Abstract
The association between inflammation and vitamin A (VA) metabolism and status assessment has been documented in multiple studies with animals and humans. The relation between inflammation and carotenoid status is less clear. Nonetheless, it is well known that carotenoids are associated with certain health benefits. Understanding these relations is key to improving health outcomes and mortality risk in infants and young children. Hyporetinolemia, i.e., low serum retinol concentrations, occurs during inflammation, and this can lead to the misdiagnosis of VA deficiency. On the other hand, inflammation causes impaired VA absorption and urinary losses that can precipitate VA deficiency in at-risk groups of children. Many epidemiologic studies have suggested that high dietary carotenoid intake and elevated plasma concentrations are correlated with a decreased risk of several chronic diseases; however, large-scale carotenoid supplementation trials have been unable to confirm the health benefits and in some cases resulted in controversial results. However, it has been documented that dietary carotenoids and retinoids play important roles in innate and acquired immunity and in the body’s response to inflammation. Although animal models have been useful in investigating retinoid effects on developmental immunity, it is more challenging to tease out the effects of carotenoids because of differences in the absorption, kinetics, and metabolism between humans and animal models. The current understanding of the relations between inflammation and retinoid and carotenoid metabolism and status are the topics of this review.
Keywords: biomarkers, cytokines, infection, retinol, retinol-binding protein, sequestration, urinary loss
Introduction
Many factors influence the plasma transport, tissue uptake, and metabolism of vitamin A (VA)9 and carotenoids, including nonmodifiable factors such as age, sex, and genetic predisposition (1–3) and modifiable factors such as smoking, diet, and exercise. Inflammation is a “hard-wired” systemic response to infection or injury but may also be modified by anti-inflammatory hormones, cytokines, and drugs. Inflammation is a component of injury response that is necessary for tissue repair and represents a program of metabolic changes necessary for survival (4), but if the process is prolonged or excessive, it becomes potentially life-threatening. Acute inflammation may be triggered by an infection or a sterile stimulus such as LPS that can be used to induce inflammation experimentally.
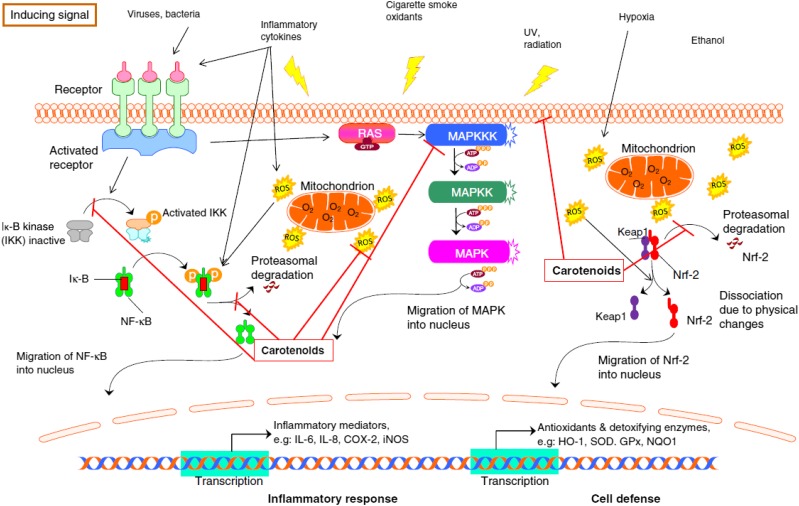
Inflammation (acute or chronic) relies on several mediators that transmit signals in the bloodstream or other fluids. These mediators include a variety of cytokines (e.g., ILs, interferons, chemokines), NO, intercellular adhesion molecules, or other messenger molecules, such as prostaglandins. Many mediators are regulated further upstream by transcription factors. Among those involved in inflammation are NF-κB, which is related to the release of several cytokines (e.g., IL-6, IL-8, TNF-α, IL-1β) and MAPKs (Figure 1). In addition, because of the interactions between oxidative stress and inflammation, nuclear factor (erythroid-derived 2)–like 2 (Nrf2) appears to play a fundamental role, strengthening the body’s antioxidant enzymatic defenses (5), which include superoxide dismutase, glutathione peroxidase, catalase, and heme oxygenase 1.
FIGURE 1.
The current understanding of the role of carotenoids in the mitigation of negative effects of ROS in response to insults. COX-2, cyclooxygenase 2; GPx, glutathione peroxidase; HO-1, heme oxygenase 1; IKK, IκB kinase; iNOS, inducible NO synthase; NQO1, NADPH quinone dehydrogenase 1; Nrf-2, nuclear factor (erythroid-derived 2)–like 2; RAS, proteins with intrinsic GTPase activity involved in cellular signal transduction; ROS, reactive oxygen species; SOD, superoxide dismutase. Reproduced from reference 5 with permission.
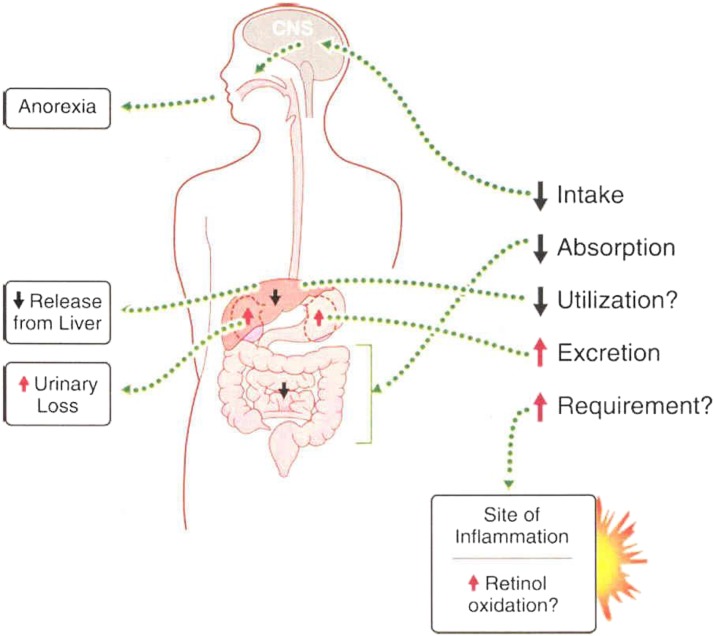
VA intake and metabolism are seriously altered during the acute-phase response (APR) to inflammation (Figure 2) (6). The APR is a systemic metabolic response to infection, trauma, or tissue injury that induces fever, increases the synthesis of inflammatory cytokines, and enhances white blood cell production. The APR is well conserved among vertebrate species (7). This review discusses VA and carotenoid metabolism and biomarkers during inflammation, with a particular emphasis on infants and children in relation to animal models.
FIGURE 2.
Inflammation can negatively affect vitamin A balance through decreased dietary intake, reduced intestinal absorption, and increased urinary excretion. Inflammation may also cause the sequestration of vitamin A in the liver, which leads to hyporetinolemia. Reproduced from reference 6 with permission.
Current Status of Knowledge
Inflammation as a modifier of vitamin A transport and metabolism
Retinol-binding protein (RBP) and transthyretin form the plasma transport complex for retinol; both proteins are chiefly synthesized in the liver (8), the major organ involved in the APR (9). The APR results in dramatic alterations in protein synthesis and energy metabolism (10). A principal characteristic of the APR is the hepatic synthesis and secretion of acute-phase proteins (APPs) that play anti-infective roles and, consequently, function in response to tissue injury after trauma or infection (9). The leading regulators of the APR in hepatocytes are IL-1 and IL-6 (11). The kinetics of APP responses vary; C-reactive protein (CRP) changes most rapidly and increases dramatically in plasma within 8 h of APR induction. Other APPs, such as α1-acid glycoprotein (AGP), increase more slowly and remain elevated during convalescence.
Both RBP and transthyretin R are negative APPs because their concentrations decline during the APR. Retinol binds nearly stoichiometrically to RBP; thus, they are equally affected. The reduction in plasma holo-RBP (RBP bound to retinol) occurs quickly, even before CRP and AGP have reached peak concentrations. The rapidity of the response may be caused by the inherently short half-life of RBP (∼12 h in adults) (12, 13), which must be continuously synthesized to maintain normal holo-RBP concentrations (14). The APR may reduce plasma amino acid concentrations (15), which further inhibits RBP synthesis, a process that is sensitive to protein and calorie malnutrition (16).
Low serum retinol (SR), i.e., hyporetinolemia, has been reported in children and adults in association with acute infections (e.g., measles, malaria, diarrhea, HIV), multiple morbidities (17), and trauma (18). Several studies have provided evidence that retinol and RBP concentrations are inversely correlated with serum concentrations of IL-6, the major regulator of the APR because it induces the gene expression of many APPs (9).
Animal studies of hyporetinolemia during inflammation.
The most common experimental models of inflammation induce the APR with LPS, which is a natural component of the outer wall of gram-negative bacteria that causes sterile inflammation (19). The response to LPS is dose-dependent, wherein lower doses induce an APR and higher doses result in vascular collapse and death. LPS signals through toll-like receptor 4, which triggers signal transduction cascades that result in the activation and nuclear translocation of NF-κB, which induces the release of proinflammatory cytokines TNF-α, IL-1, IL-6, and type 1 interferons (20). LPS acts rapidly, triggering the APR within hours (21). In the liver, IL-6 alone is sufficient to initiate similar changes by signaling through glycoprotein 130 and signal transducer and activator of transcription 3 pathways (11, 21). In rats treated with low-dose LPS sufficient to elevate body temperature but not cause serious lethargy or sickness, SR concentrations reached a nadir between 12 and 24 h, with a reduction ≥50% (22, 23). Serum RBP declined with similar kinetics (22), and although liver RBP synthesis was substantially reduced by 24-h post-LPS treatment, liver retinol concentration was maintained. RBP 4 (Rbp4) mRNA concentrations in the liver were reduced to 50% of the control value by 12 h after LPS treatment. This work provided evidence that inflammation-induced hyporetinolemia is caused by a reduction in liver RBP synthesis that was initiated by the reduced transcription of Rbp4. Similar results were obtained in a rat model of inflammation induced by recombinant human IL-6 that caused a more dramatic and prolonged decline in SR, possibly caused by persistent inflammation (24).
Although RBP was substantially reduced in the liver and kidney, these organs responded differently regarding Rbp4 mRNA. Rbp4 mRNA was reduced in the liver but did not change in the kidney, which is normally between ∼5% and 10% of the liver concentration (8). Because the decrease in Rbp4 mRNA in the liver was coincident with or preceded the decrease in serum RBP, a reduced rate of transcription may account for the hyporetinolemia (22). The reduction in RBP but not its mRNA in the kidney may signify that the reduction in RBP is caused by reduced uptake, low RBP in serum, or the reduced reuptake of RBP after filtration, which occurs normally (25).
The “look-alike” problem.
