Abstract
The adult RDA is defined as the average daily level of intake sufficient to meet the nutrient requirements of nearly all healthy people. The RDA for protein for adults ≥18 y of age (0.8 g/kg) has been essentially unchanged for >70 y. In practice, the RDA for protein was derived to estimate the minimum amount of protein that must be eaten to avoid a loss of body nitrogen. The Acceptable Macronutrient Distribution Range (AMDR) (10–35% of calories as protein) was developed to express dietary recommendations in the context of a complete diet. It is noteworthy that the lowest level of protein intake reflected in the AMDR is higher than that of the RDA. Furthermore, recent studies, particularly in older individuals, suggest specific health benefits at levels of protein intake that significantly exceed the RDA. Translation of protein intake recommendations for the general adult population into dietary guidance for individuals requires an understanding of the derivation and intended use of both the protein RDA and AMDR. The following discussion will describe limitations to the derivation and practical application of the RDA compared with the use of the AMDR to help maximize health benefits associated with higher protein intake by using flexible calories inherent in different dietary patterns.
Keywords: protein, nitrogen balance, RDA, AMDR, dietary pattern
Introduction
Dietary protein recommendations have been made for almost 100 y (1). Nonetheless, the answer to the seemingly simple question of “how much protein should we eat?” remains unclear. Protein constitutes a vital portion of body composition, and is required for growth and development (2). In addition, dietary protein is required throughout life to replace irreversibly oxidized amino acids that cannot be synthesized in the body [i.e., the essential amino acids (EAAs)7] (2). There also appears to be a requirement for a certain amount of protein intake to provide amino acids that can be produced in the body, but not at a rate to satisfy all requirements (2).
The cornerstone of macronutrient recommendations in the United States comes from the Food and Nutrition Board (FNB) of the Institute of Medicine. On a periodic basis, the FNB convenes meetings of experts to determine or update a set of reference values for both micro- and macronutrients. A variety of values are derived, as sufficient data allows, for each macronutrient. These values include the Estimated Average Requirement (EAR), the RDA, and the Tolerable Upper Intake Level (UL). These values are collectively referred to as the Dietary Reference Intake (DRI). The DRI specifies macronutrient intake requirements in absolute terms (i.e., grams per day) by age and sex group or, in the case of protein, as a function of body weight. In this paper we will focus on recommendations for protein intake in adults, who are defined in the DRI as individuals ≥18 y. The RDA for protein does not distinguish sex or age group beyond the classification of adult. In addition to the RDA, recommendations for macronutrient intake are also provided in the context of a complete diet as the Acceptable Macronutrient Distribution Range (AMDR). The AMDR expresses intake recommendations as a percentage of total caloric intake. The DRI serves as a reference point in the development of food-based dietary guidance for the general population, specifically, the Dietary Guidelines for Americans (DGA) (3), commissioned by the USDA and the Department of Health and Human Services. Food-based dietary guidance is presented in the DGA via healthy eating patterns and expressed in MyPlate guidance as MyPlate Daily Checklists (4) designed to guide individual food choices to promote healthful eating.
Although the focus of this paper is the specific recommendations promulgated by agencies in the United States, the general principles related to dietary protein intake are relevant to all dietary recommendations of which we are aware. Dietary recommendations of agencies such as a joint the FAO/WHO committee, as well as recommendations of other countries, do not differ markedly from the US recommendations for macronutrient intake, although many of these other agencies do not express an AMDR.
The DRI states that dietary protein recommendations are based on high-quality proteins. However, the definition of “high-quality” is not provided. The FAO/WHO has convened consultations to provide qualitative rankings of protein. The initial scoring system was termed the Protein Digestibility Corrected Amino Acid Score (5). Most recently, the FAO/WHO released the description of the improved Digestible Indispensable Amino Acid Score (DIAAS) (6). The DIAAS for a dietary protein is based on the proportion and profile of EAAs in the protein and the true ileal digestibility of the protein (6). The basic concept is that a high-quality protein should provide an abundance of EAAs in a profile that closely resembles the profile of individual EAA requirements, and that the protein should be readily digestible. Ultimately, the goal of the DIAAS would be to incorporate protein quality into dietary planning, and to assist the consumer in that endeavor by enabling the inclusion on package labeling of not only the protein content but also the protein quality. However, this goal is not currently feasible because of insufficient data on a variety of proteins; therefore, protein quality is not considered in depth in this article. We recently published a detailed discussion of the DIAAS that included an evaluation of the underlying assumptions (7).
Current Status of Knowledge
DRI and dietary guidance.
Currently, the EAR and the RDA for protein are 0.66 and 0.80 g ⋅ kg−1 ⋅ d−1, respectively (Table 1) (2). The AMDR is 10–35% of caloric intake as protein, which is 1.05–3.67 g ⋅ kg−1 ⋅ d−1 when reference body weights of 57 and 70 kg are assumed for women and men, respectively, and a mean estimated energy requirement of 36.5 kcal ⋅ kg−1 ⋅ d−1 is used (2) (Table 1). The actual energy requirement of an individual depends on sex, body weight and lean body mass (LBM), activity level, and other factors. The value of 36.5 kcal ⋅ kg−1 ⋅ d−1 is estimated for a 30-y-old man with low activity levels weighing 70 kg (2). For such an individual, the lowest acceptable protein intake according to the AMDR is ∼10% greater than that of the RDA. For a higher rate of energy expenditure, the lowest acceptable value would exceed the RDA by even more (8). The recommended protein intake from MyPlate is more in accord with the AMDR than is the RDA, being equivalent to 17–21% calories (4). This is equivalent to 1.78–2.20 g protein ⋅ kg−1 ⋅ d−1 if mean estimated energy requirements and body weight are assumed (Table 1). MyPlate recommendations are consistent with the usual protein intake in the United States, which ranges from 13% to 16% of total calories, depending on age and sex, or ∼1.60 g ⋅ kg−1 ⋅ d−1 for a healthy adult (9). These values highlight the value of the AMDR, which is defined as “a range of intakes for a particular energy source that is associated with reduced risk of chronic diseases while providing adequate intakes of essential nutrients” (2). The AMDR is thus meant to be a target for dietary macronutrient intake in the context of a complete diet and potentially variable nutritional goals of the individual. However, the RDA, rather than the AMDR, is widely considered to be the appropriate target for protein intake. For example, the Health Statistics division of the CDC's National Center for Health Statistics cites the recommended daily intake of protein as 0.8 g ⋅ kg−1 ⋅ d−1, and states on its health blog that “Adults’ daily protein intake is much more than recommended,” because mean protein intake exceeds the RDA (10). This representative statement is not only at odds with both the AMDR and MyPlate recommendations, as well as current dietary practice in the United States, but also the FNB’s position regarding the RDA for protein. In discussing the RDA for protein, the FNB points out that there may be benefits to eating amounts of dietary protein greater than that in the RDA, and it is extensively documented in the same chapter that no UL for protein intake beyond which adverse effects may result could be identified (2). Confusion regarding the relation between the RDA and the optimal amount of dietary protein intake in all adults may in part stem from semantic issues. The term “RDA” suggests to the average consumer not familiar with the technical definition of the RDA that it is recommended that the RDA be eaten, and that any level of protein intake above the RDA will exceed that which is allowed. Thus, there is an inherent possibility of misinterpretation of the meaning of the RDA when planning the desired amount of dietary protein intake. In this regard, the pertinent issue is whether there is an amount of dietary protein in excess of the RDA but within the AMDR that provides beneficial outcomes.
