Abstract
For the past 45 y, the National Center for Health Statistics at the CDC has carried out nutrition surveillance of the US population by collecting anthropometric, dietary intake, and nutritional biomarker data, the latter being the focus of this publication. The earliest biomarker testing assessed iron and vitamin A status. With time, a broad spectrum of water- and fat-soluble vitamins was added and biomarkers for other types of nutrients (e.g., fatty acids) and bioactive dietary compounds (e.g., phytoestrogens) were included in NHANES. The cross-sectional survey is flexible in design, and biomarkers may be measured for a short period of time or rotated in and out of surveys depending on scientific needs. Maintaining high-quality laboratory measurements over extended periods of time such that trends in status can be reliably assessed is a major goal of the testing laboratories. Physicians, health scientists, and policy makers rely on the NHANES reference data to compare the nutritional status of population groups, to assess the impact of various interventions, and to explore associations between nutritional status and health promotion or disease prevention. Focusing on the continuous NHANES, which started in 1999, this review uses a “lessons learned” approach to present a series of challenges that are relevant to researchers measuring biomarkers in NHANES and beyond. Some of those challenges are the use of multiple related biomarkers instead of a single biomarker for a specific nutrient (e.g., folate, vitamin B-12, iron), adhering to special needs for specimen collection and handling to ensure optimum specimen quality (e.g., vitamin C, folate, homocysteine, iodine, polyunsaturated fatty acids), the retrospective use of long-term quality-control data to correct for assay shifts (e.g., vitamin D, vitamin B-12), and the proper planning for and interpretation of crossover studies to adjust for systematic method changes (e.g., folate, vitamin D, ferritin).
Keywords: biochemical indicator, national nutrition survey, cutoff, biological specimen, water-soluble vitamin, fat-soluble vitamin, iron-status indicator
Introduction
Nutritional biomarkers can be used in different ways, but their most common applications are to validate dietary intake instruments, to serve as a surrogate indicator of dietary intake, or to represent an integrated measure of nutritional status for a nutrient. A 2003 supplement publication of The Journal of Nutrition addressed the use of biomarkers of exposure in nutritional epidemiology (1) and discussed biological and methodologic issues (2), methodologic and statistical considerations (3), and laboratory issues (4). More recent articles covered general aspects of nutritional biomarkers (5) and biomarkers in conjunction with anthropometric and dietary intake data to assess nutritional status (6). The BOND (Biomarkers of Nutrition for Development) project, led by the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, recently published review articles on iodine, folate, vitamin A, and zinc (7–10) to provide evidence-based advice to researchers with an interest in the role of nutrition in health; articles on iron and vitamin B-12 are in progress.
Although the generation and interpretation of nutritional biomarker data in the NHANES faces many of the same issues that are relevant to nutritional epidemiology in general, this large and highly complex nationally representative cross-sectional US survey faces numerous specific challenges. Earlier reports covered important information about nutritional biomarkers for NHANESs conducted up to the mid-1990s and for the first 4 y of the continuous survey starting in 1999 (NHANES 1999+), although nutritional biomarkers were not the primary focus of these reports (11–13). Recently, this journal published a comprehensive update on NHANES dietary data (14), but there is a lack of updated information on NHANES nutritional biomarkers. This article reviews general and specific considerations that go into the planning, execution, interpretation, and use of nutritional biomarkers in NHANES (Table 1). We use the term “nutritional biomarkers” broadly to include both nutrient and nonnutrient biomarkers of food-based substances with biological activity, so-called bioactive dietary compounds (e.g., phytoestrogens, caffeine, and metabolites). We applied a “lessons learned” approach to highlight specific challenges that have emerged in NHANES, with a special focus on the continuous NHANES 1999+.
TABLE 1.
Overview of the sections covered in this review article
| Section | Content |
| History of nutritional biomarkers in NHANES | Evolution of nutritional biomarkers from the early 1970s to the continuous NHANES (1999+) |
| NHANES nutritional biomarker selection | Scientific considerations addressing the quality and validity of the biomarker |
| Logistic considerations addressing methodologic issues of data generation | |
| NHANES preanalytic, analytic, and postanalytic phases | Specimen collection, processing, and storage considerations |
| Laboratory measurement considerations | |
| Data release considerations | |
| NHANES nutritional biomarker interpretation | Scientific considerations addressing methodologic issues of data analysis |
| Logistic considerations addressing the quality and validity of data | |
| Use of NHANES nutritional biomarker data | Generate normative data for the US population |
| Assess a potential public health problem | |
| Monitor the impact of nutritional interventions | |
| Address potential emerging scientific or public health focus areas | |
| Allow the translation of research findings |
Current Status of Knowledge
History of nutritional biomarkers in NHANES
The sequence of national surveys that assessed the health and nutrition status of the US population is summarized in Table 2. The health examination surveys conducted in the 1960s did not assess nutrition status other than through anthropometric measurements (15). The Ten State Nutrition Survey conducted in 1968–1970 was a first attempt to provide Congress with information about the prevalence of undernutrition and related conditions (16). Subsequently, nutrition surveillance at the national level was incorporated into the National Health Examination Survey, and in 1971 the survey was renamed the NHANES (17). At that time, a central laboratory was formed at the CDC that has measured the majority of nutritional biomarkers in NHANES since then.
TABLE 2.
NHANESs carried out in the United States1
| Survey | Dates | Ages |
| NHES I | 1960–1962 | 18–79 y |
| NHES II | 1963–1965 | 6–11 y |
| NHES III | 1966–1970 | 12–17 y |
| NHANES I | 1971–1975 | 1–74 y |
| NHANES II | 1976–1980 | 6 mo–74 y |
| HHANES | 1982–1984 | 6 mo–74 y |
| NHANES III | 1988–1994 | ≥2 mo |
| NHANES 1999+ | 1999–present | All ages |
HHANES, Hispanic Health and Nutrition Examination Survey; NHES, National Health Examination Survey.
Between 1971 and 1994, NHANES conducted a series of cross-sectional surveys on a periodic basis, each of which measured a selection of nutritional biochemical markers to assess iron, vitamin, and trace elements status (11, 13). NHANES I (1971–1974) assessed iron status by measuring serum iron, iron-binding capacity, and transferrin saturation and vitamin A status by measuring serum retinol (11). In NHANES II (1976–1980), erythrocyte protoporphyrin and serum ferritin (subsample) were added as additional markers of iron status, and a few trace elements (zinc and copper) and a few vitamin status biomarkers [serum and RBC folate (subsample) and serum vitamin C] were also added (11). In addition, “blind” quality-control (QC) pools were introduced as an additional quality assurance measure during the laboratory analysis. The Hispanic Health and Nutrition Examination Survey (1982–1984) covered nearly the same nutritional biomarkers as NHANES II, except for serum vitamin C and the trace elements. Serum vitamin E was measured for the first time in this survey, and HPLC made its debut as an analytical technique (11). Finally, in NHANES III (1988–1994), additional nutritional biomarkers were added: serum vitamin B-12, serum 25-hydroxyvitamin D [25(OH)D]8, serum carotenes, and urine iodine (Supplemental Table 1). Barcoded labels were also introduced to facilitate sample logistics and to reduce labeling errors.
In 1999, NHANES became a continuous survey conducted in 2-y cycles with planning, data collection, and public release of data and key reports of findings for each 2-y survey cycle. Depending on public health needs and availability of resources, nutritional biomarkers were cycled in and out of the continuous NHANES 1999+ (Table 3). However, a few key nutrients have been covered nearly continuously since 1999: iron, folate, and vitamin D. NHANES 2003–2006 represented a peak, with the greatest number of nutrients being covered by biomarker tests. The number of actual biomarkers measured has increased from 34 in 1999–2002, to 58 in 2003–2010, and to 61 in 2011–2014. Furthermore, the number of results produced (∼300,000 in 1999–2002, ∼290,000 in 2003–2006, ∼430,000 in 2007–2010, and ∼700,000 in 2011–2014) has increased. This is because several multianalyte panel tests such as FAs (24–30 analytes) and caffeine and metabolites (15 analytes) were added to NHANES in more recent years (Table 3).
TABLE 3.
