Abstract
The study of glycan function is a major frontier in biology that could benefit from small molecules capable of perturbing carbohydrate structures on cells. The widespread role of sulfotransferases in modulating glycan function makes them prime targets for small-molecule modulators. Here, we report a system for conditional activation of Golgi-resident sulfotransferases using a chemical inducer of dimerization. Our approach capitalizes on two features shared by these enzymes: their requirement of Golgi localization for activity on cellular substrates and the modularity of their catalytic and localization domains. Fusion of these domains to the proteins FRB and FKBP enabled their induced assembly by the natural product rapamycin. We applied this strategy to the GlcNAc-6-sulfotransferases GlcNAc6ST-1 and GlcNAc6ST-2, which collaborate in the sulfation of l-selectin ligands. Both the activity and specificity of the inducible enzymes were indistinguishable from their WT counterparts. We further generated rapamycin-inducible chimeric enzymes comprising the localization domain of a sulfotransferase and the catalytic domain of a glycosyltransferase, demonstrating the generality of the system among other Golgi enzymes. The approach provides a means for studying sulfate-dependent processes in cellular systems and, potentially, in vivo.
Keywords: chemical inducer of dimerization, l-selectin, rapamycin
Chemical approaches have emerged as powerful allies to traditional genetic and biochemical strategies for probing biological function (1). Small molecules that modulate a protein's function, either by inhibition or activation, can be used to effect biological processes in a temporal, reversible, and tunable fashion. The notion of developing small-molecule modulators of protein function at the systems level is embodied in the modern field of chemical genetics (2, 3). Two notable achievements in this field are convergent protein/inhibitor engineering strategies for the development of selective kinase inhibitors (4) and the development of chemical inducers of dimerization for conditional activation of proteins via domain assembly (5, 6). These chemical approaches involve the use of drug-like small molecules that target a single protein within a cellular system. Target singularity is ensured by engineering the protein to respond specifically to the small molecule “switch.”
To date, chemical approaches have had limited representation among the repertoire of techniques used by the glycobiologist. Natural product inhibitors of glycan assembly, such as tunicamycin, the nojirimycins, and swainsonine, are the most widely used, but their toxicity and lack of selectivity complicate experimental interpretation (7). Several groups have sought to design inhibitors of glycosyltransferases and sulfotransferases, the enzymes that assemble and modify glycans within the Golgi compartment. Most of these efforts have yielded polar and/or charged compounds that mimic the enzymes' nucleotide- or carbohydrate-based substrates. As a result, the handful of reported glycosyltransferase (8, 9) and carbohydrate sulfotransferase (10) inhibitors lack selectivity for a single target or cannot function in cells because of poor bioavailability.
Given the role of these enzymes in synthesizing the glycans essential for cell–cell interactions, we have focused on developing a general chemical strategy for modulating their activity in cell-based systems. One commonality among a majority of Golgi-resident enzymes is their modular domain architecture. The majority of glycosyltransferases (11) and all known carbohydrate sulfotransferases (12) have two functional domains, an N-terminal domain that encodes localization among the Golgi cisternae (Loc) and a C-terminal domain that encodes catalytic activity (Cat) (Fig. 1A). Golgi localization of the Cat domain is often required for activity on cellular substrates (13, 14). When physically separated from the Loc domain, the Cat domain is secreted from the cell and can no longer colocalize with its substrates. We reasoned that the induced assembly of these domains could rescue enzyme activity, providing the means for a small-molecule switch (Fig. 1B). This concept was realized in the context of the glycosyltransferase fucosyltransferase 7 (FucT7) (15). We fused the rapamycin binding proteins FRB and FKBP, using a system developed by Schreiber and coworkers (16) and used by others (17), to the Cat and Loc domains of FucT7, respectively. Cells transfected with these constructs expressed FucT7 activity in the presence of rapamycin, the chemical inducer of dimerization, but not in its absence. Because this chemical approach targets Golgi localization rather than a specific aspect of substrate binding or catalysis, we speculated that the strategy could be applied in a systemwide manner to any Golgi enzyme, irrespective of the reaction it catalyzes.
Fig. 1.
A chemical approach for modulating Golgi sulfotransferase activity. (A) The domain structure of the carbohydrate sulfotransferases. The enzymes are type II transmembrane proteins with an N-terminal cytosolic tail, single pass transmembrane domain, luminal stem region, and a C-terminal catalytic domain. The tail, transmembrane domain, and stem (abbreviated Loc) are usually sufficient to confer Golgi localization. (B) Small-molecule-mediated domain assembly as a means to control sulfotransferase activity in cells. The Cat and Loc domains of the enzymes are separated and fused to FRB and FKBP, respectively. Rapamycin induces association of FKBP and FRB, thereby localizing the Cat domain in the Golgi where it can encounter its substrates. (C) The structure of sulfoadhesin. Two antibodies used in this study bind to discrete components of sulfoadhesin. G72 recognizes 6-sulfo sLex, shown in blue, whereas MECA-79 recognizes a sulfated extension of the lower core 1 branch, shown in green.