TABLE 1.
Recommendations for dietary protein intake1
It is our assertion that current dietary guidance is presented in a way that may lead to confusion between nutritional scientists, nutrition practitioners, and the general public as to how much protein we should eat. Translation of protein intake recommendations for the general population to dietary food-based guidance for individuals requires an understanding of the derivation and intended use of both the RDA for protein and the AMDR for protein. We will discuss the limitations of use of only the RDA for protein to develop dietary guidance, highlight the evidence that there are benefits to an intake of dietary protein in excess of the RDA in many circumstances, and provide examples of how dietary food-based guidance can be developed with the use of the AMDR.
Determination of DRI for protein.
The EAR and RDA for adults ≥18 y of age have been determined by the single endpoint of the amount of protein intake required to maintain nitrogen equilibrium, or nitrogen balance (NB). It is stated in the DRI that “the criterion of adequacy of the EAR is based on the lowest continuing intake of dietary protein that is sufficient to achieve body nitrogen equilibrium (zero balance)” (2). The RDA is determined as being 2 SDs above the EAR, meaning that the RDA is an estimate of the lowest dietary protein intake level that meets the protein requirement of nearly all (97–98%) healthy individuals. Thus, approximately one-half of individuals consuming the EAR for protein will satisfy minimal protein requirements to maintain nitrogen equilibrium, whereas one-half will fail to maintain a zero NB while consuming the EAR for protein. In contrast, most individuals who consume the RDA for protein will be satisfying their minimal requirement for protein as defined by NB.
There is a reason, however, to question reliance on results from NB studies as the sole criterion for determination of recommendations for dietary protein. Even if the NB technique is accepted as the appropriate methodology, there is debate as to how the EAR and RDA are calculated from NB data (2). The NB technique to determine protein requirements is a conceptually straightforward approach with a quantitative endpoint that has been used as a standard by which to assess protein nutrition for >100 y. As a consequence of the long-standing use of the NB technique, there have been a large number of NB studies performed that provide an abundance of data upon which to base the EAR. On the other hand, there are shortcomings in the NB method for determining protein requirements. There is considerable variability in the determination of NB, in part because it is difficult to accurately measure all sources of nitrogen excretion. The calculation of NB also is variable because NB is a small number resulting from the difference in 2 large numbers (nitrogen intake and nitrogen excretion), each of which has its own sources of variability. Furthermore, NB is dependent on the length of time at a particular level of nitrogen intake, and there is no consensus on the acceptable duration of experimental control. In addition, differences in protein quality that are not accounted for, as well as the nature and amount of nonprotein macronutrients in the diet (i.e., carbohydrate and fat), can affect the observed NB data.
Conceptually, NB has generally been presumed to be a surrogate for measurement of changes in LBM over a defined period of time, but to our knowledge, there are few studies documenting the fact that changes in NB correspond to measured changes in LBM. In fact, the assumed relation between NB and changes in LBM is limited to circumstances of negative NB. A progressively positive NB results as nitrogen intake exceeds nitrogen excretion. However, positive NB values have been assumed to be artifacts, and therefore are not accounted for in the estimation of the EAR (2). Ignoring these positive NB values has been justified by the fact that LBM does not change in healthy adults in the short duration of most NB studies, so that the apparent increase in NB at levels of protein intake greater than the EAR must be artifactual (2). In our opinion, this justification is weak. Data cited in the following section give reason to believe that higher rates of protein consumption in fact often lead to increased LBM.
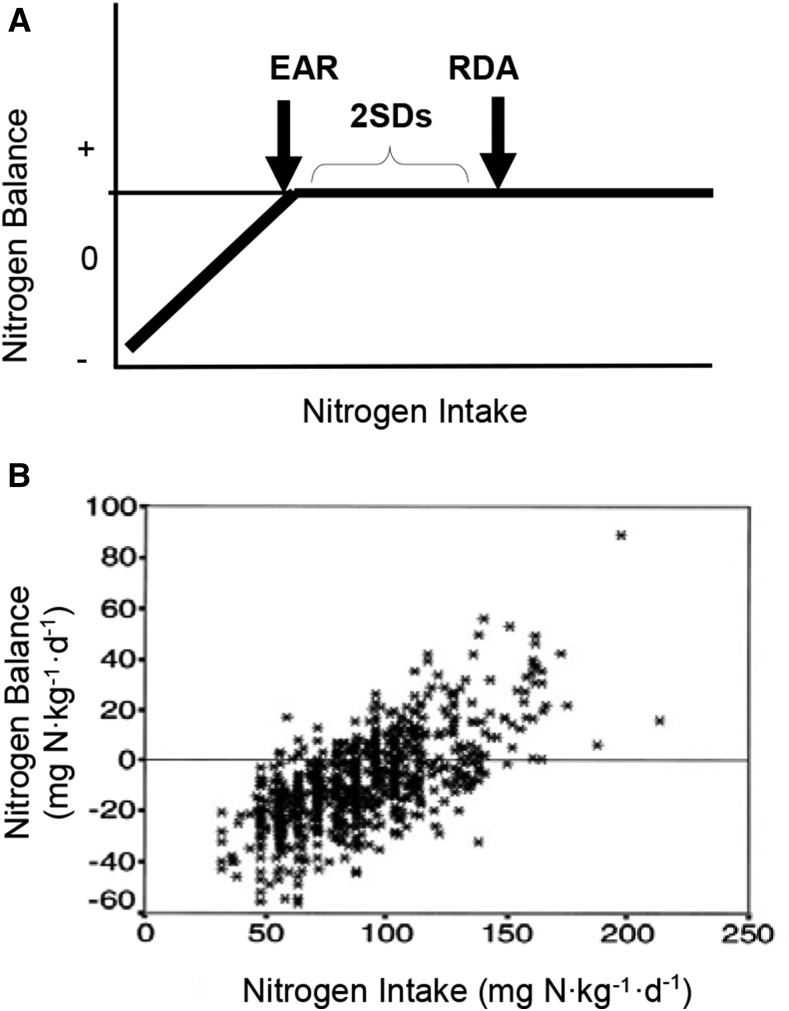
Even if it is stipulated that there is physiologic relevance to the amount of protein intake required for zero NB, the means of estimating the values for EAR and RDA from existing NB data can be questioned. The theory of the calculation of the EAR and RDA from NB data is illustrated schematically in Figure 1A. Once NB (i.e., zero balance) has been achieved, further increases in protein intake are assumed to not result in corresponding increases in NB. This assumption would appear to be at odds with the actual data used to calculate the EAR (Figure 1B) (11). When calculation of EAR and RDA was more inclusive of all NB data, values were calculated (0.93 and 1.2 g protein ⋅ kg−1 ⋅ d−1 for EAR and RDA, respectively) that were ∼50% greater than the DRI values (12). Assessment of the validity of one statistical approach compared with the other is beyond the scope of this paper, but the different results add to the uncertainty of the quantification of protein requirements from NB studies.