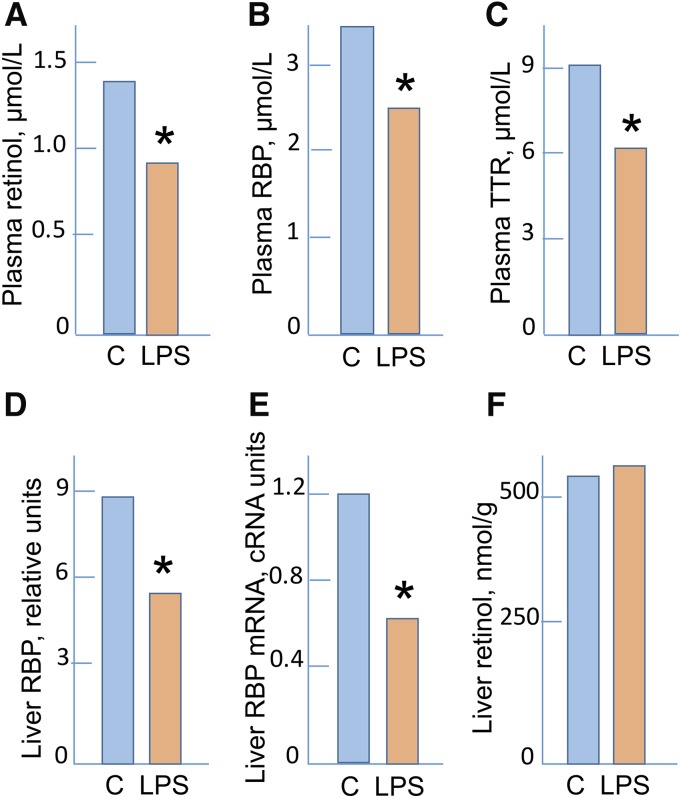
A practical concern is that if SR and RBP concentrations are reduced by inflammation, the results lend a false impression regarding VA status. An experimental illustration is shown in Figure 3 for an animal study in which low SR resulting from dietary restriction was quantitatively similar to hyporetinolemia induced by LPS in animals fed adequate VA. Whereas in experimental settings causality is known, in human settings, in which dietary intake often is uncertain or seasonal, low SR concentrations could easily be attributed to nutritional inadequacy when, in fact, they are caused by inflammation. Although this look-alike problem is now well recognized, the appropriate interventions remain uncertain. One opinion is that low SR, regardless of etiology, signifies that the uptake of retinol by tissues might be restricted, and the low values should receive intervention. Another opinion is that low SR values might be adjustable by measuring markers of inflammation, such as CRP or AGP, with the use of these factors as covariates to assess what the adjusted SR concentrations would be in the absence of inflammation (26). In this approach, inflammation is viewed as an interference to the assessment of whether VA liver stores may or may not be adequate. How one assesses these approaches depends on the question being addressed. In either case, understanding the etiology of low SR is critical for properly addressing the underlying problem.
FIGURE 3.
Plasma retinol (A), RBP (B), and TTR (C) and liver RBP (D), RBP mRNA (E), and retinol (F) in control rats and in rats after the induction of inflammation by the administration of LPS. Data are means, n = 5. Results are for 24 h after the administration of LPS except for panel E, which represents results at 12 h. Data are from reference 22. *Different from C, P < 0.02. C, control group; RBP, retinol-binding protein; TTR, transthyretin.
An NIH-sponsored working group, Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence, reviewed the literature on inflammation and biomarkers of micronutrient status for several micronutrients (27). The review provides useful guidance to clinicians, researchers, and programmatic planners with regard to the impact of inflammation on micronutrient biology and biomarkers. The findings are intended to be integrated into the Biomarkers of Nutrition for Development project (28). Researchers in European countries have conducted a similar review on the impact of inflammation on biomarkers (29).
Redistribution of retinol during the APR.
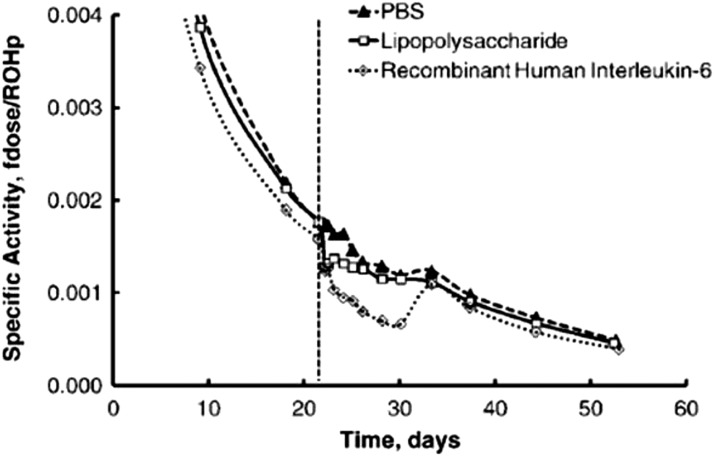
In well-nourished humans, SR concentrations return to normal values during the convalescent/resolution stage of infection. This suggests that the APR involves a redistribution of retinol from plasma to other body compartments, from which it is later able to return to plasma. A redistribution rather than a net loss may also be inferred from the results of animal studies in which the urinary loss of retinol during infection was quantitatively small compared with normal VA turnover (23). In the VA-adequate state, the plasma retinol pool is small compared with the body reserves with which it equilibrates (30). Consequently, determining the “missing plasma retinol” in tissues can be challenging. Nevertheless, the use of [3H]retinol tracer kinetics together with mathematical modeling of the data have shed light on the trafficking of retinol during inflammation (31). In rats in which [3H]retinol was orally administered and time elapsed for equilibration, the induction of inflammation resulted within 1 d in a downward deviation of the plasma [3H]retinol decay curve that was of greater magnitude in recombinant human IL-6-treated rats than in LPS-treated rats. However, during the resolution of the APR (∼10 d), the plasma [3H]retinol curve increased and followed the trajectory established before inflammation induction (Figure 4). Mathematical modeling indicated a 79% reduction in the hepatic mobilization of retinol within 15 h after LPS administration and a 75% reduction by 5.6 h after the IL-6 injection. The results imply that retinol exits plasma transiently during inflammation and accumulates in the liver, but based on its reappearance there is not an appreciable irreversible retinol loss (24). It was hypothesized that inflammation-induced hyporetinolemia reflects a sequestration of plasma retinol that is associated with impaired mobilization of retinol caused by reduced RBP synthesis.
FIGURE 4.
The specific activity, calculated as the fraction of the dose divided by the plasma retinol concentration, in rat plasma during an insult with PBS (n = 2 for 3 and 7 d), LPS (n = 5 for 3 d), or recombinant human IL-6 (n = 5 for 7 d). The dashed line is the time that the insult was initially administered. The model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. fdose; fraction of dose; ROHp; plasma retinol. This research was originally published in the Journal of Lipid Research (31). Gieng SH, Green MH, Green JB, Rosales FJ. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J Lipid Res 2007;48:904–13. ©2007 the American Society for Biochemistry and Molecular Biology. Reproduced from reference 31 with permission.
Inflammation during VA deficiency and repletion.
VA deficiency and inflammation may interact to result in a quantitatively greater reduction in plasma retinol than from either condition alone (23). When marginally VA-deficient rats were supplemented with an oral dose of VA to simulate a relative dose response (RDR) test (32), the increase in plasma retinol after the oral dose in rats with inflammation was less than that of VA-marginal rats without inflammation (23, 33). Thus, LPS-induced inflammation limited but did not completely prevent the ability of VA supplementation to improve plasma retinol concentrations. In addition, the physiologic response of plasma retinol to a VA supplement provided for repletion may be altered qualitatively in an induced state of inflammation, whereas retinyl esters accumulate in the plasma (23). This finding suggests that the hydrolysis of newly absorbed VA, transported as retinyl ester in chylomicra or their remnants, was delayed. Lipoprotein lipase activity is known to be reduced by LPS (34, 35). Consistent with the hypothesized delay in chylomicron clearance, liver retinyl esters were lower in the LPS- and VA-supplemented group (23). Reduced hepatic metabolism has also been suggested by the reduction in the liver of mRNA concentrations for several genes involved in VA uptake, β-carotene and retinol metabolism (e.g., β-carotene oxygenase 1, lecithin retinol acyltransferase and dehydrogenase/reductase 3), and retinoic acid (RA) oxidation (e.g., cytochrome P450 family 26) (36). In addition, studies in other models have shown that liver injury results in the gradual mobilization or loss of retinyl esters from stellate cells, which can eventually lead to liver fibrosis (37). Inflammation also alters the distribution and metabolism of RA, the major active metabolite of VA. When inflammation was induced by low-dose LPS in rats with marginal VA status, [3H]RA uptake and metabolism to polar metabolites were considerably reduced (38, 39).
Infections increase the risk of VA deficiency
Nutritionists have recognized for several decades that infectious diseases can increase the risk of malnutrition in children (40). Diarrhea and lower respiratory tract infections have a particularly pronounced effect on infant growth (41), presumably because of a combination of illness severity, such as pneumonia, and increased stool frequency (e.g., diarrhea is a common childhood illness in regions with inadequate sanitation). Relatively few studies have demonstrated the effects of infection on the risk of specific nutritional deficiencies. With regard to VA, an observational study in Indonesia demonstrated an association between respiratory infections and diarrhea on the increased risk for xerophthalmia, the principal clinical manifestation of VA deficiency (42). Common infections (e.g., chickenpox, respiratory infections) have been implicated in the depletion of liver stores or failure of VA intake to maintain liver stores (43, 44). The observation that common childhood infections increases the risk of VA deficiency is an important public health concern in many areas of the world because deficiency can lead to blindness (45) and death (46).
Infections increase the risk of malnutrition by a variety of mechanisms that were originally described for vitamin B-12 (47) and reviewed for VA (6). Although the specific categories vary among authors, they generally include decreased food intake, impaired nutrient absorption, direct nutrient loss, altered transport to target tissues, and increased metabolic requirements or catabolic losses. Indeed, acute infections during childhood cause decreased food intake, which is often lower with higher illness severity. In community-based studies of generally healthy children, the occurrence of acute respiratory infections decreased caloric intake by 8% relative to periods when children were asymptomatic; the decrease was 11% for children with malaria (48) and 18% with diarrheal illness (49). Measles generally cause a more severe infection, and a study showed a caloric deficit of 75% compared with intake during recovery (50), although the intake during recovery might be slightly higher than normal. Interestingly, a community-based study of infants that quantified breastmilk and food intake showed that although the total energy intake from nonbreastmilk sources was decreased by diarrhea and fever, the intake of breastmilk was not affected (51), offering further benefits for breastfeeding.
Enteric infections can decrease the absorption of many nutrients. Enteric infections damage the intestinal epithelium and decrease the expression of brush-border enzymes such as lactase, as shown in a piglet model of neonatal diarrhea (52). Intestinal barrier damage during mild Ascaris infections of children also decreases lactose absorption, which recovers upon antiworm treatment (53). For VA provided as β-carotene, absorption may be improved by roundworm treatment (54). The absorption of physiologic doses of preformed VA is generally quite high (∼99%) but is lower (70–80%) in children with diarrhea, an Ascaris infection, and nonenteric infections such as pneumonia (55, 56). Although the mechanisms of this absorptive defect are unclear, impaired absorption contributes to an increased risk of VA deficiency in children with low dietary intakes.
