' solution (32.5°C, 2.5-3 mL/min) gassed with a 95% O2/5% CO2 mixture. The composition of the solution was (in millimolars) 126 NaCl, 2.5 KCl, 1.3 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, and 18 NaHCO3. Complete replacement of the medium in the chamber took 30-60 sec.
Optical set up
Cholinergic interneurons were identified according to their characteristic shape and size up to 100 μm beneath the surface of the slice and impaled under visual guidance. Individual neurons were visualized in situ using a differential interference contrast (DIC, Nomarski) optical system combined with an infrared (IR) filter, a monochrome CCD camera (Cohu 4912, Cohu). A mechanical switch allowed shifting from IR-DIC to UV for microfluorometric measurements.
Electrophysiological and microfluorometric recordings
An Axoclamp 2B amplifier (Axon Instruments) was utilized for sharp microelectrode recordings. Traces were displayed on an oscilloscope (465B, Tektronics), acquired, and analyzed with pClamp8 software (Axon Instruments). For synaptic stimulation, a bipolar electrode was located within the striatum. Intrastriatal stimulation is thought to preferentially activate thalamostriatal fibers rather than the corticostriatal pathway (Lapper and Bolam 1992; Pisani et al. 2000). Evoked synaptic potentials were digitally stored for successive analysis. For combined optical and electrical recordings, the tip of the recording electrode was filled with a solution of 1 mM bis-fura-2 (hexapotassium salt, Molecular Probes) in 100 mM KCl. The shank of the recording electrode was backfilled with 2 M of KCl solution. In this experimental condition, the GABAA-mediated component of postsynaptic potential was recorded as a depolarizing event. After cell impalement, cells were loaded with bis-fura-2 by injecting 0.1-0.5 nA negative current for 10-15 min. Fluorescence of bis-fura-2 was excited by epi-illumination with 340 or 380 nm light provided by a 150 W Xenon lamp and selected by a monochromator (Polychrome IV, TILL Photonics). Emission light passed a barrier filter (500 nm) and was detected by a charge-coupled device (CCD) camera (C6790-81, Hamamatsu). No measurable dye bleaching was detected at the excitation intensities used. Pairs of 340 and 380 nm images were acquired and analyzed off-line with IAS software (Delta Sistemi). For experiments of synaptic stimulation at low frequency, a rate of acquisition of one ratio image/1-2 sec was utilized. During the HFS experiments, a rate of acquisition of three ratio images/second was utilized. This rate of acquisition was considered reliable to avoid a gross underestimation of the peaks of the [Ca2+]i transients. Ratio images were calculated from pairs of 340 and 380 nm images corrected for background fluorescence (measured from regions free of dye fluorescence). Time courses of ratio values (R) were calculated from regions that included the cell bodies (region of interest [ROI], defined as those pixels that exhibit at least 20%-30% of maximal-specific fluorescence). The architectural complexity of acute striatal slices and the difficulty of filling the dendrites with the Ca2+ dye by means of sharp recording microelectrodes determined the choice of the somatic region for the measurements of [Ca2+]i. The somatic areas selected for [Ca2+]i measurements were relatively small, to allow fast sampling.
Values in the text are expressed as mean ± S.E.M. Student's t, and Mann Whitney tests were used for statistical analysis; P-value was set at 0.05.
Drug source and application
Drugs were bath applied by switching the solution to one containing known concentrations of drugs. Drugs solutions entered the chamber within ∼30 sec after turning a three-ways tap on. (+)-Bicuculline (BIC), 1,4-dihydro-2,6-dimethyl-4-(2-nitro-phenyl)-3,5-pyridinedicarboxylic acid dimethyl ester (nifedipine) were from Sigma; (+)-MK 801 maleate (MK801), 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), D-(±)-2-amino-5-phosphonopentanoic acid (APV), (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydro-chloride (SCH23390) were from Tocris Cookson.
Acknowledgments
This work was supported by grants from Ministero della Salute, Regione Lazio and of Ministero dell'Istruzione, dell'Università e della Ricerca (FIRB) to A.P. and G.B. We thank Mr. Massimo Tolu and Mr. Franco Lavaroni for technical assistance.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.82104.