FIGURE 1.
Nitrogen balance as a tool to determine protein requirements. A simplified schematic of the model used to calculate the EAR and RDA for protein from nitrogen balance studies (A). Positive nitrogen balance values are considered to be artifacts. Results from the meta-analysis of nitrogen balance data that was used by the Food and Nutrition Board to calculate the EAR and the RDA (B). This represents the relation between individual nitrogen balances, corrected for dermal and miscellaneous losses, and nitrogen intake in healthy adults. In some cases the same individuals were tested at different levels of protein intake, whereas in other cases the subject was tested at only one level of protein intake. The values for EAR and RDA depend on the means by which they are calculated from this data set (11). EAR, Estimated Average Requirement. Panel B reproduced from reference 11 with permission.
The DRI report recognizes the shortcomings of the NB technique. The report states that “due to the shortcomings of the NB method noted earlier, it is recommended that the use of NB should no longer be regarded as the ‘gold standard’ for the assessment of the adequacy of protein intake and that alternative means should be sought” (2). Nonetheless, all other evidence regarding dietary protein was ignored in the derivation of the RDA. If the shortcomings of the NB technique are disregarded and the RDA is considered to be the best available estimate of protein intake to meet minimal requirements for most individuals, there remains the important issue of whether the terminology “RDA” is an accurate reflection of its true meaning. It is our opinion that the protein RDA would more appropriately be termed the “recommended minimum intake.” Although we recognize the impracticality of a new expression’s being accepted in lieu of that used in the DRI, we feel it is worthwhile to consider the proposed new nomenclature in the context of this paper to better understand the true meaning of the RDA.
Is there an optimal level of protein intake that is greater than the RDA?
Many more factors than simply maintaining LBM should be considered when determining recommendations for dietary protein intake. Maintaining or increasing LBM has been the major focus of studies designed to address the question of whether there is a benefit to dietary protein intake at rates greater than those of the RDA. It has been consistently shown that increasing the level of nitrogen intake corresponds to increases in LBM or a surrogate of LBM, such as NB or net protein synthesis. NB progressively increases as nitrogen intake increases above the RDA, as shown in Figure 1B. Whereas many discount the validity of those NB results for reflecting actual changes in LBM, the NB results are consistent with the results of stable isotope tracer studies that show that net protein synthesis increases linearly with increasing protein or amino acid intake (13–15).
Consistent with the results from short-term tracer studies of protein synthesis and breakdown, observable changes in outcomes over longer periods are achieved with higher levels of protein intake than those in the RDA. For example, both our own study (16) and a study from a different group (17) showed improvements in LBM, strength, and functional tests when the normal diet was supplemented 2 times/d with 11 g of a mixture of EAAs. We also showed that the decrease in functional capacity that normally occurs in healthy elderly individuals confined to bed rest could be minimized by supplementation of the diet with EAAs (18). Similarly, the loss of LBM and muscle strength that occurred with 28 d of bed rest in healthy young subjects was ameliorated by supplementation with a mixture of EAAs and a small amount of carbohydrate (19). The results from the bed rest studies are particularly relevant because all known factors other than total protein intake that might potentially affect LBM changes, including activity and other macronutrient intake, were completely controlled. The fact that acute isotopic studies at the onset and end of the bed rest interval agreed quantitatively with the measured changes in LBM over that 28 d period support the validity of isotopic studies as one approach to assessing the response to protein or amino acid nutrition.
Muscle mass has been shown to be improved in other studies in elderly subjects (20), as well as in patients with heart failure and cachexia (21). Tieland et al. (22) showed that supplementation of the diet of frail elderly with 30 g milk protein for 24 wk significantly improved physical performance compared with a placebo control. The results of the prospective Healthy Aging and Body Composition Study (23) also provide support for the benefit of higher protein intake in preserving LBM in a large group of >2000 elderly subjects studied over 3 y. Loss of LBM was lowest in the quintile consuming the most protein (18.6% of caloric intake), whereas the quintile consuming the lowest amount of protein (10.9% of caloric intake) lost 40% more LBM than did the highest quintile (23). These results are consistent with a survey of the dietary habits of 142 older adults, which reported a positive correlation between dietary protein intake and midarm muscle area (24).