After absorption, several infections can cause direct nutrient loss, perhaps from intestinal “leakiness” resulting in protein-losing enteropathy, which occurs with postmeasles diarrhea (57), or by the direct loss of blood, which occurs during hookworm infection and leads to iron-deficiency anemia (58). Considerable amounts of VA can be lost in the urine as a result of proximal tubular dysfunction in the kidney (6). Low-molecular-weight plasma proteins, including RBP, filtered through the glomerulus are normally reabsorbed in the proximal tubule (6). One hospital-based study (59) found that adults with severe infections, such as pneumonia or sepsis, excreted a mean of 223 μg retinol/d (presumably bound to RBP), which is 25% of the RDA for men and 32% for women. In that study, 24% excreted >1 RDA/d, indicating that severe infections could result in substantial urinary losses. (It should be noted that the use of aminoglycoside antibiotics, potentially toxic to kidney tubular epithelium, can be a contributing factor to urinary VA loss). Children with sepsis also excrete substantial VA in the urine, whereas children with pneumonia and diarrhea excrete lower amounts (60). Losses may continue for several days (61) and are associated with a high fever and evidence of kidney tubular dysfunction (e.g., increased urinary concentrations of β2 microglobulin).
During the APR, the decrease in SR concentration caused in part by the decreased mobilization from the liver (31) suggests that retinol availability in peripheral tissues may be diminished. In the eye, the retina is sensitive to low SR concentrations because it expresses stimulated by retinoic acid 6, the cell-surface binding receptor for RBP (62). Clinical and genetic data (6) have suggested that low SR during the APR may decrease retina sensitivity to light, perhaps as a result of decreased availability of retinal for rhodopsin formation. It is not known whether other tissues have specific effects from retinol limitation during the APR (e.g., for producing the RA needed for regulating gene expression). It is possible that limited VA availability in the immune system might be beneficial, perhaps by altering the type of immune response to particular pathogens, because RA is produced by immune cells and directly regulates the differentiation and survival of particular subsets of immune cells, particularly T lymphocytes (63). Carotenoid concentrations may also be lower during the APR (64), but the implications of this lowered concentration are unclear. It is not known whether the decline in carotenoid concentrations is transient, as for retinol, or if it represents “lost” carotenoids from increased catabolism or diminished intakes during illness.
During infection, requirements for some nutrients may increase because of increased utilization or catabolism. The resting metabolic rate is increased during HIV infection (65), which demands increased caloric intake to prevent weight loss at equivalent levels of activity. In addition, classical studies of model infections of human volunteers have shown increased nitrogen loss as a result of protein catabolism (66). With regard to VA during the APR, consistent evidence of increased metabolism or catabolism has not been demonstrated. However, tissue carotenoid pools decrease during the induction of an APR in chickens (67), suggesting catabolic losses. Interestingly, tissue carotenoid pools are more resistant to change at low dietary carotenoid concentrations. Similar data are not available from humans, but oxidative metabolism induced during inflammation might result in decreased tissue carotenoids. Consequently, altered metabolism of provitamin A carotenoids during infection could adversely affect VA status.
Assessment of VA status during inflammation
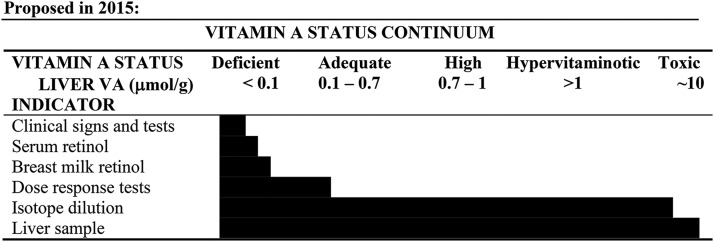
Methods used to assess VA status are often affected by the APR. The most common methods used to assess VA status include the clinical evaluation of eye symptoms, serum and breastmilk retinol concentrations, dose-response tests, and isotope dilution assays (Figure 5) (68). This section describes how each method may be affected during an APR.
FIGURE 5.
The current understanding of the relation of total liver reserves (expressed as μmol retinol/g liver) and the dynamic working range of the listed biomarker. Clinical signs of deficiency and depressed serum retinol concentrations occur during severe vitamin A depletion. Breastmilk retinol is a unique indicator for lactating women and can be extrapolated to infants. The dose-response tests offer more information on liver reserves than serum retinol concentrations alone. Isotope dilution is the only indirect biomarker that has utility along the entire continuum of reserves compared with a liver biopsy, which has limited feasibility but is considered the gold standard. Reproduced from reference 68 with permission.
Biological and functional clinical tests.
A close association exists between xerophthalmia (e.g., Bitot’s spots, nightblindness) and the APR assessed with the use of AGP and CRP (69, 70). Supplementation with VA increased plasma retinol concentrations during deficiency but did not affect AGP and CRP concentrations in children with and without xerophthalmia (70). Furthermore, corneal involvement in children who have an acute infection, such as measles, is commonly observed (71). Measles is a known precipitating factor for the development of xerophthalmia in children with VA deficiency (72). In fact, the WHO still recommends giving 2 high-dose VA supplements 24 h apart for children in developing countries who have measles. VA supplements reduce the number of measles-related deaths, replenish body stores, and prevent xerophthalmia (73).
SR concentrations.
Both RBP and transthyretin are depressed during infection and inflammation. In the kidney, RBP becomes uncoupled from transthyretin, and during a high fever holo-RBP is not reabsorbed but excreted in the urine (6). SR concentrations are a static measure usually performed on previously collected samples. The release of plasma retinol bound to RBP from the liver is homeostatically controlled, and therefore it does not change over a wide range of liver reserves (74). As a biomarker of VA status, it may not respond to interventions (68, 75). In rats, SR was actually higher after VA withdrawal in the group that received marginal daily VA (76) than in the groups that received more than the daily requirement (76, 77). In children, SR concentrations did not differ after treatment with high-dose supplements (78) or after feeding with high β-carotene–biofortified maize (79, 80).
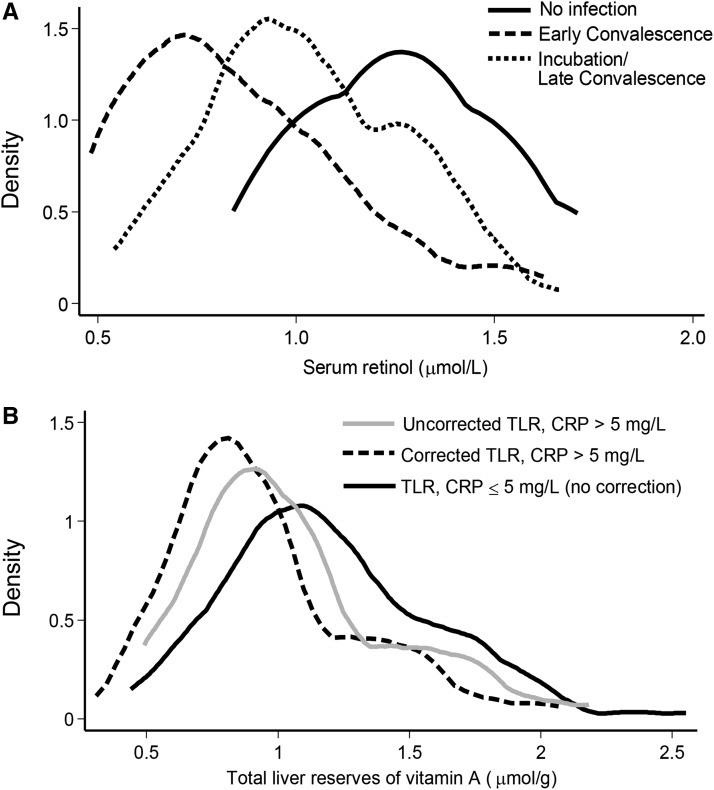
Measuring CRP and AGP is recommended in surveys to evaluate the impact of inflammation on SR concentrations (79, 81). In Zambian children, negative shifts in SR concentrations became apparent based on the degree of inflammation (68, 81) (Figure 6A). By correcting for inflammation to lower the threshold of acceptable SR concentrations (82), the percentage of individuals with SR concentrations <0.7 μmol/L decreased from 17% to 3% (81).
FIGURE 6.
The impact of elevated acute-phase proteins on serum retinol concentrations in children (A). The curve on the far right reflects the distribution in children without inflammation. The middle curve reflects children who are in late convalescence (only AGP elevated), and the curve on the far left demonstrates serum retinol concentrations during early convalescence when both CRP and AGP are elevated. (B) The influence of adding an extra correction factor in the calculations for total liver retinol reserves in Zambian children with elevated CRP. If nonsymptomatic inflammation affects absorption, the relation would be the opposite; i.e., TLRs would be overestimated. Kernel density estimations produce a smoothed curve of data. AGP, α1-acid glycoprotein; CRP, C-reactive protein; TLR, total liver reserve. Panel A is reproduced from reference 68 with permission.
Breastmilk retinol concentrations.
The degree to which inflammation affects breastmilk retinol concentrations is not currently known to our knowledge. One report from rural Zambian mothers did not find an association in a preliminary analysis (83). Considering that breastmilk retinol is derived from RBP and chylomicron delivery, this topic needs further evaluation. In rats, chylomicra contribute ∼40% of milk retinol (84), and in lactating sows 13–26% of tracer doses were either detected in milk or measured in nursing offspring (85, 86). It is likely that RBP-delivered retinol accounts for 60–85% of breastmilk retinol in humans. If SR is depressed during inflammation, breastmilk concentrations may become depressed, especially in women who have low dietary intakes.
Dose-response tests.
Two dose-response tests are available for assessing status in surveys and intervention studies. The RDR and modified RDR (MRDR) tests are based on the principle of accumulated RBP during VA depletion released after a challenge dose (87). The major difference is that the RDR uses retinyl ester, whereas the MRDR uses 3,4-didehydroretinyl acetate (68). Attributes of dose-response tests are that they only require HPLC for analysis and provide more information than SR regarding liver VA reserves (Figure 5). The RDR uses 2 blood samples—at baseline and 5 h after dosage—and may be affected by inflammation, as observed in Peruvian children (88). The MRDR measures newly ingested didehydroretinol in a single blood sample and is therefore distinguishable from circulating retinol. Three separate trials in infants and children did not detect an influence of the APR on MRDR values (79, 89, 90).
The dose-response tests are qualitative measures of liver VA reserves (68). Although infection and inflammation may reduce dose absorption (55, 56), the response depends more on the accumulation of RBP in the liver than on the absolute dose absorbed. This is in contrast to retinol isotope dilution (RID) tests that rely on the amount of dose absorbed for accurate calculations.
RID tests.
RID tests are the most sensitive methods for assessing VA status because they provide information on liver reserves from deficiency through hypervitaminosis A (68). A baseline blood sample is typically taken before dosage with either deuterated or [13C]retinyl acetate, followed by another blood sample 3–21 d after dosage depending on the application (91, 92). The impact of inflammation on RID test accuracy is currently a subject of field-based research. The factors that may be affected by inflammation include the amount of tracer absorbed (55, 56) and specific activity of retinol in the serum compared with the liver (31).
In India, radioactive tracer dose absorption was reduced from 99.2% in healthy children to 74.3% in children with infections (55). In Zambia, the absorption of a 5-mg dose was 81.2% in 3 children without illness and 62.4% in a child who was ill (93). Therefore, it may be appropriate to decrease the amount of tracer absorbed in the equation used to calculate total body stores (80, 92). However, when this assumption was applied to Zambian children, the distribution curve of calculated VA reserves (micromoles per gram of liver) in children with a CRP >5 mg/L was slightly lower than in those without elevated CRP (Figure 6B). If these children had reduced dose absorption, liver reserve calculations should have been artificially elevated. By correcting the equation for decreased tracer absorption in children with asymptomatic inflammation, the distribution curve shifted them to even lower calculated reserves (Figure 6B). None of the children enrolled had a fever or serious illness because these were exclusion criteria (80).
