Abstract
A large body of evidence suggests that the first 1000 d from conception is a critical window in which interventions to address malnutrition will be most effective, but little is known about the impact on linear growth of nutritional interventions in children ≥2 y of age. The aim of this analysis was to evaluate the effectiveness of several nutrition-based interventions, specifically iron, zinc, calcium, iodine, vitamin A, multiple (≥2) micronutrients, protein, and food, at improving growth in children ≥2 y of age. A systematic search of MEDLINE and EMBASE retrieved 7794 articles. A total of 69 studies met prespecified inclusion criteria. Baseline height-for-age z score, age, nutrient dose, and study duration were examined as potential sources of heterogeneity. Zinc (mean effect size: 0.15; 95% CI: 0.06, 0.24), vitamin A (0.05; 95% CI: 0.01, 0.09), multiple micronutrients (0.26; 95% CI: 0.13, 0.39), and protein (0.68; 95% CI: 0.30, 1.05) had significant positive effects on linear growth, with baseline height-for-age z score as a significant inverse predictor of the effect size. Iron, calcium, iodine, and food-based interventions had no significant effect on growth. Age at baseline, study duration, and dose were not related to effect size for any nutrient examined. These findings suggest that zinc, vitamin A, multiple micronutrients, and protein interventions delivered after 24 mo of age can have a positive effect on linear growth, especially in populations that have experienced growth failure.
Keywords: zinc, iron, calcium, vitamin A, protein, multiple micronutrient, stunting, 1000 days
Introduction
The global prevalence of linear growth stunting has steadily declined from 1990 (40%) to 2014 (24%), yet ∼159 million children <5 y old remain affected (1). This highlights the critical need to identify effective strategies to address the global burden of linear growth failure.
The etiology of linear growth faltering is multifactorial (2). More than 2 billion people and 800 million people suffer from vitamin and mineral deficiencies and energy deficiency, respectively (3, 4). Growth faltering may stem from deficiencies in single nutrients, multiple micronutrients (MMs)6, macronutrients, energy, or more commonly, a combination of many nutritional deficiencies.
Increasingly, the foci of nutritional interventions are pregnancy and the first 2 y of life. This period, also coined the first 1000 d, is widely accepted as a window of opportunity in which interventions will affect child growth, with interventions outside of this window often considered unlikely to have any significant effect. However, based on evidence suggesting that catch-up growth occurs even in the absence of interventions, an opportunity to promote catch-up growth beyond the first 1000 d has been proposed (5, 6).
Most previous meta-analyses have restricted their analysis to single nutrients and to children <5 y of age (including both children <2 y of age and those ≥2 y of age), thereby limiting the conclusions that can be made regarding the impact of interventions on growth after age 24 mo. To address these issues, we conducted a systematic review and meta-analysis of clinical trials to evaluate the effect of a range of nutrition-based interventions beyond the first 2 y of life on child growth.
Methods
Data sources and searches.
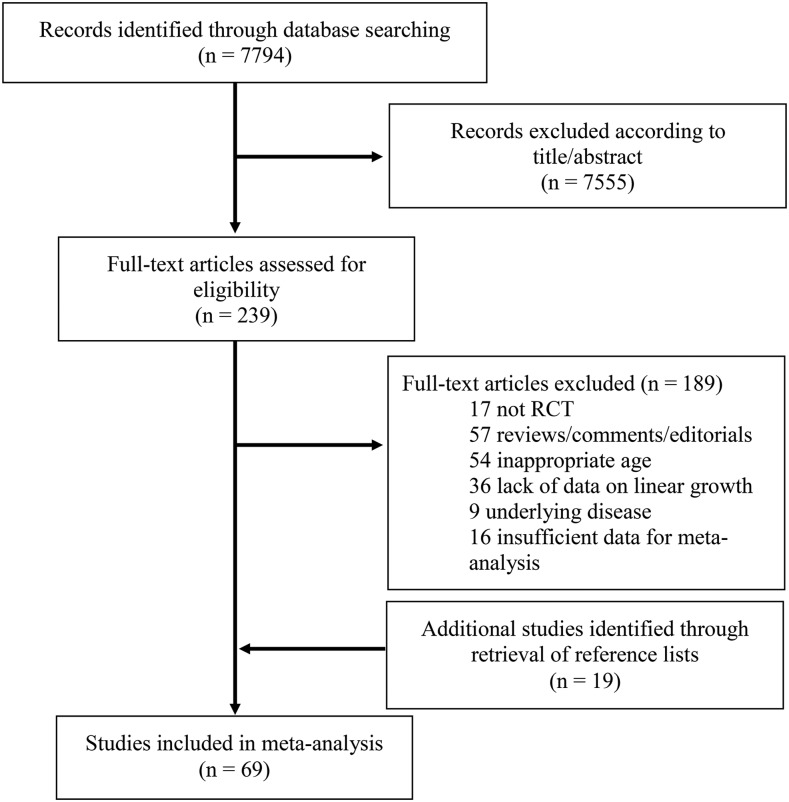
We searched MEDLINE (1966–2016) and EMBASE (1947–2016) on 14 January 2016 and 26 February 2016, respectively. For MEDLINE, the search (“linear growth or height” and “intervention or child nutritional physiological phenomena”) was used. For EMBASE, the search “nutrition and height and growth” was used. To be included, trials had to meet the following criteria: 1) study subjects were humans; 2) the study was available in English; 3) subjects were children aged ≥2 y but <20 y; 4) subjects were free of chronic disease, such as Crohn’s disease and HIV; 5) the study was a randomized controlled trial or quasirandomized controlled trial; 6) the intervention was nutritional; and 7) height was measured and reported both before and after intervention. Titles and abstracts of retrieved studies were scanned to exclude irrelevant studies. Full texts of remaining articles were reviewed, and studies that met inclusion criteria were included. Reference lists of included articles and relevant literature reviews were hand-searched for additional relevant articles. The search process is illustrated in Figure 1.
FIGURE 1.
Flowchart illustrating the selection of trials for inclusion in the systematic review and meta-analysis. RCT, randomized controlled trial.
Review of studies and extraction of summary data.
Key descriptive data, including the sample size, location of intervention, baseline and endline characteristics of the study subjects, dose and duration of intervention, height outcome, and significance of findings were extracted into a standardized form. The reported sample size corresponds to the number of subjects who completed the intervention. The included studies were grouped into 8 intervention categories: 1) iron, 2) zinc, 3) calcium, 4) iodine, 5) vitamin A, 6) multiple MMs (≥2 MMs), 7) protein, and 8) food. Studies with >1 intervention group were treated as independent studies. For studies that did not report baseline height-for-age z score (HAZ) or stunting prevalence but provided mean age and height data, HAZ was calculated by using WHO Anthro software (version 3.2.2) from reference data for the majority sex by using the mean age. In studies that used a delivery schedule other than daily, the average dose provided was converted to a daily dose. The Jadad test (a validated instrument that independently assesses the method of randomization, blinding, and report of subject withdrawal and dropout) was used to assess methodological quality, which was classified as high (score 5), moderate (score 4), or low (scores 1–3) (7). All data were entered twice and inconsistencies resolved.
Data synthesis and analysis.
The primary response variable was change in height, expressed in centimeters or HAZ. We utilized adjusted estimates of linear growth when available. Interquartile ranges, 95% CIs, and SEs were converted to SDs by using standard equations provided in the Cochrane Handbook for Systematic Reviews of Interventions (8). If only the baseline and endline values were reported, we calculated the crude mean change in height by subtracting baseline mean from the endline mean. For studies that did not report the SD for linear growth changes, we calculated the SD for change, assuming the baseline and endline were highly correlated (ρ = 0.8). In studies with multiple intervention groups and a single control group, the sample size of the control group was divided equally by the number of intervention groups while retaining the same value for the change in linear growth and its SD to avoid multiple counting of the control group.
A sensitivity analysis to assess the effect of assumptions regarding correlation between growth measures pre- and postintervention was conducted by using the following: 1) assuming a correlation of 0.5, 0.8, or 0.9; 2) assuming no correlation; and 3) calculating effect size only for the subsample of studies that reported the changes and SD of change (Supplemental Table 1). Additional sensitivity analyses were conducted by excluding studies in which there was no stunting (mean study baseline HAZ >−2) and in which dietary intake was adequate.
Effect sizes for each individual study were computed by dividing the mean change in the intervention and control groups by the pooled SD. Data were consolidated by using an inverse variance method. I2 statistics were used to measure the heterogeneity of the studies. If the I2 value was <50%, a fixed-effects model was applied. If the I2 value was >50%, a random-effects model was used (8). We visually assessed funnel plots to investigate potential publication bias (9). Data were analyzed by using RevMan (version 5.0.25), and figures were prepared by using RevMan and GraphPad Prism (version 5.01) software.
Results
Study selection
The literature search yielded 7794 references (Figure 1), of which 7555 were excluded on the basis of title and abstract, and 189 studies were excluded following full review. An additional 19 articles were identified from reference lists. A total of 69 articles were included in the systematic review and meta-analysis.
Baseline characteristics and risk of bias
The baseline characteristics for single-micronutrient and other nutrition-based interventions are reported in Tables 1 and 2, respectively. Using the Jadad test, 37 studies were classified as high quality, 19 were of medium quality, and 13 were of low quality. Symmetric funnel plots suggested a low chance of publication bias in zinc, iodine, and food-based analysis. Asymmetrical funnel plots for iron, calcium, vitamin A, MMs, and protein suggest potential publication bias (Supplemental Figure 1). The key effects of nutritional interventions administered after age 24 mo on linear growth are summarized in Table 3.
TABLE 1.