Nutritional biomarkers measured in NHANES III (1988–1994) and in the continuous NHANES 1999+1
| Survey cycle and nutritional biomarker (number of analytes if >1) | Matrix | Age or population studied | Laboratory method |
| 1999–2002 | |||
| Folate, total (2) | Serum, WB | ≥3 y | BioRad radio assay |
| Folate forms (2) | Surplus serum | ≥60 y | HPLC-electrochemical detection |
| Vitamin B-12 | Serum | ≥3 y | BioRad radio protein binding assay |
| tHcy | Plasma | ≥3 y | Abbott fluorescence polarization immunoassay |
| MMA | Plasma | ≥3 y | GC-MS |
| Ferritin | Serum | ≥1 y | BioRad radioassay |
| sTfR | Surplus serum | Pregnant women | Roche immunoturbidimetry |
| Iron and TIBC (2) | Serum | ≥1 y | Colorimetric assay |
| Protoporphyrin | WB | ≥1 y | Fluorometric assay |
| Vitamin A and retinyl esters (2) | Serum | ≥3 y | HPLC-UV/vis |
| Vitamin E (2) | Serum | ≥3 y | HPLC-UV/vis |
| Carotenoids (6)2 | Serum | ≥3 y | HPLC-UV/vis |
| 25(OH)D2 | Serum | ≥6 y | DiaSorin radioassay |
| Phytoestrogens (6) | Urine | ≥6 y (one-third sample) | HPLC-tandem MS |
| Iodine2 | Urine | ≥6 y (one-third sample) | Inductively coupled plasma mass spectroscopy |
| Selenium3 | Serum | 3–11 y | Atomic absorption spectroscopy |
| 2003–2006 | |||
| Folate, total (2) | Serum, WB | ≥1 y | BioRad radioassay |
| Vitamin B-6 (1 or 2): | Serum | ≥1 y | |
| PLP (2003–2004) | A/C Diagnostics enzymatic assay | ||
| PLP + 4PA (2005–2006) | HPLC-fluorometric detection | ||
| Vitamin B-12 | Serum | ≥1 y | BioRad radioassay |
| tHcy (2003–2004) | Plasma | ≥3 y | Abbott fluorescence polarization immunoassay |
| tHcy (2005–2006) | ≥20 y | ||
| MMA3 | Plasma | ≥3 y | GC-MS |
| Vitamin C | Serum | ≥6 y | HPLC-electrochemical detection |
| Ferritin | Serum | 1–5 y; F: 12–49 y | Roche immunoturbidimetry |
| sTfR | Serum | 1–5 y; F: 12–49 y | Roche immunoturbidimetry |
| Iron and UIBC (2) | Serum | 3–5 y; F: 12–49 y | Beckman colorimetric assay |
| Protoporphyrin | WB | 3–5 y; F: 12–49 y | Fluorometric assay |
| Vitamin A and retinyl esters (2) | Serum | ≥3 y | HPLC-UV/vis |
| Vitamin E (2003–2004) (3) | Serum | ≥3 y | HPLC-UV/vis |
| Vitamin E (2005–2006) (2) | Serum | ≥3 y | HPLC-UV/vis |
| Carotenoids (2003–2004) (11) | Serum | ≥3 y | HPLC-UV/vis |
| Carotenoids (2005–2006) (7) | Serum | ≥3 y | HPLC-UV/vis |
| 25(OH)D | Serum | ≥1 y | DiaSorin RIA |
| FAs (24)3 | Surplus plasma | ≥20 y (fasted) | GC-MS |
| Phytoestrogens (6) | Urine | ≥6 y (one-third sample) | HPLC-tandem MS |
| Iodine | Urine | ≥6 y (one-third sample) | Inductively coupled MS |
| Selenium3 | Serum | ≥40 y | Inductively coupled MS |
| 2007–2010 | |||
| Folate, total (2) | Serum, WB | ≥1 y | Microbiological assay |
| Folate forms (2)3 | Serum | ≥1 y (one-third sample) | HPLC-tandem MS |
| Vitamin B-6 (2) | Serum | ≥1 y | HPLC-fluorescence detection |
| Ferritin | Serum | 1–5 y; F: 12–49 y | Roche immunoturbidimetry |
| sTfR | Serum | 1–5 y; F: 12–49 y | Roche immunoturbidimetry |
| 25(OH)D metabolites (4) | Serum | ≥1 y | HPLC-tandem MS |
| Phytoestrogens (6) | Urine | ≥6 y (one-third sample) | HPLC-tandem MS |
| Caffeine and metabolites (15)2 | Urine | ≥6 y (one-third sample) | HPLC-tandem MS |
| Iodine | Urine | ≥6 y | Inductively coupled MS |
| 2011–2014 | |||
| Folate | WB | ≥1 y | Microbiological assay |
| Folate forms (6) | Serum | ≥1 y | HPLC-tandem MS |
| Vitamin B-12 | Serum | ≥20 y | Roche electrochemiluminescence |
| MMA | Serum | ≥20 y | HPLC-tandem MS |
| 25(OH)D metabolites (4) | Serum | ≥1 y | HPLC-tandem MS |
| FAs (30) | Serum | 1–11 y, ≥12 y (fasted) | GC-MS |
| Caffeine and metabolites (15) | Ferritin | ≥6 y (one-third sample) | HPLC-tandem MS |
| Electrolytes, Na/K/Cl (3)4 | Urine (24-h) | 20–69 y (one-half sample) | Roche ion selective electrode |
| 2015–2016 | |||
| Folate | WB | ≥1 y | Microbiologic assay |
| Folate forms (6) | Serum | ≥1 y | HPLC-tandem MS |
| Ferritin | Serum | 1–5 y; F: 12–49 y | Roche immunoturbidimetry |
| sTfR | Serum | 1–5 y; F: 12–49 y | Roche immunoturbidimetry |
| 25(OH)D metabolites (4) | Serum | ≥1 y | HPLC-tandem MS |
MMA, methylmalonic acid; PLP, pyridoxal-5′-phosphate; sTfR, soluble transferrin receptor; tHcy, total homocysteine; TIBC, total iron binding capacity; UIBC, unsaturated iron binding capacity; UV/vis, UV/visible detection; WB, whole blood; 25(OH)D, 25-hydroxyvitamin D; 4PA, 4-pyridoxic acid.
Measured during last 2 y only.
Measured during first 2 y only.
Measured only during 2014.
Nutritional biomarker selection
Ahluwalia et al. (14) provided an overview of the NHANES design and operations, including information on what the major components of NHANES were and what population groups have been oversampled in different survey cycles to produce more reliable estimates. The selection of nutritional biomarkers for inclusion in NHANES is subject to numerous scientific (“what” and “why” questions) and logistic (“how to” questions) considerations (Table 4). Periodically, expert panels are assembled to review and advise on the appropriate use of nutritional biomarkers in NHANES. Several Life Sciences Research Office reports were produced in conjunction with NHANES II and III to address analytical problems so as to improve future NHANES biomarker measurements (18–22). These reports are valuable in showing the evolution in the assessment of nutritional biomarkers. More recent examples of expert panels providing input on the selection of nutritional biomarkers are from 1999 when nutritional biomarkers included in the continuous NHANES 1999+ were discussed, from 2005 when iron biomarkers were reviewed to decide whether NHANES should change its approach to assess iron status (AC Looker, personal communication, 2006), from 2009 when laboratory methodologies for the measurement of 25(OH)D were reviewed (23), and from 2010 when biomarkers for folate (24) and vitamin B-12 (25) status and laboratory methodologies were reviewed.
TABLE 4.
Scientific and logistic considerations that are part of NHANES nutritional biomarker selection and interpretation
| Category for consideration | Questions to be considered |
| Biomarker selection—scientific issues (quality and validity of a biomarker) | What does the biomarker represent (short-term status, long-term status, intake, function)? |
| Is the sensitivity or specificity of the biomarker appropriate for the intended purpose? | |
| Are multiple biomarkers per nutrient needed to improve sensitivity or specificity? | |
| What are relevant biological confounders? | |
| What is the biological variation of the biomarker? | |
| Has the biomarker been qualified for clinical or public health use? | |
| Biomarker selection—logistic issues (methodologic issues of data generation) | Can an appropriate specimen be collected, processed, and stored? |
| What preanalytic factors need to be considered? | |
| Is a reliable and validated laboratory method available? | |
| Is the laboratory method precise enough relative to the biological variation? | |
| Can the laboratory method handle the sample throughput in a reasonable time? | |
| Are resources available for specimen collection and laboratory analysis? | |
| Biomarker interpretation—scientific issues (methodologic issues of data analysis) | Are reference intervals or cutoffs available? |
| Are cutoffs or reference intervals relevant to groups or individuals of interest? | |
| Do any exclusion criteria need to be applied? | |
| What sample sizes are required to assess status in subgroups of the population? | |
| Biomarker interpretation—logistic issues (quality and validity of data) | Is sufficient information available to judge the quality of the data? |
| Have laboratory methods been standardized to yield comparable results? | |
| Have laboratory methods changed over time? | |
| Is data adjustment needed for comparability? |
Biomarker selection—scientific considerations.
Scientific issues for biomarker selection primarily address the quality and validity of the biomarker and often include biological factors that need to be considered, such as confounders (Table 4). A few well-known biological confounders for nutritional biomarkers are age, sex, race/ethnicity, fasting status [e.g., serum folate (26)], impaired renal function [e.g., plasma total homocysteine (tHcy) and serum methylmalonic acid (MMA) (26)], inflammation [e.g., serum ferritin (27) and serum pyridoxal-5′-phosphate (PLP) as a measure of vitamin B-6 status (28)], smoking [e.g., serum vitamin C (29)], and obesity [e.g., folate (30)]. Pregnancy is another biological confounder that affects many nutritional biomarkers, likely mainly due to hemodilution (31). To allow meaningful interpretation of a single measurement result, the index of individuality [ratio between within-person (CVI) and between-person (CVG) variation] has to be low (<0.6), which was the case for the majority of nutritional biomarkers in NHANES 1999–2002 (32). The use of multiple related biomarkers instead of a single biomarker for a specific nutrient [e.g., serum vitamin B-12 and MMA or serum ferritin and soluble transferrin receptor (sTfR)] can sometimes improve problems with sensitivity and specificity of an individual biomarker and thus more accurately describe nutritional status. Another important consideration is that related biomarkers are monitored together as part of the same NHANES survey cycle and on the same (sub)sample to allow meaningful interpretation (e.g., biomarkers of water-soluble B-vitamin status, biomarkers of antioxidant status, availability of serum C-reactive protein data to help interpret serum ferritin). Finally, the expected population prevalence of the health condition of interest needs to be high enough to warrant monitoring by NHANES. That said, some vitamin deficiencies (e.g., vitamins A and E) are <1% in the US population (33), yet periodic monitoring (1999–2006 and 2017–2018) is warranted to ensure that concentrations are similar to earlier years.
Biomarker selection—lessons learned.
The assessment of folate, vitamin B-12, and iron status represents examples for the use of multiple nutritional biomarkers in NHANES. Serum folate is a short-term status biomarker, highly influenced by recent dietary folate intake, whereas RBC folate is a long-term status biomarker that integrates folate intakes over the 90- to 120-d life span of an RBC. The 2010 expert panel roundtable discussed whether the measurement of both folate biomarkers should be continued in NHANES after the BioRad radioassay was discontinued in 2006, given that public health concerns have shifted in the postfortification era to the safety of high folic acid intakes (24). The panel recognized that, in addition to serum total folate, the measurement of individual serum folate forms—in particular that of unmetabolized folic acid—is of great interest postfortification. The panel recommended that, if possible, both serum and RBC biomarkers should be measured because reducing the proportion of women of reproductive age who have lower RBC folate concentrations is one of the Healthy People 2020 objectives (34). In addition, in 2015, the WHO recommended an RBC folate population cutoff for folate insufficiency in women of reproductive age to represent elevated risk of neural tube defects (35). The availability of RBC folate data generated with the microbiologic assay during NHANES 2006–2012 allowed an immediate assessment of folate insufficiency status in US women (36).
Serum vitamin B-12 and holotranscobalamin are both biomarkers of circulating vitamin B-12 concentrations, whereas serum MMA and tHcy are functional biomarkers of vitamin B-12 status. Although elevated MMA is specific for low vitamin B-12 status, elevated tHcy also occurs in response to inadequate folate, vitamin B-2, or vitamin B-6 status. Because of problems with the sensitivity and specificity of individual vitamin B-12 biomarkers (37), the 2010 expert panel recommended that ≥1 biomarker of each category be included in NHANES (25). Thus, serum vitamin B-12 and MMA data for adults aged ≥20 y are currently being generated for 2 survey cycles in NHANES 2011–2014. Once available, these will be the first nationally representative data for 2 race/ethnic groups in the United States: Hispanics and non-Hispanic Asians. Previous serum vitamin B-12 and MMA data from NHANES 1999–2004 showed a different prevalence of low vitamin B-12 status depending on which biomarker and/or cutoff was used (38). Furthermore, modeling of an MMA-derived cutoff for serum vitamin B-12 revealed 3 distinct slopes for the curve describing the relation between serum vitamin B-12 and MMA, challenging the conventional use of 1 cutoff to classify vitamin B-12 status (39).
