Abstract
Studies have shown that supplementation of infant formula with bovine milk fat globule membranes (MFGMs) may substantially narrow the gap in health outcomes between formula-fed and breastfed infants. In one study, consumption of a formula supplemented with a lipid-rich MFGM concentrate between 2 and 6 mo of age improved cognitive performance at 24 wk of age. In another study, a formula supplemented with a protein-rich MFGM concentrate given between 2 and 6 mo of age improved cognitive performance at 12 mo of age, decreased infectious morbidity until 6 mo of age, and yielded serum cholesterol concentrations closer to those of breastfed infants. A third study that assessed the safety of supplementing infant formula with a lipid-rich or a protein-rich MFGM concentrate found a noninferior weight gain for both groups compared with a nonsupplemented formula. In this study, there was an increased risk of eczema in the protein-rich group, but no serious adverse events. Infant formulas with supplemental MFGMs have been launched on the market in several countries. However, the evidence base must still be considered quite limited. Based on 3 randomized controlled trials that are not comparable, the intervention seems safe, but there is not enough evidence for a general recommendation on which MFGM fraction to use and at what concentration as formula supplement for a given outcome.
Keywords: MFGM, infant formula, neurodevelopment, cognition, infection, otitis, cholesterol
Introduction
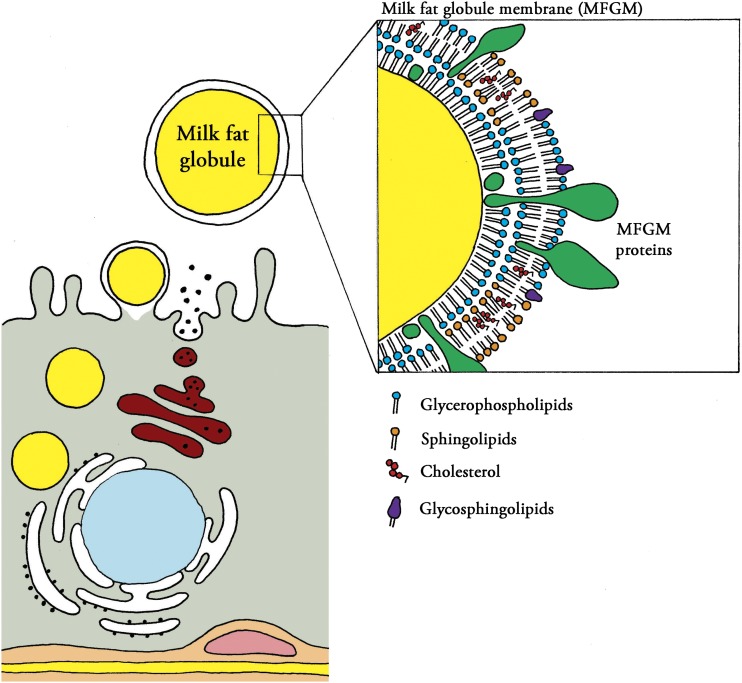
A growing number of studies have reported health benefits from oral supplementation with bovine milk fat globule membranes (MFGMs)5 in humans of different age groups, including infants (1). The MFGM is the membrane surrounding the secreted fat droplets in milk. It is released by a unique mechanism in the mammary gland and is composed of a triple phospholipid and cholesterol layer with incorporated proteins and glycoproteins (2). A schematic drawing of fat release and MFGM composition is shown in Figure 1. The genes regulating MFGM synthesis are conserved across species, indicating a functional benefit of the fraction in milk (3), even if the exact MFGM composition varies between species. Phospholipids make up 30% of the total milk lipid weight, with sphingomyelins, phosphatidylcholines, and phosphatidylethanolamines contributing approximately one-third each (2). Almost all milk gangliosides are also located in the MFGM (4). The proteome of the human MFGM includes 191 different identified proteins, including mucins, butyrophilin, lactoferrin, and lactadherin (5). In a study of bovine MFGM-rich fractions, 244 proteins were identified in a whey protein concentrate and 133 in a buttermilk protein concentrate (6). MFGM is also rich in sialic acid as part of the gangliosides (4) and glycosylated proteins.
FIGURE 1.
Schematic picture of the release of the milk fat globule and composition of the milk fat globule membrane. Illustration by Erik Domellöf. Reproduced from reference 1 with permission.
