Abstract
The perinatal period is a window of heightened plasticity that lays the groundwork for future anatomic, physiologic, and behavioral outcomes. During this time, maternal diet plays a pivotal role in the maturation of vital organs and the establishment of neuronal connections. However, when perinatal nutrition is either lacking in specific micro- and macronutrients or overloaded with excess calories, the consequences can be devastating and long lasting. The brain is particularly sensitive to perinatal insults, with several neurologic and psychiatric disorders having been linked to a poor in utero environment. Diseases characterized by learning and memory impairments, such as autism, schizophrenia, and Alzheimer disease, are hypothesized to be attributed in part to environmental factors, and evidence suggests that the etiology of these conditions may date back to very early life. In this review, we discuss the role of the early-life diet in shaping cognitive outcomes in offspring. We explore the endocrine and immune mechanisms responsible for these phenotypes and discuss how these systemic factors converge to change the brain’s epigenetic landscape and regulate learning and memory across the lifespan. Through understanding the maternal programming of cognition, critical steps may be taken toward preventing and treating diseases that compromise learning and memory.
Keywords: epigenetics, perinatal, learning and memory, psychiatric disease, nutritional programming, leptin, inflammation, IUGR, overnutrition
Introduction
Beginning at fertilization, the perinatal period lasts through gestation and lactation until weaning. In utero, maternal-fetal interactions are mediated via the placenta, an organ that regulates the exchange of oxygen and nutrients. The placenta also serves as a barrier that controls which endocrine and immune factors from maternal circulation reach the fetus. After birth, offspring continue to rely on the mother throughout lactation as their primary source of nutrition before completely transitioning to solid food. A majority of brain development occurs during the perinatal period. In humans, the brain begins to form ∼2 wk postconception and reaches 80% of its adult size by age 2 y (1). Early gestation is marked by neuronal proliferation, differentiation, and migration. Synaptogenesis then continues throughout gestation, peaks between gestational week 34 and postnatal year 1, and tapers off just before adolescence. Apoptosis and pruning concurrently counteract proliferation and synaptogenesis by eliminating redundant and inappropriate connections to fine-tune the neuronal circuitry.
Both human and animal studies have investigated the effects of perinatal nutrition on a wide variety of disease outcomes, but to our knowledge, relatively few have focused on intellect and cognitive performance. Not only are several neurologic and psychiatric diseases characterized by impaired cognition, but learning and memory are essential skills at all stages of life. Thus, understanding the biological mechanisms of cognitive programming is important both in the realm of clinical medicine and in the general population.
Perinatal Diet and Offspring Phenotype
Although perinatal diet has been shown to alter peripheral function, relatively little is known about how these nutritional conditions affect the central nervous system. Because of its involvement in hunger, satiety, and energy homeostasis, the hypothalamus has received the most attention. Perinatal dietary perturbations have been shown to alter neuropeptide Y (NPY) , pro-opiomelanocortin (POMC)6, leptin signaling, and insulin signaling in the arcuate nucleus of the hypothalamus, and therefore contribute to altered patterns of food intake and energy homeostasis (2).
Transgenerational programming of cognitive function has also been examined. Learning and memory is a broad category of cognition that consists of several subdomains, such as episodic, declarative, and procedural memory. Specific brain regions have been implicated in these processes. For instance, the hippocampus is necessary for spatial navigation learning, whereas the cerebellum is heavily responsible for motor skills–based procedural memory. Moreover, communication between regions increases the computational capacity and allows for greater information integration, as is seen in the connections between the hippocampus, parahippocampus, and neocortex (3). Such brain regions have been shown to undergo morphologic and functional changes in response to maternal diet, suggesting that higher cognitive processing may be shaped by perinatal nutrition (Table 1).
TABLE 1.