Sulfation of glycans by Golgi-resident sulfotransferases is now recognized as a widespread regulatory modification that can convert the underlying molecule from one functional state to another (18). Analogies have been drawn between sulfation and phosphorylation, a well recognized modification that regulates protein activity. Glycan sulfation is known to affect glycoprotein hormone pharmacokinetics (19), growth factor and cytokine activity (20), and viral (21) and bacterial (22) adhesion. A dramatic example is found in the recruitment of leukocytes to peripheral lymph nodes and sites of chronic inflammation (23). This process is initiated by the interaction of the leukocyte adhesion molecule l-selectin with glycoprotein ligands on specialized endothelial cells that line the blood vessel wall. The l-selectin ligands are a family of sulfated polysaccharides represented by the structure shown in Fig. 1C, termed sulfoadhesin (24, 25). This structure comprises a biantennary O-linked glycan with two sialyl Lewis x (sLex) capping groups elaborated from a core 1 GlcNAc residue on the lower branch and a core 2 GlcNAc residue on the upper branch. Both GlcNAc residues can be sulfated at the 6-position, a modification that is required for physiological l-selectin binding. Two related sulfotransferases, GlcNAc6ST-1 and -2, are capable of performing this modification, and mice deficient in GlcNAc6ST-2 lack l-selectin ligands in their peripheral lymph nodes (26–28). The sulfotransferases are therefore thought to play a regulatory role in the process by generating a unique epitope recognized by l-selectin.
Here, we demonstrate that the sulfotransferases GlcNAc6ST-1 and -2 can be engineered for control by the chemical inducer of dimerization rapamycin. This small-molecule switch allowed for temporal and tunable control of enzyme activity in cells and modulation of cell surface expression of l-selectin ligands. In addition, sulfation of a secreted glycoprotein by GlcNAc6ST-2 could be controlled by the drug. We exploited the modularity of the system to assemble chimeric enzymes comprising the Loc domains of the sulfotransferases and the Cat domain of the glycosyltransferase FucT7. These results underscore the potential generality of the approach across the Golgi enzyme superfamily.
Experimental Procedures
Generation of the Cat-FRB3 and Loc-FKBP Constructs. The plasmids with the genes encoding FKBP-myc and FRB3-hemagglutinin (HA) tag have been reported (15). To generate genes encoding 1Loc-FKBP and 2Loc-FKBP, the sequences corresponding to the first 114 residues of GlcNAc6ST-1 and the first 40 residues of GlcNAc6ST-2 were inserted into the plasmid encoding FKBP-myc. To generate the Cat-FRB3 domains, the DNA sequences corresponding to residues 46–483 of GlcNAc6ST-1 and residues 25–386 of GlcNAc6ST-2 were inserted into the plasmid encoding FRB3-HA. A detailed description of plasmid construction is provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
General Procedure for Testing Inducible Expression. CHO cells (2.2 × 106) plated in 10-cm dishes were transfected in OptiMEM (GIBCO) by using Lipofectamine PLUS (Invitrogen) with plasmids encoding the Cat-FRB3 and Loc-FKBP domains, then allowed to recover for 12 h. The cells were plated into six-well dishes at 500,000 cells per well and incubated with rapamycin. After 24 h, the cells were processed for flow cytometry. For the rapamycin titration and ascomycin experiments, cells were plated in 12-well dishes at a density of 250,000 cells per well and treated with the appropriate drugs for 24 h before being processed for flow cytometry.
Analysis of GlyCAM-Ig Sulfation. CHO cells were transfected with the plasmids encoding 2Loc-FKBP, 2Cat-FRB3, Core1-β3GlcNAcT, and GlyCAM-Ig. After 24 h, the cells were washed and incubated in OptiMEM containing various concentrations of rapamycin for 4 d. The media were collected, and the GlyCAM-Ig was purified by using Protein A-agarose as described (29).
Microscopy. Procedures for fluorescence microscopy and image processing are provided in Supporting Materials and Methods.