Baseline study characteristics of single-micronutrient interventions in children ≥2 y of age1
| Study | Year | Country | Subjects, n | Mean initial age, mo | Dose | Duration, mo | Mean initial HAZ | Quality |
| Iron | ||||||||
| Aguayo (10) | 2000 | Bolivia | 64 | 111 | 3 mg/kg bw | 4 | −1.48 | High |
| Angeles et al. (11) | 1993 | Indonesia | 76 | 37 | 30 mg | 2 | −2.26 | Medium |
| Bhatia and Seshadri 1 (12) | 1992 | India | 105 | 47 | 40 mg | 6 | −2.3 | Medium |
| Bhatia and Seshadri 2 (12) | 1992 | India | 51 | 54 | 40 mg | 6 | −1.56 | Medium |
| Chen et al. (13) | 2008 | China | 132 | 48 | 8.57 mg | 6 | −0.23 | Medium |
| Chwang et al. (14) | 1988 | Indonesia | 41 | 128 | 10 mg/kg bw | 3 | −1.52 | Medium |
| Dossa et al. (15) | 2001 | Benin | 68 | 47 | 60 mg | 3 | −2.26 | Medium |
| Hettiarachchi et al. (16) | 2008 | Sri Lanka | 374 | 167 | 35.71 mg | 6 | −1.19 | Medium |
| Latham et al. (17) | 1990 | Kenya | 55 | 96 | 285.7 mg | 1 | −0.88 | High |
| Lawless et al. (18) | 1994 | Kenya | 86 | 105 | 150 mg | 3 | −1.23 | High |
| Mwanri et al. 1 (19) | 2000 | Tanzania | 68 | 132 | 85.7 mg | 3 | −2.2 | High |
| Mwanri et al. 2 (19) | 2000 | Tanzania | 68 | 126 | 85.7 mg | 3 | −1.56 | High |
| Palupi et al. (20) | 1997 | Indonesia | 187 | 42 | 4.29 mg | 2 | −1.82 | Medium |
| Pereira et al. (21) | 1979 | India | 44 | 42 | 10 mg | 5 | — | Low |
| Rahman et al. (22) | 1999 | Bangladesh | 147 | 34 | 15 mg | 12 | −2.2 | High |
| Sungthong et al. 1 (23) | 2002 | Thailand | 200 | 116 | 300 mg | 4 | −1.55 | High |
| Sungthong et al. 2 (23) | 2002 | Thailand | 190 | 116 | 42.86 mg | 4 | −1.49 | High |
| Zinc | ||||||||
| Castillo-Durán et al. 1 (24) | 1994 | Chile | 19 | 169 | 10 mg | 12 | −2.58 | High |
| Castillo-Durán et al. 2 (24) | 1994 | Chile | 18 | 138 | 10 mg | 12 | −2.61 | High |
| Cavan et al. (25) | 1993 | Guatemala | 156 | 82 | 7.14 mg | 6 | −1.38 | High |
| Friis et al. (26) | 1997 | Zimbabwe | 276 | 134 | 40 mg | 12 | −1.18 | High |
| Gibson et al. (27) | 1989 | Canada | 60 | 75 | 10 mg | 12 | −1.39 | High |
| Hettiarachchi et al. (16) | 2008 | Sri Lanka | 382 | 161 | 10 mg | 6 | −1.1 | High |
| Kaseb and Fallah (28) | 2013 | Iran | 95 | 146 | 5 mg | 4 | −0.6 | High |
| Kikafunda et al. (29) | 1998 | Uganda | 113 | 56 | 10 mg | 6 | −0.7 | High |
| Mozaffari-Khosravi et al. 1 (30) | 2009 | Iran | 47 | 39 | 5 mg | 6 | −1.58 | Medium |
| Mozaffari-Khosravi et al. 2 (30) | 2009 | Iran | 38 | 40 | 5 mg | 6 | −1.65 | Medium |
| Nakamura et al. (31) | 1993 | Japan | 21 | 70 | 5 mg | 6 | −2.44 | Low |
| Ronaghy et al. (32) | 1969 | Iran | 27 | 156 | 28 mg | 5 | −2.73 | Low |
| Ronaghy et al. (33) | 1974 | Iran | 39 | 156 | 40 mg | 12 | −3.02 | Low |
| Rosado et al. (34) | 1997 | Mexico | 95 | 29 | 20 mg | 12 | −1.7 | High |
| Ruz et al. (35) | 1997 | Chile | 53 | 40 | 10 mg | 14 | −0.52 | High |
| Sayeg Porto et al. (36) | 2000 | Brazil | 18 | 116 | 5 mg/kg bw | 6 | −2.67 | Medium |
| Sempértegui et al. (37) | 1996 | Ecuador | 48 | 42 | 10 mg | 2 | −2 | High |
| Taneja et al. (38) | 2010 | India | 421 | 27 | 20 mg | 4 | — | High |
| Walravens et al. (39) | 1983 | United States | 40 | 50 | 10 mg | 12 | −2.07 | High |
| Calcium | ||||||||
| Bass et al. 1 (40) | 2007 | Australia | 47 | 109 | 800 mg | 9 | 0.47 | High |
| Bass et al. 2 (40) | 2007 | Australia | 41 | 109 | 800 mg | 9 | 0.36 | High |
| Bonjour et al. (41) | 1997 | Switzerland | 108 | 95 | 850 mg | 12 | 0.3 | High |
| Cameron et al. (42) | 2004 | Australia | 48 | 124 | 1200 mg | 24 | 0.3 | High |
| Chevalley et al. (43) | 2005 | Switzerland | 174 | 89.3 | 850 mg | 12 | 0.2 | High |
| Courteix et al. 1 (44) | 2005 | France | 54 | 119.3 | 800 mg | 12 | — | High |
| Courteix et al. 2 (44) | 2005 | France | 31 | 119.3 | 800 mg | 12 | — | High |
| Dibba et al. (45) | 2000 | Gambia | 160 | 124 | 714.3 mg | 12 | −1.09 | High |
| Ekbote et al. (46) | 2011 | India | 58 | 33 | 357.1 mg | 12 | −1.96 | High |
| Iuliano-Burns et al. 1 (47) | 2003 | Australia | 34 | 106 | 434 mg | 9 | −0.08 | High |
| Iuliano-Burns et al. 2 (47) | 2003 | Australia | 32 | 106 | 434 mg | 9 | 0.27 | High |
| Lloyd et al. (48) | 1993 | United States | 94 | 143 | 500 mg | 18 | −0.23 | High |
| Nowson et al. (49) | 1997 | Australia | 56 | 168 | 1000 mg | 18 | −0.9 | High |
| Pettifor et al. (50) | 1981 | South Africa | 60 | 121 | 500 mg | 3 | −1.09 | Medium |
| Specker and Binkley 1 (51) | 2003 | United States | 90 | 48 | 714.3 mg | 12 | 0 | High |
| Specker and Binkley 2 (51) | 2003 | United States | 88 | 39 | 714.3 mg | 12 | −0.15 | High |
| Iodine | ||||||||
| Zimmerman et al. 1 (52) | 2007 | Albania | 310 | 137 | 400 mg2 | 6 | −1.13 | Medium |
| Zimmerman et al. 2 (52) | 2007 | South Africa | 188 | 110 | 200 mg3 | 6 | −0.54 | Medium |
| Vitamin A | ||||||||
| Fawzi et al. 1 (53) | 1997 | Sudan | 2166 | 24 to <364 | 200,000 IU | 17 | — | High |
| Fawzi et al. 2 (53) | 1997 | Sudan | 2033 | 36 to <484 | 200,000 IU | 17 | — | High |
| Fawzi et al. 3 (53) | 1997 | Sudan | 1975 | 48 to <604 | 200,000 IU | 17 | — | High |
| Fawzi et al. 4 (53) | 1997 | Sudan | 1756 | 60 to <724 | 200,000 IU | 17 | — | High |
| Fawzi et al. 5 (53) | 1997 | Sudan | 2079 | >604 | 200,000 IU | 17 | — | High |
| Fawzi et al. 6 (53) | 1997 | Sudan | 1991 | 24 to <364 | 200,000 IU | 17 | — | High |
| Fawzi et al. 7 (53) | 1997 | Sudan | 2023 | 36 to <484 | 200,000 IU | 17 | — | High |
| Fawzi et al. 8 (53) | 1997 | Sudan | 1985 | 48 to <604 | 200,000 IU | 17 | — | High |
| Fawzi et al. 9 (53) | 1997 | Sudan | 1875 | 60 to <724 | 200,000 IU | 17 | — | High |
| Fawzi et al. 10 (53) | 1997 | Sudan | 2120 | >604 | 200,000 IU | 17 | — | High |
| Hadi et al. 1 (54) | 2000 | Indonesia | 377 | ≥244 | 206,000 IU | 24 | — | Medium |
| Hadi et al. 2 (54) | 2000 | Indonesia | 2263 | ≥244 | 206,000 IU | 24 | — | Medium |
| Lin et al. (55) | 2009 | China | 86 | 37 | 100,000 IU | 3 | −0.46 | High |
| Mwanri et al. 1(19) | 2000 | Tanzania | 68 | 131 | 5000 IU | 3 | −2.13 | High |
| Mwanri et al. 2 (19) | 2000 | Tanzania | 68 | 127 | 5000 IU | 3 | −1.39 | High |
| Yang et al. (56) | 2002 | China | 63 | 48 | 667 IU | 12 | −1.55 | High |
bw, body weight; HAZ, height-for-age z score; IU, international unit.
Single dose.
Two doses delivered 3 mo apart.
Age range.
TABLE 2.