Evidence from animal models: neuronal dynamics and synaptic function1
| Reference | Treatment | Species | Offspring age | Effects |
| Perinatal undernutrition | ||||
| 4 | 50% caloric restriction during gestation and lactation | Rat | PND 90 | Reduced proliferation in the dentate gyrus |
| 5 | 50% caloric restriction during gestation and lactation | Rat | PND 70 | Reduced dendritic spine density in CA1 and altered spine morphology, including fewer mushroom spines |
| 6 | 50% caloric restriction during gestation and lactation | Rat | PND 70 | Lower density of nNOS-positive cells in CA1, CA3, and dentate gyrus; impaired performance on water maze task |
| 7 | 30% caloric restriction during gestation and lactation | Baboon | Embryonic day 90 | Increased proliferation and apoptosis in the SVZ, reduced BDNF at the cortical plate, and decreased gene expression of Bcl2 (antiapoptosis) and Ephb2 (axonogenesis and neurogenesis) |
| 8 | Protein restriction during gestation and lactation (6% protein) | Rat | PND 28 | Reduced cortical and hippocampal mass, decreased hippocampal BDNF concentrations, and impaired water maze performance |
| 9 | Protein restriction during gestation (6% protein) | Rat | PND 90 | Fewer neurons in CA1 |
| 10–12 | Protein restriction during gestation (6% protein) | Rat | PNDs 15, 30, 90, 120, 130–280 | Impaired LTP in the dentate gyrus |
| 13, 14 | Protein restriction during gestation (6% protein) | Rat | PND 21 | Increased frequency of miniature IPSCs in CA3 and CA1 |
| 15 | Protein restriction during gestation (6% protein) | Rat | PND 220 | Decreased serotonin sites and receptors in CA3 and dentate gyrus |
| 16 | Choline deficiency during gestation | Rat | Embryonic day 18 | Increased apoptosis in hippocampus and cortex |
| 17 | Iron deficiency during gestation until PND 7 | Rat | PND 65 | Reduced expression of Bdnf and Mbp; impaired performance on novel object recognition task |
| 18 | IUGR | Rat | PND 21 | Increased NR1 subunit, decreased NR2B, and decreased NR2A:NR2B ratio |
| 19 | IUGR | Rat | PNDs 1, 4, 8, 30 | Lower NR1 subunit expression in hippocampus |
| Perinatal overnutrition | ||||
| 20 | Prenatal exposure to 20% sucrose solution | Rat | PND 60 | Increased apoptosis in CA1, CA2, and CA3; impaired performance on water maze; altered protein expression: increased NR2B and increased caspase 3 (apoptosis) |
| 21 | 32% HFD during gestation and lactation | Mouse | PND 21 | Decreased hippocampal BDNF mRNA and protein, impaired performance on Barnes Maze |
| 22 | 32% HFD during gestation and lactation | Mouse | PNDs 21, 49, 70 | Reduced progenitor cell proliferation and neurogenesis in dentate gyrus |
| 23 | 35% HFD during gestation | Mouse | Neonatal | Increased Msi/Notch/Hes in neural stem cells |
| 24 | 45% HFD during gestation and lactation | Rat | PND 120 | Impaired performance on water maze task; lower hippocampal mRNA and protein BDNF, NGF, and ARC; lower hippocampal mRNA synaptophysin, NR2B, and NR2B:NR2A ratio |
| 25 | 60% HFD during gestation | Rat | Postnatal week 20 | Impaired retention in water maze task |
| 26 | 60% HFD during gestation | Mouse | Embryonic day 17 | Lower cell proliferation in dentate gyrus, higher proliferation in hippocampal and cortical SVZ, reduced hippocampal apoptosis, reduced early neuronal maturation |
| 27 | 60% HFD during gestation and lactation | Mouse | PND 90 | Increased BDNF in dorsal hippocampus, and increased 5-HT1A and GABAAα2 in ventral hippocampus |
ARC, activity-regulated cytoskeletal-associated protein; Bcl2, B cell lymphoma 2; BDNF, brain-derived neurotrophic factor; CA, cornu ammonis; Ephb2, Eph receptor B2; GABAAα2, γ-aminobutyric acid A α2 receptor; Hes, hairy and enhancer of split; HFD, high-fat diet; IPSC, inhibitory postsynaptic current; IUGR, intrauterine growth restriction; LTP, long-term potentiation; Mbp, myelin basic protein; Msi, musashi RNA binding protein; NGF, nerve growth factor; nNOS, neuronal nitric oxide synthase; Notch, neurogenic locus notch homolog protein; NR, N-methyl d-aspartate receptor; PND, postnatal day; SVZ, subventricular zone; 5-HT1A, serotonin receptor 1a.