The iron status of the US population has been monitored since the inception of NHANES in 1971 by using a battery of hematologic and biochemical indicators (40). In NHANES II (1976–1980), models with multiple biochemical iron status indicators were used (41). The ferritin model, also known as the 3-indicator model (serum ferritin, transferrin saturation, and erythrocyte protoporphyrin), was applied to NHANES III (1988–1994) (42) and to the first few years of the continuous NHANES (1999–2002) (43). With the use of this model, participants were categorized as iron deficient if 2 out of 3 iron status indicators were abnormal. Starting in 2003, NHANES introduced the measurement of serum sTfR and limited the sample to 2 groups of interest: children (1–5 y) and women of childbearing age (12–49 y). Serum ferritin describes tissue iron stores, but it does not reflect the severity of the progressing iron depletion. Serum sTfR, on the other hand, describes the iron functional pool and continues to rise with increasing functional iron deficiency (44). The ratio of sTfR to ferritin is a valuable measure of the extent of iron deficiency and allows the evaluation of iron status by the body iron model (45). By using data from NHANES 2003–2006, the agreement between the body iron model and the previously used ferritin model was fair to good (46). Among nonpregnant women, the body iron model produced lower estimates of iron deficiency prevalence and better predicted anemia, and it appeared to be less affected by inflammation than the ferritin model.
Biomarker selection—logistic considerations.
Logistic issues for biomarker selection address mainly methodologic issues of measurement, including special needs for specimen collection and handling and requirements regarding laboratory capacity and the analytical method (Table 4). The most accurate and precise laboratory method cannot generate high-quality data if the specimen integrity has been compromised. To ensure that high-quality data will be obtained during laboratory analysis, it is important that the laboratory has experience with “high volume” studies, maintains a functioning internal quality assurance system, operates with trained and experienced laboratory staff, participates in external quality assurance programs, and uses certified reference materials when available. Assessing trends in laboratory data between NHANES cycles requires laboratory tests with high analytical precision and minimal bias. Slight laboratory shifts can result in significant changes in estimates of prevalence. The assay precision has to be low to enable detection of small temporal trends, and the assay performance has to be stable to ensure comparability of data over time. The limit of detection (LOD) has to be low enough to ensure that the analyte can be measured in the majority of samples. Objective quality specifications for the assay based on biological variation recommend that the assay variation CVA should be a fraction of the within-person variation (CVA <0.5 CVI for desirable performance) and the assay bias should be a fraction of the sum of within- and between-person variation [CVA <0.25 (CVI2 + CVG2)1/2 for desirable performance] (47). It is beneficial if major laboratory equipment is under service contract to avoid delays in maintenance and repair. If a commercial assay is used, laboratories often have to use QC materials provided by the manufacturer as part of the assay kit to allow the manufacturer to troubleshoot when the measurement quality parameters are not met. However, it is advisable that the laboratory additionally prepares, characterizes, and regularly analyzes in-house QC pools to document assay stability over time and to allow the laboratory to troubleshoot problems independent from the manufacturer, such as lot-to-lot variability and kit reformulations. Preparing a sufficient volume of QC pools to last for several years, possibly even the entire study period, and overlapping old and new QC pools for a certain period are also good measures to document long-term assay stability. Additional laboratory issues with regard to the use of nutritional biomarkers have been reviewed previously (4).
Biomarker selection—lessons learned.
An important logistic consideration for a large national survey is how to reconcile the need for fasted specimens with the increased participant burden of an overnight fast. In NHANES, participants are randomly assigned to a morning, afternoon, or evening Mobile Examination Center (MEC) appointment with the option to switch appointments if they are unavailable at the assigned time. Those aged ≥12 y with a morning appointment are asked to undergo an overnight fast. The NHANES staff attempt to maintain a balance, with approximately half of the participants being fasted for ≥8 h. When participants arrive at the MEC they are asked when they consumed their last meal and the number of hours they fasted is recorded.
Some examples of special needs for specimen collection and handling in NHANES to ensure optimum specimen quality are the addition of meta-phosphoric acid to serum to improve the stability of vitamin C (NHANES 2003–2006) or the addition of ascorbic acid to stabilize serum folate forms (started in NHANES 2013–2014). The prompt processing of EDTA whole blood to avoid an artifactual increase in plasma tHcy as a result of homocysteine export from the erythrocytes (NHANES 1999–2006) and the use of prescreened urine collection supplies for iodine to avoid external contamination (started in NHANES 2000) are additional examples. An example of suboptimal specimen quality that resulted in a cancellation of planned analyses is the plasma cis-FA component in NHANES 2007–2008. These specimens were erroneously stored at −20°C instead of −70°C for nearly 1 y before the laboratory was ready for the analysis. Stability data generated by the laboratory as part of the assay validation revealed that the PUFAs underwent losses ≤45% under these conditions (48). The laboratory also determined that EDTA plasma samples frequently contained fibrinogen microclots that caused sample aspiration problems with the automated liquid handler. To be able to generate high-quality data on FAs, NHANES collected serum samples for the analysis of cis-FAs in NHANES 2011–2014 and specimens were stored at −70°C.
The measurement of serum PLP by an enzymatic immunoassay provides an example of suboptimal assay sensitivity leading to problems later when data from 2 methods were compared. During 2003–2004, plasma PLP was measured by an enzymatic immunoassay, which had an LOD of 10 nmol/L. During 2005–2010, serum PLP was measured by an HPLC assay, which had a much lower LOD of 0.3 nmol/L. The 2 assays were poorly correlated at low PLP concentrations that represent inadequate vitamin B-6 status (Pearson’s r = 0.21). Although a crossover study was conducted, the resulting regression equation could not be used to adjust the 2003–2004 PLP data to make them comparable to the HPLC data, because it overadjusted the data, making them even less comparable. The data user was cautioned against combining the 2003–2004 PLP data with HPLC PLP data (49).
NHANES specimen collection, laboratory measurement, and data release
Specimen collection, processing, and storage issues.
The specimen collection and processing in the MEC are conducted by fully trained field staff who follow standardized protocols under tightly controlled environmental conditions. Frozen specimens are shipped weekly on dry ice from the field to the NHANES biorepository or directly to the analyzing laboratory. The NHANES biorepository groups samples by test and serves as a temporary holding place until the laboratory is ready to receive samples for analysis. The NHANES plan and operations manual describes all aspects of survey planning, field operations, and data collection and processing (50).
Blood collected from participants aged 1–2 y (not fasted) is limited to 9 mL, whereas ≤115 mL of blood is collected from adult participants (51). Due to the multitude of laboratory tests performed for each participant, the specimen volume per assay is limited. Typical serum volumes available for a test are 0.3–0.7 mL and are generally ≤0.5 mL for children ≤5 y of age. This amount has to be sufficient to allow repeat laboratory analysis (if a sample QC variable or the batch QC are outside of specifications) and pipetting on an automated liquid handling station, which requires a higher specimen volume than manual pipetting. NHANES does not routinely collect dried blood spot samples, but during late 2012, the survey conducted a pilot Health Measures at Home Study with 130 NHANES participant volunteers to assess the feasibility of interviewers collecting physical measures in the home (52). Glycated hemoglobin, HDL cholesterol, and total cholesterol were measured from dried blood spots.
Obtaining sufficient specimen volumes for urine biomarkers is less problematic, and 1–5 mL of urine/test is usually acceptable. Starting with NHANES 2015–2016, urine has been collected from children 3–5 y of age, whereas in previous survey cycles the collection age started at age 6 y. When urine-based biomarkers are used to assess intake or exposure, collecting a 24-h urine specimen is generally considered the gold standard (53). However, NHANES has traditionally only collected spot urine samples during the MEC visit. The usefulness of spot urine samples to assess population iodine status has been accepted by a WHO committee (54); however, the population status of other nutrients, such as sodium, was first assessed through a calibration study (55). Urine sodium data from spot samples can be used to compare population means over time; however, the interpretation of the tail percentiles is more biased than 24-h urine sodium data (55). Starting with NHANES 2009–2010, the collection of urine flow rate (volume of urine sample collected divided by total time between the time of the previous urine void and the time of the urine sample collection) was introduced for the spot urine specimen collection in the MEC to allow a better estimation of the mass of the biomarker excreted in a 24-h period (56). Starting in 2010, NHANES also collected a second spot urine sample in addition to the MEC collection (first morning void urine sample collected at the participant’s home within 10 d of the MEC examination) to enable the assessment of persistent microalbuminuria in the US population by measuring the albumin-to-creatinine ratio (57). This second urine specimen allows adjustment for day-to-day variation. During 2014, NHANES collected, for the first time, 24-h urine specimens to assess sodium excretion as a biomarker of sodium intake and several other nutritional and environmental variables (58).
Sometimes it is beneficial to make use of stored specimens from NHANES. This applies to situations when the nutritional status needs to be assessed for a time period in the past, when certain variables are necessary to interpret the biomarker of interest but are only available on previous NHANES survey cycles, or when an urgent public health need arises that can be more quickly addressed with already available specimens. When possible, NHANES collects and stores additional sample aliquots for future research. Stored serum specimens are available for NHANES III (1988–1994), and stored serum, plasma, and urine specimens are available for the continuous NHANES 1999+. Stored specimens are only available for future research if the NHANES participant consented to this use of his or her specimens. Guidelines for NHANES stored biological specimens are available on the NHANES website (59).
Specimen collection, processing, and storage issues—lessons learned.
To facilitate operations in the MEC and to reduce potential sources of error and variability, the CDC Nutritional Biomarkers Laboratory provided the MEC laboratory with accurately weighed aliquots of meta-phosphoric acid for the vitamin C assay and ascorbic acid for the RBC folate assay. These aliquots were dissolved in water on the day of use to generate stabilized serum for vitamin C measurement and whole-blood hemolysates for folate measurement.
Every attempt should be made to save specimen volume for future NHANES research. This can be achieved by performing >1 test from the same vial. Such vial sharing can be logistically feasible when multiple tests are conducted in the same laboratory and the analytes of interest are stable during repeated freezing and thawing of the specimen. Examples of vial sharing in NHANES 2011–2014 are the analysis of serum vitamin B-12 and MMA from 1 vial and of serum 25(OH)D and FAs from another vial. Examples of nationally representative data that were generated from stored NHANES specimens as a result of an urgent public health need are serum aflatoxin-albumin adducts (1999–2000), plasma cis-FAs (2003–2004), and plasma trans-FAs (1999–2000).