Baseline study characteristics of multiple-micronutrient, protein, and food-based interventions in children ≥2 y of age1
| Study | Year | Country | Subjects, n | Mean initial age, mo | Form | Duration, mo | Mean initial HAZ | Quality |
| Multiple micronutrient | ||||||||
| Ash et al. (57) | 2003 | Tanzania | 750 | 120 | 5.4 mg Fe, 1750 IU vitamin A, 45 μg I, 5.25 mg Zn, 72 mg vitamin C, 0.6 mg riboflavin, 0.15 mg folic acid, 3 μg vitamin B-12, 0.7 mg pyridoxal, 10.5 mg vitamin E | 6 | −2.1 | Medium |
| Hall et al. (58) | 2007 | Vietnam | 1080 | 83 | Biscuits fortified with 18 vitamins and minerals; supplemented food provided 1400 IU retinol, 60 μg I, 5 mg Fe, 6 mg Zn | 17 | −1.47 | Low |
| Hettiarachchi et al. (16) | 2008 | Sri Lanka | 380 | 159 | 50 mg Fe, 14 mg Zn | 6 | −1.08 | Medium |
| Hyder et al. (59) | 2007 | Bangladesh | 837 | 144 | 7 mg Fe, 1296 IU vitamin A, 75 μg I, 7.5 mg Zn, 120 mg vitamin C, 0.91 mg riboflavin, 120 μg folic acid, 1 μg vitamin B-12, 1 mg vitamin B-6, 10 mg vitamin E, 5 mg niacin | 12 | −1.97 | High |
| Vinod Kumar and Rajagopalan (60) | 2006 | India | 159 | 90 | 3,000 IU vitamin A, 1 mg vitamin B-12, 1 mg Ca, 15 mg niacin, 1 mg pyridoxal, 100 μg folic acid, 1 μg vitamin B-12, 30 IU vitamin E, 30 mg vitamin C, 10 mg Fe, 250 mg lysine | 9 | −1.23 | Low |
| Lopriore et al. (61) | 2004 | Algeria | 209 | 50 | 1000 mg Ca, 1134 mg K, 635 mg P, 156 mg Mg, 42 mg Fe, 41 mg Zn, 2 mg Cu, 2000 μg vitamin A, 50 μg vitamin D, 20 mg vitamin E, 125 mg vitamin C, 4 mg thiamin, 4 mg riboflavin, 4 mg pyridoxal, 4 μg vitamin B-12, 500 μg folate, 25 mg pantothenic acid, 60 mg niacin | 6 | −2.85 | Medium |
| Manger et al. (62) | 2008 | Thailand | 563 | 111 | 5 mg Fe, 5 mg Zn, 50 μg I, 270 μg vitamin A | 7 | −1 | High |
| Muthayya et al. (63) | 2009 | India | 277 | 104 | 500 μg vitamin A, 0.9 mg riboflavin, 1 mg pyridoxal, 1.8 μg vitamin B-12, 300 μg folate, 227.1 mg vitamin C, 231 mg Ca, 100 μg I, 18 mg Fe, 10.5 mg Zn, 0.93 g α-linolenic acid, 0.10 g docosahexaenoic acid | 12 | −1.32 | High |
| Mwanri et al. (19) | 2000 | Tanzania | 68 | 143 | 5,000 IU vitamin A, 200 mg Fe | 3 | −1.9 | High |
| Ronaghy et al. (32) | 1969 | Iran | 30 | 156 | 10 g dried egg white, 6,000 IU vitamin A, 500 IU vitamin D, 50 IU α-tocopherol, 2 mg thiamin, 2.5 mg riboflavin, 25 mg niacin, 20 mg panthothenic acid, 0.3 mg biotin, 4 mg pyridoxal, 10 μg vitamin B-12, 90 mg vitamin C, 0.1 mg folic acid, 100 mg Ca, 100 mg Fe, 5 mg Mn, 0.1 mg Co, 1.1 mg Cu, 0.15 Cr, 2 mg Mb, 0.5 mg Ni, 0.1 mg Se, 0.15 mg I, 1 mg F | 20 | −2.96 | Low |
| Ronaghy et al. (33) | 1974 | Iran | 25 | 156 | 6,000 IU vitamin A, 500 IU vitamin D, 50 IU vitamin E, 2 mg thiamin, 2.5 mg riboflavin, 25 mg niacin, 20 mg panthothenic acid, 0.1 mg folic acid, 0.3 mg biotin, 4 mg pyridoxal, 10 μg vitamin B-12, 90 mg vitamin C, 100 mg Ca, 120 mg Mg, 100 mg Fe, 5 mg Mn, 0.1 mg Co, 40 mg Zn, 1.1 mg Cu, 0.15 mg Cr, 2 mg Mb, 0.5 mg Ni, 0.1 mg Se, 0.15 mg I, 1 mg F | 5 | −2.74 | Low |
| Rosado et al. (34) | 1997 | Mexico | 96 | 29 | 20 mg Fe, 20 mg Zn | 12 | −1.65 | High |
| Sarma et al. (64) | 2006 | India | 695 | 122 | 7.3 g protein, 2 g fat, 576 mg Ca, 14 mg Fe, 1.6 mg riboflavin, 2 mg pyridoxal, 1 μg vitamin B-12, 200 μg folic acid, 80 mg vitamin C, 400 μg vitamin A, 2.5 μg vitamin D, 0.7 mg thiamin, 0.9 mg niacin, 75 μg I, 2.3 mg Zn | 14 | −0.62 | High |
| Shatrugna et al. (65) | 2006 | India | 184 | 138 | 14 mg Fe, 1.6 mg riboflavin, 2 mg pyridoxal, 1 μg vitamin B-12, 200 μg folic acid, 80 mg vitamin C, 400 μg vitamin A, 2.5 μg vitamin D, 0.7 mg thiamin, 0.9 mg niacin, 400 mg Ca, 75 μg I, 2.3 mg Zn | 14 | −0.86 | High |
| Solon et al. (66) | 2003 | Philippines | 831 | 119 | 4.8 mg Fe, 700 IU vitamin A, 48 μg I, 3.75 mg Zn, 75 mg vitamin C, 0.46 mg riboflavin, 0.06 mg folic acid, 0.5 μg vitamin B-12, 0.5 mg pyridoxal, 2.5 mg vitamin E, 2.5 mg niacin | 4 | −1.81 | Medium |
| Zadik et al. (67) | 2004 | Israel | 37 | 173 | 10 mg Fe, 11 mg Zn, 10,000 IU vitamin A | 6 | −2.65 | Medium |
| Zadik et al. (68) | 2010 | Israel | 37 | 77 | 12 mg Fe, 6,000 IU vitamin A | 6 | −2.75 | Medium |
| Protein | ||||||||
| Grillenberger et al. 1 (69) | 2003 | Kenya | 178 | 91 | Ground beef added to githeri | 24 | −1.4 | Medium |
| Grillenberger et al. 2 (69) | 2003 | Kenya | 190 | 88 | Cow milk | 24 | −1.3 | Medium |
| Kabir et al. (70) | 1998 | Bangladesh | 69 | 33 | Protein-supplemented diet | 1 | −1.87 | Low |
| Lampl et al. 1 (71) | 1978 | New Guinea | 45 | 124 | Skim milk powder added to diet | 8 | −3.85 | Low |
| Lampl et al. 2 (71) | 1978 | New Guinea | 41 | 124 | Skim milk powder added to diet | 8 | −3.95 | Low |
| Larnkjær et al. 1 (72) | 2014 | Denmark | 61 | 161 | Skim milk | 3 | 0.84 | High |
| Larnkjær et al. 2 (72) | 2014 | Denmark | 53 | 161 | Casein protein | 3 | 0.78 | High |
| Larnkjær et al. 3 (72) | 2014 | Denmark | 59 | 160 | Whey protein | 3 | 0.89 | High |
| Malcolm 1 (73) | 1970 | New Guinea | 43 | 94 | Skim milk powder | 2 | −3.75 | Low |
| Malcolm 2 (73) | 1970 | New Guinea | 66 | 97 | Skim milk powder | 3 | −3.64 | Low |
| Pereira et al. (74) | 1969 | India | 35 | 42 | Rice supplemented with lysine and threonine | 6 | −2.07 | Medium |
| Pereira et al. 1 (75) | 1973 | India | 63 | 42 | Rice supplemented with lysine and threonine | 6 | −2.22 | Low |
| Pereira et al. 2 (75) | 1973 | India | 46 | 42 | Wheat supplemented with lysine | 6 | −1.88 | Low |
| Food | ||||||||
| Alarcon et al. (76) | 2003 | Philippines and Taiwan | 91 | 49 | Pediasure (Abbott Laboratories) | 3 | −1.38 | Low |
| Grillenberger et al. (69) | 2003 | Kenya | 246 | 87 | Fat added to githeri | 24 | −1.35 | Medium |
| Malcolm (73) | 1970 | New Guinea | 57 | 126 | 5 meals of taro and sweet potato instead of 3 | 3 | −3.6 | Low |
| Maleta et al. (77) | 2004 | Malawi | 61 | 51 | RTUF (high in micronutrient) or maize or soy flour | 23 | −3.1 | Low |
| Prasad Mp et al. 1 (78) | 2016 | India | 137 | 134 | Sorghum supplementation | 8 | −1.64 | Low |
| Prasad Mp et al. 2 (78) | 2016 | India | 125 | 119 | Sorghum supplementation | 8 | −1.2 | Low |
HAZ, height-for-age z score; RTUF, ready to use food; IU, international unit.
TABLE 3.
Summary estimates of the weighted mean effect sizes (95% CIs) for each nutritional intervention
| Outcome | Iron | Zinc | Calcium | Iodine | Vitamin A | MM1 | Protein | Food |
| n | 1953 | 1966 | 1175 | 498 | 22,928 | 6258 | 939 | 717 |
| Linear growth | 0.10 (−0.04, 0.24) | 0.15 (0.06, 0.24) | 0.03 (−0.09, 0.14) | 0.07 (−0.10,0.25) | 0.05 (0.01, 0.09) | 0.26 (0.13, 0.39) | 0.68 (0.30, 1.05) | 0.19 (−0.28, 0.66) |
MM, multiple micronutrient.
Effects of nutrition-based interventions on linear growth
Iron.
A total of 14 studies were included in the meta-analysis. All studies were conducted in low- and middle-income countries (LMICs) located in East Asia and the Pacific (n = 4), South Asia (n = 5), Africa (n = 4), and Latin America or the Caribbean (n = 1). The number of subjects in each study ranged from 41 to 374, with mean ages ranging from 34 to 167 mo. Approximately 82% of datasets had a baseline HAZ <−1, and 5 of 17 datasets had a baseline HAZ <−2. One study (21) did not have extractable baseline height data. The daily dose of iron ranged from 10 to 286 mg/d. In 2 studies, the daily dose of iron could not be determined because administration of the mineral was based on body weight (10, 14). The duration of supplementation lasted from 1 to 12 mo.