A common infection in some African countries is malaria. VA-deficient mice were made resistant to malaria by administering VA (94). In vitro, 9-cis-RA increased the phagocytosis of Plasmodium falciparum–parasitized erythrocytes, increased parasite clearance, and reduced proinflammatory cytokine responses (95). The association between malaria in humans and VA status is, to our knowledge, not known. In Zambian children from 4 villages, total body VA reserves were highest in the village that did not have asymptomatic malaria (96). This observation might imply that asymptomatic malaria does not affect RID test accuracy. If the other villages have a greater prevalence of malaria, which might result in a lower absorption of the tracer dose, then total body stores would be calculated to be lower in the village that did not have malaria, but this was not observed.
The specific activity of radioactive retinol to plasma retinol was affected by administering LPS or recombinant human IL-6 to rats (31) (Figure 4). Not only were SR concentrations reduced by ∼47 and ∼65% (31), respectively, but the change in serum specific activity suggested the heavier isotope was sequestered in the liver. The specific activity resolved quickly after the cessation of the inflammatory agents. Therefore, RID tests may be influenced during times of active infection. Best practice is not to enroll children who have an active fever in VA surveys or studies because the illness may affect dose absorption, retention, and the specific activity difference between serum and the liver.
Epidemiologic studies related to carotenoid metabolism and inflammation
Interrelation of carotenoids, inflammation, and oxidative stress.
Carotenoid metabolism is altered during inflammation, and carotenoid status can in return influence inflammation and oxidative stress. Several epidemiologic studies, including meta-analyses, have suggested that a high dietary intake of carotenoids and elevated plasma concentrations are correlated with a decreased risk of cardiovascular diseases (97), type 2 diabetes mellitus (98), and certain types of cancer (99, 100), often by ≤30%, including all-cause mortality (101). These findings were originally attributed to antioxidant effects because isolated carotenoids are effective free-radical scavengers in vitro (102). Some of these associations may have been misinterpreted; i.e., low carotenoid concentrations may have resulted from the diseases rather than contributory factors.
However, several large-scale supplementation trials that incorporated carotenoids were unable to confirm health benefits, including the α-Tocopherol β-Carotene Cancer Prevention trial (103) and β-Carotene and Retinol Efficacy Trial (104). In fact, increased mortality occurred from lung cancer in smokers at daily doses of 20 mg (with 50 mg α-tocopherol) and 30 mg β-carotene (with 25,000 IU VA). Likewise, meta-analyses suggested increased all-cause mortality for subjects taking β-carotene supplements (105, 106). The comparative or combined treatment of 25,000 IU VA, however, has not been evaluated to our knowledge. It has been hypothesized that higher carotenoid doses may act as prooxidants in smokers’ lungs, especially when administered in isolated form. Two long-term β-carotene supplementation trials in China did not report adverse effects; however, this may be explained by coexisting poor VA status caused by low intakes (107).
Because of limited carotenoid bioavailability [∼10–40% (108)] and low achievable plasma concentrations (∼2 μmol/L), the direct antioxidant effects may not fully account for the attributed carotenoid-related health effects. Instead, other mechanisms, such as the alteration of intracellular-signaling cascades and/or gene expression, may be more important. Carotenoids interact with several transduction cascades, reducing NF-κB and stimulating Nrf2 translocation (5). Carotenoids can block signal transduction at various stages by binding to cysteine residues (Michael adduct reaction) of nucleophilic proteins, depending on their cellular concentration and the general inflammatory state. For example, carotenoids have been reported to bind to the kinase responsible for the phosphorylation of the inhibitory protein of NF-κB, consequently blocking the ubiquitylation and dissociation of the inhibitor from the NF-κB complex and preventing the translocation of the free NF-κB subunits to the nucleus (109). Similarly, for Nrf2, carotenoids can bind to Nrf2 and/or its Kelch-like ECH-associated protein 1 inhibitor, promoting the dissociation from Nrf2, which then translocates and activates gene expression (110). However, as outlined in the next section, the effects are generally found to depend on the concentration, cell type investigated, cell inflammatory state, and type of carotenoid or carotenoid metabolite.
In vitro studies and mechanistic insights.
Multiple in vitro studies with carotenoids, their oxidation products, or their metabolites have exposed cells or tissues to high concentrations, such as Caco-2 intestinal cells, immune cells, the liver, adipose, and the retina. Despite limitations, such as studying isolated cells, using high concentrations, solubilization in organic solvents, lack of stability of carotenoids, and short-term exposures, these studies interrogated mechanistic effects without ethical restrictions (111). Several studies that used a wide range of cell models have suggested both anti-inflammatory and antioxidant effects (5). For example, lycopene at physiologic concentrations resulted in reduced TNF-α activity in inflammation-stimulated human mammary cancer and osteoblast cell lines. Interestingly, this effect was stronger for lycopene hydrophilic degradation products (after UV radiation) and its derivatives (109). In another trial, aldehyde derivatives with a carbon chain length of 12 and those having a methyl group with 3 carbon atoms from the terminal aldehyde had the strongest effects on Nrf2 translocation in the human breast and prostate cancer cells (112). In fact, studies have proposed that β-carotene oxygenase 1 and 2 cleavage products are better candidates for binding to cysteine residues of both NF-κB and Nrf2 because of their higher solubility in the cytosol and electrophilicity (109, 112).
To overcome the limitations of excluding digestive processes and studying isolated carotenoids, Caco-2 cells and a Caco-2:HT-29THP-1 triple culture (90:10 ratio coculture plus THP-1 cells in the basolateral compartment) were exposed to the digesta of plum and cabbage varieties rich in carotenoids and polyphenols. IL-6 and IL-8 release was partly reduced and was related to decreased NF-κB and Nrf2 expression and translocation (113). However, the digesta of foods low in carotenoids and polyphenols showed similar results, suggesting other bioactive compounds as causal agents.
Many carotenoids escape absorption in the small intestine, and processes regarding carotenoid metabolism and degradation in the colon have been overlooked (114, 115). In vitro studies have suggested that carotenoids are only partly recovered in the colonic fraction (10–50%) (116, 117). From other phytochemical studies with polyphenols, it is evident that microbiota can promote molecular ring fission, deglycosylation, hydrolysis, deglucuronidation, and demethylation reactions (115). No data to our knowledge are available for carotenoids. It may be speculated that potential polar degradation products could be produced, which may be bioactive.
Short-term animal and human intervention trials.
Several animal studies have confirmed the positive effects of carotenoids on inflammation markers (5). Interestingly, the bioactivity of polar degradation products has recently been studied. In a rat study with lutein-derived products created by UV irradiation, degradation products more strongly ameliorated NO, malondialdeyhde, prostaglandin E2, TNF-α, and IL-6 than lutein itself (118). Contrarily, supraphysiologic RA doses (1–10 μmol/L) were associated with prooxidative effects (119) compared with lower doses (<1 μmol/L) (5), emphasizing the importance of exposure concentration. Other carotenoid metabolites, such as apo10-lycopenoic acid, were stronger activators of RA receptor (RAR) and retinoid X receptor in animal trials than lycopene (120, 121), suggesting VA-like behavior (122). In addition, apo10-lycopenoid acid upregulated sirtuin 1 enzymatic activity in ob/ob mice, preventing fatty liver formation (123). In liver cells, several β-carotene cleavage products (β-apo14′-carotenal, β-apo14′-carotenoic acid, and β-apo13-carotenone) competed with RA binding to RAR, highlighting their involvement in cell growth and differentiation (124). Whether the metabolism of these apocarotenoids is in turn influenced by inflammation is to our knowledge currently unknown.
Contrary to long-term supplementation trials, several short- and midterm intervention trials with whole foods, especially tomato products rich in lycopene, have suggested health benefits for generally healthy and overweight people, as measured by favorable changes in markers of inflammation (IL-6, IL-8, TNF-α, IL-1β, CRP, NF-κB) and oxidative stress (e.g., improved superoxide dismutase, glutathione peroxidase, catalase, heme oxygenase 1, Nrf2) (Figure 1) (5). For example, in a randomized controlled trial, consuming a tomato beverage (16 mg carotenoids/d) for 26 d reduced plasma TNF-α concentrations in healthy subjects by 34% (125). Most studies that used carotenoid-rich foods, however, have shown limited effects in healthy subjects (125–127). Supplementation trials on subjects with chronic inflammation have been more promising. For instance, lycopene (70 mg/wk for 12 wk) improved the inflammation marker serum amyloid A in middle-aged overweight subjects by ∼30% (128), but the study was not placebo-controlled. In a placebo-controlled study, lutein (20 mg/d dissolved in oil as a gelatin capsule) taken for 3 mo by early arthritis patients improved plasma IL-6 (defined as carotid intima media thickness as >750 μm for individuals aged <59 y or >850 μm for those aged >60 y) (129) and monocyte-chemoattractant protein 1 compared with a placebo by −150 and −100 pg/mL, respectively. Several studies have highlighted rather high interindividual differences regarding carotenoid absorption, degradation, metabolism, and excretion partly because of genetic differences in several single-nucleotide polymorphisms on lutein (130), lycopene (131), and β-carotene absorption (3, 132), which may partly explain variable intervention responses.
Vitamin A, carotenoids, and inflammation in pregnancy and infancy
Dietary carotenoids and retinoids play important roles in innate and acquired immunity during pregnancy and development. In particular, VA deficiency, which affects ∼190 million children worldwide, increases the likelihood of early-childhood mortality because of common infections (133, 134). In VA-deficient or -insufficient states, the increased susceptibility to immune-mediated and inflammatory disorders is related to impaired responses to infection, impaired epithelial barrier function (6, 135), and immunologic defects. Responses to mucosal pathogens are impaired when VA stores are low partly because VA metabolites promote the functional maturation of innate immune cells (6, 63, 136, 137).
VA and cellular immunity during development.
VA-deficient animals exhibit abnormalities in the blood and splenic lymphocyte numbers. T- and occasionally B-cell populations are reduced, and myeloid lineage cells, especially granulocytes, tend to increase (138, 139). Granulocyte stimulation likely results from the insufficient RA-mediated inhibition of granulocyte-macrophage colony-stimulating factor (140), which is reversible with RA administration (139). RA signaling plays a critical role in the development of B cells, the major cell mediators of humoral immunity. VA and RA regulate B-cell maturation and differentiation at multiple combinatorial levels that control and often potentiate antibody production. VA deficiency reduces the number of fetal B-cell progenitors, whereas a pan-RAR antagonist, LE540, inhibits both fetal and adult B-cell lymphopoiesis in vitro (141). Although physiologic concentrations of RA inhibit the proliferation of normal B-cell progenitors (142), they apparently influence multiple stages of B-cell lymphopoiesis and accelerate the generation of CD19+/IgM+ B cells (143). These results suggest that RA helps to sustain the microenvironment for B-cell development and maintain a functional B-cell pool essential for the response to antigens (137).