Perinatal undernutrition
Perinatal undernutrition is often modeled by intrauterine growth restriction (IUGR), specific nutrient deficiencies, or general caloric restriction. IUGR is a condition in which the fetus does not reach full growth potential because of inadequate oxygen and nutrient supply (28, 29). IUGR produces lasting metabolic consequences, including glucose intolerance (30), hypertension (31), and impaired lipid metabolism (32). Moreover, MRI studies have demonstrated a correlation between IUGR and reduced hippocampal volume in children (33, 34). In addition to anatomical alterations, IUGR has been associated with negative cognitive outcomes, such as substandard intellectual capacity, poor spatial memory (35), lower performance in school (36), and lower intelligence quotient (37).
Animal models of IUGR mimic uteroplacental insufficiency by uterine artery ligation, effectively limiting blood supply and growth factor availability via the placenta (38). Along with nutrient deficiencies, this method also induces fetal hypoxia and hypoglycemia. Similar to observations in humans, animal studies have revealed changes in hippocampal neuron number and composition, as well as impaired learning and memory in response to IUGR (39–41).
IUGR is a well-studied fetal model, but because it incorporates hypoxia, nutrient deficiency, and stress, it is often difficult to discern whether a single factor or a combination of factors is responsible for offspring outcome. By concentrating specifically on nutrient and caloric deficits, exact mechanisms may be more clearly identified. The first alternative to IUGR is general caloric restriction. Studies have used maternal diets ranging from 15% to 50% of normal caloric intake (42, 43). Alternatively, specific nutrients may be reduced or completely excluded from the maternal diet. This includes micronutrients such as choline and iron or macronutrients such as protein. All 3 models have produced significant changes in the molecular substrates of learning and memory, as well as performance on hippocampus-dependent behavioral tests (Table 1).
Neuronal proliferation, maintenance, and apoptosis.
In humans and animal models, early childhood is marked by heightened synaptic connectivity followed by a period of synaptic pruning throughout adolescence. Any delay or imbalance in these processes can lead to undesirable cognitive outcomes later in life. Cellular processes such as neurogenesis, neuronal maintenance, and apoptosis are altered in response to early life malnutrition. For instance, general food restriction during gestation and lactation has been shown to reduce proliferation of dentate gyrus neurons in adult rat offspring (4, 9). Although less severe, this loss of proliferation was similar in rats exposed to malnutrition only during lactation, suggesting that the entire perinatal period is sensitive to nutritional insults. Furthermore, maternal undernutrition studies have reported decreases in dendritic spine density in the cornu ammonis 1 region of the hippocampus (5). Such morphologic differences have been attributed to altered levels of brain-derived neurotrophic factor (BDNF), insulin-like growth factor (IGF) 1, myelin basic protein, and glial neurotrophic factor S-100β, all of which are essential factors that enhance neuronal proliferation, growth, and maintenance (7, 8, 17).