Results
Characterization of Rapamycin-Inducible Sulfotransferases. We relied on previous work from our laboratory (29) and others (30) that identified the residues comprising functional Cat and Loc domains of GlcNAc6ST-1 and -2. For GlcNAc6ST-1, we generated constructs comprising residues 1–114 (Loc) fused to the gene encoding FKBP bearing a C-terminal myc tag (15) (final construct termed 1Loc-FKBP) and residues 46–483 (Cat) fused to three copies of the gene encoding FRB (final construct termed 1Cat-FRB3). This latter construct also possessed an N-terminal secretion signal to ensure entry into the secretory pathway, followed by an HA tag. The orientation of this system with respect to FRB and FKBP, along with the copy number of these proteins (1xFKBP and 3xFRB) were deemed optimal for rapamycin control of FucT7 activity (15) and were thus implemented for the sulfotransferases. Similar constructs were generated for GlcNAc6ST-2, comprising residues 1–40 (2Loc-FKBP) and residues 25–386 (2Cat-FRB3). For the complete sequences of both sulfotransferases, see Fig. 6, which is published as supporting information on the PNAS web site.
The constructs were introduced either singly or in pairs into CHO cells stably expressing FucT7 (FucT7-CHO) or WT CHO cells by transient transfection. FucT7 installs the critical fucose residue in sLex (31). Sulfotransferase activity was assessed by flow cytometry analysis using antibodies specific for sulfated components of sulfoadhesin. The two antibodies used in these studies were mAb G72 (32), specific for 6-sulfo-sLex, and mAb MECA-79 (33), specific for the GlcNAc-6-sulfated extension on the lower branch of sulfoadhesin (the epitopes are highlighted in Fig. 1C). Elaboration of the lower branch requires expression of the Core1-β3GlcNAcT (25), which was cotransfected alongside sulfotransferase domains in all experiments using MECA-79 as a probe. A key difference between these antibodies is that G72 will recognize 6-sulfo-sLex capping structures on any glycan subtype (both N- and O-linked), whereas the determinant recognized by MECA-79 is found only on O-linked glycans. Our previous work has shown that GlcNAc6ST-1 prefers to sulfate N-linked glycans in CHO cells, whereas GlcNAc6ST-2 prefers O-linked glycan substrates but has significant activity on N-linked structures (29). Thus, the two antibodies can provide a qualitative measure of both enzyme activity and substrate specificity.
FucT7-CHO cells expressing both GlcNAc6ST-1 domains (1Loc-FKBP and 1Cat-FRB3) showed robust G72 immunoreactivity in the presence of rapamycin, but not in its absence (Fig. 2A Upper Left). The rapamycin-dependent activity was comparable to that displayed by the full-length enzyme (Fig. 2A Lower Left). In control experiments, rapamycin treatment had no effect on the activity of the full-length enzyme, and the 1Cat-FRB3 domain alone showed only basal levels of sulfotransferase activity in the presence or absence of the drug (Fig. 2C). This basal activity may result from partial retention of the catalytic domain in the Golgi compartment, as has been observed for some enzymes (34, 35), or from the high expression levels associated with transient transfection. The rapamycin-inducible GlcNAc6ST-1 activity was also analyzed by MECA-79 staining of transiently transfected CHO cells. As shown in Fig. 2 A Upper Right and C, the reconstituted enzyme was not capable of generating the MECA-79 antigen, consistent with the preference of the full-length enzyme for N-linked structures (Fig. 2A Lower Right). In summary, both the activity and substrate specificity of the rapamycin-inducible GlcNAc6ST-1 are comparable to the WT enzyme.
Fig. 2.
Initial characterization of the inducible sulfotransferases. (A) Flow cytometry analysis of FucT7-CHO cells transfected with 1Cat-FRB3 and 1Loc-FKBP. (Left) Cells were analyzed with G72. (Upper Left) Cells were treated with 200 nM rapamycin (Rap) (gray), or they were untreated (white). (Lower Left) Cells were treated with 200 nM rapamycin (Rap) (gray), or they were transfected with full-length GlcNAc6ST-1 and treated with 200 nM rapamycin (white). (Right) Cells were cotransfected with Core1-β3GlcNAcT and analyzed with MECA-79. (Upper Right) Cells were treated with 200 nM rapamycin (Rap) (gray), or they were untreated (white). (Lower Right) Cells were treated with 200 nM rapamycin (Rap) (gray), or they were transfected with full-length GlcNAc6ST-1 and treated with 200 nM rapamycin (white). (B) Flow cytometry analysis of FucT7-CHO cells transfected with 2Cat-FRB3 and 2Loc-FKBP. Panels are represented as in A, with comparison to full-length GlcNAc6ST-2 in the lower left and right. (C) Mean fluorescence intensities (MFI) from flow cytometry data obtained as in A. Error bars represent the standard deviation of triplicate data points. (D) Mean fluorescence intensities (MFI) from flow cytometry data obtained as in B. Error bars are as in C.(E) Effects of increasing rapamycin on the activity of inducible sulfotransferases. (F) Effect of ascomycin on rapamycin-dependent sulfotransferase activity.