Laboratory measurements.
The Centers for Medicare and Medicaid Services regulates all laboratory testing (except for research) performed in humans in the United States through the Clinical Laboratory Improvement Amendments (CLIA) (60). Laboratories analyzing human biological specimens for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment, or the assessment of human health, must be CLIA-certified. NHANES requires the use of CLIA-certified laboratories for results reported to NHANES participants, but also prefers CLIA-certified laboratories for nonreported results. The majority of nutritional biomarker tests in NHANES fall under the category of high-complexity testing because they are mostly manual or semi-automated procedures with multiple steps in sample or reagent preparation and operator intervention is generally required during the analytical process. Tests conducted on fully automated clinical analyzers with the use of commercial test kits fall under the moderate-complexity testing category (e.g., serum vitamin B-12, ferritin, sTfR). The laboratory requirements for CLIA certification cover personnel standards (education, training, responsibilities of staff at various levels), patient test management (as defined in the test procedure manual, through standard operating procedures, and the organization’s policies and procedures manual), quality assurance (program designed to monitor and evaluate the ongoing and overall quality of the total testing process), QC (written procedures to verify the validity of results), proficiency testing (PT; participation in an official or alternative PT program), inspection (unannounced or announced every 2 y), and enforcement. Proper documentation, continuous staff training to achieve and maintain competency for a test, and remedial actions to correct current and to avoid future problems are central to compliance with CLIA regulations. For example, the yearly competency evaluation of an analyst covers 6 areas: observation of test performance, recording of test results, review of test results, performance of instrument maintenance, performance with “blind” QC, and problem-solving skills (60).
Extensive online documentation is available for the nutritional biomarker tests used in NHANES (61), and researchers often use that information to set up the test of interest in their laboratory. To ensure that an up-to-date laboratory protocol is implemented, it is best to check the most recent NHANES cycle in which the test was performed. At the end of each analytical procedure manual, researchers can also find relevant method performance information for a survey cycle, such as the summary statistics for each QC pool and a Shewhart plot showing QC pool performance for that survey.
NHANES requires laboratories to submit results in a standardized format to the NHANES program staff responsible for data collection. Nutritional biomarker results with clinical relevance (e.g., serum and RBC folate, serum vitamin B-12, serum ferritin, low or high concentrations of serum vitamin A, low concentrations of serum vitamin C) are reported to the NHANES participants for follow-up with their health care provider. Some reportable analytes have to be measured by the laboratory and reported to NHANES program staff within 21 d of sample collection for an early report of findings. Other reportable analytes have to be included on the final report of findings within 8–12 wk of sample collection. This is especially challenging for chromatographic assays that require multistep sample preparation, analyses that use complex instrumentation, and data review including chromatographic peak integrations. Although the 1970s and 1980s were dominated by immunoassays, NHANES 1988–1994 used a few chromatography-based assays to measure vitamin C as well as fat-soluble vitamins and micronutrients. The continuous NHANES 1999+ integrated progressively more HPLC- and GC-based assays, often coupled to tandem MS to achieve a high degree of selectivity and specificity (Table 3). The continuous evolution of and improvement in methods over time and the flexibility of NHANES to use the best and most suitable method available at the time are major goals of NHANES. However, NHANES needs to ensure that appropriate method crossover studies are conducted to allow comparability of data over time.
Laboratory measurements—lessons learned.
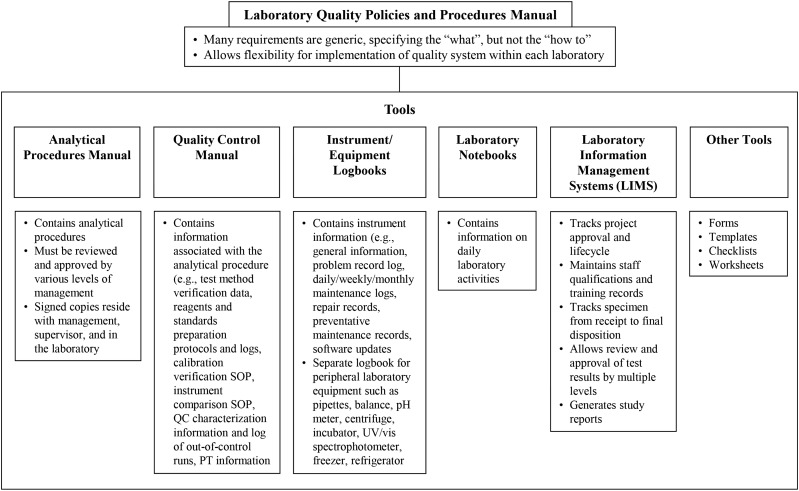
The CDC Nutritional Biomarkers Laboratory is part of the larger environmental health laboratory in the Division of Laboratory Sciences, which conducts a wide array of biomonitoring testing related to human health assessment. The Division of Laboratory Sciences has a comprehensive quality systems architecture anchored in a laboratory policies and procedures manual that is accompanied by various tools to ensure the generation of high-quality data (Figure 1). Requirements for quality assurance are summarized in Table 5.
FIGURE 1.
Tools used to ensure the quality of laboratory data with an example from the CDC Division of Laboratory Sciences Policies and Procedures Manual. pH, potential of hydrogen; PT, proficiency testing; QC, quality control; SOP, standard operating procedures; UV/vis, UV/visible detection.
TABLE 5.
Requirements for laboratory quality assurance
| Quality assurance component | Steps taken to ensure the quality of laboratory measurements |
| Method validation | Validate a new laboratory test for key parameters such as trueness, precision, sensitivity, specificity, reportable range, and reference range. |
| Subject the final method to ruggedness testing to determine how much the accuracy of the method varies with changes in 5 method parameters that are important for that method. | |
| For updated or modified methods, carry out a split-sample analysis by the new and old method by using a minimum of 30 specimens that span the range of concentrations of interest. | |
| Evaluate method comparability by reviewing a correlation plot and Bland-Altman difference plot, as well as conducting regression analysis. | |
| Method verification | Calibration verification is required ≥2 times/y, unless each analytical run contains a multilevel (minimum 5-point) calibration curve. Calibration verification has to be performed after any change in the analytical procedure, which is likely to make a nontrivial difference in sample results. When available, certified reference materials are used for calibration verification. In lieu of that, a different lot of calibrators can be used or peer-to-peer sample exchanges can be conducted. |
| Proficiency testing is also required ≥2 times/y with a minimum of 5 samples/round. Proficiency testing samples are to be handled and analyzed the same as patient samples, and ≥80% of results have to be acceptable. Document remedial actions for unacceptable results. | |
| Instrument equivalency | If method is carried out on >1 instrument, document instrument equivalence ≥2 times/y by analyzing a minimum of 5 specimens spanning the reportable range on both instruments. |
| Instrument verification | Conduct instrument verification in the form of function checks by following the manufacturer’s recommendations, best practices, and sound scientific judgment. |
| Quality audits | The Quality Assurance Officer randomly and periodically surveys patient test management (e.g., completeness of test reports, ability to accurately regenerate test reports, barred access to test result databases without proper authorization), quality control (e.g., appropriate corrective actions and documentation of out-of-control conditions, proficiency testing results, instrument equivalence testing), and personnel records (e.g., documentation of personnel training and competency assessment). |
To manage timely analysis, review, and reporting of clinically relevant reportable results, it is helpful if the laboratory has redundancy in instrumentation and personnel, particularly for complex chromatographic assays for which instrument troubleshooting and repair may take weeks or longer. Good communication between the laboratory and NHANES is essential to keep the survey program staff abreast of potential delays and of method changes requiring crossover studies or the comparison of participant distributions pre- and post-method change.
Data release.
For the continuous NHANES 1999+, nutritional biomarker data, related documentation, and the laboratory method file are released for every 2-y survey cycle, with the first publicly available files after 9 mo of the 2-y cycle. If data are available for only 1 survey year, they cannot be publicly released due to disclosure reasons and are only available through the National Center for Health Statistics’ Research Data Center (62). Certain variables may be available only through the Research Data Center because of potentially confidential information (e.g., specific geographical data). A detailed analytical note is prepared for each data release, and data users are strongly encouraged to carefully review this documentation because it contains important information with regard to the data set, its comparability to previous data sets, and specific concerns and recommendations for data analysis and interpretation. Some NHANES data have been removed after the initial data release because of method issues or to adjust the data after reanalysis with newer methods.
A frequent question of data users is how laboratory data were treated when concentrations were less than the LOD. When nutritional biomarker data are prepared for public release, results that are less than the LOD are replaced with a “fill” value that represents the LOD divided by the square root of 2 (LOD/1.414). This approach has been shown to be a good approximation of the central tendency of results that are less than the LOD (63). In their data analysis, researchers can choose different approaches to substitute results that are less than the LOD. The LOD value for each nutritional biomarker and test can be found in the analytical note that accompanies that data set (in the more recent survey cycles) and in the analytical procedure manual (for all survey cycles). For tests with many results that are less than the LOD, the distribution of concentrations must be considered when doing statistical analysis.
Data release—lessons learned.
Examples of restricted data sets only accessible through the Research Data Center to avoid the risk of disclosure are serum 25(OH)D and urine iodine for NHANES 2000, urine electrolytes (sodium, potassium, and chloride) in spot urine samples from NHANES 2010, and urine iodine and electrolytes in 24-h specimens from NHANES 2014.
The recent re-release of HPLC–tandem MS–standardized 25(OH)D data for NHANES 1988–2006 was one of the most complicated data releases for NHANES (64). The original DiaSorin radioassay data were released in 2010, and NHANES re-released QC-harmonized 25(OH)D data for NHANES 2003–2006 to correct periodic assay shifts during that time period. Although these old data sets are still available on the public access NHANES website, the new HPLC–tandem MS–standardized 25(OH)D data are prominently featured on the public access NHANES website. A detailed analytical note and a comprehensive National Health Statistics Report that documents the complex process of arriving at the final regression equations used to adjust the DiaSorin radioassay data to HPLC–tandem MS–equivalent units have been published (64, 65).
Interpretation of nutritional biomarkers in NHANES
Once nutritional biomarker data have been released for an NHANES cycle, various scientific and logistical considerations are needed with regard to data interpretation. As mentioned earlier, the analytical note accompanying the data release provides important information relevant to interpretation; there also have been several expert panel reports on the interpretation of NHANES nutritional biomarker data, such as for folate, vitamin A, zinc, and iron biomarker data from NHANES II (18–21) and for folate data from NHANES III (22).