CD4+/CD8+ T cells differentiate in the thymus. In humans, thymic development starts before birth and ceases during puberty with thymic involution (144). As previously mentioned, VA deficiency is accompanied by immune deficiency and susceptibility to a wide range of infectious diseases (145, 146). In addition, marked atrophy of the thymus and spleen is observed in VA-deficient animals (147). A pertinent observation in developmental immunity is that RA-synthesizing enzyme activity peaks at the same time as RAR responsiveness, a time during which thymic cellularity is highest and T-cell selection is most pronounced (148). Direct data on the effects of carotenoids on thymic selection remain unavailable to our knowledge.
Lymphocyte proliferative responses to mitogens are also retinoid-dependent (149, 150). In pregnant mice, VA and β-carotene supplementation affected immune cell functions during ontogenesis (151). Beginning at conception, dams were provided with a control diet or different retinoid- and carotenoid-enriched (4500 retinol equivalents/kg) diets. The percentage and total numbers of splenic mononuclear cells were determined serially throughout gestation. VA and β-carotene supplementation variously increased lymphocyte numbers in early and midpregnancy and increased T:B-cell ratios (151). In addition, in mice, maternal VA supplementation via intraperitoneal injections increased serum IgM and T-helper cell (Th) 2–specific IgG1 concentrations in the progeny (152). Nevertheless, during the first month of lactation in a human β-carotene supplementation study neither lactation nor β-carotene supplementation affected T-cell proliferation (153).
T-effector cells differentiate into several subtypes and include Th1 (defense against intracellular bacteria and protozoa), Th2 (humoral immune stimulators against extracellular parasites), Th17 (proinflammatory autoimmunity regulators), and immunosuppressive T-regulatory cells. In postnatal development, VA regulates the Th1:Th2 switch and thereby modifies immune and inflammatory responses (152, 154).
Investigating carotenoid effects on developmental immunity in animal models has been more challenging because of pronounced differences in carotenoid absorption, kinetics, and metabolism between humans and rodents (155). Nevertheless, some investigations have suggested important roles in T-cell polarization for several presumably nonprovitamin A carotenoids. One example is fucoxanthin-mediated T-regulatory induction and Th17 inhibition, which leads to suppressed inflammation and autoimmunity (156).
Carotenoids and inflammation in pregnancy and fetal development.
The inflammatory response is tightly regulated during reproduction, embryonic and fetal development, and postnatal transition into infancy. During implantation in humans, the expression of proinflammatory cytokines such as IL-6, IL-15, and TNF-α promotes placental trophoblast invasion into the maternal endometrium, myometrium, and uterine vasculature; this tissue invasion results in the recruitment and activation of maternal immune cells. However, after the uteroplacental bed is established during the first trimester, the normal pregnancy state is characterized by immune quiescence, namely a Th2 cytokine profile, and the suppression of the maternal immunologic rejection of the “foreign” fetoplacental unit (157). A proinflammatory Th1 cytokine state is reactivated during parturition (158). In contrast, preeclampsia (a hypertensive disorder that causes substantial maternal and offspring morbidities and mortality) increases uteroplacental inflammation and the persistence of proinflammatory Th1 cytokine concentrations compared with normal pregnancy.
Preeclampsia is a state of both oxidative and inflammatory stress. Consequently, antioxidant and anti-inflammatory supplementation has been proposed to prevent or lessen its severity (159). Analyses of maternal carotenoid concentrations have suggested inverse relations between plasma lutein, α- and β-carotene, and lycopene and the risk/severity of preeclampsia and diabetes mellitus (160–164), 2 pregnancy-related pro-oxidant and proinflammatory conditions. Of course, these findings might have resulted from the disease, dietary intake, or both. In support of a salutary role in pregnancy, Lorenzoni et al. (165) observed that lutein supplementation in pregnant women with gestational diabetes reduced newborn oxidative stress, as measured by blood total hydroxyperoxide concentrations.
VA, carotenoids, and inflammation in newborns.
Intermittent or sustained systemic inflammation contributes to the pathogenesis of most disorders associated with prematurity, including brain damage and neurodevelopmental disorders (166, 167). In preterm and term infants, both the prevention of and therapy for hypoxic-ischemic brain injury increasingly emphasizes the interference with neurotoxic and neuroinflammatory cascades, with emphasis on endogenous neuroprotective mechanisms (168, 169). Both RA (170) and lutein (171) suppress neuroinflammation mediated by astrocytes and microglia, 2 cell types important in acute brain injury that accompanies preterm and term birth (172). The mechanisms of carotenoid (lutein, astaxanthin)-mediated neuroprotection include blocking the actions of NF-κB signaling on microglial and astrocyte activation, neuronal inflammation, inflammatory cytokine and chemokine release, and neuronal cell death (173).
Human milk contains various carotenoids, of which lutein is often predominant (174, 175). In term newborns, lutein supplementation suppresses measures of systemic oxidant stress (176, 177). In the retinopathy of prematurity, the principal cause of blindness in children in industrialized countries, systemic and localized neuroretinal inflammation plays a major pathogenic role (178, 179). Carotenoids, particularly lutein, suppress both systemic inflammation (measured by CRP) and retinopathy severity in preterm infants (179). It is important to note that lutein supplementation (or repletion) has similar antioxidant and anti-inflammatory effects in preterm infant and adult diabetic neovascular retinopathies (180). In experimental animal models, lutein and astaxanthin (181) suppress inflammation and improve retinal function in diabetic retinopathy. Wolfberry (goji; Lycium barbarum), an Asian fruit traditionally consumed to prevent eye diseases, is a particularly zeaxanthin-rich dietary source. In diabetic mice, wolfberry ameliorated retinopathy, suppressed inflammation, and provided retinal protection, effects that were mimicked by zeaxanthin or lutein in vitro (182).
Xanthophyll carotenoids exert anti-inflammatory and immunomodulatory activities in several mammalian systems. Mechanisms may include the inhibition of oxidative stress, inflammatory mediators, and lipid peroxidation; inhibition of proinflammatory NF-κB and MAPK signaling (Figure 1); blockage of advanced glycation end-product formation; suppression of scavenger receptor expression; suppression of lymphocyte and macrophage activation (183); and modulation of T-cell polarization, such as increasing T-regulatory and decreasing Th17 cell expansion (137). Recent data have suggested that xanthophylls are regulators of macrophage-dependent immune responses. The human dietary xanthophyll astaxanthin, which is found in pink-orange fish and crustaceans, drives IL-10 production in anti-inflammatory M2 macrophages; similarly, lutein and astaxanthin support monocyte polarization away from the “killer” M1 macrophage phenotype to M2 macrophages (LP Rubin, unpublished data, 2014). In the liver inflammatory-disorder nonalcoholic fatty liver disease, an increasingly common comorbidity of obesity, dietary xanthophylls (including β-cryptoxanthin and astaxanthin) offer important preventive and treatment strategies. Experimentally, these carotenoids decrease hepatic inflammatory damage by restraining M1 macrophage activation or by driving M2 activation (184).
Conclusions
Inflammation affects retinoid and carotenoid metabolism. The roles of VA and carotenoids as potential modulators of developmental immunity, the APR, and inflammation, especially in light of global endemic VA insufficiency and low carotenoid intakes, warrant further investigation. Infections decrease VA intake as a result of infection-induced anorexia and decreased VA absorption from the intestine. VA may also be lost in substantial amounts in the urine during infection. In addition, plasma retinol may be sequestered in tissues, leading to a reduction in SR concentrations, which implies that assessing VA status with the use of SR or RBP during inflammation is problematic. These factors account for the increased risk of VA deficiency associated with infection. However, VA requirements are currently not known to be substantially increased by infections, a phenomenon that needs further evaluation considering the losses that occur in urine during acute infections. In addition, retinol mobilization from the liver decreases during the APR, perhaps having adverse effects on VA availability to target tissues, especially the retina. Furthermore, some biomarkers of VA status are affected by the APR, and this needs to be considered in population-based assessments.
Regarding carotenoid health benefits, a discrepancy exists between epidemiologic studies that have suggested benefits related to carotenoid intake and tissue concentrations and controversial results from interventions involving β-carotene. Nevertheless, in clinical trials and animal models, carotenoid supplementation (particularly lutein) to young infants reduced systemic inflammation, the inflammatory response in retinopathy of prematurity, and the neuroinflammation that accompanies hypoxic-ischemic brain injury. In general, information on carotenoid bioactivity is incomplete, and questions related to dose response, metabolism, and synergism may explain some findings. Several studies have suggested that carotenoid metabolites are bioactive, including enzymatic cleavage products (apocarotenals), and act as better targets for transcription factors such as NF-κB and Nrf2. Lower-to-intermediate concentrations may exert positive effects on gene expression and antioxidant effects, whereas higher concentrations act pro-oxidatively. Further nuclear targets, such as the implication of RAR/retinoid X receptor and potential VA-like effects, also deserve more attention. Finally, our knowledge on carotenoid processes in the colon and interaction with the microbiota is nil. As long as these aspects remain marginally understood, our comprehension of the role of carotenoids in chronic diseases and inflammation will be far from complete.
Acknowledgments
We thank Devika Suri for preparing Figure 6. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGP, α1-acid glycoprotein; APP, acute-phase protein; APR, acute-phase response; CRP, C-reactive protein; MRDR, modified relative dose response; Nrf2, nuclear factor (erythroid-derived 2)–like 2; RA, retinoic acid; RAR, retinoic acid receptor; RBP, retinol-binding protein; Rbp4, retinol-binding protein 4 gene; RDR, relative dose response; RID, retinol isotope dilution; SR, serum retinol; Th, T-helper cell; VA, vitamin A.