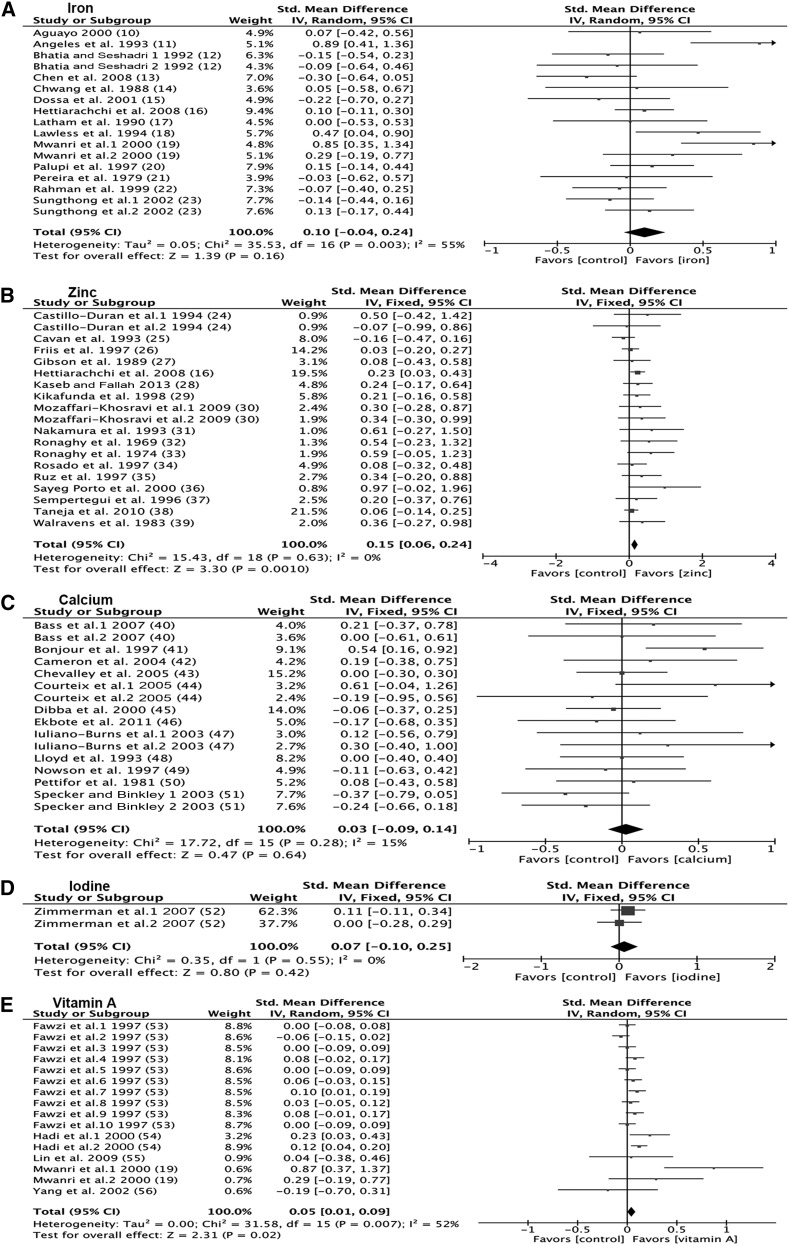
Effect sizes for change in height were calculated from 17 datasets (n = 14 studies) and ranged from −0.30 to 0.89 (Figure 2A). There was significant heterogeneity (P = 0.003). The overall standard mean effect was not statistically significant (0.10; 95% CI: −0.04, 0.24) (Figure 2A). Sensitivity analyses excluding studies in which no children were stunted (0.13; 95% CI: −0.24, 0.51) and in which serum hemoglobin was ≥110 g/L (0.27; 95% CI: −0.13, 0.67) did not alter the conclusions. Baseline HAZ (r = −0.34; P = 0.22), age (r = 0.20; P = 0.46), study length (r = −0.41; P = 0.10), dose (r = −0.03; P = 0.91), and hemoglobin (r = −0.15; P = 0.60) were not predictors of the effect size (Supplemental Figure 2A–E).
FIGURE 2.
Forest plots of the effect on linear growth of single micronutrient interventions in children ≥2 y of age. Data derived from systematic literature review of nutrient interventions administered after age 2 y. (A) Iron. (B) Zinc. (C) Calcium. (D) Iodine. (E) Vitamin A. Fixed, fixed-effects model; IV, inverse variance; random, random-effects model; std., standard.
Zinc.
We identified 17 studies that examined the effect of zinc on height. The majority of included studies were conducted in LMICs located in South Asia (n = 2), Africa (n = 2), the Middle East (n = 4), and Latin America or the Caribbean (n = 3). The number of subjects in each study ranged from 18 to 421, with mean ages of subjects ranging from 27 to 169 mo. At baseline, 74% of datasets had HAZ scores <−1, and 8 of the 17 study populations had HAZ scores <−2. One study (38) did not have extractable baseline height data for children aged ≥24 mo. The daily dose of zinc ranged from 5 to 40 mg/d for 2 to 12 mo.
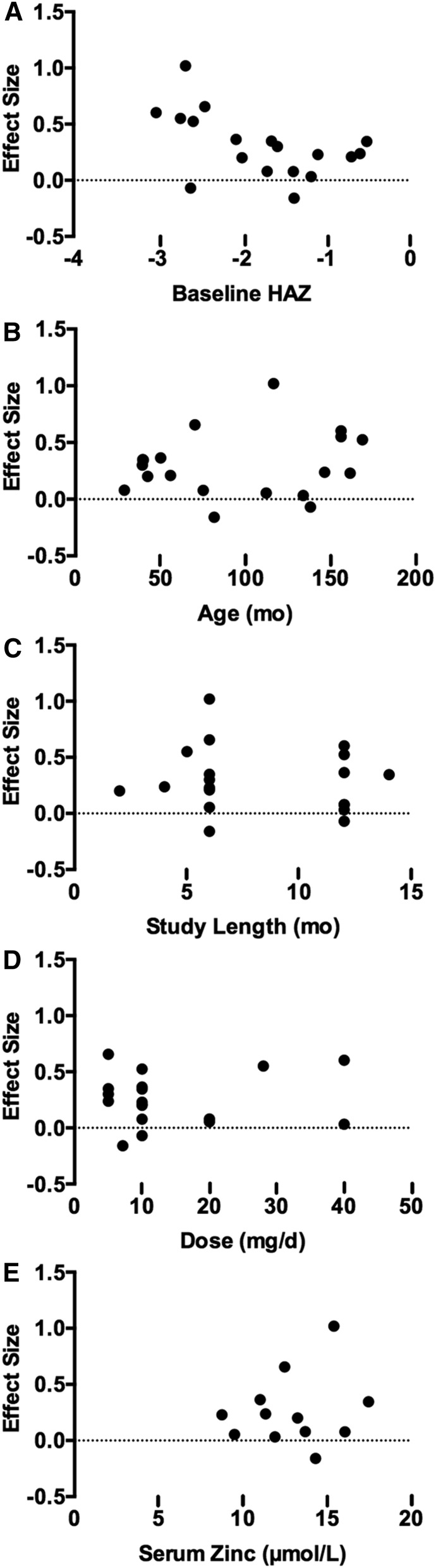
Effect sizes for change in height were calculated from 19 datasets (n = 17 studies) and ranged from −0.16 to 0.97 (Figure 2B). The majority of datasets (89%) had a positive effect size, and the overall standard mean effect was statistically significant (0.15; 95% CI: 0.06, 0.24) (Figure 2B). Sensitivity analyses excluding studies in which no children were stunted (0.49; 95% CI: 0.19, 0.79) and in which serum zinc was >12 μmol/L (0.13; 95% CI: 0.02, 0.24) did not alter the conclusions. The regression model showed a significant negative relation (r = −0.51; P = 0.03) between baseline HAZ and effect size for height gain (Figure 3A). Baseline age (r = 0.17; P = 0.48), study length (r = −0.11; P = 0.65), dose (r = 0.09; P = 0.72), and serum zinc (r = 0.19; P = 0.56) were not predictors of the effect size (Figure 3B–E).
FIGURE 3.
Relation between potential sources of heterogeneity and the standard mean effect of zinc intervention on linear growth in children ≥2 y of age. (A) Baseline HAZ was a significant predictor of the effect size (r = −0.51; P < 0.05). (B) Age (r = 0.17; P > 0.05), (C) study duration (r = −0.11; P > 0.05), (D) dose (r = 0.09; P > 0.05), and (E) serum zinc (r = 0.19; P > 0.05) were not predictors of the effect size. Each point represents one study estimate. HAZ, height-for-age z score.
Calcium.
A total of 12 studies contributed 16 data sets. Three of the studies were conducted in LMICs located in South Asia (n = 1) and Africa (n = 2). The remaining studies were conducted in high-income countries, including 2 in the United States, 4 in Australia, 2 in Switzerland, and 1 in France. The number of subjects in each study ranged from 31 to 174, with mean ages of subjects ranging from 33 to 168 mo. Four studies enrolled both boys and girls, 6 studies enrolled only girls, and 2 enrolled only boys. At baseline, 3 of 8 study populations had HAZ scores <−1, and no population had HAZ scores <−2. There was insufficient data to calculate baseline HAZ in the study by Courteix et al. (44). The daily dose of calcium ranged from 357 to 1200 mg/d for 3 to 24 mo.
Effect sizes for change in height ranged from −0.37 to 0.61 (Figure 2C). The overall standard mean effect was not statistically significant (0.03; 95% CI: −0.09, 0.14) (Figure 2C). Sensitivity analyses excluding studies in which dietary intake was ≥400 mg/d did not alter the conclusions (−0.08; 95% CI: −0.35, 0.18). Baseline HAZ (r = 0.33; P = 0.25), age (r = 0.29; P = 0.08), study length (r = −0.29; P = 0.27), dose (r = −0.07; P = 0.79), and baseline dietary calcium (r = 0.14; P = 0.63) were not predictors of the effect size (Supplemental Figure 3A–E).
Iodine.
Two studies that examined the impact of iodine supplementation on linear growth were identified. One study contributing 2 data sets was included in the meta-analysis. The data were obtained from studies conducted in Albania and South Africa, both LMICs. The number of subjects was 188 and 310, with mean ages 110 and 137 mo, respectively. Both studies had a baseline mean HAZ >−2, with 1 study having a mean HAZ <−1. Supplemental iodine was delivered as iodized oil in one large bolus (400 mg) or 2 smaller doses (200 mg every 3 mo). The duration of both studies was 6 mo.
Effect sizes for change in height were calculated from 2 datasets (n = 1 study) and were 0.00 and 0.11 (Figure 2D). The overall standard mean effect was not statistically significant (0.07; 95% CI: −0.10, 0.25) (Figure 2D). Because of limited data, effect modification by baseline HAZ, age, and study length was not examined.
Vitamin A.
We identified 5 studies that assessed the effect of vitamin A on linear growth. The studies contributed 16 datasets for analysis. The studies were conducted in Sudan (53), Tanzania (19), China (55, 56), and Indonesia (54). The study in Sudan (53) was stratified by age group and sex, the study in Tanzania (19) had 2 treatment groups, and the study in Indonesia (54) was stratified by breastfeeding status. The number of subjects in each study ranged from 63 to 2166, with mean ages of subjects ranging from 2 to 12 y. A majority (75%) of the study populations had a baseline HAZ <−1, of which 1 study population had HAZ <−2. The study by Fawzi et al. (53) did not provide sufficient data to calculate baseline HAZ for the stratified sex and age groups. The dose of vitamin A ranged from 5000 to 206,000 international units, and duration of supplementation lasted from 3 to 17 mo. Larger doses (≥100,000 international units) were administered with larger time intervals between doses (monthly to biannually); whereas smaller vitamin A supplements were administered more frequently (3 d/wk to weekly).
Effect sizes for change in height were calculated from 16 datasets (n = 5 studies) and ranged from −0.19 to 0.87 (Figure 2E). There was significant heterogeneity (P = 0.007). One dataset by Mwanri et al. (19) was identified as a potential outlier because of its effect size being 3 times higher than that of the next largest effect size. The overall standardized mean effect was statistically significant with (0.05; 95% CI: 0.01, 0.09) and without (0.04; 95% CI: 0.01, 0.07) the outlier (Figure 2E). Baseline HAZ (r = −0.62; P = 0.38), age (r = 0.83; P = 0.17), and study duration (r = −0.43; P = 0.10) were not predictors of effect size for height gain (Supplemental Figure 4A–C).