Breastfed infants have a higher intake of MFGM components than do their formula-fed counterparts because, traditionally, the MFGM fraction is discarded with the milk fat when this is replaced by vegetable oils as the fat source in infant formulas. After advances in dairy technology, bovine MFGM concentrates are now commercially available and possible to use as a supplement to foods including infant formulas. The MFGM has emerged as a complex factor that may explain some of the differences observed between breastfed and formula-fed infants.
Current Status of Knowledge
Possible biological effects of MFGM components.
The rationale for MFGM supplementation of infant formula is based on a growing number of studies that show health benefits of individual components of the MFGM, mostly in animal models, and a limited number of human studies of supplementation with different MFGM fractions.
Dietary gangliosides (7), sialic acid (8), and sphingomyelin (9) have been shown to be important for optimal brain development and function in different animal models. However, it should be noted that some of these models are disease models or models with inhibited de novo synthesis, which is far from supplementing a healthy infant. In a small study in premature infants with a birth weight <1500 g, infants receiving formula with high sphingomyelin content (20% compared with 13% of all phospholipids in milk) to cover shortages of breast milk performed better at a neurobehavioral follow-up between 6 and 18 mo corrected age (10). Furthermore, oral sphingomyelin increased maturation of the intestine in rats (11). Gangliosides have also been suggested as playing an important role in the development of intestinal microbiota composition and gut immunity, and, consequently, in the defense against infections (12). Other components of MFGMs are also involved in the defense against infections. For example, the glycoproteins butyrophilin, lactadherin, and mucins (13, 14) all have antimicrobial effects, the lipid fraction of bovine MFGMs has an antiviral effect in vitro (15), and oligosaccharides inhibit the binding of several bacteria (including pneumococci) to the mucosa (14). Both lipid and protein components of MFGMs have anticancer effects in vitro (13).
Dietary MFGMs in adults and children after weaning.
In human adults, most studies of dietary MFGMs have focused on outcomes related to different risk factors for cardiovascular disease. Buttermilk consumption reduced serum cholesterol concentrations, primarily through the inhibition of the intestinal absorption of cholesterol (16) and decreased blood pressure and angiotensin I–converting enzyme in moderately hypercholesterolemic adults (17). In contrast to milk fat without MFGMs, milk fat enclosed by MFGMs did not impair the lipoprotein profile in overweight adults (18). The addition of an MFGM fraction to a high–saturated fat meal reduced the postprandial insulinemic and inflammatory response in overweight and obese adults (19). Dietary MFGMs given to healthy adults have also been shown to have positive effects on gastrointestinal symptoms from diarrheagenic Escherichia coli (20) and on muscle strength (21).
A few randomized studies have been published on dietary MFGM supplementation in children after weaning. In a Peruvian study, an MFGM-enriched protein fraction given to healthy, primarily breastfed infants aged 6–11 mo for 6 mo decreased the longitudinal prevalence of diarrhea (3.84% compared with 4.37%, P < 0.05) and the incidence of bloody diarrhea (OR: 0.59; 95% CI: 0.34, 1.02; P = 0.025) (22). In contrast, a daily dose of milk powder supplemented with 2 g of a spray-dried ganglioside concentrate given to infants 8–24 mo of age for 12 wk in India did not affect diarrheal morbidity (23). In a Belgian study, a phospholipid-rich MFGM concentrate given daily for 4 mo to preschool children aged 2.5–6 y decreased behavioral problems and reduced days with fever during the intervention period (24).
Supplementation with MFGMs in term infants in early infancy.
Three randomized controlled trials of MFGM supplementation in infant formula given to term infants during the first 6 mo of life were identified in a search in PubMed performed on 1 September 2016 (Table 1).
TABLE 1.
Double-blind randomized controlled trials evaluating MFGM supplementation in early infancy (identified on 1 September 2016)1
| Site | Number of infants in final analysis of MFGM-supplemented/control groups | Age at intervention | MFGM supplement | Primary outcome2 | Secondary outcomes2 | Reference |
| Indonesia | 29/30 | <8 wk to 6 mo | Complex milk lipids (AnmumInfacare; Fonterra Cooperative Group) | Higher general IQ, hand and eye coordination IQ, and performance IQ on Griffiths scale at 24 wk | Higher serum gangliosides GM3 and GD3 | (25) |
| Sweden | 73/68 | <2 mo to 6 mo | Lacprodan MFGM-10 (Arla Foods Ingredients) | Higher cognitive score on Bayley-III at 12 mo | Lower incidence of otitis media; higher serum cholesterol | (26–28) |
| France and Italy | 47 (MFGM-L) + 52 (MFGM-P)/45 | 14 d to 4 mo | MFGM-L: Lipid-rich MFGM fraction (Fonterra Cooperative Group) | Noninferior weight gain for both groups ≤4 mo | Higher rate of eczema in the MFGM-P group | (29) |
| MFGM-P: Lacprodan MFGM-10 (Arla Foods Ingredients) |
Bayley-III, Bayley Scales of Infant Development, 3rd edition; IQ, intelligence quotient; MFGM, milk fat globule membrane; MFGM-L, lipid-rich milk fat globule membrane fraction; MFGM-P, protein-rich milk fat globule membrane fraction.