Not only does the preponderance of evidence indicate that maintenance of maximal LBM requires a level of protein intake greater than that of the RDA, other physiologic functions have been shown to benefit from consumption of dietary protein at a rate >0.8 g ⋅ kg−1 ⋅ d−1. The deleterious effects of chronic inflammation on muscle in elderly persons have been shown to be inversely related to the level of protein intake (25). In addition, intake of 13.4% of energy as protein compared with 8.0% (the EAR) was beneficial in terms of strength and physical function in young, healthy volunteers restrained to bed rest (26). Studies have uniformly shown that levels of protein intake greater than those in the RDA are particularly important in caloric restriction weight loss. Diets containing between 18% and 25% calories as protein have been shown to result in greater maintenance of LBM than diets containing 10–12% of calories (27). In a study of 60 obese older adults who participated in a weight program, receiver-operating characteristic curve analysis revealed a protein intake requirement of 1.2 g ⋅ kg−1 ⋅ d−1 to maintain LBM (28). This amount of protein intake may exceed 35% of caloric intake, depending on the weight of the individual and the extent of caloric restriction. Beneficial effects of higher protein diets have not only been observed in normal-weight adults, but also in children. In a study of normal-weight children aged 5–18 y who had overweight parents, a diet containing ∼20% protein improved cardiovascular disease risk markers such as blood pressure and blood lipids compared with a diet containing ∼16% of calories as protein (29). Supplementation of the normal diet of older individuals with 22 g EAAs/d not only improved physical function, but also decreased liver fat and circulating TGs (14, 30). Insulin sensitivity was improved in men with type 2 diabetes when they consumed a diet containing 30% of calories as protein as opposed to 15% of calories as protein (31). Although high-protein diets were at one time thought to cause bone resorption because of acidification of the blood, studies since then have convincingly demonstrated that higher-protein diets are in fact beneficial for bone health (32).
We believe that the overall conclusion from these various studies is that there is an optimal level of protein intake that is greater than that of the RDA. Importantly, to our knowledge, there has never been a study in which the RDA for protein intake was compared with a higher level of protein intake, and the RDA was found to be superior in terms of any endpoint. We emphasize that this discussion has focused entirely on the amount of dietary protein, and neither the quality of protein nor the protein food source has been considered.
Although there are numerous studies that have demonstrated a benefit of increasing the level of protein intake above the RDA for protein, it should be understood that, in all of these studies, the amount of protein consumed did not exceed the upper limit as defined by the AMDR (35% of caloric intake). In fact, there are few data in which subjects received >20% of calories as protein, whereas in certain circumstances, such as in training for strength events or bodybuilding, individuals may consume >20% of calories as protein. In general, a diet composed of >35% of calories as protein would be unusual and even difficult to accomplish. This means that, in all cases, nonprotein macronutrients comprise ≥65% of the diet. This amount of nonprotein macronutrient intake is adequate to supply all of the potential beneficial effects of factors, such as fiber, essential FAs, and ω-3 FAs. This discussion of the beneficial effects of dietary protein has therefore been presented in the context of the AMDR, and does not imply failing to satisfy other nutrient requirements.
Applying the AMDR.
The AMDR clearly recommends an intake of protein that exceeds that of the RDA (Table 1). The wide acceptable range expressed in the AMDR (10–35% of calories) suggests flexibility regarding exactly how much protein should be eaten in the context of a complete diet. Determination of the appropriate flexible level of protein intake to achieve optimal physiologic responses is an important area of investigation, and it is likely dependent on the dietary goals of the individual. The AMDR is particularly practical in this regard, because the AMDR provides guidance for individualizing meal plans according to specific circumstances.
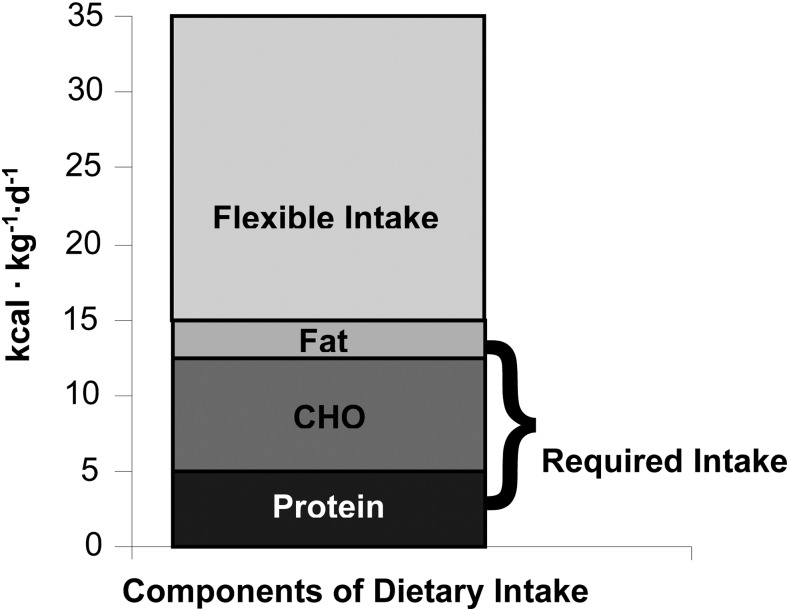
The RDA for carbohydrate is 130 g/d, and the recommended minimal intake for fat (there is no RDA for fat) is 30 g/d (2). For a 70-kg man, this would correspond to 1.86 g ⋅ kg−1 ⋅ d−1 for carbohydrate and 0.43 g ⋅ kg−1 ⋅ d−1 for fat. When the caloric equivalents of the recommended allowances for fat (3.9 kcal ⋅ kg−1 ⋅ d−1) and carbohydrate (7.4 kcal ⋅ kg−1 ⋅ d−1) are added to that for protein (3.2 kcal ⋅ kg−1 ⋅ d−1), the total (14.5 kcal ⋅ kg−1 ⋅ d−1) constitutes between 30% and 40% of the estimated energy requirement, depending upon the age, sex, and activity level of the individual (Figure 2) (2). Thus, depending on physiologic circumstances, ≥60% of caloric intake can be considered to be flexible (Figure 2). The flexible component of the diet may be the most important in terms of development of obesity, undernutrition, or diseases that are, at least in part, related to dietary habits. The issue of practical importance is the amount of protein, fat, and carbohydrate that should comprise the flexible component of caloric intake.
FIGURE 2.
The relation of the caloric contributions of the RDA for protein and carbohydrate and the recommended minimal intake of fat to total caloric requirement as estimated for a 30-y-old man with low activity levels (36.5 kcal ⋅ kg−1 ⋅ d−1) (reference 2, Tables 5–22). Although energy requirements may vary according to sex, age, activity level, and other factors, at most, the RDA of the macronutrients can account for ∼40% of energy requirement. CHO, carbohydrate.