MMs.
Seventeen trials were identified. All studies were conducted primarily in LMICs: 3 in Africa, 3 in East Asia and the Pacific, 4 in the Middle East, 6 in South Asia, and 1 in Latin America and the Caribbean. The number of subjects in each study ranged from 25 to 1080, with mean ages of subjects ranging from 29 to 173 mo. At baseline, 14 study populations had a mean HAZ <−1, of which 6 had a mean HAZ <−2. MM interventions were administered as either supplements (e.g., pills) or fortified foods and beverages. The composition and dose of the MM supplements varied among studies. All interventions contained iron, whereas the inclusion of zinc (76.5%), vitamin A (88.2%), calcium (41.2%), and iodine (52.9%) was more variable. The duration of supplementation ranged from 3 to 20 mo.
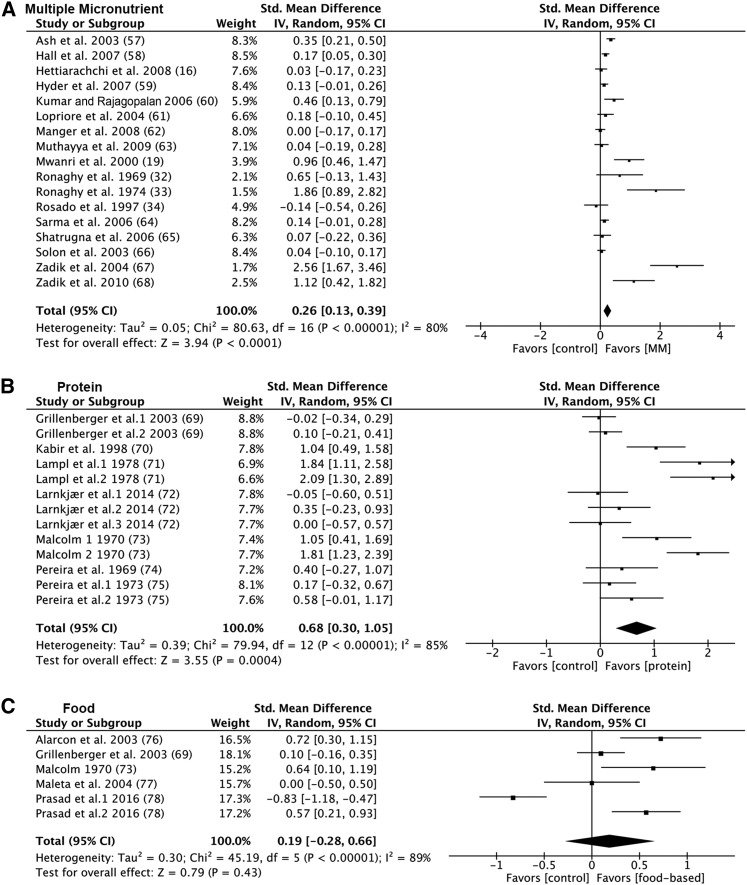
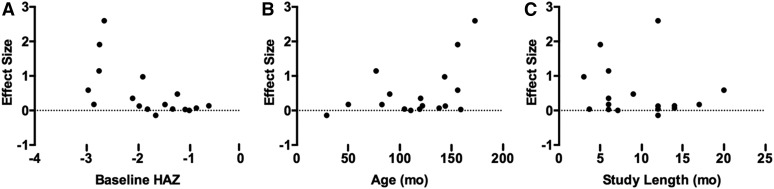
The effect sizes for change in height ranged from −0.14 to 2.56 (Figure 4A). The majority of datasets (94%) had a positive effect size, and 7 were statistically significant. There was significant heterogeneity (P < 0.00001). The studies by Zadik et al. (67, 68) and Ronaghy et al. (33) were identified as possible outliers because of extreme effect sizes. The overall weighted mean effect was 0.26 (95% CI: 0.13, 0.39) and 0.15 (95% CI: 0.06, 0.24) with and without the potential outliers, respectively. Excluding studies in which no children were stunted at baseline did not alter these conclusions (0.96; 95% CI: 0.46, 1.46). The regression model showed a significant negative relation (r = −60; P = 0.0117) between baseline HAZ and effect size for height gain (Figure 5A). Age (r = 0.45; P = 0.067) and study length (r = −0.14; P = 0.6) were not predictors of the effect size for height gain (Figure 5B–C).
FIGURE 4.
Forest plots of the effect on linear growth of multiple-micronutrient, protein, and food-based interventions in children ≥2 y of age. Data derived from systematic literature review of nutrient interventions administered after age 2 y. (A) Multiple micronutrient. (B) Protein. (C) Food-based. Fixed, fixed-effects model; IV, inverse variance; MM, multiple micronutrient; random, random-effects model; std., standard.
FIGURE 5.
Relation between potential sources of heterogeneity and the standard mean effect of multiple-micronutrient interventions on linear growth in children ≥2 y of age. (A) Baseline HAZ was a significant predictor of the effect size (r = −0.60; P < 0.05). (B) Age (r = 0.45; P > 0.05) and (C) study duration (r = −0.14; P > 0.05) were not predictors of the effect size. Each point represents 1 study estimate. HAZ, height-for-age z score.
Protein.
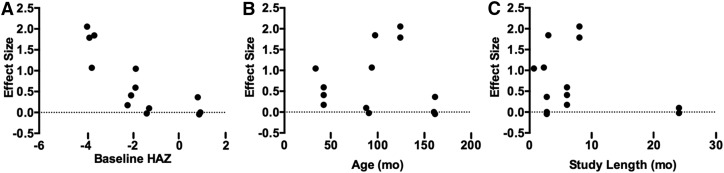
A total of 7 protein-based intervention trials that provided data to calculate an effect on linear growth were identified. Five studies contained multiple treatment groups, each providing either 2 (69, 73, 75) or 3 data sets (72). A majority of the studies were conducted in LMICs, including 1 in Africa, 2 in East Asia and the Pacific, and 3 in South Asia. The number of subjects in each study ranged from 35 to 190, with mean ages of subjects ranging from 33 to 161 mo. At baseline, 10 study populations had a mean HAZ of <−1, of which 6 had a mean HAZ <−2. Protein supplements were administered as either meat (69), cow milk (liquid or powder) (69, 71–73), amino acid–supplemented rice or wheat (74, 75), casein (72), whey (72), or high-protein diets (70). The duration of supplementation ranged from 0.69 to 24 mo.
The effect sizes for change in height ranged from −0.05 to 2.09 (Figure 4B). The majority of datasets (85%) had a positive effect size, but only 5 were statistically significant. There was significant heterogeneity (P < 0.00001). The data sets extracted from Lampl et al. (71), Malcolm (73), and 1 data set from Kabir et al. (70) were possible outliers because of the large effect size. The overall weighted mean effect was 0.68 (95% CI: 0.30, 1.05) and 0.13 (95% CI: −0.03, 0.29) with and without the potential outliers, respectively. Excluding studies in which no children were stunted at baseline did not alter the conclusions (1.21; 95% CI: 0.53, 1.88). The regression model showed a significant negative relation (r = −0.80; P = 0.001) between baseline HAZ and effect size for height gain (Figure 6A). Age (r = −0.02; P = 0.94) and study length (r = −0.29; P = 0.34) were not predictors of the effect size for height gain (Figure 6B–C).
FIGURE 6.
Relation between potential sources of heterogeneity and the standard mean effect of protein interventions on linear growth in children ≥2 y of age. (A) Baseline HAZ was a significant predictor of the effect size (r = −0.80; P < 0.05). (B) Age (r = −0.02; P > 0.05) and (C) study duration (r = −0.29; P > 0.05) were not predictors of the effect size. Each point represents 1 study estimate. HAZ, height-for-age z score.
Food-based intervention.
Sufficient data were available from 6 data sets from 5 studies (69, 73, 76–78) for calculation of the effect of food-based supplementation on height. All of the studies were conducted in LMICs, including 2 in Africa, 1 in East Asia and the Pacific, and 1 in South Asia. The number of subjects in each study ranged from 57 to 246, with mean ages of subjects ranging from 49 to 134 mo. At baseline, all study populations had a HAZ score <−1, of which 33% had a HAZ score of <−2. Food-based supplements were delivered in many forms including Pediasure (Abbott Laboratories) (76), fat (69), ready-to-use therapeutic food (77), additional meals (73), or the addition of sorghum to the diet (78).
The effect sizes for change in height ranged from −0.83 to 0.72 (Figure 4C). There was significant heterogeneity (P < 0.001). The overall weighted mean effect was not statistically significant (0.19; 95% CI: −0.28, 0.66) (Figure 4C). Furthermore, excluding studies in which no children were stunted at baseline did not alter the conclusions (0.31; 95% CI: −0.32, 0.94). Baseline HAZ (r = −0.11; P = 0.84), age (r = −0.28; P = 0.59) and study length (r = −0.23; P = 0.66) were not predictors of the effect size for height gain (Supplemental Figure 5A–C).
Discussion
This systematic review and meta-analysis was conducted to investigate the impact of nutrition-based interventions on linear growth in children aged ≥2 y. A few notable observations may have programmatic implications. Specifically, interventions containing iron, calcium, or iodine or those providing food do not improve linear growth, whereas interventions containing zinc (0.15; 95% CI: 0.06, 0.24), vitamin A (0.05; 95% CI: 0.01, 0.09), MMs (0.26; 95% CI: 0.13, 0.39), or protein (0.68; 95% CI: 0.30, 1.05) had a significant positive effect on height. A range of sensitivity analyses with the use of different assumptions for the SD for the change in height did not alter these conclusions (Supplemental Table 1).
Several observational studies have reported associations between iron-deficiency anemia and impaired linear growth that may stem from impaired immunity, appetite, and thyroid hormone metabolism (18, 79, 80). As such, the impact of iron supplementation on linear growth has been the subject of a number of systematic reviews and meta-analyses (81–83). The review by Ramakrishnan et al. (81) restricted their analysis to children <5 y old, which excludes a large number of trials included in this study. Three systematic reviews examined iron-supplementation trials conducted in all children (aged <18 y) and did not identify a statistically significant effect on linear growth (82–84). Despite methodological differences, our findings were consistent with those of earlier reviews. When we excluded studies with normal hemoglobin, the effect size increased (0.10 compared with 0.27) but did not reach statistical significance (11, 12, 15, 19, 21). Our study further contributes to the growing body of evidence suggesting that iron is ineffective at promoting linear growth.