References
- Aosaki, T. and Kawaguchi, Y. 1996. Actions of substance P on rat neostriatal neurons in vitro. J. Neurosci. 16: 5141-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki, T., Graybiel, A.M., and Kimura, M. 1994. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science 265: 412-415. [DOI] [PubMed] [Google Scholar]
- Aosaki, T., Kimura, M., and Graybiel, A.M. 1995. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J. Neurophysiol. 73: 1234-1252. [DOI] [PubMed] [Google Scholar]
- Apicella, P. 2002. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur. J. Neurosci. 16: 2017-2026. [DOI] [PubMed] [Google Scholar]
- Apicella, P., Scarnati, E., and Schultz, W. 1991. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp. Brain Res. 84: 672-675. [DOI] [PubMed] [Google Scholar]
- Bennett, B.D. and Wilson, C.J. 1998. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J. Neurosci. 18: 8539-8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, B.D. and Wilson, C.J. 1999. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J. Neurosci. 19: 5586-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, B.D., Callaway, J.C., and Wilson, C.J. 2000. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J. Neurosci. 20: 8493-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, V., Somogyi, P., and Bolam, J.P. 1997. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J. Neurosci. 17: 819-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi, P., Florio, T., Capozzo, A., Pisani, A., Calabresi, P., Siracusano, A., and Scarnati, E. 2003a. Behavioral learning-induced increase in spontaneous GABAA-dependent synaptic activity in rat striatal cholinergic interneurons. Eur. J. Neurosci. 17: 147-178. [DOI] [PubMed] [Google Scholar]
- Bonsi, P., Pisani, A., Bernardi, G., and Calabresi, P. 2003b. Stimulus frequency, calcium levels and striatal synaptic plasticity. Neuroreport 14: 419-422. [DOI] [PubMed] [Google Scholar]
- Calabresi, P., Pisani, A., Mercuri, N.B., and Bernardi, G. 1994. Post-receptor mechanisms underlying striatal long-term depression. J. Neurosci. 14: 4871-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi, P., Centonze, D., Gubellini, P., Marfia, G.A., Pisani, A., Sancesario, G., and Bernardi, G. 2000. Synaptic transmission in the striatum: From plasticity to neurodegeneration. Prog. Neurobiol. 61: 231-265. [DOI] [PubMed] [Google Scholar]
- Cho, K., Aggleton, J.P., Brown, M.W., and Bashir, Z.I. 2001. An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of the rat. J. Physiol. 532.2: 459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contant, C., Umbriaco, D., Garcia, S., Watkins, K.C., and Descarries, L. 1996. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience 71: 937-947. [DOI] [PubMed] [Google Scholar]
- Cormier, R.J., Greenwood, A.C., and Connor, J.A. 2001. Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J. Neurophysiol. 85: 399-406. [DOI] [PubMed] [Google Scholar]
- Graybiel, A.M. 1990. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 13: 244-254. [DOI] [PubMed] [Google Scholar]
- Hansel, C., Artola, A., and Singer, W. 1997. Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons. Eur. J. Neurosci. 9: 2309-2322. [DOI] [PubMed] [Google Scholar]
- Jiang, Z.G. and North, R.A. 1991. Membrane properties and synaptic responses of rat striatal neurones in vitro. J. Physiol. 443: 533-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, Y. 1992. Large aspiny cells in the matrix of the rat neostriatum in vitro: Physiological identification, relation to the compartments and excitatory postsynaptic currents. J. Neurophysiol. 67: 1669-1682. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, Y. 1993. Physiological, morphological and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 13: 4908-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, Y., Wilson, C.J., Augood, S.J., and Emson, P.C. 1995. Striatal internruones: Chemical, physiological and morphological characterization. Trends Neurosci. 18: 527-535. [DOI] [PubMed] [Google Scholar]
- Kemp, N. and Bashir, Z.I. 2001. Long-term depression: A cascade of induction and expression mechanisms. Prog. Neurobiol. 65: 339-365. [DOI] [PubMed] [Google Scholar]
- Kimura, M., Rajkowski, J., and Evarts, E. 1984. Tonically discharging putamen neurons exhibit set-dependent responses. Proc. Natl. Acad. Sci.. 81: 4998-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper, S.R. and Bolam, J.P. 1992. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51: 533-545. [DOI] [PubMed] [Google Scholar]
- Lehmann, J. and Langer, S.Z. 1983. The striatal cholinergic interneuron: Synaptic target of dopaminergic terminals? Neuroscience 10: 1105-1120. [DOI] [PubMed] [Google Scholar]
- Lisman, J. 2001. Three Ca2+ levels affect plasticity differently: The LTP zone, the LTD zone and no man's land. J. Physiol. 532.2: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, G., Larson, J., Kelso, S., Barrionuevo, G., and Schottler, F. 1983. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305: 719-721. [DOI] [PubMed] [Google Scholar]
- Malenka, R.C., Kauerm, J.A., Zucker, R.S., and Nicoll, R.A. 1988. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science 242: 81-84. [DOI] [PubMed] [Google Scholar]
- Matsumoto, N., Minamimoto, T., Graybiel, A.M., and Kimura, M. 2001. Neurons in the thalamic CM-Pf complex supply neurons in the striatum with information about behaviorally significant sensory events. J. Neurophysiol. 85: 960-976. [DOI] [PubMed] [Google Scholar]
- Mesulam, M.M., Mash, D., Hersh, L., Bothwell, M., and Geula, C. 1992. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J. Comp. Neurol. 323: 252-268. [DOI] [PubMed] [Google Scholar]
- Pisani, A., Bonsi, P., Centonze, D., Calabresi, P., and Bernardi, G. 2000. Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J. Neurosci. 20RC69: 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani, A., Bonsi, P., Catania, M.V., Giuffrida, R., Morari, M., Marti, M., Centonze, D., Bernardi, G., Kingston, A.E., and Calabresi, P. 2002. Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J. Neurosci. 22: 6176-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., Miura, M., Nishimura, K., and Aosaki, T. 2001. Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J. Neurosci. 21: 6492-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K. and Kimura, M. 1998. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J. Neurophysiol. 79: 2568-2580. [DOI] [PubMed] [Google Scholar]
- Wilson, C.J., Chang, H.T., and Kitai, S.T. 1990. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci. 10: 508-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Z. and Surmeier, D.J. 1996. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 16: 2592-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Z. and Surmeier, D.J. 1997. D5 dopamine receptors enhance Zn2+-sensitive GABAA currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron 19: 1115-1126. [DOI] [PubMed] [Google Scholar]
- Yang, S.-N., Tang, Y.-G., and Zucker, R.S. 1999. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 81: 781-787. [DOI] [PubMed] [Google Scholar]