Determining the flexible intake of protein to achieve optimal physiologic responses requires consideration of all aspects of dietary macronutrient intake, including the benefits and potential adverse effects. Adverse responses to diets high in carbohydrate and/or fat are well documented in animal studies (33–36). Epidemiologic data indicate that the relations between the amount of carbohydrate intake and adverse responses in humans are similar to those in the rat (37). Prospective studies in human subjects in which the type or amount of carbohydrate intake was altered demonstrated adverse effects on cardiovascular disease risk factors. Recently, the Canadian Trial of Carbohydrates in Diabetes reported that higher carbohydrate intake was associated with increased postprandial glucose and TG concentrations (38). The DRI for macronutrients reports no metabolic benefit of the carbohydrate component of the diet. The RDA for carbohydrate is based entirely on the estimated utilization of glucose by the central nervous system, with the caveat that a carbohydrate-free diet causes no adverse effects because the central nervous system adapts to the use of ketone bodies as energy substrates (39). Thus, there is no physiologic rationale for increasing carbohydrate intake above the RDA.
The physiologic consequences of dietary fat are less clear than those for carbohydrate. Fat intake has historically been considered to be undesirable, particularly if a substantial portion of fat intake is in the form of trans or nonstearic acid saturated fat (2). However, it has become recognized that diets that use nonhydrogenated unsaturated fats as the predominant form of fat, along with adequate ω-3 FA, can be part of a healthy diet (40). Even consumption of saturated fats, once thought to be directly linked to the development of heart disease, has since been cast in a favorable light (41). Nonetheless, even though the detrimental effects of increasing fat intake are less clear-cut than are those with carbohydrate, there is no metabolic advantage attributable, per se, to increasing the fat portion of the diet above requirement levels, other than an advantage that might be gained by a corresponding decrease in carbohydrate consumption.
A lengthy discussion of the voluminous data on the pros and cons of dietary fat and carbohydrate is beyond the scope of this paper. Relative to determining the optimal level of protein intake, it is sufficient to recognize that a diet containing ≥65% nonprotein calories has adequate flexibility to accommodate all potential beneficial effects of both dietary fat and carbohydrate. To meet this level of nonprotein intake, it is not necessary for the sum total of fat and carbohydrate to comprise a large portion of flexible caloric intake.
In contrast to the scenario of increasing either the fat or carbohydrate portion of the diet to satisfy the total requirement for energy intake, consumption of dietary protein in excess of the RDA but within the AMDR guidelines is not known to cause adverse responses. Areas of potential concern with a higher protein intake that were discussed in depth in the DRI included renal function, formation of kidney stones, bone health, and the development of cardiovascular disease (2). Evidence indicates that a higher intake of protein by patients with renal disease may contribute to the deterioration of kidney function (42). However, in healthy individuals, there is no evidence linking reduced glomerular filtration with increased protein intake (43). No clear conclusions can be drawn between the intake of protein and the formation of kidney stones (2). Evaluations of the effect of protein intake on bone health concluded that dietary intake at levels in excess of the recommended dietary intake would be beneficial (44). With regard to cardiovascular disease, evidence from human studies indicates that diets with a higher proportion of protein are beneficial (45, 46). These observations are consistent with analysis of epidemiologic data from the Nurses’ Health Study of >80,000 women (47), which reported an inverse correlation between occurrence of ischemic heart disease and the level of protein intake.
How much protein should we eat? A menu modeling exercise based on the AMDR for protein.
Given that no adverse metabolic consequences of a higher protein diet within the AMDR range have been identified, the question of nutrient balance of other key nutrients is logical to consider. The 2015 DGA provides 12 USDA food patterns, based on age and sex, to model dietary plans that would provide the basis for nutrient adequacy (3). All 12 USDA food patterns meet or exceed the majority of nutrient adequacy goals, although some nutrients in the food patterns of the DGA, including choline, vitamin E, potassium, and vitamin D, are below the RDA or Adequate Intake for several age and sex groups (3). A 2000-kcal, healthy US-style eating pattern is used to demonstrate how a nutrient-dense diet can be created with the use of the types and proportions of foods commonly consumed by Americans to meet most nutrient needs. This healthy US-style eating pattern includes daily servings of vegetable, fruit, grain, dairy, protein, and oil food groups, and allows for a limited amount of flexible calories from added sugars and saturated fats. The protein food group servings allocated in the 2000-kcal healthy US-style eating pattern are 5.5 ounce–equivalent protein food servings, which translates to ∼18% energy or 91 g protein/d (Table 2). This amount is aligned with the AMDR, but exceeds the RDA by providing 163% and 198% of the estimated protein RDA for adult women and men (48), respectively.
TABLE 2.
Selected nutrients in food patterns modeled with the use of the AMDR for protein1
| AMDR modeling |
|||
| DGA 20152 | 18% protein | 30% protein | |
| Macronutrients | |||
| Protein, g/d | 91.0 | 94.0 | 147 |
| % of RDA3 | 0.0 | 192 | 300 |
| % of calories | 18.2 | 18.8 | 29.4 |
| Total lipid (fat), g/d | 72.0 | 64.5 | 51.3 |
| % of calories | 32.0 | 29.0 | 23.0 |
| Cholesterol, mg/d | 215 | 128 | 226 |
| % of goal (<300 mg/d) | 72.0 | 43.0 | 75.0 |
| SFAs, g/d | 18.7 | 14.0 | 11.3 |
| % of calories | 8.4 | 6.3 | 5.1 |
| Carbohydrate, g/d | 256 | 278 | 250 |
| % of calories | 51.0 | 55.7 | 50.0 |
| Added sugars | 30.0 | 32.0 | 20.0 |
| % of goal4 | 100 | 106 | 67.0 |
| Total dietary fiber, g/d | 31.0 | 27.8 | 32.1 |
| % of goal (14 g/1000 kcal) | 111 | 99.0 | 104 |
| Minerals | |||
| Calcium, mg/d | 1274 | 1420 | 1275 |
| % of RDA3 | 127 | 142 | 124 |
| % of UL | 51.0 | 57.0 | 51.0 |
| Iron, mg/d | 17.0 | 16.2 | 18.0 |
| % of RDA3 | 94.0 | 90.0 | 101 |
| % of UL | 38.0 | 36.0 | 40.0 |
| Magnesium, mg/d | 352 | 442 | 518 |
| % of RDA3 | 110 | 138 | 159 |
| % of UL5 | NA | NA | NA |
| Potassium, mg/d | 3348 | 4236 | 4500 |
| % of AI | 71.0 | 90.0 | 96.0 |
| Sodium, mg/d | 1787 | 1932 | 1928 |
| % of goal4 (<2300 mg) | 78.0 | 84.0 | 84.0 |
| Vitamins | |||
| Vitamin A, μg RAE/d | 898 | 1480 | 1464 |
| % of RDA3 | 128 | 211 | 209 |
| % of UL | 30.0 | 49.0 | 49.0 |
| Vitamin E, mg α-tocopherol/d | 10.2 | 6.8 | 10.5 |
| % of RDA3 | 68.0 | 45.0 | 70.0 |
| % of UL6 | 1.0 | 0.7 | 1.0 |
| Vitamin C, mg/d | 117 | 165 | 153 |
| % of RDA3 | 156 | 219 | 204 |
| % of UL | 5.8 | 8.2 | 7.6 |
| Vitamin D, IU/d | 274 | 410 | 403 |
| % of RDA3 | 46.0 | 68.0 | 68.0 |
| % of UL | 6.8 | 10.0 | 10.0 |
| Folate, μg DFE/d | 586 | 441 | 373 |
| % of RDA3 | 146 | 112 | 93 |
| % of UL | 59.0 | 44.0 | 37.0 |
| Choline, mg/d | 349 | 294 | 365 |
| % of AI | 82.0 | 69.0 | 86.0 |
| % of UL | 10.0 | 8.4 | 10.0 |
All based on the USDA Health Eating Pattern and 2000 kcal/d. AI, Adequate Intake; AMDR, Acceptable Macronutrient Distribution Range; DFE, dietary folate equivalent; DGA, Dietary Guidelines for Americans; IU, international unit; NA, not applicable; RAE, retinol activity equivalent; UL, tolerable upper intake level.