Zinc is an essential transition metal that plays a critical role in normal linear growth via mechanisms involving growth-hormone release, insulin-like growth factor I, chondrogenesis, collagen synthesis, osteoblast function, and calcification of bone (85). Thus, it is no surprise that moderate-to-severe zinc deficiency in children depresses growth and skeletal maturation (85). For this reason, the effect of zinc supplementation on linear growth has been the topic of several meta-analyses (2, 81, 86–88). In contrast to our study, the systematic reviews by Ramakrishnan et al. (81), Stammers et al. (86), and Das et al. (87) reported no significant effect of zinc supplementation on height; however, it is not appropriate to compare our review and the aforementioned studies because of considerable variability in inclusion criteria. Our findings confirm those of Brown et al. (88) who found a statistically significant positive effect (0.35; 95% CI: 0.19, 0.51) of zinc supplementation on linear growth in children aged <12 y. Interestingly, this systematic review reported a weighted mean effect size that was 2-fold higher than the standard mean difference of our review (0.35 compared with 0.15), likely a consequence of differing inclusion criteria and statistical techniques. Only baseline HAZ was a significant inverse predictor of the effect of zinc supplementation on height. We also did not find any evidence that linear growth was more responsive to zinc supplementation in individuals with baseline serum zinc ≤12 μmol/L. However, the small number of trials with baseline zinc deficiency likely limited this analysis (16, 26, 28, 38, 39). Collectively, our study suggests that zinc supplementation after age 24 mo will have a positive effect on linear growth, principally in stunted children.
Bone is the principal reservoir for body calcium, where it is stored as a component of hydroxyapatite. Calcium deficiency will induce bone resorption, which may impair linear growth. The majority of the studies included in this analysis measured height secondary to bone outcomes, the primary variable of interest. It is also likely that baseline calcium intake can influence the effect of calcium supplementation on height; however, few studies included in this review had a low baseline calcium intake (<400 mg/d) (45, 46), which limits our ability to assess effect modification by baseline status. Overall, our findings confirm those of Winzenberg et al. (89), who found no significant effect of calcium supplementation on linear growth in children aged <18 y. Taken together, there is no evidence to support the use of calcium supplements in children ≥2 y of age as a public health strategy to reduce stunting.
Iodine deficiency remains a major public health problem, despite successful strategies to prevent deficiency (e.g., fortification of salt). Iodine is an essential component of thyroid hormones that are required for skeletal growth (90). There were few studies that met our inclusion criteria. One study that provided 2 datasets was included in the meta-analysis, which showed no effect of iodine supplementation on linear growth. The sparsity of randomized, placebo-controlled iodine intervention trials is a limitation of this analysis, and the conclusions that can be made regarding the impact of iodine supplementation in children ≥2 y of age on height remain limited.
Vitamin A is essential for growth, and clinical vitamin A deficiency has been linked to poor growth performance (91). Supplemental vitamin A is thought to promote growth by reducing infection and diarrhea, both predictors of growth faltering. Our results suggest that vitamin A supplementation does have an effect on linear growth in children ≥2 y of age. Our findings contradict those of 2 meta-analyses by Ramakrishnan et al. (84, 81) in which the authors conclude that vitamin A supplementation does not have a positive effect on height gain. These conflicting findings likely stem from different inclusion criteria, because the reviews by Ramakrishnan et al. (81, 84) included children aged <2 y. Taken together, the evidence suggests that vitamin A supplementation may be a strategy to address linear-growth faltering in children.
Nutrient deficiencies are unlikely to occur in isolation; thus, MM supplementation has been promoted as a more holistic strategy to address malnutrition and stunting. Interestingly, our findings (0.26; 95% CI: 0.13, 0.39) were nearly identical to the effect of MM interventions reported in a meta-analysis of four studies conducted by Ramakrishnan et al. (84) (0.28; 95% CI: 0.16, 0.41). However, in a subsequent review by Ramakrishnan et al. (81), the effect of MMs on height in children aged <5 y was considerably smaller, although the results remained statistically significant (0.09; 95% CI: 0.01, 0.17). Similar to zinc, baseline HAZ was a significant predictor of the effect of MM supplementation on height. It is important to note that the studies by Zadik et al. (67, 68) and Ronaghy et al. (33) had individual effect sizes that were considerably higher than the other included studies. A possible explanation for the large effect sizes is that the subjects in the studies by Zadik et al. (67, 68) were diagnosed with constitutional delay of growth and puberty and born small-for-gestational age, respectively, whereas the subjects in the study by Ronaghy et al. (33) were malnourished. Excluding these studies from our analysis reduced the overall standardized mean effect size (0.15; 95%: CI: 0.06, 0.24), but it remained statistically significant. Overall, our results are consistent with previous meta-analyses and provide evidence in support of MM supplementation after age 24 mo as a strategy to promote linear growth during childhood.
It is well documented that severe protein malnutrition in children mechanistically drives linear growth retardation through a reduction of insulin-like growth factor I (92). The results of our review suggest a statistically significant positive effect of protein supplementation on linear growth in children ≥2 y of age. Baseline HAZ was a significant inverse predictor of the effect of protein supplementation on height. The studies by Lampl et al. (71), Malcolm (73), and Kabir et al. (70) had individual effect sizes that were nearly double that of the next highest effect, and excluding those studies from our analysis substantially reduced the overall standardized mean effect size (0.13; 95% CI: −0.03, 0.29). The subjects in the studies by Lampl et al. (71) and Malcolm (73) were severely stunted (HAZ <−3) at baseline and had a deficiency of 14–25 g protein/d, whereas those in the study by Kabir et al. (70) were recovering from shigellosis infection, which may explain the large effect of protein supplementation on linear growth. Nonetheless, we believe that the studies should remain in the meta-analysis because they both represent situations in which protein malnutrition would be present. Overall, our findings provide evidence in support of protein-based interventions after age 24 mo as an effective strategy to address stunting, especially in children who are severely stunted.
Growth faltering is also associated with overall poor diet quality that includes low intake of animal foods and high intakes of foods that contain inhibitors that impede the bioavailability of nutrients essential for growth. However, the results from our systematic review suggest that food interventions are ineffective at improving linear growth. This analysis is limited by the small number of studies (n = 6) and suffered from significant heterogeneity, which is to be expected because food-based interventions can take many forms. Additional well-designed trials are warranted to truly elucidate the growth-promoting potential of food-based interventions.
This review highlights several limitations of the extant literature. Approximately half of the included trials were of medium-to-low quality as assessed by Jadad scoring. However, influence analyses, or the effect of omitting single studies, did not reveal an overwhelming effect of any single trial, suggesting that no one study was influencing the results. A second limitation is the duration of interventions and length of follow-up of the included studies. Stunting is indicative of long-term nutritional status; the short duration of most trials (≤12 mo) may have corrected the nutritional deficiency but may not have been sufficiently long enough to observe a significant effect on height. Future intervention trials should have longer durations and/or include extended periods of subject follow-up. It is also worth noting that the inclusion of females >16 y and males >18 y of age may have reduced the potential impact of intervention effect because of the cessation of linear growth after the pubertal growth spurt. Across studies included in this review, the oldest mean age of subjects was 14 y. Furthermore, the studies reported that children aged >13 y showed no evidence of puberty, had recently reached menarche, or had not completed pubertal development as assessed by Tanner stages. Thus, the inclusion of older children is unlikely to have influenced our results. Lastly, consistent data on baseline nutritional status of subjects were available only for iron, zinc, and calcium interventions. Restricting our analysis to subjects deficient in the respective nutrient did not alter our conclusions but may have been limited by the small number of studies with baseline deficiency. Ultimately, our ability to draw conclusions regarding conditions that are more responsive to nutritional interventions is limited by a lack of extractable data and represents a critical gap that should be addressed in future trials.
In conclusion, this review indicates that nutritional interventions, namely zinc, vitamin A, MMs, and protein, delivered to children ≥2 y of age have the potential to improve linear growth, particularly in children who have experienced growth failure. The results suggest that the window of opportunity to address stunting does not completely close at 2 y of age.
Acknowledgments
We thank Barbara Abu-Zied (Emory University, Atlanta, GA) for her assistance with development of the search strategy. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: HAZ, height-for-age z score; LMICs, low- and middle-income countries; MM, multiple micronutrient.
References
- 1.UNICEF, WHO, World Bank. 2014 Joint child malnutrition estimates: levels and trends 2014. [cited 2016 Jun 17]. Available from: http://www.who.int/nutgrowthdb/jme_brochure2015.pdf?ua=1.