Early life malnutrition disrupts apoptotic processes. During development, widespread neuronal apoptosis is particularly important in eliminating excessive neurons and glia. Data from the rhesus macaque cortex suggests a 25% reduction in dendritic spine density between postnatal weeks 8 and 12 (44). This decrease is sustained at postnatal week 36 and further into adulthood. When synaptic connections are not scaled down, cognitive deficits can surface, as is the case in fragile X syndrome and other intellectual disability disorders (45, 46). However, although early childhood pruning is necessary, excessive or inappropriate timing of apoptosis, especially during gestation, may result in insufficient or ineffective neuronal connections. Prenatal undernutrition has been demonstrated to increase apoptosis during fetal development. Fetal rats on a maternal choline-deficient diet showed higher amounts of cortical and hippocampal apoptosis than did controls (16). Similar results were seen in offspring of baboon mothers on a 30% calorically restricted diet (7). Caloric restriction resulted in reduced neuronal density and increased apoptosis in the subventricular zone of fetal brains collected halfway through gestation. This suggests an altered developmental time course, which may potentially lead to cognitive impairment later in life. Together, these studies indicate that perinatal malnutrition skews the balance between neurogenesis, maintenance, and apoptosis in the developing brain.
Synaptic function.
Several studies have investigated the effects of maternal undernutrition on synaptic dynamics in offspring. Synaptic transmission in the hippocampus is mediated primarily by excitatory glutamatergic synapses. In addition to the primary ionotropic glutamate receptors N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), the synapse involves numerous scaffolding proteins and neurotransmitters. Perinatal nutrient restriction has been shown to impair synaptic transmission by blunting long-term potentiation (LTP) and NMDA-independent post-tetanic potentiation (5, 10–12). This may have implications for cognition, because LTP is commonly recognized as the molecular basis of learning. Another study proposed that nutrition-induced deficits in synaptic transmission may be attributed to a decrease in synaptic NO, a retrograde mediator of LTP (6). Upon NMDA receptor activation, NO is synthesized in the postsynaptic neuron and diffuses into the synaptic cleft, where it then interacts with the presynaptic neuron by facilitating neurotransmitter release and mediating vesicle recycling. The observed reduction in hippocampal NO in response to maternal caloric restriction was accompanied by impaired spatial memory.
In addition to retrograde neurotransmitters, perinatal environment also affects glutamate neurotransmission via AMPA and NMDA receptors in the hippocampus. NMDA receptors are particularly important for synaptic plasticity, because they allow for a high influx of postsynaptic calcium, which can act as a second messenger to modify synapses (47). The NMDA receptor is a tetramer consisting of 2 N-methyl D-aspartate receptor (NR) 1 subunits, along with 2 NR2 or NR3 subunits. The NR2 subunit comes in 4 varieties, but the most well studied are the NR2A and NR2B varieties. These subunits differ in their calcium permeability, synaptic localization, and protein-binding partners. Over the course of the lifetime, the ratio of NR2A to NR2B increases and is believed to modulate synaptic plasticity (19, 48, 49). Several studies have shown that IUGR alters the composition and function of NMDA receptors in the hippocampus (18, 50). Such experiments suggest that a decrease in NR1 subunit expression may impair NMDA receptor availability and function. Studies also report changes in the ratio of NR2A to NR2B subunits in adolescent offspring exposed to IUGR (18). This may point to an altered developmental time course and subsequent learning and memory deficits.
Although the aforementioned studies have focused on synaptic changes within the hippocampus itself, hippocampal neurons also receive modulatory subcortical inputs primarily via inhibitory interneurons (51). Afferents from the raphe nuclei, the locus coeruleus, and the medial septum synapse onto intermediate γ-aminobutyric acid (GABA) neurons, which then project to the hippocampus. Previous studies have indicated that prenatal protein malnutrition enhances GABAergic inputs to the hippocampus and suggest that altered GABAergic function may be caused by changes in serotonergic inputs (13, 14). Indeed, protein-restricted rats showed a downregulation in the serotonin fiber density and uptake sites in the dentate gyrus and cornu ammonis 3 (15). These studies postulate that the decrease in inhibitory serotonergic inputs to GABA interneurons allows the GABA neurons to more effectively inhibit primary hippocampal neurons. Together, these studies suggest that maternal undernutrition impairs neurotransmitter and receptor efficacy within the hippocampus itself and increases inhibitory GABAergic inputs.