We performed similar experiments with the domains of GlcNAc6ST-2. FucT7-CHO cells expressing both 2Loc-FKBP and 2Cat-FRB3 expressed 6-sulfo-sLex (G72 antigen) in the presence of rapamycin but not in its absence (Fig. 2B Upper Left). This activity was comparable to that of the full-length enzyme (Fig. 2B Lower Left). Control experiments confirmed that rapamycin has no effect on WT enzyme activity and that, in this case, the 2Cat-FRB3 domain alone showed no detectable activity in the presence or absence of rapamycin (Fig. 2D). Flow cytometry analysis of transiently transfected CHO cells with MECA-79 showed robust activity of the rapamycin-reconstituted enzyme on O-linked glycoproteins (Fig. 2 B Upper Right and D). This activity was within 2-fold of that produced by the full-length enzyme (Fig. 2 B Lower Right and D). Thus, the rapamycin-inducible GlcNAc6ST-2 is active on both N- and O-linked glycoproteins in CHO cells, similar to the WT enzyme.
To investigate the tunability of the system, we analyzed the effects of various doses of rapamycin on the expression density of 6-sulfo-sLex (G72 antigen). FucT7-CHO cells were transiently transfected with either 1Loc-FKBP and 1Cat-FRB3 or 2Loc-FKBP and 2Cat-FRB3 in the presence of increasing concentrations of rapamycin, then stained with G72 and analyzed by flow cytometry. As shown in Fig. 2E, both reconstituted enzymes showed dose-dependent activity characterized by EC50 values of 0.8 nM for GlcNAc6ST-1 and 0.1 nM for GlcNAc6ST-2. The specificity of the response was confirmed by competition experiments with the drug ascomycin, which binds to FKBP but not to FRB and thus prevents formation of the rapamycin/FKBP/FRB complex (15, 36). Ascomycin inhibited the rapamycin-dependent response of both sulfotransferases in a dose-dependent manner (Fig. 2F).
Rapamycin-Induced Golgi Localization of the Cat Domain Is Required for Cellular Activity. We hypothesized that rapamycin exerts its effects by retaining the Cat domain in the Golgi compartment in association with the Loc domain. To test this hypothesis, we investigated the effects of rapamycin on the cellular distribution of 1Cat-FRB3 and 2Cat-FRB3 by immunofluorescence microscopy, exploiting their N-terminal HA tags. In the absence of rapamycin, anti-HA staining of CHO cells transfected with either 1Cat-FRB3 or 2Cat-FRB3 and the respective loc domains shows a diffuse reticulated signal and nuclear envelope staining (Fig. 3A). This observation is consistent with a fraction of the protein being nonspecifically retained in the endoplasmic reticulum, possibly because of overexpression or misfolding. However, in the presence of rapamycin, the HA immunoreactivity was observed in punctate juxtanuclear structures consistent with Golgi localization (Fig. 3A). Furthermore, colocalization studies with the known Golgi enzyme mannosidase II (MannII) confirmed that the catalytic domains are Golgi-resident in the presence of rapamycin (Fig. 3B, rows 1 and 2 for GlcNAc6ST-1 and -2, respectively). By contrast, in the absence of rapamycin, the catalytic domains showed no colocalization with MannII (Fig. 3B, rows 3 and 4 for GlcNAc6ST-1 and -2, respectively). To verify that Golgi retention of the Cat domain correlates with expression of the enzymatic product 6-sulfo-sLex, we performed three-color immunofluorescence microscopy studies with G72, anti-Giantin (a Golgi-resident protein), and anti-HA. As shown in Fig. 3C, expression of the G72 antigen on FucT7-CHO cells transfected with 2Loc-FKBP and 2Cat-FRB3 correlated with HA immunoreactivity in the Golgi compartment, and both depended on the presence of rapamycin.
Fig. 3.
Effect of rapamycin (Rap) on Cat domain localization and sulfotransferase activity. (A) FucT7-CHO cells were transfected with the GlcNAc6ST-1 and –2 Cat and Loc domains in the presence or absence of 200 nM rapamycin. The cells were fixed, permeabilized, and stained with an anti-HA antibody followed by an Alexa546-conjugated secondary antibody (red). The cells were also stained with DAPI to highlight the nucleus (blue). (Scale bar = 10 μm.) (B) FucT7-CHO cells were transfected with either 1Cat-FRB3 and 1Loc-FKBP (denoted 1 in rows 1 and 3) or 2Cat-FRB3 and 2Loc-FKBP (denoted 2 in rows 2 and 4) in the presence or absence of 200 nM rapamycin. The cells were fixed, permeabilized, and stained with anti-HA antibody and anti-MannII sera, followed by Alexa546-conjugated (against anti-HA) and Alexa647-conjugated secondary antibodies. Panels in the top two rows show single sections of a deconvolved data set with the signal from the HA tag and MannII shown in monochrome in the first and second columns. The third column shows three color overlays with the HA tag in green, MannII in red, and the nuclear stain DAPI in blue. Panels in the bottom two rows are 3D projections containing the maximum pixel intensities of a deconvolved data set. The DAPI-stained nucleus is shown for the purpose of orientation. The color scheme is identical to that in the top two rows. (Scale bar = 5 μm.) (C) FucT7-CHO cells were transfected with 2Cat-FRB3 and 2Loc-FKBP in the presence (Upper) or absence (Lower) of 200 nM rapamycin. The cells were fixed, permeabilized, and stained with anti-HA, G72, and anti-Giantin, followed by the corresponding secondary antibodies (Alexa546-, Alexa488-, and Alexa647-conjugated secondary antibodies, respectively). (Left) HA signal is shown in red, the G72 signal is shown in green, and the DAPI signal is shown in blue. (Right) The G72 signal is shown in green, the Giantin signal is shown in red, and the DAPI signal is shown in blue. (Scale bar = 10 μm.)