References
- 1.Krasinski SD, Russell RM, Otradovec CL, Sadowski JA, Hartz SC, Jacob RA, McGandy RB. Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl esters, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: increased plasma retinyl esters among vitamin A-supplement users. Am J Clin Nutr 1989;49:112–20. [DOI] [PubMed] [Google Scholar]
- 2.Dever J. Host factors: gender and body composition. In: Tanumihardjo SA, editor. Carotenoids and human health. New York: Springer Science and Business Media; 2013. p. 141–52. [Google Scholar]
- 3.Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J 2009;23:1041–53. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Real JM. Genetic predispositions to low-grade inflammation and type 2 diabetes. Diabetes Technol Ther 2006;8:55–66. [DOI] [PubMed] [Google Scholar]
- 5.Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 2014;34:907–29. [DOI] [PubMed] [Google Scholar]
- 6.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 7.Klasing KC. Nutrition and the immune system. Br Poult Sci 2007;48:525–37. [DOI] [PubMed] [Google Scholar]
- 8.Soprano DR, Blaner WS. Plasma retinol-binding protein. In: Sporn MB, Roberts AB, Goodman DS, editors. The retinoids: biology, chemistry and medicine. New York: Raven Press; 1994. p. 257–81. [Google Scholar]
- 9.Baumann H, Gauldie J. The acute phase response. Immunol Today 1994;15:74–80. [DOI] [PubMed] [Google Scholar]
- 10.Birch HE, Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem 1986;261:8077–80. [PubMed] [Google Scholar]
- 11.Bode JG, Albrecht U, Håussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kB-dependent signaling. Eur J Cell Biol 2012;91:496–505. [DOI] [PubMed] [Google Scholar]
- 12.Goodman DS. Plasma retinol-binding protein. In: Sporn MB, Roberts AB, Goodman DS, editors. The retinoids. Vol. 2 Orlando (FL): Academic Press; 1984. p. 42–88. [Google Scholar]
- 13.Thurnham DI. Micronutrients and immune function: some recent developments. J Clin Pathol 1997;50:887–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felding P, Fex G. Rates of synthesis of prealbumin and retinol-binding protein during acute inflammation in the rat. Acta Physiol Scand 1985;123:477–83. [DOI] [PubMed] [Google Scholar]
- 15.Friis H, Gomo E, Koestel P, Ndhlovu P, Nyazema N, Krarup H, Michaelsen KF. HIV and other predictors of serum beta-carotene and retinol in pregnancy: a cross-sectional study in Zimbabwe. Am J Clin Nutr 2001;73:1058–65. [DOI] [PubMed] [Google Scholar]
- 16.Smith FR, Suskind R, Thanangkul O, Leitzmann C, Goodman DS, Olson RE. Plasma vitamin A, retinol-binding protein and prealbumin concentrations in protein-calorie malnutrition. III. Response to varying dietary treatments. Am J Clin Nutr 1975;28:732–8. [DOI] [PubMed] [Google Scholar]
- 17.Rosales FJ, Topping JD, Smith JE, Shankar AH, Ross AC. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am J Clin Nutr 2000;71:1582–8. [DOI] [PubMed] [Google Scholar]
- 18.Moody BJ. Changes in the serum concentrations of thyroxine-binding prealbumin and retinol-binding protein following burn injury. Clin Chim Acta 1982;118:87–92. [DOI] [PubMed] [Google Scholar]
- 19.Munford RS. Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect Immun 2008;76:454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008;42:145–51. [DOI] [PubMed] [Google Scholar]
- 21.Beutler B. Science review: key inflammatory and stress pathways in critical illness - the central role of the Toll-like receptors. Crit Care 2003;7:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res 1996;37:962–71. [PubMed] [Google Scholar]
- 23.Rosales FJ, Ross AC. Acute inflammation induces hyporetinemia and modifies the plasma and tissue response to vitamin A supplementation in marginally vitamin A-deficient rats. J Nutr 1998;128:960–6. [DOI] [PubMed] [Google Scholar]
- 24.Gieng SH, Raila J, Rosales FJ. Accumulation of retinol in the liver after prolonged hyporetinolemia in the vitamin A-sufficient rat. J Lipid Res 2005;46:641–9. [DOI] [PubMed] [Google Scholar]
- 25.Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK. Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 1999;10:685–95. [DOI] [PubMed] [Google Scholar]
- 26.Thurnham DI, Mburu AS, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 2005;64:502–9. [DOI] [PubMed] [Google Scholar]
- 27.Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J Nutr 2015;145:1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasantwisut E, Neufeld L. Use of nutritional biomarkers in program evaluation in the context of developing countries. J Nutr 2012;142:186S–90S. [DOI] [PubMed] [Google Scholar]
- 29.Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, Holgate ST, Jönsson LS, Latulippe ME, Marcos A, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr 2013;109(Suppl 1):S1–34. [DOI] [PubMed] [Google Scholar]
- 30.Green MH, Green JB. Quantitative and conceptual contributions of mathematical modeling to current views on vitamin A metabolism, biochemistry, and nutrition. Adv Food Nutr Res 1996;40:3–24. [DOI] [PubMed] [Google Scholar]
- 31.Gieng SH, Green MH, Green JB, Rosales FJ. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J Lipid Res 2007;48:904–13. [DOI] [PubMed] [Google Scholar]
- 32.Tanumihardjo SA. Assessing vitamin A status: past, present and future. J Nutr 2004;134:290S–3S. [DOI] [PubMed] [Google Scholar]
- 33.Rosales FJ, Ross AC. A low molar ratio of retinol-binding protein to transthyretin indicates vitamin A deficiency during inflammation: studies in rats and a posteriori analysis of vitamin A-supplemented children with measles. J Nutr 1998;128:1681–7. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami M, Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med 1981;154:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casanovas A, Carrascal M, Abián J, López-Tejero MD, Llobera M. Lipoprotein lipase is nitrated in vivo after lipopolysaccharide challenge. Free Radic Biol Med 2009;47:1553–60. [DOI] [PubMed] [Google Scholar]
- 36.Zolfaghari R, Chen Q, Ross AC. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am J Physiol Gastrointest Liver Physiol 2012;303:G578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirakami Y, Lee SA, Clugston RD, Blaner WS. Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta 2012;1821:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cifelli CJ, Ross AC. All-trans-retinoic acid distribution and metabolism in vitamin A-marginal rats. Am J Physiol Gastrointest Liver Physiol 2006;291:G195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zolfaghari R, Cifelli CJ, Lieu SO, Chen Q, Li NQ, Ross AC. Lipopolysaccharide opposes the induction of CYP26A1 and CYP26B1 gene expression by retinoic acid in the rat liver in vivo. Am J Physiol Gastrointest Liver Physiol 26007;292:G1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Geneva (Switzerland): WHO; 1968. [PubMed] [Google Scholar]
- 41.Rowland MG, Rowland SG, Cole TJ. Impact of infection on the growth of children from 0 to 2 years in an urban West African community. Am J Clin Nutr 1988;47:134–8. [DOI] [PubMed] [Google Scholar]
- 42.Sommer A, Tarwotjo I, Katz J. Increased risk of xerophthalmia following diarrhea and respiratory disease. Am J Clin Nutr 1987;45:977–80. [DOI] [PubMed] [Google Scholar]
- 43.Campos FA, Flores H, Underwood BA. Effect of an infection on vitamin A status of children as measured by the relative dose response (RDR). Am J Clin Nutr 1987;46:91–4. [DOI] [PubMed] [Google Scholar]
- 44.Rahman MM, Mahalanabis D, Alvarez JO, Wahed MA, Islam MA, Habte D, Khaled MA. Acute respiratory infections prevent improvement of vitamin A status in young infants supplemented with vitamin A. J Nutr 1996;126:628–33. [DOI] [PubMed] [Google Scholar]
- 45.Sommer A. Nutritional blindness: xerophthalmia and keratomalacia. New York: Oxford University Press; 1982. [Google Scholar]
- 46.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 47.Herbert V. The five possible causes of all nutrient deficiency: illustrated by deficiencies of vitamin B12. Am J Clin Nutr 1973;26:77–86. [DOI] [PubMed] [Google Scholar]
- 48.Bresnahan KA, Chileshe J, Tanumihardjo SA. Quantification of food and nutrient intakes in Zambian children with and without malaria under controlled feeding conditions. Exp Biol Med (Maywood) 2014;239:45–51. [DOI] [PubMed] [Google Scholar]
- 49.Martorell R, Yarbrough C, Yarbrough S, Klein RE. The impact of ordinary illnesses on the dietary intakes of malnourished children. Am J Clin Nutr 1980;33:345–50. [DOI] [PubMed] [Google Scholar]
- 50.Duggan MB, Milner RD. Energy cost of measles infection. Arch Dis Child 1986;61:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown KH, Stallings RY, de Kanashiro HC, Lopez de Romana G, Black RE. Effects of common illnesses on infants’ energy intakes from breast milk and other foods during longitudinal community-based studies in Huascar (Lima), Peru. Am J Clin Nutr 1990;52:1005–13. [DOI] [PubMed] [Google Scholar]
- 52.Zijlstra RT, Donovan SM, Odle J, Gelberg HB, Petschow BW, Gaskins HR. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J Nutr 1997;127:1118–27. [DOI] [PubMed] [Google Scholar]
- 53.Carrera E, Nesheim MC, Crompton DW. Lactose maldigestion in Ascaris-infected preschool children. Am J Clin Nutr 1984;39:255–64. [DOI] [PubMed] [Google Scholar]
- 54.Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr 1998;68:623–9. [DOI] [PubMed] [Google Scholar]
- 55.Sivakumar B, Reddy V. Absorption of labelled vitamin A in children during infection. Br J Nutr 1972;27:299–304. [DOI] [PubMed] [Google Scholar]
- 56.Sivakumar B, Reddy V. Absorption of vitamin A in children with ascariasis. J Trop Med Hyg 1975;78:114–5. [PubMed] [Google Scholar]
- 57.Sarker SA, Wahed MA, Rahaman MM, Alam AN, Islam A, Jahan F. Persistent protein losing enteropathy in post measles diarrhoea. Arch Dis Child 1986;61:739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma V, Gunjan D, Chhabra P, Sharma R, Rana SS, Bhasin DK. Gastrointestinal bleeding in the tropics: look for the hookworm. Trop Doct 2017;47:48–51. [DOI] [PubMed] [Google Scholar]
- 59.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI Jr, Gammon RB Jr. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 1994;60:388–92. [DOI] [PubMed] [Google Scholar]
- 60.Mitra AK, Alvarez JO, Stephensen CB. Increased urinary retinol loss in children with severe infections. Lancet 1998;351:1033–4. [DOI] [PubMed] [Google Scholar]
- 61.Mitra AK, Alvarez JO, Guay-Woodford L, Fuchs GJ, Wahed MA, Stephensen CB. Urinary retinol excretion and kidney function in children with shigellosis. Am J Clin Nutr 1998;68:1095–103. [DOI] [PubMed] [Google Scholar]
- 62.Kelly M, Widjaja-Adhi MA, Palczewski G, von Lintig J. Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J 2016;30:2985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Y, Brown C, Ortiz C, Noelle RJ. Leukocyte homing, fate, and function are controlled by retinoic acid. Physiol Rev 2015;95:125–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cser MA, Majchrzak D, Rust P, Sziklai-Laszlo I, Kovacs I, Bocskai E, Elmadfa I. Serum carotenoid and retinol levels during childhood infections. Ann Nutr Metab 2004;48:156–62. [DOI] [PubMed] [Google Scholar]
- 65.Mittelsteadt AL, Hileman CO, Harris SR, Payne KM, Gripshover BM, McComsey GA. Effects of HIV and antiretroviral therapy on resting energy expenditure in adult HIV-infected women—a matched, prospective, cross-sectional study. J Acad Nutr Diet 2013;113:1037–43. [DOI] [PubMed] [Google Scholar]
- 66.Powanda MC, Beisel WR. Metabolic effects of infection on protein and energy status. J Nutr 2003;133:322S–7S. [DOI] [PubMed] [Google Scholar]
- 67.Koutsos EA, Calvert CC, Klasing KC. The effect of an acute phase response on tissue carotenoid levels of growing chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Integr Physiol 2003;135:635–46. [DOI] [PubMed] [Google Scholar]
- 68.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development (BOND)—vitamin A review. J Nutr 2016;146:1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christian P, Schulze K, Stoltzfus RJ, West KP Jr. Hyporetinolemia, illness symptoms, and acute phase protein response in pregnant women with and without night blindness. Am J Clin Nutr 1998;67:1237–43. [DOI] [PubMed] [Google Scholar]
- 70.Semba RD, Muhilal, West KP Jr, Natadisastra G, Eisinger W, Lan Y, Sommer A. Hyporetinolemia and acute phase proteins in children with and without xerophthalmia. Am J Clin Nutr 2000;72:146–53. [DOI] [PubMed] [Google Scholar]
- 71.Inua M, Duggan MB, West CE, Whittle HC, Kogbe OI, Sandford-Smith JH, Glover J. Post-measles corneal ulceration in children in northern Nigeria: the role of vitamin A, malnutrition and measles. Ann Trop Paediatr 1983;3:181–91. [DOI] [PubMed] [Google Scholar]
- 72.Hussey GD, Klein M. Measles-induced vitamin A deficiency. Ann N Y Acad Sci 1992;669:188–94. [DOI] [PubMed] [Google Scholar]
- 73.WHO. Measles. [Internet]. [cited 2016 Jul 5]. Available from: http://www.who.int/entity/mediacentre/factsheets/fs286/en.