Perinatal overnutrition
Despite residing at opposite ends of the spectrum, maternal undernutrition and overnutrition produce strikingly similar cognitive outcomes in offspring (Table 1). As with perinatal malnutrition, maternal overweight and obesity have been linked to lower cognitive scores and delayed mental development in children (52–56). Animal models recapitulate these associations, showing that exposure to a perinatal high-fat diet (HFD) impairs hippocampal-dependent spatial memory and performance on the Morris Water Maze and the Barnes Maze (20, 21, 25). Maternal overnutrition in animal models induces hyperphagia by ad libitum consumption of a highly palatable HFD in which 32–60% of total energy is derived from fat. As with IUGR, obesity is a systemic state that elicits a wide variety of immune and endocrine changes that combine to produce lasting cognitive effects.
Neuronal proliferation, maintenance, and apoptosis.
Similar to perinatal undernutrition, maternal overnutrition has been shown to hinder nascent neuronal growth by altering differentiation and neurogenesis (22, 23). Downregulation of neurogenesis was reported at 3, 7, and 10 wk of age in mice exposed to an HFD in utero (22). Another study speculated that prenatal HFD-induced inhibition of neuronal differentiation was a consequence of upregulated neurogenic locus notch homolog protein (Notch) signaling and consequent suppression of proneural gene expression (23).
In addition to new neurons, perinatal nutrition may also affect maintenance of mature neurons. A maternal HFD has been linked to decreased concentrations of BDNF and nerve growth factor, both of which promote dendritic survival and synapse maintenance (21, 24). Indeed, the lower concentrations of BDNF in HFD-exposed offspring correlated with reduced total dendritic length and density. Furthermore, studies have observed disruptions in apoptotic processes in rat and mouse offspring that had been exposed in utero to a high-calorie maternal diet. Studies have found decreased pruning of mature neurons in the hippocampus during gestation (26), as opposed to an upregulation of apoptotic-related proteins after weaning (20). These findings may suggest that these animals are either unable to scale down excessive synapses or that they are unable to maintain important connections at the proper time points during development. Together, this indicates that the delicate balance between synaptic formation and pruning during the perinatal period is disrupted by overnutrition.
Synaptic function.
Perinatal HFD exposure alters synaptic function in the hippocampus. A recent study by Page et al. (24) examined the effects of both a perinatal and postweaning HFD on learning and memory in Sprague-Dawley rats. The offspring received either an HFD or an unpurified diet during the perinatal period followed by either an HFD or an unpurified diet after weaning. On postnatal day (PND) 110, offspring exposed to an in utero HFD performed worse on a Morris Water Maze regardless of their diet during adulthood. qPCR revealed that offspring of HFD-fed dams had reduced expression of neurotrophins in the hippocampus. Furthermore, the offspring showed a decrease in synaptophysin, an important presynaptic protein involved in vesicle docking and neurotransmitter release. They also displayed a lower ratio of NMDA receptor subunit NR2B to NR2A, which has been associated with altered channel conductance and long-term memory deficits (57). This suggests that a maternal HFD may interfere with learning and memory processes by altering dendritic spine maturation and synaptic transmission. In addition, the study highlights the permanence of perinatal phenotype, because the postweaning dietary intervention was unable to reverse the impaired behavioral performance.
In addition to the primary glutamatergic receptors, GABA receptors are also dysregulated in response to a perinatal HFD. Peleg-Raibstein et al. (27) found increases in GABAA receptor α2 subunit in the hippocampus in response to an HFD during gestation and lactation. This may suggest heightened subcortical inhibition in the hippocampus in response to a perinatal HFD. Multiple changes in receptor function and synaptic transmission within the hippocampus indicate that early life overnutrition can severely interfere with normal neuronal processes.