Biomarker interpretation—scientific considerations.
Scientific issues for biomarker interpretation mainly address methodologic and statistical issues of data analysis (Table 4). Furthermore, it is important to distinguish between nutritional biomarkers that can only be interpreted on a population basis compared with those that can be interpreted clinically for each NHANES participant as well as on a population basis. NHANES data are often used for individual assessments, particularly in correlation and regression analyses and in epidemiologic follow-up studies. In these cases, misclassification can become an important concern.
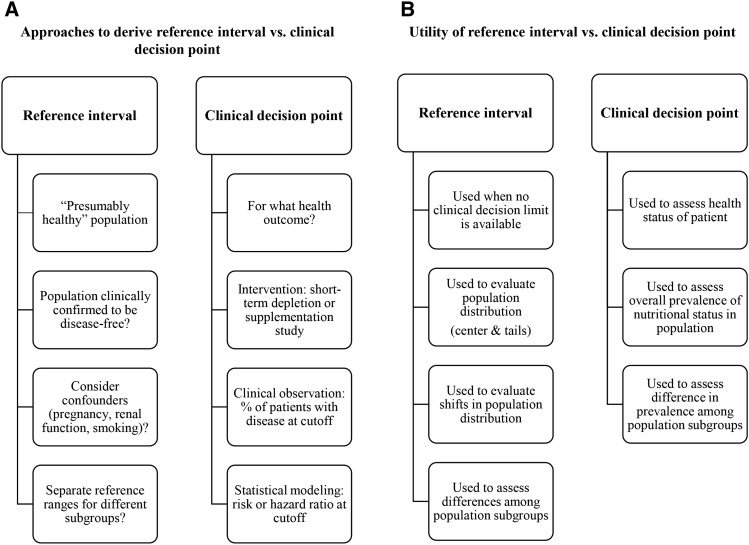
The most important tools in the interpretation of nutritional biomarker data are reference intervals and clinical decision points. These 2 concepts, although not the same, are often discussed in the scientific literature interchangeably. According to the Clinical Laboratory Standards Institute “Harmonized Terminology Database” (66), reference intervals (or reference ranges) represent “the range of test values expected for a designated population of individuals” they are mostly statistically derived (i.e., by using specific percentiles in the population distribution). On the other hand, clinical decision points (critical values) are “decision limits determined on the basis of scientific and/or medical knowledge, often based on a medical condition” (i.e., values used for diagnosis and treatment). We summarized approaches to derive reference intervals and clinical decision points and the utility of these tools (Figure 2). A recent review article discussed common issues related to cutoffs and reference limits for nutritional biomarkers (67). Because clinical decision points are health-based, it is important to specify the health outcome when using a particular cutoff. The most important cutoffs for nutritional biomarkers measured in NHANES are summarized in Table 6.
FIGURE 2.
Approaches to derive reference intervals and clinical decision points (A) and the utility of these tools (B).
TABLE 6.
Commonly used cutoffs for nutritional biomarkers measured in NHANES1
| Nutritional biomarker and matrix | Cutoff (reference) | Unit | Age or population | Interpretation | Comment |
| Folate, total | |||||
| Serum | <7 (26) | nmol/L | All ages | Risk of megaloblastic anemia | Assay used for cutoff was traditional microbiological assay |
| RBC | <305 (26) | nmol/L | |||
| Serum | <10 (68) | nmol/L | All ages | Possible deficiency based on elevated metabolic marker tHcy | Assay used for cutoff was BioRad radioassay |
| RBC | <340 (68) | nmol/L | |||
| RBC | <906 (35) | nmol/L | WRA | Insufficiency based on elevated neural tube defect risk | Assay used for cutoff was updated microbiological assay using folic acid calibrator |
| PLP | |||||
| Serum | <20 (26) | nmol/L | All ages | Low status; basis for Estimated Average Requirement | Cutoff may overestimate vitamin B-6 requirement for health maintenance of more than half the group |
| Vitamin B-12 | |||||
| Serum | <74 (69) | pmol/L | All ages | Deficient | Suggested criteria based on combination of vitamin B-12 with metabolic markers MMA and tHcy (70) |
| <148 (26) | pmol/L | All ages | Moderately low | “Clinical” deficiency: vitamin B-12 low (<148 pmol/L), often very low (<74 pmol/L) and metabolic abnormalities present, often severe (MMA >1000 nmol/L, tHcy >50 μmol/L) | |
| 148–222 (26) | pmol/L | All ages | Low normal | “Subclinical” deficiency: vitamin B-12 low (<148 pmol/L) or low normal (185–258 pmol/L) and ≥1 metabolic abnormality present, usually mild (MMA 300–800 nmol/L, tHcy 15–25 μmol/L) | |
| tHcy | |||||
| Plasma | >12–14 (26) | μmol/L | All ages | Low folate, vitamin B-2, vitamin B-6, or vitamin B-12 status | May need separate cutoffs by age or sex; impaired renal function is a confounder (increased tHcy) |
| MMA | |||||
| Plasma | >271 (26, 71) or >376 (71) | nmol/L | All ages | Low vitamin B-12 status | Statistically derived (2 or 3 SDs); may need separate cutoffs by age; impaired renal function is a confounder (increased MMA) |
| Vitamin C | |||||
| Serum | <11.4 (72) | μmol/L | All ages | Clinical deficiency | Risk of scurvy |
| 11.4–23 (72) | μmol/L | All ages | Low status | ||
| Vitamin A | |||||
| Serum | <0.70 (27) | μmol/L | All ages | Risk of deficiency in population | Prevalence of low serum retinol to define public health problem: 2–9% (mild), 10–19% (moderate), ≥20% (severe) (73); inflammation is a confounder (decreased serum retinol) |
| Vitamin E | |||||
| Serum | <14 (72) | μmol/L | All ages | Risk of deficiency | |
| 25(OH)D | |||||
| Serum | <30 (74) | nmol/L | All ages | Risk of deficiency | Cutoffs developed by using radioassay are in use with HPLC-tandem MS and other methods; new cutoffs may need to be developed |
| 30 to <50 (74) | nmol/L | All ages | Risk of insufficiency | ||
| <40 (74) | nmol/L | All ages | Risk of inadequate intake | ||
| >125 (74) | nmol/L | All ages | Risk of excess | ||
| Ferritin | |||||
| Serum | <12 (75) | μg/L | 1–5 y | Depleted iron stores | Inflammation is a confounder (increased serum ferritin) |
| <15 (75) | μg/L | >5 y | |||
| >150 (75) | μg/L | Women | Risk of iron overload | Other indicators should be included in clinical evaluation | |
| >200 (75) | μg/L | Men | |||
| sTfR | |||||
| Serum | >6.0 (76) | mg/L | 1–5 y | Functional iron deficiency | Statistically derived (97.5th percentile) from NHANES 2003–2010; assay-specific cutoffs |
| >5.33 (76) | mg/L | WRA2 |
MMA, methylmalonic acid; PLP, pyridoxal-5′-phosphate; sTfR, soluble transferrin receptor; tHcy, total homocysteine; WRA, women of reproductive age (12–49 y); 25(OH)D, 25-hydroxyvitamin D.
Nonpregnant women.
The variability in reference intervals derived from different studies can be explained by differences in how the intervals were defined, but also by differences in the laboratory methods, particularly for nutritional biomarkers in which measurements have not been standardized. When defining reference intervals, investigators often poorly describe the population characteristics (e.g., age ranges, exclusion and inclusion criteria) and they use different statistical approaches. Small research studies often use the mean ± 2 SDs or mean ± 3 SDs to define a “normal range,” depending on whether they opt for more false positives (higher sensitivity) or false negatives (higher specificity). When using this approach, it is important to verify that the data are normally distributed and, if not, to transform the data to normality before using parametric statistics. Population surveys generally use percentiles to define a “normal range,” most commonly as the central 95% reference interval (2.5th to 97.5th percentiles). Sometimes 1-sided percentiles are used (5th or 95th percentile). In some cases, an asymmetrical location of the reference interval or another size [central 90% reference interval (5th to 95th percentiles) or central 80% reference interval (10th to 90th percentiles)] could be more appropriate. Ideally, laboratories throughout a homogeneous population area should use the same reference intervals to ensure consistent and comparable patient and population evaluation. If the assay bias is less than one-quarter of the sum of within- and between-person variation, this has been shown to be achievable (77). However, the difficult question is how to assess assay bias when validated reference points for laboratory assays are missing.
Sample size is another important consideration when data from population surveys are used to interpret the status of population subgroups. A full sample of a 2-y NHANES survey cycle consists of ∼9000 participants of all ages. Although this represents many individuals, the sample size rapidly reduces to ∼250 and ∼100 participants, respectively, when a 2-level (e.g., age and sex) or a 3-level (e.g., age, sex, and race/ethnicity) stratification is applied to the data. Depending on what tail percentiles are of interest, the above sample size may be too small to produce a robust estimate. For example, ≥112 persons have to be represented to allow estimation of the 10th and 90th percentiles, 224 persons for the 5th and 95th percentiles, and 448 persons for the 2.5th and 97.5th percentiles, if an average survey design effect of 1.4 is assumed (78). Therefore, if the distribution of concentrations is different in population subgroups, necessitating separate central 95% reference intervals for different subgroups, the 2.5th and 97.5th percentiles can often only be generated when data from multiple survey periods are combined (e.g., 4 or 6 y of NHANES data).
Biomarker interpretation—lessons learned.
A prime example of confusion in the scientific community with regard to the interpretation of cutoffs is folate status. The risk of folate deficiency based on megaloblastic anemia has a different cutoff and interpretation than does the risk of possible deficiency based on increasing tHcy as a metabolic indicator, or insufficiency based on an elevated risk of neural tube defects (8, 79). Although the first 2 stages of folate deficiency are applicable to persons of all ages, the risk of neural tube defects can only be assessed on a population level for women of reproductive age with the use of RBC folate data (35). Furthermore, the cutoffs for these different stages of folate deficiency have been derived differently (experimental compared with epidemiologic data) by using different laboratory methods that do not provide comparable results (microbiological assay compared with BioRad radioassay), a point that is often neglected. If the assay used to derive the cutoff is not comparable to the assay used to generate the study data, either the cutoff or the study data need to be adjusted to avoid an over- or underestimation of the prevalence (79).