- 74.Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst 1984;73:1439–44. [PubMed] [Google Scholar]
- 75.WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Vitamin and mineral nutrition information system [Internet]. [cited 2016 Jul 6]. Available from: http://www.who.int/vmnis/indicators/retinol.pdf.
- 76.Tanumihardjo SA. Vitamin A status assessment in rats using 13C4-retinyl acetate and gas chromatography-combustion isotope ratio mass spectrometry (GCCIRMS). J Nutr 2000;130:2844–9. [DOI] [PubMed] [Google Scholar]
- 77.Green MH, Green JB. Vitamin A intake and status influence retinol balance, utilization and dynamics in rats. J Nutr 1994;124:2477–85. [DOI] [PubMed] [Google Scholar]
- 78.Tanumihardjo SA, Permaesih D, Muherdiyantiningsih, Rustan E, Rusmil K, Fatah AC, Wilbur S, Muhilal, Karyadi D, Olson JA. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr 1996;126:451–7. [DOI] [PubMed] [Google Scholar]
- 79.Bresnahan KA, Chileshe J, Arscott SA, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affects traditional measures of micronutrient status in rural Zambian children during a randomized controlled feeding trial. J Nutr 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 80.Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014;100:1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr 2015;102:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. [Internet]. [cited 2017 Feb 2]. Available from: http://www.who.int/nutrition/publications/micronutrients/background_paper4_report_assessment_vitAandIron_status.pdf.
- 83.Palmer A, Chileshe J, Barffour M, Hall A, West K Jr, Haskell M.. Influence of inflammation on breast milk retinol concentrations of apparently healthy women in rural Zambia. FASEB J 2015;29:39.4. [Google Scholar]
- 84.Ross AC, Pasatiempo AM, Green MH. Chylomicron margination, lipolysis, and vitamin A uptake in the lactating rat mammary gland: implications for milk retinoid content. Exp Biol Med (Maywood) 2004;229:46–55. [DOI] [PubMed] [Google Scholar]
- 85.Dever JT, Surles RL, Davis CR, Tanumihardjo SA. α-Retinol is distributed through serum retinol-binding protein-independent mechanisms in the lactating sow-nursing piglet dyad. J Nutr 2011;141:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Surles RL, Hutson PR, Valentine AR, Mills JP, Tanumihardjo SA. 3, 4-Didehydroretinol (DR) kinetics differ during lactation in sows on a retinol (R) depletion regimen and the serum to milk DR to R ratios are correlated. J Nutr 2011;141:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972;247:2542–50. [PubMed] [Google Scholar]
- 88.Stephensen CB, Franchi LM, Hernandez H, Campos M, Colarossi A, Gilman RH, Alvarez JO. Assessment of vitamin A status with the relative-dose-response test in Peruvian children recovering from pneumonia. Am J Clin Nutr 2002;76:1351–7. [DOI] [PubMed] [Google Scholar]
- 89.Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]
- 90.Whitehead RD Jr, Perrine CG, Flores-Ayala RC, Tanumihardjo SA, Dahal P, Mebrahtu S, Subedi GR, Jefferds ME. Use of modified relative dose-response to examine sensitivity and specificity of retinol binding protein as an indicator of vitamin A deficiency among Nepali children 6–23 mo of age. FASEB J 2016;30:892.4. [Google Scholar]
- 91.Green MH. Evaluation of the “Olson equation,” an isotope dilution method for estimating vitamin A stores. Int J Vitam Nutr Res 2014;84(Suppl 1):9–15. [DOI] [PubMed] [Google Scholar]
- 92.Gannon BM, Tanumihardjo SA. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015;145:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aklamati EK, Mulenga M, Dueker SR, Buchholz BA, Peerson JM, Kafwembe E, Brown KH, Haskell MJ. Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. J Nutr 2010;140:1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krishnan S, Krishnan AD, Mustafa AS, Talwar GP, Ramalingaswami V. Effect of vitamin A and undernutrition on the susceptibility of rodents to a malarial parasite Plasmodium berghei. J Nutr 1976;106:784–91. [DOI] [PubMed] [Google Scholar]
- 95.Serghides L, Kain KC. Mechanism of protection induced by vitamin A in falciparum malaria. Lancet 2002;359:1404–6. [DOI] [PubMed] [Google Scholar]
- 96.Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory JF III, Tanumihardjo SA. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am J Clin Nutr 2015;102:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Manson JE, Willett WC. Dietary carotenoids and risk of coronary artery disease in women. Am J Clin Nutr 2003;77:1390–9. [DOI] [PubMed] [Google Scholar]
- 98.Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens 2007;25:2361–9. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Cui R, Xiao Y, Fang J, Xu Q. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. PLoS One 2015;10:e0137427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S. Carotenoid intake from natural sources and head and neck cancer: a systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol Biomarkers Prev 2015;24:1003–11. [DOI] [PubMed] [Google Scholar]
- 101.Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, de Groot LC. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the survey in Europe on nutrition and the elderly, a concerted action (SENECA). Am J Clin Nutr 2005;82:879–86. [DOI] [PubMed] [Google Scholar]
- 102.Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med 1989;7:617–35. [DOI] [PubMed] [Google Scholar]
- 103.The Alpha-Tocopherol Beta-Carotene Cancer Prevention Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35. [DOI] [PubMed] [Google Scholar]
- 104.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, et al. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J Natl Cancer Inst 1996;88:1550–9. [DOI] [PubMed] [Google Scholar]
- 105.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297:842–57. [DOI] [PubMed] [Google Scholar]
- 106.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: mortality rates by vitamin-mineral intervention group. Am J Clin Nutr 1995;62:1424S–6S. [DOI] [PubMed] [Google Scholar]
- 108.Bohn T. Provitamin A carotenoids: occurrence, intake, and bioavailability. In: Preedy VR, editor. Vitamin A and carotenoids: chemistry, analysis, function and effects (Food and nutritional components in focus). London: RSC Publishing; 2012. [Google Scholar]
- 109.Linnewiel-Hermoni K, Motro Y, Miller Y, Levy J, Sharoni Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic Biol Med 2014;75:105–20. [DOI] [PubMed] [Google Scholar]
- 110.He X, Ma Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol Pharmacol 2009;76:1265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Biehler E, Bohn T. Methods for assessing aspects of carotenoid bioavailability. Curr Nutr Food Sci 2010;6:44–69. [Google Scholar]
- 112.Linnewiel K, Ernst H, Caris-Veyrat C, Ben-Dor A, Kampf A, Salman H, Danilenko M, Levy J, Sharoni Y. Structure activity relation of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic Biol Med 2009;47:659–67. [DOI] [PubMed] [Google Scholar]
- 113.Kaulmann A, Legay S, Schneider Y-J, Hoffmann L, Bohn T. Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastro-intestinal digestion. Mol Nutr Food Res 2016;60:992–1005. [DOI] [PubMed] [Google Scholar]
- 114.Alminger M, Aura AM, Bohn T, Dufour C, El SN, Gomes A, Karakaya S, Martínez-Cuesta MC, McDougall GJ, Requena T, et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr Rev Food Sci Food Saf 2014;13:413–36. [DOI] [PubMed] [Google Scholar]
- 115.Bohn T, McDougall GJ, Alegria A, Alminger M, Arrigoni E, Aura AM, Brito C, Cilla A, El SN, Karakaya S, et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—a position paper focusing on carotenoids and polyphenols. Mol Nutr Food Res 2015;59:1307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaulmann A, Andre CM, Schneider YJ, Hoffmann L, Bohn T. Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food Chem 2016;197(Pt A):325–32. [DOI] [PubMed] [Google Scholar]
- 117.Goñi I, Serrano J, Saura-Calixto F. Bioaccessibility of beta-carotene, lutein, and lycopene from fruits and vegetables. J Agric Food Chem 2006;54:5382–7. [DOI] [PubMed] [Google Scholar]
- 118.Nidhi B, Sharavana G, Ramaprasad TR, Vallikannan B. Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct 2015;6:450–60. [DOI] [PubMed] [Google Scholar]
- 119.Tan KP, Kosuge K, Yang M, Ito S. NRF2 as a determinant of cellular resistance in retinoic acid cytotoxicity. Free Radic Biol Med 2008;45:1663–73. [DOI] [PubMed] [Google Scholar]
- 120.Aydemir G, Kasiri Y, Birta E, Beke G, Garcia AL, Bartók EM, Rühl R. Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene’s anti-cancer potential. Mol Nutr Food Res 2013;57:739–47. [DOI] [PubMed] [Google Scholar]
- 121.Harrison EH, dela Sena C, Eroglu A, Fleshman MK. The formation, occurrence, and function of beta-apocarotenoids: beta-carotene metabolites that may modulate nuclear receptor signaling. Am J Clin Nutr 2012;96:1189S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caris-Veyrat C, Garcia AL, Reynaud E, Lucas R, Aydemir G, Rühl R. Lycopene-induced nuclear hormone receptor signalling in inflammation and lipid metabolism via still unknown endogenous apo-10´-lycopenoids. Int J Vitam Nutr Res 2016. (Epub ahead of print; DOI: 10.1024/0300-9837/a000289). [DOI] [PubMed] [Google Scholar]
- 123.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. Apo-10′-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr 2012;142:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW Jr, Harrison EH. Naturally occurring eccentric cleavage products of provitamin A carotene β-carotene function as antagonists of retinoic acid receptors. J Biol Chem 2012;287:15886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Riso P, Visioli F, Grande S, Guarnieri S, Simonetti P, Porrini M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem 2006;54:2563–6. [DOI] [PubMed] [Google Scholar]
- 126.Biddle MJ, Lennie TA, Bricker GV, Kopec RE, Schwartz SJ, Moser DK. Lycopene dietary intervention: a pilot study in patients with heart failure. J Cardiovasc Nurs 2015;30:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watzl B, Bub A, Blockhaus M, Herbert BM, Lührmann PM, Neuhäuser-Berthold M, Rechkemmer G. Prolonged tomato juice consumption has no effect on cell-mediated immunity of well-nourished elderly men and women. J Nutr 2000;130:1719–23. [DOI] [PubMed] [Google Scholar]
- 128.McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, McMaster C, Thies F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J Nutr Biochem 2013;24:163–8. [DOI] [PubMed] [Google Scholar]
- 129.Xu XR, Zou ZY, Xiao X, Huang YM, Wang X, Lin XM. Effects of lutein supplement on serum inflammatory cytokines, ApoE and lipid profiles in early atherosclerosis population. J Atheroscler Thromb 2013;20:170–7. [DOI] [PubMed] [Google Scholar]