Rapamycin Can Modulate GlcNAc6ST-2 Activity on a Secreted Glycoprotein in a Tunable Fashion. Both membrane-associated and secreted glycoprotein ligands for l-selectin have been identified. Indeed, the first ligand characterized in the mouse was the secreted mucin-like glycoprotein GlyCAM-1, which is produced by peripheral lymph node endothelial cells with the same sulfated polysaccharides found on their membrane-bound glycoproteins (24, 37). The WT sulfotransferases are capable of sulfating both membrane-bound and secreted l-selectin ligands, prompting us to inquire whether the inducible enzymes have a comparable substrate spectrum. To address this question, we coexpressed the domains of GlcNAc6ST-2 (and the gene encoding Core1-β3GlcNAcT) with the gene encoding GlyCAM-1 fused to the Fc region of human IgG1 (GlyCAM-Ig) (38). The CHO cells were simultaneously treated with various doses of rapamycin. The secreted GlyCAM-Ig was purified on Protein A-agarose and then analyzed by Western blot probing with MECA-79 or anti-human IgG. As shown in Fig. 4, GlyCAM-Ig demonstrated MECA-79 immunoreactivity in a manner that depended on the dose of rapamycin, with a minimal effective concentration of 10 nM. Thus, the chemically modulated GlcNAc6ST-2 can act on secreted in addition to membrane-bound glycoproteins in a tunable fashion.
Fig. 4.
Tunable activity of GlcNAc6ST-2 on the secreted glycoprotein GlyCAM-Ig. CHO cells were transfected with GlyCAM-Ig, 2Cat-FRB3, 2Loc-FKBP, and Core1-β3GlcNAcT. The cells were then incubated with rapamycin for 4 d. The secreted GlyCAM-Ig was captured on Protein A-agarose and analyzed by Western blot probing with MECA-79 (Upper) or anti-human IgG (Lower). Far right lane shows GlyCAM-Ig isolated from CHO cells transfected with full-length GlcNAc6ST-2. Molecular mass marker in both blots equals 64 kDa.
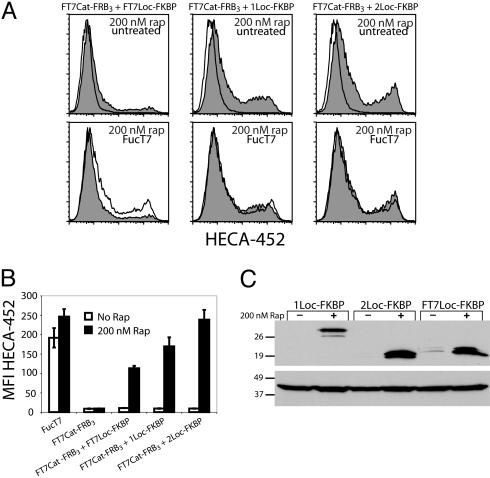
Heterologous Cat and Loc Domains Can Be Functionally Assembled by Rapamycin. The modularity of Golgi enzymes is underscored by numerous previous studies in which functional chimeric enzymes were generated comprising heterologous catalytic and localization domains (29, 39). We therefore sought to address whether such chimeras could be generated with chemical inducibility. Toward this end, we exploited our previous FucT7 constructs comprising the catalytic domain fused to three copies of FRB (FT7Cat-FRB3) and the localization domain fused to FKBP (FT7Loc-FKBP) (15). FT7Cat-FRB3 was introduced in CHO cells alongside either FT7Loc-FKBP, 1Loc-FKBP, or 2Loc-FKBP. The activity of the catalytic domain was assessed by flow cytometry analysis using the anti-sLex antibody HECA-452. As shown in Fig. 5, all combinations demonstrated rapamycin-dependent activity within 2-fold of that produced by full-length FucT7. The most robust activity was observed with the localization domain of GlcNAc6ST-2, which produced a signal similar to that of full-length FucT7. There are several possible explanations for the apparent differences in activity. First, the various localization domains may have different stabilities in cells or may be expressed at different levels. Second, the localization domains may have subtly different distributions among the Golgi cisternae that are more or less favorable with respect to substrate access. Finally, the rapamycin-assembled complexes may experience different geometrical constraints that affect their substrate access or catalytic activities. We tested the effect of rapamycin on the stability of the heterologous pairs of Cat and Loc domains in CHO cells by determining the quantity of Loc domain in the presence or absence of the drug. As shown in Fig. 5C, the presence of rapamycin dramatically increased the stability of all three Loc domains, suggesting that complex formation blocks degradation of the membrane-bound domain. 1Loc-FKBP possesses an extended stem region that may be especially susceptible to proteolysis, which may explain its lower steady-state levels in the absence of the drug.