Another example of a cutoff issue is urine iodine. Data can only be used to interpret the population iodine status by comparing the median urine iodine concentration with the WHO categories representing insufficiency, sufficiency, and excess (54). It is not appropriate to calculate the prevalence below or above each WHO category level.
Biomarker interpretation—logistic considerations.
Logistic issues pertaining to biomarker interpretation address the quality and validity of the data (Table 4). To date, a considerable number of certified reference materials for nutritional biomarkers exist (5). The use of commercial QC materials with assigned values for nutritional biomarkers should not be confused with the use of certified reference materials available from metrologic agencies such as the National Institute of Standards and Technology (NIST). There are continued issues with the comparability of data across methods and laboratories (80). A major source of information for method comparability comes from PT programs. These programs often modify the testing materials (e.g., addition of preservatives or stabilizers, supplementation with nonnative forms of the analyte, use of animal blood or outdated human blood from blood banks), which sometimes makes the materials noncommutable for certain assays (i.e., the assay responds differently to the modified material than with native material) (80). Method comparability information derived from PT programs thus needs to be interpreted with caution.
As part of the internal laboratory quality assurance system, having data from ≥2, preferably 3, “bench” QC pools in each analytical batch is essential to be able to interpret the quality of study data. If the QC samples were treated the same way as the unknown study samples, the QC data can shed light on issues with assay precision, bias, and long-term assay stability. The inclusion of so-called blind QC pools (suggested rate of 1 blind QC in 20 unknown study samples) in addition to the above-mentioned “bench” QC pools is an additional tool that helps to detect assay problems (e.g., shifts, sample mix-up errors). The identity of the “blind” QC sample is concealed to the analyst either by having the same physical appearance as NHANES participant samples or by being part of several “blind” QC pools with similar analyte concentrations, making it difficult to know which pool has been selected (open-label blind QC).
NHANES also uses “field-split” participant samples as part of an external quality assurance system to detect clerical errors and method or analyst problems. For 1.5–2.9% of eligible (aged ≥12 y) participant samples, the MEC generates 2 identical sample aliquots with different participant IDs, which appear like samples from different participants to the laboratory. The mean imprecision CV derived from these field-split samples is compared with the laboratory internal “bench” QC imprecision CV and field-split samples with discrepant results are brought to the attention of the laboratory for further investigation.
NHANES 1999+ is now continuous, and method or instrument changes occur inevitably with improvement in methods or changes in manufacturers’ reagents or instruments. Sometimes the same assay is maintained, but it undergoes shifts or fluctuations. In each of these cases, well-designed crossover studies to “bridge” data from different time periods are essential to derive regression equations and to mathematically adjust the data. The types of regressions used for crossover studies are numerous and need to be tested by using the NHANES participant distributions. The distributions may need to be transformed for normality before applying the regression. When trending data over a long time, there can be multiple crossover studies that have associated regressions and combined regression errors. Sometimes a crossover study needs to be conducted in a short period of time, when the method may be biased high or low, and does not reflect the method performance over a longer period of time. In addition, in less than ideal cases, the crossover study may cover a more limited concentration range than that observed in NHANES participants. Thus, the crossover regression may not match expected participant distributions pre- and postmethod change. When possible, forward (new method as dependent variable) and backward (old method as dependent variable) regressions should be reported for the crossover study.
Biomarker interpretation—lessons learned.
A classic example of a field error that occurs during specimen preparation in the MEC and leads to unusable laboratory data was for serum vitamin C as part of NHANES I (13). At that time, a small amount of ascorbic acid was added to the serum folate vial to enhance the stability of folate. However, the open serum vitamin C vial was next to the serum folate vial, leading to cross-contamination with ascorbic acid and making the serum vitamin C data uninterpretable. This was an important lesson that showed how critical the preanalytical phase is as part of the overall process of generating reliable laboratory results.
Over the past few years, the CDC Nutritional Biomarkers Laboratory has migrated for most serum-based tests from concealed blind QC to open-label blind QC pools. This has several advantages: pools can be shared across multiple tests and multiple studies, making it easier to maintain a larger number of pools to cover the expected concentration range; the analyst can incorporate the open-label blind QC vials into the analytical run by using coded vials that have been preselected by the supervisor; and the coded vials can be used for other purposes, such as troubleshooting the assay or in-house PT when no formal PT program is available.
The 2010 harmonization of serum 25(OH)D data generated with the DiaSorin radioassay relied on long-term QC information from the laboratory, which was used retrospectively to correct periodic assay shifts during certain years between 2003 and 2006 (23). Because the same 5 QC pools were used over several years in each analytical run, pre- and postassay shift data were used to generate regression equations that were then used to adjust the NHANES participant data generated during the assay shifts. Similarly, serum vitamin B-12 data in NHANES 2013–2014 were adjusted for a periodic shift of the Roche electrochemiluminescence assay with the use of the same approach as described for 25(OH)D (81). These examples emphasize the importance of the laboratory analyzing their in-house QC materials as part of each analytical run to document and, if necessary, correct fluctuations of commercial assays that are often due to lot-to-lot variations or calibration shifts.
A fair number of methodologic crossover studies to “bridge” nutritional biomarker data from different time periods have been conducted as part of the continuous NHANES 1999+ (Supplemental Table 2). Two examples of extensive and well-documented method crossover studies are vitamin D and folate (65, 82–85). These crossover studies resulted in several important lessons learned (Table 7). Most important, if the data had not been adjusted, folate and vitamin D status would have been interpreted incorrectly. By properly adjusting the data generated with the old method to be as equivalent as possible to the data generated with the new method, the assessment of long-term trends was possible (83, 85). An example in which the crossover regression did not match expected participant distributions pre- and postmethod change was for serum ferritin during NHANES 2003–2004. During 2003, the BioRad radioassay was used, whereas an immunoturbidimetric assay on the Roche Hitachi 912 clinical analyzer was used during 2004 after the discontinuation of the BioRad radioassay assay. The Roche assay produced higher serum ferritin results. A crossover study showed overall good correlation between the 2 methods; however, the correlation was poor at low ferritin concentrations. In this case, the BioRad radioassay data were adjusted to match the Roche data by comparing percentile values of the 2 participant distributions and deriving a regression equation (86). The correlation for this regression was much improved compared with that seen for the crossover study, especially at low ferritin concentrations.
TABLE 7.
Lessons learned from the folate and vitamin D NHANES crossover studies1
| Topic | Lessons learned |
| Folate | Use of HPLC-tandem MS method and comparison to international reference materials revealed systematic bias for BioRad radioassay |
| BioRad radioassay was stable over time and precise (<5% CV) and it showed a high correlation to the microbiological assay; this led to robust regression equation used to adjust data | |
| Several regression models were evaluated to find the best fit for the data, paying particular attention at the tails of the distribution where prevalence for low or high folate status is derived | |
| Relation between BioRad radioassay and microbiological assay varied by matrix | |
| Trending data over time needs to be based on adjusted assay data | |
| Vitamin D | DiaSorin radioassay shifted over time, masking the ability to monitor “true” population trends over time |
| Separate regression equations linking the DiaSorin radioassay and HPLC-tandem MS method had to be derived for different time periods | |
| Several regression models were evaluated to find the best fit for the data, paying particular attention at the tails of the distribution where prevalence for low or high vitamin D status is derived | |
| Use of in-house QC materials in each analytical run provided information on method shifts; this was used initially to QC-adjust the data and thus “smooth out” method shifts | |
| Trending data over time needs to be based on adjusted assay data |
QC, quality control.
Uses of NHANES nutritional biomarker data
The main rationale for the inclusion of nutritional biomarkers in NHANES is the need to obtain nationally representative data to generate normative data for the US population (11). Specific uses of NHANES data are to assess a potential public health problem [e.g., iron deficiency in women and children (41–43, 46, 76)], to monitor the impact of nutritional interventions [e.g., introduction of folic acid fortification of cereal-grain products in 1998 (85), “ban” on trans-FAs in foods in 2004 (87)], to address potential emerging scientific or public health focus areas [e.g., vitamin D deficiency (83), reduction in sodium intake (56, 88)], and to allow the translation of research findings from intervention studies and more narrowly focused epidemiologic studies to broader public health applications. Another important use of NHANES data is by researchers from other countries who compare nutritional biomarker data from their country-specific nutrition survey with data obtained for the US population (89–91).
Nutritional biomarker data are continuously published in the peer-reviewed literature. An overview of 50 y of NHANES contribution to public health includes some key examples that use nutritional biomarkers (92). To provide researchers with easy access to descriptive data on the vast collection of nutritional biomarkers measured as part of the continuous NHANES 1999+, the CDC Nutritional Biomarkers Laboratory developed the National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population (Nutrition Report) (33). This report is a serial publication that provides ongoing assessment of the population’s nutritional status in a consistent format to allow for comparisons across demographic subgroups and biomarkers. The Second Nutrition Report was released in 2012 and contains data on 58 biochemical indicators measured as part of NHANES 2003–2006 (93). The Third Nutrition Report will contain data for >80 biochemical indicators measured as part of NHANES 1999–2010 and is currently in production. A collection of articles that used the NHANES 2003–2006 data studied the associations between sociodemographic, lifestyle, and physiologic covariates and nutritional biomarkers to show patterns of influential covariates for different classes of nutrients (94–99).
Future nutritional biomarker challenges in NHANES
One of the biggest challenges for the future of nutritional biomarkers is the need to improve the comparability of analytical methods. This requires the availability of reference materials that have been certified by NIST or other metrologic agencies by using validated high-order reference measurement procedures. As a result of collaborative efforts between different scientific and public health agencies such as the NIH, NIST, and CDC, a considerable number of reference materials have already been developed, but there is a growing need for more materials, particularly in the area of folate research.
The past 10 y have shown a shift away from immuno-based assays to LC–tandem MS (e.g., folate and vitamin D), which provides greater specificity and selectivity. Because many vitamins are found in the human body in >1 form, assessing patterns of these vitamin forms by LC–tandem MS may provide further insight into metabolism or reveal important clues with regard to health associations. However, the measurement of multiple vitamin forms also presents challenges, such as the need for authentic, well-characterized primary standards and associated isotopically labeled internal standards for each form, the increasing complexity of an assay that needs to be validated and maintained for multiple instead of just one compound, and the need to measure all biologically active forms of the vitamin to allow the calculation of a sum variable that represents the total bioactive concentration. As was recently shown with MeFox, an oxidation product of 5-methyltetrahydrofolate, it may be difficult to determine whether a breakdown product occurs in vivo or in vitro and thus whether it should be included into the sum variable (100). Furthermore, these more sophisticated methods are not universally available, especially in the clinical setting. This highlights the need for comparison studies among commonly used methods and newer methods.