For MFGM-supplemented groups in relation to the formula-fed control group.
In a double-blind randomized controlled trial (DBRCT) in Indonesia, Gurnida et al. (25) evaluated the impact on cognitive function of feeding a standard infant formula enriched with bovine milk gangliosides, provided as a complex bovine milk lipid fraction (AnmumInfacare; Fonterra Cooperative Group), in which the ganglioside content was increased from 6 to 9 mg/L, compared with the same formula without enrichment (control group), from 2 to 8 wk (baseline) until 24 wk of age. A total of 70 healthy term infants were randomly assigned to either the ganglioside-supplemented formula (n = 35; 29 completed the study) or the control formula (n = 35; 30 completed the study). A breastfed reference group was also recruited (n = 40; 32 completed the study). The primary outcome was the Griffiths Mental Developmental Scale at 24 wk of age and the secondary outcome was serum ganglioside concentrations. After adjustment for socioeconomic background variables, hand-eye coordination intelligence quotient (IQ) (129.5 compared with 122.0, P = 0.006), performance IQ (131.1 compared with 123.2, P < 0.001), and general IQ (125.4 compared with 120.6, P = 0.041) were higher in the ganglioside-supplemented group than in the control group, and the ganglioside-supplemented group did not differ from the breastfed reference group.
In a DBRCT in Umeå, Sweden, formula-fed healthy term infants were randomly assigned to receive an experimental formula supplemented with a protein-rich MFGM fraction (Lacprodan MFGM-10; Arla Foods Ingredients) (n = 80; 73 completed the study) or a control formula (n = 80; 68 completed the study) from <2 to 6 mo of age. The experimental formula had a lower energy density (60 kcal/100 mL compared with 66 kcal/100 mL) and protein concentration (1.20 g/100 mL compared with 1.27 g/100 mL), and MFGM proteins made up 4% (wt:wt) of the total protein content of the formula. A breastfed reference group was also recruited (n = 80; 72 fulfilled the study). Primary outcomes were weight at 6 mo of age and psychological assessment with the Bayley Scales of Infant Development, 3rd edition. The formula-fed infants regulated their ingested volumes by increasing meal size, resulting in no differences in energy intake, protein intake, blood urea nitrogen, serum insulin, or growth, including body fat percentage, ≤12 mo of age (26, 30). At 12 mo of age, the MFGM-supplemented group received higher mean ± SD scores in the cognitive domain of the Bayley Scales of Infant Development, 3rd edition (105.8 ± 9.2) than did the control group (101.8 ± 8.0, P = 0.008), and did not differ from the breastfed reference group (106.4 ± 9.5, P = 0.73) (26). There were no observed differences in socioeconomic background factors between the MFGM-supplemented and control formula groups. During the intervention, the MFGM-supplemented group had a lower incidence of acute otitis media than the control group (1% compared with 9%, P = 0.034), a lower incidence and longitudinal prevalence of antipyretic use, and lower serum concentrations of IgG against pneumococci after vaccination (27). During the intervention, the MFGM-supplemented group gradually reached higher serum cholesterol concentrations than did the control group, and did not differ from the breastfed reference group at 6 mo of age (28).
In a multicenter noninferiority DBRCT on 199 healthy term infants (149 completed the intervention), Billeaud et al. (29) evaluated the safety of 2 infant formulas enriched with a lipid-rich or a protein-rich bovine MFGM fraction. At 14 d of age, the infants were randomly assigned to receive standard infant formula (control), standard formula enriched with the lipid-rich MFGM (Fonterra Co-operative Group), or standard formula enriched with protein-rich MFGM (Lacprodan MFGM-10; Arla Foods Ingredients) until 4 mo of age. The primary outcome, weight gain, was noninferior in the lipid-rich MFGM and protein-rich MFGM groups compared with in the control group. Among secondary and exploratory outcomes, few between-group differences were observed. Adverse events and morbidity rates were similar across groups, except for a higher rate of eczema in the protein-rich MFGM group (13.9% compared with 1.4% in the lipid-rich MFGM group and 3.5% in the control group, P = 0001). However, the limited number of observations and the lack of a systematic eczema scoring system make this result uncertain. In the Swedish study, no increased risk of rash was observed (31).