- 2.Bhandari N, Bahl R, Taneja S. Effect of micronutrient supplementation on linear growth of children. Br J Nutr 2001;85 Suppl 2:S131–7. [PubMed] [Google Scholar]
- 3.WHO, FAO. Guidelines on food fortification with micronutrients. In: Allen L, de Benoist B, Dary O, Hurrell R, editors. Geneva (Switzerland): WHO; 2009. [Google Scholar]
- 4.FAO, IFAD, WFP. The state of food insecurity in the world 2015. Meeting the 2015 international hunger targets: taking stock of uneven progress. Rome (Italy): FAO; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, Prentice A. Critical windows for nutritional interventions against stunting. Am J Clin Nutr 2013;97:911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundeen EA, Behrman JR, Crookston BT, Dearden KA, Engle P, Georgiadis A, Penny ME, Stein AD. Growth faltering and recovery in children aged 1–8 years in four low- and middle-income countries: Young Lives. Public Health Nutr 2014;17:2131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.2. London: The Cochrane Collaboration, 2009. [Google Scholar]
- 9.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguayo VM. School-administered weekly iron supplementation–effect on the growth and hemoglobin status of non-anemic Bolivian school-age children: a randomized placebo-controlled trial. Eur J Nutr 2000;39:263–9. [DOI] [PubMed] [Google Scholar]
- 11.Angeles IT, Schultink WJ, Matulessi P, Gross R, Sastroamidjojo S. Decreased rate of stunting among anemic Indonesian preschool children through iron supplementation. Am J Clin Nutr 1993;58:339–42. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia D, Seshadri S. Growth performance in anemia and following iron supplementation. Indian Pediatr 1992;30:195–200. [PubMed] [Google Scholar]
- 13.Chen L, Liu YF, Gong M, Jiang W, Fan Z, Qu P, Chen J, Liu YX, Li TY. Effects of vitamin A, vitamin A plus zinc, and multiple micronutrients on anemia in preschool children in Chongqing, China. Asia Pac J Clin Nutr 2008;21:3–11. [PubMed] [Google Scholar]
- 14.Chwang LC, Soemantri AG, Pollitt E. Iron supplementation and physical growth of rural Indonesian children. Am J Clin Nutr 1988;47:496–501. [DOI] [PubMed] [Google Scholar]
- 15.Dossa RAM, Ategbo EAD, de Koning FLHA, van Raaij JMA, Hautvast JGAJ. Impact of iron supplementation and deworming on growth performance in preschool Beninese children. Eur J Clin Nutr 2001;55:223–8. [DOI] [PubMed] [Google Scholar]
- 16.Hettiarachchi M, Liyanage C, Wickremasinghe R, Hilmers DC, Abrams SA. The efficacy of micronutrient supplementation in reducing the prevalence of anaemia and deficiencies of zinc and iron among adolescents in Sri Lanka. Eur J Clin Nutr 2008;62:856–65. [DOI] [PubMed] [Google Scholar]
- 17.Latham MC, Stephenson LS, Kinoti SN, Zaman MS, Kurz KM. Improvements in growth following iron supplementation in young Kenyan school children. Nutrition 1990;6:159–65. [PubMed] [Google Scholar]
- 18.Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr 1994;124:645–54. [DOI] [PubMed] [Google Scholar]
- 19.Mwanri L, Worsley A, Ryan P, Masika J. Supplemental vitamin A improves anemia and growth in anemic school children in Tanzania. J Nutr 2000;130:2691–6. [DOI] [PubMed] [Google Scholar]
- 20.Palupi L, Schultink W, Achadi E, Gross R. Effective community intervention to improve hemoglobin status in preschoolers receiving once-weekly iron supplementation. Am J Clin Nutr 1997;65:1057–61. [DOI] [PubMed] [Google Scholar]
- 21.Pereira SM, Begum A, Mathan VI, Baker SJ. A preliminary simulated iron fortification trial in South Indian preschool children. Br J Nutr 1979;41:413–7. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MM, Akramuzzaman SM, Mitra AK, Fuchs GJ, Mahalanabis D. Long-term supplementation with iron does not enhance growth in malnourished Bangladeshi children. J Nutr 1999;129:1319–22. [DOI] [PubMed] [Google Scholar]
- 23.Sungthong R, Mo-suwan L, Chongsuvivatwong V, Geater AF. Once weekly is superior to daily iron supplementation on height gain but not on hematological improvement among schoolchildren in Thailand. J Nutr 2002;132:418–22. [DOI] [PubMed] [Google Scholar]
- 24.Castillo-Durán C, García H, Venegas P, Torrealba I, Panteón E, Concha N, Pérez P. Zinc supplementation increases growth velocity of male children and adolescents with short stature. Acta Paediatr 1994;83:833–7. [DOI] [PubMed] [Google Scholar]
- 25.Cavan KR, Gibson RS, Grazioso CF, Isalgue AM, Ruz M, Solomons NW. Growth and body composition of periurban Guatemalan children in relation to zinc status: a longitudinal zinc intervention trial. Am J Clin Nutr 1993;57:344–52. [DOI] [PubMed] [Google Scholar]
- 26.Friis H, Ndhlovu P, Mduluza T, Kaondera K, Sandstrom B, Michaelsen KF, Vennervald BJ, Christensen NO. The impact of zinc supplementation on growth and body composition: a randomized, controlled trial among rural Zimbabwean schoolchildren. Eur J Clin Nutr 1997;51:38–45. [DOI] [PubMed] [Google Scholar]
- 27.Gibson RS, Vanderkooy PD, MacDonald AC, Goldman A, Ryan BA, Berry M. A growth-limiting, mild zinc-deficiency syndrome in some southern Ontario boys with low height percentiles. Am J Clin Nutr 1989;49:1266–73. [DOI] [PubMed] [Google Scholar]
- 28.Kaseb F, Fallah R. Efficacy of zinc supplementation on improvement of weight and height growth of healthy 9–18 year children in Iran. World Appl Sci J 2013;26:89–93. [Google Scholar]
- 29.Kikafunda JK, Walker AF, Allan EF, Tumwine JK. Effect of zinc supplementation on growth and body composition of Ugandan preschool children: a randomized, controlled, intervention trial. Am J Clin Nutr 1998;68:1261–6. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffari-Khosravi H, Shakiba M, Eftekhari M-H, Fatehi F. Effects of zinc supplementation on physical growth in 2–5-year-old children. Biol Trace Elem Res 2009;128:118–27. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Nishiyama S, Futagoishi-Suginohara Y, Matsuda I, Higashi A. Mild to moderate zinc deficiency in short children: effect of zinc supplementation on linear growth velocity. J Pediatr 1993;123:65–9. [DOI] [PubMed] [Google Scholar]
- 32.Ronaghy H, Fox MRS, Garn SM, Israel H, Harp A, Moe PG, Halsted JA. Controlled zinc supplementation for malnourished school boys: a pilot experiment. Am J Clin Nutr 1969;22:1279–89. [DOI] [PubMed] [Google Scholar]
- 33.Ronaghy HA, Reinhold JG, Mahloudji M, Ghavami P, Fox MRS, Halsted JA. Zinc supplementation of malnourished schoolboys in Iran: increased growth and other effects. Am J Clin Nutr 1974;27:112–21. [DOI] [PubMed] [Google Scholar]
- 34.Rosado JL, López P, Muñoz E, Martinez H, Allen LH. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am J Clin Nutr 1997;65:13–9. [DOI] [PubMed] [Google Scholar]
- 35.Ruz M, Castillo-Duran C, Lara X, Codoceo J, Rebolledo A, Atalah E. A 14-mo zinc-supplementation trial in apparently healthy Chilean preschool children. Am J Clin Nutr 1997;66:1406–13. [DOI] [PubMed] [Google Scholar]
- 36.Sayeg Porto MA, Oliveira HP, Cunha AJ, Miranda G, Guimarães MM, Oliveira WA, Santos DM. Linear growth and zinc supplementation in children with short stature. J Pediatr Endocrinol Metab 2000;13:1121–8. [DOI] [PubMed] [Google Scholar]
- 37.Sempértegui F, Estrella B, Correa E, Aguirre L, Saa B, Torres M, Navarrete F, Alarcón C, Carrión J, Rodríguez A, et al. Effects of short-term zinc supplementation on cellular immunity, respiratory symptoms, and growth of malnourished Equadorian children. Eur J Clin Nutr 1996;50:42–6. [PubMed] [Google Scholar]
- 38.Taneja S, Strand TA, Sommerfelt H, Bahl R, Bhandari N. Zinc supplementation for four months does not affect growth in young North Indian children. J Nutr 2010;140:630–4. [DOI] [PubMed] [Google Scholar]
- 39.Walravens PA, Krebs NF, Hambidge KM. Linear growth of low income preschool children receiving a zinc supplement. Am J Clin Nutr 1983;38:195–201. [DOI] [PubMed] [Google Scholar]
- 40.Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Briganti EM, Hume C, Nowson C. Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res 2007;22:458–64. [DOI] [PubMed] [Google Scholar]
- 41.Bonjour JP, Carrie AL, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest 1997;99:1287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron MA, Paton LM, Nowson CA, Margerison C, Frame M, Wark JD. The effect of calcium supplementation on bone density in premenarcheal females: a co-twin approach. J Clin Endocrinol Metab 2004;89:4916–22. [DOI] [PubMed] [Google Scholar]
- 43.Chevalley T, Bonjour JP, Ferrari S, Hans D, Rizzoli R. Skeletal site selectivity in the effects of calcium supplementation on areal bone mineral density gain: a randomized, double-blind, placebo-controlled trial in prepubertal boys. J Clin Endocrinol Metab 2005;90:3342–9. [DOI] [PubMed] [Google Scholar]
- 44.Courteix D, Jaffré C, Lespessailles E, Benhamou L. Cumulative effects of calcium supplementation and physical activity on bone accretion in premenarchal children: a double-blind randomised placebo-controlled trial. Int J Sports Med 2005;26:332–8. [DOI] [PubMed] [Google Scholar]
- 45.Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EME. Effect of calcium supplementation on bone mineral accretion in Gambian children accustomed to a low-calcium diet. Am J Clin Nutr 2000;71:544–9. [DOI] [PubMed] [Google Scholar]
- 46.Ekbote VH, Khadilkar AV, Chiplonkar SA, Hanumante NM, Khadilkar VV, Mughal MZ. A pilot randomized controlled trial of oral calcium and vitamin D supplementation using fortified laddoos in underprivileged Indian toddlers. Eur J Clin Nutr 2011;65:440–6. [DOI] [PubMed] [Google Scholar]
- 47.Iuliano-Burns S, Saxon L, Naughton G, Gibbons K, Bass SL. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res 2003;18:156–62. [DOI] [PubMed] [Google Scholar]
- 48.Lloyd T, Andon MB, Rollings N, Martel JK, Landis JR, Demers LM, Eggli DF, Kieselhorst K, Kulin HE. Calcium supplementation and bone mineral density in adolescent girls. JAMA 1993;270:841–4. [PubMed] [Google Scholar]
- 49.Nowson CA, Green RM, Hopper JL, Sherwin AJ, Young D, Kaymakci B, Guest CS, Smid M, Larkins RG, Wark JD. A co-twin study of the effect of calcium supplementation on bone density during adolescence. Osteoporos Int 1997;7:219–25. [DOI] [PubMed] [Google Scholar]