Future emphasis will likely be placed on the relation of nutritional biomarkers with genetic background. For example, folate and tHcy concentrations and polymorphisms of genes coding for the folate pathway enzyme 5,10-methylenetetrahydrofolate reductase were examined by using NHANES III data, before folic acid fortification of flour in the United States (101). Several of these polymorphisms are common; they vary by race/ethnicity, influence folate metabolism, and ultimately affect disease risk. NHANES data provided an opportunity to assess the frequencies of the polymorphisms and to study some of these associations.
Another area of research that could pose future challenges is the question of whether nutritional biomarker concentrations should be adjusted for within-person variability when estimating the prevalence of a condition in a population. To conduct such an adjustment, replicate measures in at least a subgroup of the studied population and algorithms designed specifically for this purpose are needed. The adjustment is intended to reflect an individual's ongoing average biomarker concentration over several weeks or longer, taking into account day-to-day variations. For groups, it typically results in a narrower distribution, which provides a more accurate estimate of prevalence of inadequacy or excess than does a population distribution based only on a single measurement in a given individual. This was shown for biomarkers of iron status with the use of data from the Hispanic Health and Nutrition Examination Survey (102).
Conclusions
This review covers a broad range of examples and lessons learned from the nutritional biomarkers component of NHANES, with a special focus on the continuous NHANES 1999+. We have learned a tremendous amount since NHANES I and, although some mistakes have been made along the way, the lessons learned and attention to detail have resulted in high-quality data that have withstood the intense review by scientists and proved invaluable for public health and nutrition policy uses.
Acknowledgments
CMP designed the overall research project, wrote the initial draft, and had primary responsibility for all content; DAL, RLS, CLJ, and EAY contributed “lessons learned” examples and provided feedback for modification to the initial draft of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CLIA, Clinical Laboratory Improvement Amendments; LOD, limit of detection; MEC, Mobile Examination Center; MMA, methylmalonic acid; NIST, National Institute of Standards and Technology; PLP, pyridoxal-5′-phosphate; PT, proficiency testing; QC, quality control; sTfR, soluble transferrin receptor; tHcy, total homocysteine; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Potischman N, Freudenheim JL. Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr 2003;133:873S–4S. [DOI] [PubMed] [Google Scholar]
- 2.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr 2003;133(Suppl):875S–80S. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JR. Methodologic and statistical considerations regarding use of biomarkers of nutritional exposure in epidemiology. J Nutr 2003;133(Suppl):881S–7S. [DOI] [PubMed] [Google Scholar]
- 4.Blanck HM, Bowman BA, Cooper GR, Myers GL, Miller DT. Laboratory issues: use of nutritional biomarkers. J Nutr 2003;133:888S–94S. [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer CM, Schleicher RL, Caldwell KL. Biochemical indices. In: Caballero B, editor. Enyclopedia of human nutrition. Vol. 1, 3rd ed Waltham (MA): Academic Press; 2013. p. 156–74. [Google Scholar]
- 6.Elmadfa I, Meyer AL. Developing suitable methods of nutritional status assessment: a continuous challenge. Adv Nutr 2014;5:590S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, Raiten DJ. Biomarkers of nutrition for development—iodine review. J Nutr 2014;144(Suppl):1322S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF III, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, et al. Biomarkers of nutrition for development—folate review. J Nutr 2015;145(Suppl):1636S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ. Biomarkers of nutrition for development—zinc review. J Nutr 2016;146(Suppl):858S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanumihardjo SA, Russel RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development—vitamin A review. J Nutr 2016;146(Suppl):1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yetley E, Johnson C. Nutritional applications of the Health and Nutrition Examination Surveys (HANES). Annu Rev Nutr 1987;7:441–63. [DOI] [PubMed] [Google Scholar]
- 12.Wright JD, Borrud LG, McDowell MA, Wang C-Y, Radimer K, Johnson CL. Nutrition assessment in the National Health and Nutrition Examination Survey 1999–2002. J Am Diet Assoc 2007;107:822–9. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C, Lacher D, Lewis B, McQuillan G. Challenges in collecting survey-based biomarker and genetic data: the NHANES experience. In: Proceedings of the Ninth Conference on Health Survey Research Methods. Hyattsville (MD): National Center for Health Statistics; 2010. [Google Scholar]
- 14.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr 2016;7:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woteki CE. Integrated NHANES: uses in national policy. J Nutr 2003;133(Suppl):582S–4S. [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health, Education, and Welfare. Ten-state nutrition survey 1968–1970. Washington (DC): US Government Printing Office; 1972. DHEW Publication No. (HSM) 72-8134.
- 17.Porter DV. A National Nutrition Monitoring System: background and legislative mandate. Washington (DC): Science Policy Research; 1991. Congressional Research Service Report No.: 91–785.
- 18.Senti FR, Pilch SM. Assessment of folate data from the second National Health and Nutrition Survey (NHANES II). J Nutr 1985;115:1398–402. [DOI] [PubMed] [Google Scholar]
- 19.Pilch SM. Analysis of vitamin A data from the health and nutrition examination surveys. J Nutr 1987;117:636–40. [DOI] [PubMed] [Google Scholar]
- 20.Pilch SM, Senti FR. Analysis of zinc data from the second National Health and Nutrition Examination Survey (NHANES II). J Nutr 1985;115:1393–7. [DOI] [PubMed] [Google Scholar]
- 21.Expert Scientific Working Group. Summary of a report on assessment of the iron nutritional status of the United States population. Am J Clin Nutr 1985;42:1318–30. [DOI] [PubMed] [Google Scholar]
- 22.Raiten DJ, Fisher KD. Assessment of folate methodology used in the third National Health and Nutrition Survey (NHANES 1988–1994). J Nutr 1995;125(Suppl):1371S–98S. [DOI] [PubMed] [Google Scholar]
- 23.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr 2010;140(Suppl):2030S–45S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94(Suppl):303S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin RL, Durazo-Arvizu RA, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94(Suppl):313S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 27.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicone, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 28.Sakakeeny L, Roubenoff R, Obin M, Fontes JD, Benjamin EJ, Bujanover Y, Jacques PF, Selhub J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J Nutr 2012;142:1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009;90:1252–63. [DOI] [PubMed] [Google Scholar]
- 30.Tinker SC, Hamner HC, Berry RJ, Bailey LB, Pfeiffer CM. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defect Res A Clin Mol Teratol 2012;94:749–55. [DOI] [PubMed] [Google Scholar]
- 31.Young DS. Effects of preanalytical variables on clinical laboratory tests. 2nd ed Washington (DC): AACC Press; 1997. [Google Scholar]
- 32.Lacher DA, Hughes JP, Carroll MD. Biological variation of laboratory analytes based on the 1999–2002 National Health and Nutrition Examination Survey. Natl Health Stat Report 2010; 21:1–7. [PubMed] [Google Scholar]
- 33.Pfeiffer CM, Sternberg MR, Schleicher RL, Haynes MBH, Rybak ME, Pirkle JL. The CDC’s second national report on biochemical indicators of diet and nutrition in the US population is a valuable tool for researchers and policy makers to assess nutritional status. J Nutr 2013;143(Suppl):938S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Healthy People 2020. Maternal, Infant, and Child Health (MICH-15). Reduce the proportion of women of childbearing potential who have lower red blood cell folate concentrations [Internet] [cited 2016 Jun 19]. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives.
- 35.WHO. Guideline: optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects [Internet]. Geneva (Switzerland): World Health Organization; 2015. [cited 2016 Nov 4]. Available from: http://apps.who.int/iris/bitstream/10665/161988/1/9789241549042_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 36.Tinker SC, Hamner HC, Qi YP, Crider KS. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res A Clin Mol Teratol 2015;103:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmel R. Biomarkers of cobalamin (vitamin B12) status in the epidemiologic setting: a critival overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94(Suppl):348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B12 nutritional status in the United States using plasma methylmalonic acid and serum vitamin B12. Am J Clin Nutr 2011;94:552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey RL, Durazo-Arvizu RA, Carmel R, Green R, Pfeiffer CM, Sempos CT, Carriquiry A, Yetley EA. Modeling a methylmalonic acid-derived change point for serum vitamin B-12 for adults in the National Health and Nutrition Examination Survey. Am J Clin Nutr 2013;98:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Looker AC, Gunter EW, Johnson CL. Methods to assess iron status in various NHANES surveys. Nutr Rev 1995;53:246–54. [DOI] [PubMed] [Google Scholar]
- 41.Pilch S. Assessment of iron nutritional status of the U.S. population based on data collected in the second National Health and Nutrition Examination Survey, 1976–1980. Bethesda (MD): Federation of American Societies for Experimental Biology; 1984. [Google Scholar]
- 42.Looker AC, Dallman PR, Carroll M, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 43.CDC. Iron deficiency—United States, 1999–2000. MMWR Morb Mortal Wkly Rep 2002;51:897–9. [PubMed] [Google Scholar]
- 44.Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 1990;75:1870–6. [PubMed] [Google Scholar]
- 45.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 46.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr 2009;89:1334–42. [DOI] [PubMed] [Google Scholar]
- 47.Fraser CG, Hyltoft Petersen P, Libeer JC, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem 1997;34:8–12. [DOI] [PubMed] [Google Scholar]
- 48.Powers CD, Chen H, Sternberg MR, Momin SS, Schleicher SL. Stability of 24 plasma fatty acids stored at -20°C. Clin Chem 2011;57 S10:B-140. [Google Scholar]
- 49.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey: 2005–2006 data documentation, codebook, and frequencies. Vitamin B6 (Vit_B6_D) [Internet] [cited 2016 Jun 23]. Available from: http://wwwn.cdc.gov/Nchs/Nhanes/2005–2006/VIT_B6_D.htm.
- 50.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 1 2013;56:1–37 [cited 2016 Apr 11]. Available from: http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. [PubMed] [Google Scholar]
- 51.CDC, National Center for Health Statistics. NHANES: MEC laboratory procedures manual, January 2013 [Internet] [cited 2016 Jun 19]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_MEC_Laboratory_Procedures_Manual.pdf.
- 52.Gindi RM, Zipf G, Galinsky AM, Miller IM, Nwankwo T, Terry AL. Comparison of in-home collection of physical measurements and biospecimens with collection in a standardized setting: the health measures at home study. Vital Health Stat 2 2014;2(164):1–16. [PubMed] [Google Scholar]
- 53.Institute of Medicine. Strategies to reduce sodium intake in the United States. Washington (DC): National Academies Press; 2010. [Google Scholar]
- 54. WHO. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva (Switzerland): WHO; 2007.