Systemic Physiologic Mechanisms
The maternal diet directs development by providing specific nutrients as building blocks for growth. However, nutrients do not act solely on the brain, but rather induce systemic changes that may also influence cognitive health. For example, HFD intake can alter hormone concentrations by promoting adiposity and increasing circulating leptin concentrations. Undernutrition may also affect endocrine function by inducing a stress response characterized by high glucocorticoid production (58). Furthermore, both maternal over- and undernutrition are associated with immunologic abnormalities that can interfere with offspring development. Nutrients themselves and the systems they activate then converge to modify the epigenome, changing the way genes and proteins are expressed in childhood and throughout adulthood.
Leptin
During pregnancy, maternally produced leptin and leptin produced by the placenta are transported to the fetus. Leptin receptors are expressed in the fetal brain as early as embryonic day 15 in mice and can bind leptin to promote brain development in utero (59). After birth, leptin concentrations in the newborn are in part dictated by the high concentrations of leptin in breast milk. However, it is unknown whether this leptin is biologically active or how much the offspring is able to absorb (60).
In the brain, leptin is perhaps best known for its role as a long-term satiety peptide. Leptin activates hypothalamic anorexigenic POMC/cocaine and amphetamine–related transcript pathways and inhibits orexigenic NPY/agouti-related protein neurons (61). Although the hypothalamus hosts the highest density of leptin receptors in the brain, leptin receptors are widely expressed in other areas, including the hippocampus and neocortex. Leptin also serves as a crucial neurotrophic factor during development and in adulthood (62). During the neonatal period, leptin concentrations surge between PNDs 4 and 15 in mice and rats, peaking at day 10 (63, 64). In mice, the leptin surge is critical in establishing connections in the hypothalamus and hippocampus (65–67). In animal models, leptin has been shown to enhance dendritic and synapse morphology, promote NMDA receptor-dependent synaptic transmission, and possibly improve learning and memory in adulthood (68, 69). Cortical development and neurogenesis are also dependent on leptin signaling (70). Here, leptin promotes axon development via MAPK and AKT pathways, such that the inhibition of MAPK or AKT activation prevents leptin-mediated axonal outgrowth (71). Leptin receptors at the axonal growth cone also regulate the expression of growth-associated protein 43 and proper axon formation.
Extreme leptin concentrations may have adverse effects on the developing brain. Both prolonged and intermittent caloric restriction result in lower leptin concentrations (72). Perinatal undernutrition has been shown to blunt the postnatal leptin surge in rat pups (73). Given leptin’s critical role in neurodevelopment, it is no surprise that the leptin deficiency observed in perinatal protein restriction results in altered hypothalamic connections, including a reduction in innervation of the paraventricular nucleus by agouti-related protein and α-melanocyte stimulating hormone fibers (74). Conversely, elevated caloric intake is marked by hyperleptinemia. Although leptin facilitates hippocampal growth and plasticity, the literature indicates that a maternal HFD is not beneficial to offspring cognition. It appears that the concentration and duration of leptin exposure determine molecular and behavioral outcomes. Moderate concentrations of leptin have been shown to improve memory, whereas high concentrations impair memory and synaptic transmission (75, 76). In addition, whereas acute neonatal leptin treatment has been shown to improve neurogenesis (77), the effect of long-term leptin exposure on the hippocampus and other brain regions remains largely unknown.
Further evidence suggests that, as in the hypothalamus, high leptin concentrations induce leptin insensitivity or even resistance in the hippocampus. In the hypothalamus, leptin resistance is attributed to reduced leptin transport across the blood-brain barrier, impaired leptin receptor trafficking, and aberrant leptin receptor signaling (78–83). Rat models suggest that overactivation of leptin pathways may produce similar effects in the hippocampus. Consumption of an HFD resulted in an elevation in hippocampal dendritic spine density, but impaired episodic memory measured by poor performance on a novel object recognition task. Despite the dendritic hypertrophy, offspring of HFD-fed dams displayed lower hippocampal AKT phosphorylation in response to a leptin challenge, suggesting a desensitization of this pathway (84). Thus, hyperleptinemia may desensitize signaling in the pathways necessary for proper neuronal morphology and subsequent learning and memory.