Fig. 5.
Chimeric enzymes comprising Cat and Loc domains of different proteins are functionally assembled by rapamycin (rap). (A) CHO cells were cotransfected with FT7Cat-FRB3 and one of the following Loc domains: FT7Loc-FKBP (Left), 1Loc-FKBP (Center), or 2Loc-FKBP (Right). (Upper) The cells were treated with 200 nM rapamycin (gray) or untreated (white). (Lower) Comparison of the rapamycin-induced enzyme (gray) with full-length FT7 (white). The cells were stained with mAb HECA-452 and analyzed by flow cytometry. (B) Graphical representation of the mean fluorescence intensities (MFI) from flow cytometry data generated as in A. Error bars represent the standard deviation of triplicate data points. (C) Cells transfected as in A were lysed, and the expression levels of the different Loc domains were compared by Western blot probing with an anti-myc mAb (Upper). The blot was stripped and reprobed with an anti-actin antibody to verify equivalent protein loading (Lower).
Discussion
We have demonstrated that Golgi-resident sulfotransferases responsible for l-selectin ligand biosynthesis can be engineered to respond to a small-molecule switch. The inducible enzymes possess activities and substrate specificities that are comparable to their native counterparts. Because all Golgi sulfotransferases share the same domain organization, this approach for modulating their activity may be applicable across the superfamily. Specific sulfoforms of heparan sulfate bind to growth factors and chemokines and influence their receptor signaling activity (40). These sulfoforms reflect the expression of discrete sulfotransferases, the regulation of which would provide an unprecedented level of control over processes such as angiogenesis, Wnt signaling, and wound repair. Similarly, the sulfotransferases that modify chondroitin sulfates govern processes such as development, nerve regeneration, and lymphocyte homing (41, 42). The ability to control sulfotransferases with small molecules will provide mechanisms for studying these events.
There are several questions that can be uniquely addressed with this experimental system. First, the chemically controlled enzymes are tunable. Their cellular activity can be titrated as a function of the dose of rapamycin. Thus, the system permits a high level of control over the density of sulfated glycans displayed on the cell surface. A single cell line can be induced to display various epitope densities, which can be altered in a dynamic fashion by simply changing the drug concentration. Furthermore, the response time to the drug is limited only by its diffusion rate and the transit time of newly synthesized glycans through the secretory pathway. By contrast, genetic approaches to modulating enzyme activity, such as inducible promoters (i.e., tetracycline), gene silencing (i.e., RNA silencing or antisense), and gene disruption do not offer such control.
Second, the system offers a convenient means to assess the impact of Golgi distribution on the activity of catalytic domains. As demonstrated by the GlcNAc6ST-FucT7 chimeras, a single catalytic domain can be functionally assembled with various localization domains by the chemical inducer of dimerization. Localization domains that reside in different Golgi subcompartments could be used to position a single catalytic domain in those cisternae for comparison of activity on various cellular substrates. By contrast, direct fusion of the catalytic domain to various localization domains could produce structural perturbations that preclude direct comparisons of activity.
One of the most exciting potential applications is the temporally controlled induction/suppression of enzyme activity in living organisms. Conventional gene knockouts are often plagued by lethal phenotypes or compensatory up-regulation of related genes during development of the organism. The inducible system described here may serve as an alternative means for down-regulating enzyme activity after an organism has developed in its presence. It is notable that rapamycin and a related natural product FK506 have been successfully used as modulators of protein activity in vivo (43, 44). Furthermore, the chemical approach we describe could be used to titrate enzyme activity levels in vivo as a means to assess the roles of specific sulfated glycans during development or disease progression.
Supplementary Material
Acknowledgments
This paper is dedicated to Steven D. Rosen on the occasion of his 60th birthday. We thank Jennifer Czlapinski for a critical reading of the manuscript. C.L.d.G. was supported by a National Science Foundation Predoctoral Fellowship. J.J.K. was supported by American Cancer Society Postdoctoral Fellowship TBE-101932. This research was supported by National Institutes of Health Grant GM59907.