- 55.Cogswell ME, Wang CY, Chen TC, Pfeiffer CM, Elliott P, Gillespie CD, Carriquiry AL, Sempos CT, Liu K, Perrine CG, et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am J Clin Nutr 2013;98:1502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CDC, National Center for Health Statistics. NHANES: laboratory procedures manual, July 2009 [Internet] [cited 2016 Jun 19]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/lab.pdf.
- 57.CDC, National Center for Health Statistics. NHANES: home urine collection, 2009 [Internet] [cited 2016 Nov 4]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/HomeUrine.pdf.
- 58.CDC, National Center for Health Statistics. NHANES: 2013–2014 data documentation, codebook, and frequencies. Electrolytes—24-hour urine–first collection (U1LT_H_R) [Internet] [cited 2016 Nov 4]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/limited_access/U1LT_H_R.htm.
- 59.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey Biospecimen Program [Internet] [cited 2016 Nov 4]. Available from: http://www.cdc.gov/nchs/nhanes/biospecimens/biospecimens.htm.
- 60.Centers for Medicare and Medicaid Services. Clinical Laboratory Improvement Amendments (CLIA) [Internet] [cited 2016 May 31]. Available from: https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html.
- 61.CDC, National Center for Health Statistics. NHANES: 2013–2014 lab methods [Internet] [cited 2016 Nov 4]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2013–2014/lab_methods_13_14.htm.
- 62.CDC, National Center for Health Statistics. NHANES data release and access policy [Internet] [cited 2016 Jun 23]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_release_policy.pdf.
- 63.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5:46–51. [Google Scholar]
- 64.CDC, National Center for Health Statistics. Analytical note for 25-hydroxyvitamin D data analysis using NHANES III (1988–1994), NHANES 2001–2006, and NHANES 2007–2010 October 2015 [Internet] [cited 2016 May 31]. Available from: http://wwwn.cdc.gov/Nchs/Nhanes/VitaminD/AnalyticalNote.aspx?h=http://wwwn.cdc.gov/Nchs/Nhanes/20092010/VID_F.htm&t=VID_F%20Doc.
- 65.Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, et al. A method bridging study for serum 25-hydroxyvitamin D: predicting standardized liquid chromatography-tandem mass spectrometry assay-equivalent concentrations from historical RIA data. Hyattsville (MD): National Center for Health Statistics; 2016. National Health Statistics Report No.: 93. [PubMed] [Google Scholar]
- 66.Clinical Laboratory Standards Institute. Harmonized terminology database [Internet] [cited 2016 Apr 13]. Available from: http://htd.clsi.org/.
- 67.Raghavan R, Ashour FS, Bailey R. A review of cutoffs for nutritional biomarkers. Adv Nutr 2016;7:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 2008;29:S238–44. [DOI] [PubMed] [Google Scholar]
- 69.Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 1990. [Google Scholar]
- 70.Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology Am Soc Hematol Educ Program 2003;2003:62–81. [DOI] [PubMed] [Google Scholar]
- 71.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol 1990;34:90–8. [DOI] [PubMed] [Google Scholar]
- 72.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 73.WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Vitamin and Mineral Nutrition Information System [Internet] [cited 2016 Jun 25] Geneva (Switzerland): WHO; 2011. Available from: http://www.who.int/vmnis/indicators/retinol.pdf.
- 74.Institute of Medicine, Food and Nutrition Board. Dietary Reference Iintakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [Google Scholar]
- 75.WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System [Internet] [cited 2016 Jun 25]. Geneva (Switzerland): WHO; 2011. Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf.
- 76.Mei Z, Pfeiffer CM, Looker AC, Flores-Ayala RC, Lacher DA, Mirel LB, Grummer-Strawn LM. Serum soluble transferrin receptor concentrations in US preschool children and non-pregnant women of childbearing age from the National Health and Nutrition Examination Survey 2003–2010. Clin Chim Acta 2012;413:1479–84. [DOI] [PubMed] [Google Scholar]
- 77.Gowans EMS, Hyltoft Petersen P, Blaabjerg O, Horder M. Analytical goals for the acceptance of common reference intervals for laboratories throughout a geographical area. Scand J Clin Lab Invest 1988;48:757–64. [DOI] [PubMed] [Google Scholar]
- 78.CDC. NHANES analytic guidelines, the third National Health and Nutrition Examination Survey, NHANES III (1988–94) [Internet] [cited 2015 Sep 4]. Hyattsville (MD): National Center for Health Statistics; October 1996. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf.
- 79.Pfeiffer CM, Sternberg MR, Hamner HC, Crider KS, Lacher DA, Rogers LM, Bailey RL, Yetley EA. Applying inappropriate cutpoints leads to misinterpretation of folate status in the US population. Am J Clin Nutr 2016;104:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bock JL, Eckfeldt JH. Advances in standardization of laboratory measurement procedures: implications for measuring biomarkers of folate and vitamin B-12 status in NHANES. Am J Clin Nutr 2011;94(Suppl):332S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.CDC, National Center for Health Statistics. NHANES: 2013–2014 data documentation, codebook, and frequencies. Vitamin B12 (VITB12_H) [Internet] [cited 2016 Sep 9]. Available from: http://wwwn.cdc.gov/Nchs/Nhanes/2013–2014/VITB12_H.htm.
- 82.Pfeiffer CM, Schleicher RL, Johnson CL, Coates PM. Assessing vitamin status in large population surveys by measuring biomarkers and dietary intake—two case studies: folate and vitamin D. Food Nutr Res 2012;56:5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr 2016;104:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfeiffer CM, Hughes JP, Durazo-Arvizu RA, Lacher DA, Sempos CT, Zhang M, Yetley EA, Johnson CL. Changes in measurement procedure from a radioassay to a microbiologic assay necessitates adjustment of serum and RBC folate concentrations in the U.S. population from the NHANES 1988–2010. J Nutr 2012;142:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, Yetley EA, Rader JI, Sempos CT, Johnson CL. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr 2012;142:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.CDC, National Center for Health Statistics. NHANES: 2003–2004 data documentation, codebook, and frequencies. Ferritin & transferrin receptor (L06TFR_C) [Internet] [cited 2016 Jun 19]. Available from: http://wwwn.cdc.gov/Nchs/Nhanes/2003–2004/L06TFR_C.htm.
- 87.Vesper HW, Kuiper HC, Mirel LB, Johnson CL, Pirkle JL. Levels of plasma trans-fatty acids in non-Hispanic white adults in the United States in 2000 and 2009. JAMA 2012;307:562–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfeiffer CM, Hughes JP, Cogswell ME, Burt VL, Lacher DA, LaVoie DJ, Rabinowitz DJ, Johnson CL, Pirkle JL. Urine sodium excretion increased slightly among U.S. adults between 1988 and 2010. J Nutr 2014;144:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colapinto CK, Tremblay MS, Aufreiter S, Bushnik T, Pfeiffer CM, O’Connor DL. The direction of the difference between Canadian and American red blood cell folate concentrations is dependent on the assay method employed: a comparison of the Canadian Health Measures Survey and National Health and Nutrition Examination Survey. Br J Nutr 2014;112:1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradbury KE, Williams SM, Mann JI, Brown RC, Parnell W, Skeaff CM. Estimation of serum and erythrocyte folate concentrations in the New Zealand adult population within a background of voluntary folic acid fortification. J Nutr 2014;144:68–74. [DOI] [PubMed] [Google Scholar]
- 91.Hopkins SM, Gibney MJ, Nugent AP, McNulty H, Molloy AM, Scott JM, Flynn A, Strain JJ, Ward M, Walton J, et al. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B-12 in Irish adults. Am J Clin Nutr 2015;101:1163–72. [DOI] [PubMed] [Google Scholar]
- 92.CDC, National Center for Health Statistics. NHANES: 50 years of contribution to public health [Internet] [cited 2016 Aug 8]. Available from: http://www.cdc.gov/nchs/video/nhanes50th_contributions/nhanes_contributions.htm.
- 93.CDC, National Center for Environmental Health. Second national report on biochemical indicators of diet and nutrition in the U.S. population 2012 [Internet] [cited 2016 Jun 23]. Available from: http://wwwn.cdc.gov/nutritionreport.
- 94.Pfeiffer CM, Sternberg MR, Schleicher RL, Rybak ME. Dietary supplement use and smoking were strongly related to biomarkers of water-soluble vitamin status after adjusting for socioeconomic and lifestyle factors in a representative sample of US adults. J Nutr 2013;143(Suppl):957S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schleicher RL, Sternberg MR, Pfeiffer CM. Race-ethnicity is a strong correlate of circulating fat-soluble nutrient concentrations in a representative sample of the U.S. population. J Nutr 2013;143(Suppl):966S–76S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfeiffer CM, Sternberg MR, Caldwell KL, Pan Y. Race-ethnicity is related to biomarkers of iron and iodine status after adjusting for sociodemographic and lifestyle factors in a representative sample of US adults. J Nutr 2013;143(Suppl):977S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rybak ME, Sternberg MR, Pfeiffer CM. Sociodemographic and lifestyle variables are compound- and class-specific correlates of urine phytoestrogen concentrations in the US population. J Nutr 2013;143(Suppl):986S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vesper HW, Sternberg MR, Pfeiffer CM. Among ten sociodemographic and lifestyle factors, smoking was most strongly associated with biomarkers of acrylamide exposure in a representative sample of the US population. J Nutr 2013;143(Suppl):995S–1000S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haynes BMH, Pfeiffer CM, Sternberg MR, Schleicher RL. Selected pre-analytical and physiological factors are weakly to moderately associated with twenty-nine biomarkers of diet and nutrition, NHANES 2003–2006. J Nutr 2013;143(Suppl):1001S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pfeiffer CM, Sternberg MR, Fazili Z, Lacher DA, Zhang M, Johnson CL, Hamner HC, Bailey RL, Rader JI, Yamini S, et al. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2. Br J Nutr 2015;113:1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Q-H, Botto LD, Gallagher M, Friedman JM, Sanders CL, Koontz D, Nikolova S, Erickson JD, Steinberg K. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: findings from the third National Health and Nutrition Examination Survey DNA Bank. Am J Clin Nutr 2008;88:232–46. [DOI] [PubMed] [Google Scholar]
- 102.Looker AC, Sempos CT, Liu K, Johnson CL, Gunter EW. Within-person variance in biochemical indicators of iron status: effects on prevalence estimates. Am J Clin Nutr 1990;52:541–7. [DOI] [PubMed] [Google Scholar]