From reference 3, Table E3.1.A6.
Assumes a woman aged 31–50 y with a body weight of 61.5 kg.
As described in reference 3, Table E3.1.
Established only for dietary supplements and pharmaceutical preparations.
Applies to synthetic forms obtained from fortified foods, supplements, or a combination of the 2.
The 2015 DGA states that the 2000-kcal healthy US-style eating plan consists of 1732 essential kilocalories, foods consumed in nutrient-dense forms without additional solid fats or added sugars, and 271 nonessential kilocalories, e.g., calories that can be obtained from solid fats or added sugars. If those nonessential or flexible calories would be allocated to protein instead, it would translate to ∼68 g additional protein (159 g total protein or 32% protein energy) in the eating plan. This amount is significantly above the RDA for the age and sex groups, but remains within the AMDR. It is therefore theoretically possible to incorporate a relatively high percentage of protein into the diet while staying fundamentally within the context of the DGA.
Building on this concept, to determine whether a higher-protein eating pattern (30% protein energy) can be consistent with the 2000-kcal healthy US-style eating pattern that has ∼18% protein energy in nutrient adequacy and meets USDA food group intake recommendations, menu modeling exercises were conducted. We chose to model diets composed of only 2000 kcal to be consistent with the healthy US-style eating pattern, recognizing that, for most Americans, individual caloric balance would require an intake of >2000 kcal/d (2). In addition to the fact that our model conformed to the published healthy US-style eating pattern, our rationale in using such a low rate of energy consumption in our modeling was that if higher protein intake can be successfully incorporated into a 2000-kcal diet, then it would not be a problem to do the same with a higher-calorie diet. Similarly, we did not model a diet consisting of <18% protein, because if all other nutrients and food groups could be successfully incorporated into a diet containing 18% protein, then this certainly could be done with a diet containing <18% protein.
Two single-day menus were created, each consistent with the USDA food group serving recommendations for a 2000-kcal healthy US-style eating pattern (Supplemental Tables 1 and 2). Results of the modeling exercise demonstrated that a higher-protein version (30% protein energy) of the 2000-kcal healthy US-style eating pattern could be achieved without compromising food group serving intake recommendations for fruits, vegetables, grains (including whole grains), and dairy foods, all while maintaining or improving nutrient content (Table 3). A variety of sources of high-quality proteins were used in these meal plans, with a preponderance of animal sources of protein. Note that in the DGA, those animal sources of protein are referred to as “meat equivalents,” which consists of a variety of protein food sources that include fish, poultry, milk, and cheese, in addition to meat. It was necessary to rely on meat equivalents as sources of protein within the limits of a 2000-kcal meal plan, because the protein density relative to total calories in vegetable protein sources generally is low. Vegetable protein sources could be used more extensively in a meal plan targeting a higher caloric intake. The main point of the meal plans was to demonstrate that a higher level of protein intake than that of the RDA could be incorporated into a meal that was consistent with the healthy USDA eating pattern referred to in the DGA, and also fall within the limits of the AMDR.
TABLE 3.
Daily food group amounts for USDA Healthy Eating Pattern modeled with the use of the AMDR for protein1
| Protein level of pattern, % |
|||
| Food group | 18.22 | 18.83 | 30.03 |
| Vegetables,4 cup equivalents | 2.5 | 2.5 | 2.5 |
| Dark-green subgroup | NS5 | 0.5 | 0.5 |
| Red and orange subgroup | NS5 | 1.0 | 1.0 |
| Starchy subgroup | NS5 | 1.0 | 1.0 |
| Fruits,6 cup equivalents | 2.0 | 2.0 | 2.0 |
| Grains,7 ounce equivalents | 6.0 | 6.0 | 6.0 |
| Whole grains | 3.0 | 3.0 | 6.0 |
| Refined grains | 3.0 | 3.0 | 0.0 |
| Dairy,8 cup equivalents | 3.0 | 3.0 | 3.0 |
| Protein foods,9 ounce equivalents | 5.5 | 5.5 | 12.0 |
All based on 2000 kcal/d. AMDR, Acceptable Macronutrient Distribution Range; NS, not specified.
DGA reference pattern reported in reference 3, Table A3–1.
Patterns modeled for current manuscript exercise.
One cup of raw, canned, frozen, or cooked vegetables; 2 cups of raw, leafy vegetables; 1 cup of cooked beans and peas; or 1 cup of 100% vegetable juice are defined as 1 cup equivalent of vegetables; cup weights range from 80 to 245 g, depending on vegetable selected.
Subgroups reported in the Dietary Guidelines for Americans on a weekly basis only.
One cup of raw, canned, or frozen fruit or 1 cup of 100% fruit juice is defined as 1 cup equivalent of fruit. The weights of 1 cup equivalents range from 70 to 250 g, depending on fruit selected.
For grain products made with flour (e.g., cakes and breads), each 16 g flour in the food defines 1 ounce equivalent of grain; for intact grains (e.g., oats, pasta, and rice), 28.35 g defines 1 ounce of grain equivalent.
One cup equivalent of dairy ranges in weight from 125 to 245 g for fluid products and from 28 to 127 g for solid or semisolid cheese.