Author contributions: C.L.d.G. and C.R.B. designed research; C.L.d.G., S.T.L., and J.J.K. performed research; C.L.d.G. analyzed data; and C.L.d.G. and C.R.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FucT7, fucosyltransferase 7; sLex, sialyl Lewis x; HA, hemagglutinin; MannII, mannosidase II.
References
- 1.Specht, K. M. & Shokat, K. M. (2002) Curr. Opin. Cell Biol. 14, 155–159. [DOI] [PubMed] [Google Scholar]
- 2.Mayer, T. U. (2003) Trends Cell Biol. 13, 270–277. [DOI] [PubMed] [Google Scholar]
- 3.Yeh, J. R. & Crews, C. M. (2003) Dev. Cell 5, 11–19. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, A., Buzko, O., Heyeck-Dumas, S., Jung, I., Kraybill, B., Liu, Y., Shah, K., Ulrich, S., Witucki, L., Yang, F., et al. (2000) Annu. Rev. Biophys. Biomol. Struct. 29, 577–606. [DOI] [PubMed] [Google Scholar]
- 5.Paulmurugan, R., Massoud, T. F., Huang, J. & Gambhir, S. S. (2004) Cancer Res. 64, 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, K., Bleczinski, C., Lin, H., Salazar-Jimenez, G., Sengupta, D., Krane, S. & Cornish, V. W. (2002) Proc. Natl. Acad. Sci. USA 99, 16537–16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbien, A. D. (1987) Annu. Rev. Biochem. 56, 497–534. [DOI] [PubMed] [Google Scholar]
- 8.Lee, L. V., Mitchell, M. L., Huang, S. J., Fokin, V. V., Sharpless, K. B. & Wong, C. H. (2003) J. Am. Chem. Soc. 125, 9588–9589. [DOI] [PubMed] [Google Scholar]
- 9.Eason, P. D. & Imperiali, B. (1999) Biochemistry 38, 5430–5437. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong, J. I., Portley, A. R., Chang, Y. T., Nierengarten, D. M., Cook, B. N., Bowman, K. G., Bishop, A., Gray, N. S., Shokat, K. M., Schultz, P. G. & Bertozzi, C. R. (2000) Angew. Chem. Int. Ed. 39, 1303–1306. [DOI] [PubMed] [Google Scholar]
- 11.Munro, S. (1998) Trends Cell Biol. 8, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunwell, J. R. & Bertozzi, C. R. (2002) Biochemistry 41, 13117–13126. [DOI] [PubMed] [Google Scholar]
- 13.Grabenhorst, E., Nimtz, M., Costa, J. & Conradt, H. S. (1998) J. Biol. Chem. 273, 30985–30994. [DOI] [PubMed] [Google Scholar]
- 14.Zhu, G., Allende, M. L., Jaskiewicz, E., Qian, R., Darling, D. S., Worth, C. A., Colley, K. J. & Young, W. W., Jr. (1998) Glycobiology 8, 831–840. [DOI] [PubMed] [Google Scholar]
- 15.Kohler, J. J. & Bertozzi, C. R. (2003) Chem. Biol. 10, 1303–1311. [DOI] [PubMed] [Google Scholar]
- 16.Spencer, D. M., Wandless, T. J., Schreiber, S. L. & Crabtree, G. R. (1993) Science 262, 1019–1024. [DOI] [PubMed] [Google Scholar]
- 17.Otto, K. G., Jin, L., Spencer, D. M. & Blau, C. A. (2001) Blood 97, 3662–3664. [DOI] [PubMed] [Google Scholar]
- 18.Honke, K. & Taniguchi, N. (2002) Med. Res. Rev. 22, 637–654. [DOI] [PubMed] [Google Scholar]
- 19.Baenziger, J. U., Kumar, S., Brodbeck, R. M., Smith, P. L. & Beranek, M. C. (1992) Proc. Natl. Acad. Sci. USA 89, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lortat-Jacob, H., Grosdidier, A. & Imberty, A. (2002) Proc. Natl. Acad. Sci. USA 99, 1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla, D., Liu, J., Blaiklock, P., Shworak, N. W., Bai, X., Esko, J. D., Cohen, G. H., Eisenberg, R. J., Rosenberg, R. D. & Spear, P. G. (1999) Cell 99, 13–22. [DOI] [PubMed] [Google Scholar]
- 22.Pethe, K., Alonso, S., Biet, F., Delogu, G., Brennan, M. J., Locht, C. & Menozzi, F. D. (2001) Nature 412, 190–194. [DOI] [PubMed] [Google Scholar]