- 50.Pettifor JM, Ross P, Moodley G, Shuenyane E. The effect of dietary calcium supplementation on serum calcium, phosphorus, and alkaline phosphatase concentrations in a rural black population. Am J Clin Nutr 1981;34:2187–91. [DOI] [PubMed] [Google Scholar]
- 51.Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res 2003;18:885–92. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann MB, Jooste PL, Mabapa NS, Mbhenyane X, Schoeman S, Biebinger R, Chaouki N, Bozo M, Grimci L, Bridson J. Treatment of iodine deficiency in school-age children increases insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations and improves somatic growth. J Clin Endocrinol Metab 2007;92:437–42. [DOI] [PubMed] [Google Scholar]
- 53.Fawzi WW, Herrera MG, Willett WC, Nestel P, El Amin A, Mohamed KA. The effect of vitamin A supplementation on the growth of preschool children in the Sudan. Am J Public Health 1997;87:1359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadi H, Stoltzfus RJ, Dibley MJ, Moulton LH, West KP Jr, Kjolhede CL, Sadjimin T. Vitamin A supplementation selectively improves the linear growth of Indonesian preschool children: results from a randomized controlled trial. Am J Clin Nutr 2000;71:507–13. [DOI] [PubMed] [Google Scholar]
- 55.Lin J, Lai X, Qin J, Song F, Zhang Y, Yao P, Yang X, Liu L. Effect of beta-carotene supplementation on health and growth of vitamin A deficient children in China rural villages: a randomized controlled trial. e-SPEN 2009;4:e17-e21. [Google Scholar]
- 56.Yang YX, Han JH, Shao XP, He M, Bian LH, Wang Z, Wang GD, Men JH. Effect of micronutrient supplementation on the growth of preschool children in China. Biomed Environ Sci 2002;15:196–202. [PubMed] [Google Scholar]
- 57.Ash DM, Tatala SR, Frongillo EA Jr, Ndossi GD, Latham MC. Randomized efficacy trial of a micronutrient-fortified beverage in primary school children in Tanzania. Am J Clin Nutr 2003;77:891–8. [DOI] [PubMed] [Google Scholar]
- 58.Hall A, Hanh TTM, Farley K, Quynh TPN, Valdivia F. An evaluation of the impact of a school nutrition programme in Vietnam. Public Health Nutr 2007;10:819–26. [DOI] [PubMed] [Google Scholar]
- 59.Hyder SMZ, Haseen F, Khan M, Schaetzel T, Jalal CSB, Rahman M, Lönnerdal B, Mannar V, Mehansho H. A multiple-micronutrient-fortified beverage affects hemoglobin, iron, and vitamin A status and growth in adolescent girls in rural Bangladesh. J Nutr 2007;137:2147–53. [DOI] [PubMed] [Google Scholar]
- 60.Vinod Kumar M, Rajagopalan S. Impact of a multiple-micronutrient food supplement on the nutritional status of schoolchildren. Food Nutr Bull 2006;27:203–10. [DOI] [PubMed] [Google Scholar]
- 61.Lopriore C, Guidoum Y, Briend A, Branca F. Spread fortified with vitamins and minerals induces catch-up growth and eradicates severe anemia in stunted refugee children aged 3–6 y. Am J Clin Nutr 2004;80:973–81. [DOI] [PubMed] [Google Scholar]
- 62.Manger MS, McKenzie JE, Winichagoon P, Gray A, Chavasit V, Pongcharoen T, Gowachirapant S, Ryan B, Wasantwisut E, Gibson RS. A micronutrient-fortified seasoning powder reduces morbidity and improves short-term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. Am J Clin Nutr 2008;87:1715–22. [DOI] [PubMed] [Google Scholar]
- 63.Muthayya S, Eilander A, Transler C, Thomas T, van der Knaap HC, Srinivasan K, van Klinken BJW, Osendarp SJ, Kurpad AV. Effect of fortification with multiple micronutrients and n−3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children’s Health and Mental Performance Influenced by Optimal Nutrition) study. Am J Clin Nutr 2009;89:1766–75. [DOI] [PubMed] [Google Scholar]
- 64.Sarma KVR, Udaykumar P, Balakrishna N, Vijayaraghavan K, Sivakumar B. Effect of micronutrient supplementation on health and nutritional status of schoolchildren: growth and morbidity. Nutrition 2006; 22(1 Suppl)S8–14. [DOI] [PubMed] [Google Scholar]
- 65.Shatrugna V, Balakrishna N, Krishnaswamy K. Effect of micronutrient supplement on health and nutritional status of schoolchildren: bone health and body composition. Nutrition 2006; 22(1 Suppl)S33–9. [DOI] [PubMed] [Google Scholar]
- 66.Solon FS, Sarol JN, Bernardo ABI, Solon JAA, Mehansho H, Sanchez-Fermin LE, Wambangco LS, Juhlin KD. Effect of a multiple-micronutrient-fortified fruit powder beverage on the nutrition status, physical fitness, and cognitive performance of schoolchildren in the Philippines. Food Nutr Bull 2003; 24(4 Suppl 2)S129–40. [DOI] [PubMed] [Google Scholar]
- 67.Zadik Z, Sinai T, Zung A, Reifen R. Vitamin A and iron supplementation is as efficient as hormonal therapy in constitutionally delayed children. Clin Endocrinol (Oxf) 2004;60:682–7. [DOI] [PubMed] [Google Scholar]
- 68.Zadik Z, Sinai T, Zung A, Golander A, Reifen R. “Functional food” for acceleration of growth in short children born small for gestational age. J Pediatr Endocrinol Metab 2010;23:435–41. [DOI] [PubMed] [Google Scholar]
- 69.Grillenberger M, Neumann CG, Murphy SP, Bwibo NO. Van’T Veer P, Hautvast JGAJ West CE. Food supplements have a positive impact on weight gain and the addition of animal source foods increases lean body mass of Kenyan schoolchildren. J Nutr 2003; 133(11 Suppl 2)3957S–64S. [DOI] [PubMed] [Google Scholar]
- 70.Kabir I, Rahman MM, Haider R, Mazumder RN, Khaled MA, Mahalanabis D. Increased height gain of children fed a high-protein diet during convalescence from shigellosis: a six-month follow-up study. J Nutr 1998;128:1688–91. [DOI] [PubMed] [Google Scholar]
- 71.Lampl M, Johnston FE, Malcolm LA. The effects of protein supplementation on the growth and skeletal maturation of new Guinean school children. Ann Hum Biol 1978;5:219–27. [DOI] [PubMed] [Google Scholar]
- 72.Larnkjær A, Arnberg K, Michaelsen KF, Jensen SM, Mølgaard C. Effect of milk proteins on linear growth and IGF variables in overweight adolescents. Growth Horm IGF Res 2014;24:54–9. [DOI] [PubMed] [Google Scholar]
- 73.Malcolm LA. Growth retardation in a new Guinea boarding school and its response to supplementary feeding. Br J Nutr 1970;24:297–305. [DOI] [PubMed] [Google Scholar]
- 74.Pereira SM, Begum A, Jesudian G, Sundararaj R. Lysine-supplemented wheat and growth of preschool children. Am J Clin Nutr 1969;22:606–11. [DOI] [PubMed] [Google Scholar]
- 75.Pereira SM, Jones S, Jesudian G, Begum A. Feeding trials with lysine- and threonine-fortified rice. Br J Nutr 1973;30:241–50. [DOI] [PubMed] [Google Scholar]
- 76.Alarcon PA, Lin L-H, Noche M, Hernandez VC, Cimafranca L, Lam W, Comer GM. Effect of oral supplementation on catch-up growth in picky eaters. Clin Pediatr (Phila) 2003;42:209–17. [DOI] [PubMed] [Google Scholar]
- 77.Maleta K, Kuittinen J, Duggan MB, Briend A, Manary M, Wales J, Kulmala T, Ashorn P. Supplementary feeding of underweight, stunted Malawian children with a ready-to-use food. J Pediatr Gastroenterol Nutr 2004;38:152–8. [DOI] [PubMed] [Google Scholar]
- 78.Prasad Mp R, Benhur D, Kommi K, Madhari R, Rao MV, Patil JV. Impact of Sorghum supplementation on growth and micronutrient status of school going children in Southern India — a randomized trial. Indian J Pediatr 2016;83:9–14. [DOI] [PubMed] [Google Scholar]
- 79.Rao KVRG, Raju SV. Association of growth status and the prevalence of anaemia in preschool children. Indian J Med Res 1980;71:237–46. [PubMed] [Google Scholar]
- 80.Beard J, Haas J, Gomez LH. The relationship of nutritional status to oxygen transport and growth in highland Bolivian children. Hum Biol 1983;55:151–64. [PubMed] [Google Scholar]
- 81.Ramakrishnan U, Nguyen P, Martorell R. Effects of micronutrients on growth of children under 5 y of age: meta-analyses of single and multiple nutrient interventions. Am J Clin Nutr 2009;89:191–203. [DOI] [PubMed] [Google Scholar]
- 82.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public Health Nutr 2006;9:904–20. [DOI] [PubMed] [Google Scholar]
- 83.Vucic V, Berti C, Vollhardt C, Fekete K, Cetin I, Koletzko B, Gurinovic M, van’t Veer P. Effect of iron intervention on growth during gestation, infancy, childhood, and adolescence: a systematic review with meta-analysis. Nutr Rev 2013;71:386–401. [DOI] [PubMed] [Google Scholar]
- 84.Ramakrishnan U, Aburto N, McCabe G, Martorell R. Multimicronutrient Interventions but not vitamin A or iron interventions alone improve child growth: results of 3 meta-analyses. J Nutr 2004;134:2592–602. [DOI] [PubMed] [Google Scholar]
- 85.Hambridge KM, Casey CE, Krebs NF. Zinc In: Mertz W, editor. Trace elements in human and animal nutrition. New York: Academic Press; 1986. [Google Scholar]
- 86.Stammers AL, Lowe NM, Medina MW, Patel S, Dykes F, Perez-Rodrigo C, Serra-Majam L, Nissensohn M, Moran VH. The relationship between zinc intake and growth in children aged 1–8 years: a systematic review and meta-analysis. Eur J Clin Nutr 2015;69:147–53. [DOI] [PubMed] [Google Scholar]
- 87.Das JK, Kumar R, Salam RA, Bhutta ZA. Systematic review of zinc fortification trials. Ann Nutr Metab 2013;62 Suppl 1:44–56. [DOI] [PubMed] [Google Scholar]
- 88.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2002;75:1062–71. [DOI] [PubMed] [Google Scholar]
- 89.Winzenberg T, Shaw K, Fryer J, Jones G. Calcium supplements in healthy children do not affect weight gain, height, or body composition. Obesity (Silver Spring) 2007;15:1789–98. [DOI] [PubMed] [Google Scholar]
- 90.Markou KB, Tsekouras A, Anastasiou E, Vlassopoulou B, Koukkou E, Vagenakis GA, Mylonas P, Vasilopoulos C, Theodoropoulou A, Rottstein L, et al. Treating iodine deficiency: long-term effects of iodine repletion on growth and pubertal development in school-age Children. Thyroid 2008;18:449–54. [DOI] [PubMed] [Google Scholar]
- 91.West KP Jr, LeClerq SC, Shrestha SR, Wu LSF, Pradhan EK, Khatry SK, Katz J, Adhikari R, Sommer A, Pokhrel RP, et al. Effects of vitamin a on growth of vitamin A-deficient children: field studies in Nepal. J Nutr 1997;127:1957–65. [DOI] [PubMed] [Google Scholar]
- 92.Estívariz CF, Ziegler TR. Nutrition and the insulin-like growth factor system. Endocrine 1997;7:65–71. [DOI] [PubMed] [Google Scholar]