One ounce equivalent of protein food is defined as 28.35 g cooked lean portion of meat, poultry, or seafood, 50 g egg, 14 g nuts and seeds, 16 g nut butters, or 175 g legumes.
In the case of many nutrients (e.g., iron, vitamin E, and saturated fat), a higher-protein diet manages to meet intake recommendations by replacing flexible calories from potential saturated fats and added sugars with protein foods, which provide a more favorable nutrient profile (Table 3). More specifically, the 18% protein pattern provides 65 g total fat (29% of total calories), 14 g saturated fat (∼6.5% of total calories; 70% of daily limit), and 128 mg cholesterol (43% of daily limit; goal ≤300 mg/d), whereas the 30% protein energy menu provides less total fat at 51 g (23% of total calories), less saturated fat at 11.3 g (∼5.0% of total calories; 55% of daily limit) and slightly more cholesterol at 226 mg, but still within daily limits (76% of daily limit). Sodium intake is below intake limit recommendations for both the 30% protein energy menu and the 18% protein energy menu at 84% of the recommended goal. The 30% protein menu is higher in several vitamins (e.g., riboflavin, niacin, vitamin B-6, vitamin B-12, and pantothenic acid) and minerals (e.g., magnesium, manganese, phosphorus, selenium, zinc, and iron), although both menus contain enough of these nutrients to meet or exceed the RDA without exceeding ULs. Biotin and iron quantities in the 30% protein energy menu are high enough to meet daily intake recommendations, but not in the 18% protein energy menu. With respect to iron, the 30% protein energy menu meets iron recommendations at 101% for a woman aged 31–50 y, whereas the 18% protein energy menu falls slightly short at 89%; adequate iron is lacking in the diets of adolescent girls and women aged 19–50 y (3), and increasing the intake of select protein foods, such as lean beef, may help achieve recommendations, as demonstrated in this modeling exercise. The 30% protein menu is lower in folate (providing 93% of daily folate recommendations), whereas the 18% protein energy menu meets folate recommendations. All other nutrients assessed are at adequate levels to meet or exceed daily intake recommendations and/or are provided in comparable quantities (<10% difference between the 2 menus). It is important to note that this exercise provides a single-day menu, whereas a healthful eating plan is part of a lifestyle in which nutrient adequacy is obtained and maintained over an extended period of time. Thus, the results of the menu exercise are intended to provide an example of how recommended nutrient and food group servings can be met in an eating pattern that incorporates more protein foods. An additional limitation of this exercise is the reliance on individual food items when USDA food patterns are traditionally based on composites. Foods selected for the current exercise may or may not be representative of foods included in USDA composites, but by no means are they considered to be unusual for the US diet.
Conclusions
The ultimate goal of dietary guidance should be to inform the public of optimal nutrient intake. We propose that the RDA represents an acceptable estimate of the minimal level of protein intake required, the limitations of the methodology used to determine it notwithstanding. The flexible amount of dietary protein may vary according to the physiologic circumstance, and thus there may be no unique optimal proportion of protein in the flexible caloric intake. However, the absence of definitive studies precisely defining optimal protein intake levels does not imply that we should rely solely on recommendations for total protein intake based on the “recommended minimal intake”. Ample data exist from a variety of studies that use more modern techniques than NB, including body composition analysis and stable isotope tracer studies, as well as functional and health outcomes, to make informed recommendations regarding the optimal amount of protein to include in the flexible portion of the diet.
There is little doubt that some component of the flexible dietary intake should be composed of protein. Not only are there documented benefits of higher protein intake, but also, adverse consequences of increased protein intake are minimal, particularly when compared with an excess intake of carbohydrate and/or fat. Future DRI committees might provide additional terminology or explanation to help highlight the differences between the RDA and the AMDR for practitioners. Future DGA might provide more recognition of the AMDR for protein and encourage nutrition professionals to consider this macronutrient range the goal or target for formulating individual dietary patterns rather than the RDA.
Acknowledgments
We thank Orsolya M Palacios for her contribution to the dietary pattern modeling exercise. RRW and I-YK developed the ideas for the paper together through discussions; RRW did the majority of writing and was responsible for integrating all the sections and producing the final draft; I-YK provided comments on the text and produced the figures; AMC and GK completed the dietary pattern modeling at 18% and 30% of the Acceptable Macronutrient Distribution Range for protein; and all authors participated in Protein Summit 2.0 and were involved in the writing and editing of this manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AMDR, Acceptable Macronutrient Distribution Range; DGA, Dietary Guidelines for Americans; DIAAS, Digestible Indispensable Amino Acid Score; DRI, Dietary Reference Intake; EAA, essential amino acid; EAR, Estimated Average Requirement; FNB, Food and Nutrition Board; LBM, lean body mass; NB, nitrogen balance; UL, Tolerable Upper Intake Level.
References
- 1.Sherman HC. The protein requirement of maintenance in man. Proc Natl Acad Sci USA 1920;6:38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine of the National Academies. Dietary Reference Intakes for energy, carbohydrates, fiber, fat, protein and amino acids (macronutrients). Washington (DC): The National Academies Press; 2002/2005. [Google Scholar]
- 3.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 dietary guidelines for Americans [Internet]. 8th ed. [cited 2016 Feb 9]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 4.MyPlate Daily Checklist [Internet]. [cited 2016 Jan 26]. Available from: http://www.choosemyplate.gov/MyPlate-Daily-Checklist.
- 5. FAO/WHO [Internet]. [cited 2016 Jan 26]. Protein quality evaluation: report of the joint FAO/WHO expert consultation, FAO Food and Nutrition 1999; paper 51. Rome (Italy); 1991. [Google Scholar]
- 6.Food and Agriculture Organization of the United Nations Dietary protein quality evaluation in human nutrition. FAO Food and Nutrition 2013; paper 92. Rome (Italy).
- 7.Wolfe RR, Rutherfurd SM, Kim IY, Moughan PJ. Protein quality as determined by the digestible indispensable amino acid score (DIAAS): evaluation of factors underlying the calculation. Nutr Rev 2016;74:584–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilsborough S, Mann N. A review of issues of dietary protein intake in humans. Int J Sports Nutr and Ex 2006;16:129–52. [DOI] [PubMed] [Google Scholar]
- 9.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey. Am J Clin Nutr 2008;87:1554S–7S. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Blog [Internet]. [cited 2016 Jan 26]. Available from: http://nchstats.com/2010/03/03/adults%E2%80%99-daily-protein-intake-much-more-than-recommended/.