- 23.Rosen, S. D. (2004) Annu. Rev. Immunol. 22, 129–156. [DOI] [PubMed] [Google Scholar]
- 24.Hemmerich, S., Leffler, H. & Rosen, S. D. (1995) J. Biol. Chem. 270, 12035–12047. [DOI] [PubMed] [Google Scholar]
- 25.Yeh, J. C., Hiraoka, N., Petryniak, B., Nakayama, J., Ellies, L. G., Rabuka, D., Hindsgaul, O., Marth, J. D., Lowe, J. B. & Fukuda, M. (2001) Cell 105, 957–969. [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka, N., Petryniak, B., Nakayama, J., Tsuboi, S., Suzuki, M., Yeh, J. C., Izawa, D., Tanaka, T., Miyasaka, M., Lowe, J. B. & Fukuda, M. (1999) Immunity 11, 79–89. [DOI] [PubMed] [Google Scholar]
- 27.Bistrup, A., Bhakta, S., Lee, J. K., Belov, Y. Y., Gunn, M. D., Zuo, F. R., Huang, C. C., Kannagi, R., Rosen, S. D. & Hemmerich, S. (1999) J. Cell Biol. 145, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemmerich, S., Bistrup, A., Singer, M. S., van Zante, A., Lee, J. K., Tsay, D., Peters, M., Carminati, J. L., Brennan, T. J., Carver-Moore, K., et al. (2001) Immunity 15, 237–247. [DOI] [PubMed] [Google Scholar]
- 29.de Graffenried, C. L. & Bertozzi, C. R. (2003) J. Biol. Chem. 278, 40282–40295. [DOI] [PubMed] [Google Scholar]
- 30.Uchimura, K., El-Fasakhany, F. M., Hori, M., Hemmerich, S., Blink, S. E., Kansas, G. S., Kanamori, A., Kumamoto, K., Kannagi, R. & Muramatsu, T. (2002) J. Biol. Chem. 277, 3979–3984. [DOI] [PubMed] [Google Scholar]
- 31.Natsuka, S., Gersten, K. M., Zenita, K., Kannagi, R. & Lowe, J. B. (1994) J. Biol. Chem. 269, 16789–16794. [PubMed] [Google Scholar]
- 32.Mitsuoka, C., Sawada-Kasugai, M., Ando-Furui, K., Izawa, M., Nakanishi, H., Nakamura, S., Ishida, H., Kiso, M. & Kannagi, R. (1998) J. Biol. Chem. 273, 11225–11233. [DOI] [PubMed] [Google Scholar]
- 33.Streeter, P. R., Rouse, B. T. & Butcher, E. C. (1988) J. Cell Biol. 107, 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho, S. K. & Cummings, R. D. (1997) J. Biol. Chem. 272, 13622–13628. [DOI] [PubMed] [Google Scholar]
- 35.Opat, A. S., Houghton, F. & Gleeson, P. A. (2000) J. Biol. Chem. 275, 11836–11845. [DOI] [PubMed] [Google Scholar]
- 36.Mootz, H. D., Blum, E. S., Tyszkiewicz, A. B. & Muir, T. W. (2003) J. Am. Chem. Soc. 125, 10561–10569. [DOI] [PubMed] [Google Scholar]
- 37.Lasky, L. A., Singer, M. S., Dowbenko, D., Imai, Y., Henzel, W. J., Grimley, C., Fennie, C., Gillett, N., Watson, S. R. & Rosen, S. D. (1992) Cell 69, 927–938. [DOI] [PubMed] [Google Scholar]
- 38.Tangemann, K., Bistrup, A., Hemmerich, S. & Rosen, S. D. (1999) J. Exp. Med. 190, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabenhorst, E. & Conradt, H. S. (1999) J. Biol. Chem. 274, 36107–36116. [DOI] [PubMed] [Google Scholar]
- 40.Esko, J. D. & Selleck, S. B. (2002) Annu. Rev. Biochem. 71, 435–471. [DOI] [PubMed] [Google Scholar]
- 41.Bradbury, E. J., Moon, L. D., Popat, R. J., King, V. R., Bennett, G. S., Patel, P. N., Fawcett, J. W. & McMahon, S. B. (2002) Nature 416, 636–640. [DOI] [PubMed] [Google Scholar]
- 42.Fieger, C. B., Sassetti, C. M. & Rosen, S. D. (2003) J. Biol. Chem. 278, 27390–27398. [DOI] [PubMed] [Google Scholar]
- 43.Stankunas, K., Bayle, J. H., Gestwicki, J. E., Lin, Y. M., Wandless, T. J. & Crabtree, G. R. (2003) Mol. Cell 12, 1615–1624. [DOI] [PubMed] [Google Scholar]
- 44.Spencer, D. M., Belshaw, P. J., Chen, L., Ho, S. N., Randazzo, F., Crabtree, G. R. & Schreiber, S. L. (1996) Curr. Biol. 6, 839–847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.