Conclusions
Two DBRCTs on MFGM supplementation of infant formula have shown promising effects on neurodevelopment and 1 DBRCT has shown promise with respect to infectious morbidity. The findings are supported by the known effects of individual components of MFGMs on neurodevelopment and protection against infection, mostly based on in vitro and/or animal studies. Furthermore, all 3 identified DBRCTs concluded that the intervention was safe. Even if the results are promising, the scientific base of knowledge must still be considered to be quite limited. One of the studies had a small number of study subjects (25), and the study population in the other study (26) was from a homogenous, highly educated population with high socioeconomic standards. Extrapolations of the results to a general population worldwide must be made with caution. The studies were performed with different MFGM concentrates. Different concentrates have different composition and each product should be tested for efficacy and safety.
It is important that more high-quality randomized controlled trials with formulas supplemented with different types and concentrations of bovine MFGMs including those already used be performed and that results, positive or negative, be published to increase the scientific body of evidence. A systematic scoring system should be included in future studies to assess the occurrence of eczema (32).
MFGM supplementation of infant formula may be an important step toward narrowing the gap between formula-fed and breastfed infants with respect to neurodevelopment, infectious diseases, and cholesterol metabolism. Infant formulas supplemented with bovine MFGMs have already been launched on several markets, but there is not enough evidence for a general recommendation for MFGM supplementation of infant formula.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DBRCT, double-blind randomized controlled trial; IQ, intelligence quotient; MFGM, milk fat globule membrane.
References
- 1.Hernell O, Timby N, Domellöf M, Lönnerdal B. Clinical benefits of milk fat globule membranes for infants and children. J Pediatr 2016;173 Suppl:S60–5. [DOI] [PubMed] [Google Scholar]
- 2.Kanno C. Secretory membranes of the lactating mammary gland. Protoplasma 1990;159:184–208. [Google Scholar]
- 3.German JB. Dietary lipids from an evolutionary perspective: sources, structures and functions. Matern Child Nutr 2011;7 Suppl 2:2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rueda R, Maldonado J, Narbona E, Gil A. Neonatal dietary gangliosides. Early Hum Dev 1998;53 Suppl:S135–47. [DOI] [PubMed] [Google Scholar]
- 5.Liao Y, Alvarado R, Phinney B, Lönnerdal B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J Proteome Res 2011;10:3530–41. [DOI] [PubMed] [Google Scholar]
- 6.Alexy U, Kersting M, Sichert-Hellert W, Manz F, Schoch G. Macronutrient intake of 3- to 36-month-old German infants and children: results of the DONALD Study. Dortmund Nutritional and Anthropometric Longitudinally Designed Study. Ann Nutr Metab 1999;43:14–22. [DOI] [PubMed] [Google Scholar]
- 7.Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P. The role of gangliosides in neurodevelopment. Nutrients 2015;7:3891–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Brand-Miller J. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr 2007;85:561–9. [DOI] [PubMed] [Google Scholar]
- 9.Oshida K, Shimizu T, Takase M, Tamura Y, Yamashiro Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr Res 2003;53:589–93. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Hosozawa M, Kudo N, Yoshikawa N, Hisata K, Shoji H, Shinohara K, Shimizu T. The pilot study: sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev 2013;35:45–52. [DOI] [PubMed] [Google Scholar]
- 11.Motouri M, Matsuyama H, Yamamura J, Tanaka M, Aoe S, Iwanaga T, Kawakami H. Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. J Pediatr Gastroenterol Nutr 2003;36:241–7. [DOI] [PubMed] [Google Scholar]
- 12.Rueda R. The role of dietary gangliosides on immunity and the prevention of infection. Br J Nutr 2007;98 Suppl 1:S68–73. [DOI] [PubMed] [Google Scholar]
- 13.Spitsberg VL. Invited review: bovine milk fat globule membrane as a potential nutraceutical. J Dairy Sci 2005;88:2289–94. [DOI] [PubMed] [Google Scholar]