- 11.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr 2003;77:109–27. [DOI] [PubMed] [Google Scholar]
- 12.Elango R, Humayun MA, Ball RO, Pencharz PB. Evidence that protein requirements have been significantly underestimated. Curr Opin Clin Nutr Metab Care 2010;13:52–7. [DOI] [PubMed] [Google Scholar]
- 13.Deutz NE, Wolfe RR. Is there a maximal response to protein intake with a meal? Clin Nutr 2013;32:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab 2015;308:E21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim IY, Schutzler S, Schrader A, Spencer H, Azhar G, Ferrando AA, Wolfe RR. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab 2016;310:E73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Børsheim E, Bui Q-UT, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr 2008;27:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab 2009;94:1630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 2010;29:18–23. [DOI] [PubMed] [Google Scholar]
- 19.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 2004;89:4351–8. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier S, Gougeon R, Nayar K, Morais JA. Frailty amplifies the effects of aging on protein metabolism: role of protein intake. Am J Clin Nutr 2003;78:422–9. [DOI] [PubMed] [Google Scholar]
- 21.Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar-Zadeh K, Polonski L, Anker SD. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle 2010;1:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, de Groot LC. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720–6. [DOI] [PubMed] [Google Scholar]
- 23.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 24.Asp ML, Richardson JR, Collene AL, Droll KR, Belury MA. Dietary protein and beef consumption predict for markers of muscle mass and nutrition status in older adults. J Nutr Health Aging 2012;16:784–90. [DOI] [PubMed] [Google Scholar]
- 25.Bartali B, Frongillo EA, Stipanuk MH, Bandinelli S, Salvini S, Palli D, Morais JA, Volpato S, Guralnik JM, Ferrucci L. Protein intake and muscle strength in older persons: does inflammation matter? J Am Geriatr Soc 2012;60:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart CA, Shaangraw RE, Peters EJ, Wolfe RR. Effect of dietary protein on bed rest-related changes in whole body protein synthesis. Am J Clin Nutr 1990;52:509–14. [DOI] [PubMed] [Google Scholar]
- 27.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr 2008;87(5):1558S–61S. [DOI] [PubMed] [Google Scholar]
- 28.Weijs PJ, Wolfe RR. Exploration of the protein requirement during weight loss in obese adults. Clin Nutr 2016;35:394–8. [DOI] [PubMed] [Google Scholar]
- 29.Damsgaard CT, Papadaki A, Jensen SM, Ritz C, Dalskov S-M, Hlavaty P, Saris WH, Martinez JA, Handjieva-Darlenska T, Anderson MR, et al. Higher protein diets consumed ad libitum improve cardiovascular risk markers in children of overweight parents from eight European countries. J Nutr 2013;143:810–7. [DOI] [PubMed] [Google Scholar]
- 30.Børsheim E, Bui QU, Tissier S, Cree MG, Ronsen O, Morio B, Ferrando AA, Kobayashi H, Newcomer BR, Wolfe RR. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition 2009;25:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuttall FQ, Gannon MC. The metabolic response to high-protein, low carbohydrate diet in men with type 2 diabetes mellitus. Metabolism 2006;55:243–51. [DOI] [PubMed] [Google Scholar]
- 32.Kerstetter JE, Mitnick ME, Gundberg CM, Caseria DM, Ellison AF, Carpenter TO, Insogna KL. Changes in bone turnover in young women consuming different levels of dietary protein. J Clin Endocrinol Metab 1999;84:1052–5. [DOI] [PubMed] [Google Scholar]
- 33.Even PC, Nadkarni NA, Chaumontet C, Azzout-Marniche D, Fromentin G, Tome D. Identification of behavioral and metabolic factors predicting adiposity sensitivity to both high fat and high carbohydrate diets in rats. Front Physiol 2011;2:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poudyal H, Panchal SK, Ward LC, Waanders J, Brown L. Chronic high-carbohydrate, high-fat feeding in rats induces reversible metabolic, cardiovascular, and liver changes. Am J Physiol Endocrinol Metab 2012;302:E1472–82. [DOI] [PubMed] [Google Scholar]
- 35.Panchal SK, Ward L, Brown L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr 2013;52:559–68. [DOI] [PubMed] [Google Scholar]
- 36.Chun MR, Lee YJ, Kim KH, Kim YW, Park SY, Lee KM, Kim JY, Park YK. Differential effects of high-carbohydrate and high-fat diet composition on muscle insulin resistance in rats. J Korean Med Sci 2010;25:1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu CJ, Liu S, Willett WC, Wolever TM, Brand-Miller JC, Barclay AW, Taylor A. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr Rev 2011;69:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolever TM, Gibbs AL, Chiasson JL, Connelly PW, Josse RG, Leiter LA, Maheux P, Rabasa-Lhoret R, Rodger NW, Ryan EA. Altering source or amount of dietary carbohydrate has acute and chronic effects on postprandial glucose and triglycerides in type 2 diabetes: Canadian trial of Carbohydrates in Diabetes (CCD). Nutr Metab Cardiovasc Dis 2013;23:227–34. [DOI] [PubMed] [Google Scholar]
- 39.Cahill GF. The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes 1971;20:785–99. [DOI] [PubMed] [Google Scholar]
- 40.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–78. [DOI] [PubMed] [Google Scholar]
- 41.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate and cardiovascular disease. Am J Clin Nutr 2010;91:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson S, Brenner BM. The aging kidney: structure, function, mechanisms, and therapeutic implications. J Am Geriatr Soc 1987;35:590–3. [DOI] [PubMed] [Google Scholar]
- 43.Walser M. The relationship of dietary protein to kidney disease. In: Liepa GU, Beitz DC, Beynen AC, Gorman MA, editors. Dietary proteins: how they alleviate disease and promote better health. Champaign (IL): Am Oil Chem Soc Monograph; 1992. p. 168–178. [Google Scholar]
- 44.Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr 2009;90:1674–92. [DOI] [PubMed] [Google Scholar]
- 45.Obarzanek E, Velletri PA, Cutler JA. Dietary protein and blood pressure. JAMA 1996;275:1598–603. [DOI] [PubMed] [Google Scholar]
- 46.Appel LJ. The effects of protein intake on blood pressure and cardiovascular disease. Curr Opin Lipidol 2003;14:55–9. [DOI] [PubMed] [Google Scholar]
- 47.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr 1999;70:221–7. [DOI] [PubMed] [Google Scholar]
- 48.The Food Processor 11.0.103: database structure, version 11.0.2. Salem (OR): ESHA Research.