- 130.Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr 2014;100:168–75. [DOI] [PubMed] [Google Scholar]
- 131.Borel P, Desmarchelier C, Nowicki M, Bott R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic Biol Med 2015;83:238–44. [DOI] [PubMed] [Google Scholar]
- 132.Borel P, Desmarchelier C, Nowicki M, Bott R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary beta-carotene bioavailability in healthy men. J Nutr 2015;145:1740–7. [DOI] [PubMed] [Google Scholar]
- 133.WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO global database on vitamin A deficiency. Geneva (Switzerland): WHO; 2009. [Google Scholar]
- 134.Cediel G, Olivares M, Brito A, Lòpez de Romaña D, Cori H, La Frano MR. Interpretation of serum retinol data from Latin America and the Caribbean. Food Nutr Bull 2015; 36(2 Suppl):S98–108. [DOI] [PubMed] [Google Scholar]
- 135.Biesalski HK, Nohr D. New aspects in vitamin A metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia. J Nutr 2004;134:3453S–7S. [DOI] [PubMed] [Google Scholar]
- 136.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000;12:721–7. [DOI] [PubMed] [Google Scholar]
- 137.Gong X, Rubin LP. Carotenoids in early life. In: Tanumihardjo SA, editor. Carotenoids in human health. New York: Springer Science and Business Media; 2013. p. 167–79. [Google Scholar]
- 138.Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, De Luca LM, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 2000;95:3349–56. [PubMed] [Google Scholar]
- 139.Zhao Z, Ross AC. Retinoic acid repletion restores the number of leukocytes and their subsets and stimulates natural cytotoxicity in vitamin A-deficient rats. J Nutr 1995;125:2064–73. [DOI] [PubMed] [Google Scholar]
- 140.Smeland EB, Rusten L, Jacobsen SE, Skrede B, Blomhoff R, Wang MY, Funderud S, Kvalheim G, Blomhoff HK. All-trans retinoic acid directly inhibits granulocyte colony-stimulating factor-induced proliferation of CD34+ human hematopoietic progenitor cells. Blood 1994;84:2940–5. [PubMed] [Google Scholar]
- 141.Chen X, Welner RS, Kincade PW. A possible contribution of retinoids to regulation of fetal B lymphopoiesis. Eur J Immunol 2009;39:2515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW. Retinoids accelerate B lineage lymphoid differentiation. J Immunol 2008;180:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fahlman C, Jacobsen SE, Smeland EB, Lomo J, Naess CE, Funderud S, Blomhoff HK. All-trans- and 9-cis-retinoic acid inhibit growth of normal human and murine B cell precursors. J Immunol 1995;155:58–65. [PubMed] [Google Scholar]
- 144.Sen J. Signal transduction in thymus development. Cell Mol Biol 2001;47:197–215. [PubMed] [Google Scholar]
- 145.Reifen R. Vitamin A as an anti-inflammatory agent. Proc Nutr Soc 2002;61:397–400. [DOI] [PubMed] [Google Scholar]
- 146.Semba RD. Vitamin A, immunity, and infection. Clin Infect Dis 1994;19:489–99. [DOI] [PubMed] [Google Scholar]
- 147.West KP Jr, Howard GR, Sommer A. Vitamin A and infection: public health implications. Annu Rev Nutr 1989;9:63–86. [DOI] [PubMed] [Google Scholar]
- 148.Kiss I, Ruhl R, Szegezdi E, Fritzsche B, Toth B, Pongracz J, Perlmann T, Fesus L, Szondy Z. Retinoid receptor-activating ligands are produced within the mouse thymus during postnatal development. Eur J Immunol 2008;38:147–55. [DOI] [PubMed] [Google Scholar]
- 149.Wang W, Ballow M. The effects of retinoic acid on in vitro immunoglobulin synthesis by cord blood and adult peripheral blood mononuclear cells. Cell Immunol 1993;148:291–300. [DOI] [PubMed] [Google Scholar]
- 150.Ballow M, Wang W, Xiang S. Modulation of B-cell immunoglobulin synthesis by retinoic acid. Clin Immunol Immunopathol 1996;80:S73–81. [DOI] [PubMed] [Google Scholar]
- 151.Garcia AL, Ruhl R, Herz U, Koebnick C, Schweigert FJ, Worm M. Retinoid- and carotenoid-enriched diets influence the ontogenesis of the immune system in mice. Immunology 2003;110:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guzman JJ, Caren LD. Effects of prenatal and postnatal exposure to vitamin A on the development of the murine immune system. Life Sci 1991;49:1455–62. [DOI] [PubMed] [Google Scholar]
- 153.Gossage C, Deyhim M, Moser-Veillon PB, Douglas LW, Kramer TR. Effect of beta-carotene supplementation and lactation on carotenoid metabolism and mitogenic T lymphocyte proliferation. Am J Clin Nutr 2000;71:950–5. [DOI] [PubMed] [Google Scholar]
- 154.Rühl R, Hänel A, Garcia AL, Dahten A, Herz U, Schweigert FJ, Worm M. Role of vitamin A elimination or supplementation diets during postnatal development on the allergic sensitisation in mice. Mol Nutr Food Res 2007;51:1173–81. [DOI] [PubMed] [Google Scholar]
- 155.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW Jr. Review of animal models in carotenoid research. J Nutr 1999;129:2271–7. [DOI] [PubMed] [Google Scholar]
- 156.Kawashima T. A marine carotenoid, fucoxanthin, induces regulatory T cells and inhibits Th17 cell differentiation in vitro. Biosci Biotechnol Biochem 2011;75:2066–9. [DOI] [PubMed] [Google Scholar]
- 157.Chaouat G. Reconsidering the Medawar paradigm placental viviparity existed for eons, even in vertebrates; without a “problem”: why are Tregs important for preeclampsia in great apes? J Reprod Immunol 2016;114:48–57. [DOI] [PubMed] [Google Scholar]
- 158.Barrera D, Díaz L, Noyola-Martínez N, Halhali A. Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutrients 2015;7:6465–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cohen JM, Beddaoui M, Kramer MS, Platt RW, Basso O, Kahn SR. Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: a systematic review and meta-analysis. PLoS One 2015;10:e0135192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cohen JM, Kahn SR, Platt RW, Basso O, Evans RW, Kramer MS. Small-for-gestational-age birth and maternal plasma antioxidant levels in mid-gestation: a nested case-control study. BJOG 2015;122:1313–21. [DOI] [PubMed] [Google Scholar]
- 161.Azar M, Basu A, Jenkins AJ, Nankervis AJ, Hanssen KF, Scholz H, Henriksen T, Garg SK, Hammad SM, Scardo JA, et al. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: a longitudinal study. Diabetes Care 2011;34:1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Williams MA, Woelk GB, King IB, Jenkins L, Mahomed K. Plasma carotenoids, retinol, tocopherols, and lipoproteins in preeclamptic and normotensive pregnant Zimbabwean women. Am J Hypertens 2003;16:665–72. [DOI] [PubMed] [Google Scholar]
- 163.Sharma JB, Kumar A, Kumar A, Malhotra M, Arora R, Prasad S, Batra S. Effect of lycopene on pre-eclampsia and intra-uterine growth retardation in primigravidas. Int J Gynaecol Obstet 2003;81:257–62. [DOI] [PubMed] [Google Scholar]
- 164.Palan PR, Mikhail MS, Romney SL. Placental and serum levels of carotenoids in preeclampsia. Obstet Gynecol 2001;98:459–62. [DOI] [PubMed] [Google Scholar]
- 165.Lorenzoni F, Giampietri M, Ferri G, Lunardi S, Madrigali V, Battini L, Boldrini A, Ghirri P. Lutein administration to pregnant women with gestational diabetes mellitus is associated to a decrease of oxidative stress in newborns. Gynecol Endocrinol 2013;29:901–3. [DOI] [PubMed] [Google Scholar]
- 166.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 2014;75:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leviton A, Allred EN, Fichorova RN, Kuban KC, O’Shea TM, Dammann O. Systemic inflammation on postnatal days 21 and 28 and indicators of brain dysfunction 2 years later among children born before the 28th week of gestation. Early Hum Dev 2016;93:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Robertson NJ, Tan S, Groenendaal F, van Bel F, Juul SE, Bennet L, Derrick M, Back SA, Valdez RC, Northington F, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr 2012;160:544–552.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed 2015;100:F541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Neerven S, Nemes A, Imholz P, Regen T, Denecke B, Johann S, Beyer C, Hanisch UK, Mey J. Inflammatory cytokine release of astrocytes in vitro is reduced by all-trans retinoic acid. J Neuroimmunol 2010;229:169–79. [DOI] [PubMed] [Google Scholar]
- 171.Gong X, Sambalingam D, Rubin LP. Lutein suppresses effects of brain injury in a neonatal rat model: implications for nutritional neuroscience. Proceedings of the Carotenoids Gordon Research Conference; 2016 May 22–27; Lucca (Italy).
- 172.Lingam I, Avdic-Belltheus A, Robertson NJ. Using animal models to improve care of neonatal encephalopathy. Arch Dis Child Educ Pract Ed 2016;101:271–6. [DOI] [PubMed] [Google Scholar]
- 173.Zhou X, Zhang F, Hu X, Chen J, Wen X, Sun Y, Liu Y, Tang R, Zheng K, Song Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol Behav 2015;151:412–20. [DOI] [PubMed] [Google Scholar]
- 174.Sherry CL, Oliver JS, Renzi LM, Marriage BJ. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J Nutr 2014;144:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lipkie TE, Morrow AL, Jouni ZE, McMahon RJ, Ferruzzi MG. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS One 2015;10:e0127729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perrone S, Longini M, Marzocchi B, Picardi A, Bellieni CV, Proietti F, Rodriguez A, Turrisi G, Buonocore G. Effects of lutein on oxidative stress in the term newborn: a pilot study. Neonatology 2010;97:36–40. [DOI] [PubMed] [Google Scholar]
- 177.Perrone S, Tei M, Longini M, Santacroce A, Turrisi G, Proietti F, Felici C, Picardi A, Bazzini F, Vasarri P, Buonocore G. Lipid and protein oxidation in newborn infants after lutein administration. Oxid Med Cell Longev 2014;2014:781454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, Hougaard D, Shankaran S, Carlo W. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 2010;67:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rubin LP, Chan GM, Barrett-Reis BM, Fulton AB, Hansen RM, Ashmeade TL, Oliver JS, Mackey AD, Dimmit RA, Hartmann EE, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol 2012;32:418–24. [DOI] [PubMed] [Google Scholar]
- 180.Gong X, Rubin LP. Role of macular xanthophylls in prevention of common neovascular retinopathies: retinopathy of prematurity and diabetic retinopathy. Arch Biochem Biophys 2015;572:40–8. [DOI] [PubMed] [Google Scholar]
- 181.Yeh PT, Huang HW, Yang CM, Yang WS, Yang CH. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One 2016;11:e0146438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lo HM, Chen CL, Yang PH, Tsou CJ, Chiang KW, Wu WB. The carotenoid lutein enhances matrix metalloproteinase-9 production and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RARβ activation in murine macrophages. J Leukoc Biol 2013;93:723–35. [DOI] [PubMed] [Google Scholar]
- 184.Ni Y, Zhuge F, Nagashimada M, Ota T. Novel action of carotenoids on non-alcoholic fatty liver disease: macrophage polarization and liver homeostasis. Nutrients 2016;8:E391. [DOI] [PMC free article] [PubMed] [Google Scholar]