- 14.Peterson JA, Patton S, Hamosh M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol Neonate 1998;74:143–62. [DOI] [PubMed] [Google Scholar]
- 15.Fuller KL, Kuhlenschmidt TB, Kuhlenschmidt MS, Jimenez-Flores R, Donovan SM. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J Dairy Sci 2013;96:3488–97. [DOI] [PubMed] [Google Scholar]
- 16.Conway V, Couture P, Richard C, Gauthier SF, Pouliot Y, Lamarche B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr Metab Cardiovasc Dis 2013;23:1255–62. [DOI] [PubMed] [Google Scholar]
- 17.Conway V, Couture P, Gauthier S, Pouliot Y, Lamarche B. Effect of buttermilk consumption on blood pressure in moderately hypercholesterolemic men and women. Nutrition 2014;30:116–9. [DOI] [PubMed] [Google Scholar]
- 18.Rosqvist F, Smedman A, Lindmark-Månsson H, Paulsson M, Petrus P, Straniero S, Rudling M, Dahlman I, Riserus U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am J Clin Nutr 2015;102:20–30. [DOI] [PubMed] [Google Scholar]
- 19.Demmer E, Van Loan MD, Rivera N, Rogers TS, Gertz ER, German JB, Smilowitz JT, Zivkovic AM. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J Nutr Sci 2016;5:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ten Bruggencate SJ, Frederiksen PD, Pedersen SM, Floris-Vollenbroek EG, Lucas-van de Bos E, van Hoffen E, Wejse PL. Dietary milk-fat-globule membrane affects resistance to diarrheagenic Escherichia coli in healthy adults in a randomized, placebo-controlled, double-blind study. J Nutr 2016;146:249–55. [DOI] [PubMed] [Google Scholar]
- 21.Soga S, Ota N, Shimotoyodome A. Dietary milk fat globule membrane supplementation combined with regular exercise improves skeletal muscle strength in healthy adults: a randomized double-blind, placebo-controlled, crossover trial. Nutr J 2015;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavaleta N, Kvistgaard AS, Graverholt G, Respicio G, Guija H, Valencia N, Lönnerdal B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J Pediatr Gastroenterol Nutr 2011;53:561–8. [DOI] [PubMed] [Google Scholar]
- 23.Poppitt SD, McGregor RA, Wiessing KR, Goyal VK, Chitkara AJ, Gupta S, Palmano K, Kuhn-Sherlock B, McConnell MA. Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in northern Indian infants. J Pediatr Gastroenterol Nutr 2014;59:167–71. [DOI] [PubMed] [Google Scholar]
- 24.Veereman-Wauters G, Staelens S, Rombaut R, Dewettinck K, Deboutte D, Brummer RJ, Boone M, Le Ruyet P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012;28:749–52. [DOI] [PubMed] [Google Scholar]
- 25.Gurnida DA, Rowan AM, Idjradinata P, Muchtadi D, Sekarwana N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum Dev 2012;88:595–601. [DOI] [PubMed] [Google Scholar]
- 26.Timby N, Domellöf E, Hernell O, Lönnerdal B, Domellöf M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr 2014;99:860–8. [DOI] [PubMed] [Google Scholar]
- 27.Timby N, Hernell O, Vaarala O, Melin M, Lönnerdal B, Domellöf M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr 2015;60:384–9. [DOI] [PubMed] [Google Scholar]
- 28.Timby N, Lönnerdal B, Hernell O, Domellöf M. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr Res 2014;76:394–400. [DOI] [PubMed] [Google Scholar]
- 29.Billeaud C, Puccio G, Saliba E, Guillois B, Vaysse C, Pecquet S, Steenhout P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: a randomized controlled multicenter non-inferiority trial in healthy term infants. Clin Med Insights Pediatr 2014;8:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timby N, Hernell O, Lönnerdal B, Domellöf M. Parental feeding control in relation to feeding mode and growth pattern during early infancy. Acta Paediatr 2014;103:1072–7. [DOI] [PubMed] [Google Scholar]
- 31.Timby N, Domellöf M, Lönnerdal B, Hernell O. Comment on “Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: a randomized controlled multicenter non-inferiority trial in healthy term infants.” Clin Med Insights Pediatr 2015;9:63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, Dohil M, Apfelbacher C, Singh JA, Chalmers J, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800–7. [DOI] [PubMed] [Google Scholar]