Another possibility is that poor perinatal nutrition inappropriately alters the timing of leptin-induced neurodevelopment in learning and memory–associated brain regions. Ugadawa et al. (85) suggested that low leptin concentrations maintained neural progenitor cells, whereas high concentrations promoted neuronal differentiation. Thus, the neonatal leptin surge may initiate differentiation and subsequent synaptic development. This hypothesis has been tested in the hypothalamus. Normally, leptin-deficient ob/ob mice display altered arcuate projections; however, this phenotype can be rescued with leptin injections from PND 4 to PND 12, suggesting that leptin serves a crucial function during this window of hypothalamic development (62). A handful of studies have investigated how the timing of the leptin surge affects neurodevelopment and brain function in the long term. In a mouse model, maternal undernutrition resulted in an earlier leptin surge, along with abnormal POMC neuron nerve fiber projections and elevated NPY and cocaine and amphetamine–related transcript nerve terminal densities in the hypothalamus (73, 86). Another rat study suggested that perinatal protein restriction delays and blunts the leptin surge and impairs neuronal projections to the paraventricular nucleus (74). Although these studies disagreed on which direction perinatal restriction shifts the leptin surge, they both demonstrated that any mistiming alters hypothalamic organization. Given the different developmental mechanisms and timelines for each brain region, it is difficult to predict whether the timing of leptin signaling would affect the hippocampus and cortical areas as severely as it does the hypothalamus. Although this avenue of research has yet to be fully explored, postnatal leptin injections in mice between PNDs 2 and 14 resulted in greater expression of synaptic proteins in the hippocampus, suggesting that an exaggerated leptin surge may indeed alter hippocampal development (87).
Inflammation
Systemic inflammation is a common consequence of obesity and HFD consumption. As adiposity increases, so does the number of macrophages present in white adipose tissue. These macrophages secrete cytokines and chemokines and inhibit the production of anti-inflammatory adiponectin, further perpetuating an inflamed state. There is also evidence that IUGR increases inflammatory response, with pregnant mothers and fetuses displaying elevated serum concentrations of inflammatory markers, including TNFα, IL-6, and C-reactive protein (88). Although peripheral inflammation is marked by a release of adipokines from white adipose tissue, neuroinflammation is mediated by microglia. Environmental disruptions and pathogens activate microglial toll-like receptors and initiate the release of cytokines, adhesion molecules, and free radicals. Although mediated by different cell types, peripheral and central inflammation are interdependent processes. Systemic inflammation has been shown to induce long-lasting neuroinflammation via TNFα and inflammatory cytokines that cross the blood-brain barrier (89). Furthermore, obesity has been demonstrated to induce the expression of cytokines and the proinflammatory transcription factor NF-κB in the hypothalamus (90).
Several inflammatory factors, including TNFα and IL-1b, mediate synaptic functions critical to learning and memory. Such factors can exert positive effects on synaptic dynamics. Specifically, TNFα mediates AMPA receptor expression and hippocampal development and function (91, 92), whereas IL-1b is necessary for LTP maintenance (93, 94). However, overactivation of microglia can have detrimental consequences at the synapse. Peripheral inflammation reduces LTP and long-term depression and alters AMPA-mediated currents in rat hippocampal slices (95). Moreover, the effects of IL-1b appear to be dose-dependent such that higher concentrations of IL-1b inhibit LTP through the activation of p38 and NF-κB signaling and reduced BDNF activity (96, 97). These molecular events correspond with impaired spatial memory and contextual fear conditioning (98, 99).
In addition to influencing neuronal function, maternal immune activation also appears to be a key player during neurodevelopment. Inflammatory cytokines have been shown to cross the placenta and the blood-brain barrier and have been measured in the uterus, fetal circulation, and the fetal brain (100). This implies that diet-induced inflammation may directly affect the developing fetus. Numerous studies have demonstrated the detrimental effects of maternal immune activation on offspring brain morphology and behavior. For instance, LPS administration during gestation has been shown to increase TNFα and IL-1b mRNA in the fetal brain and alter the glia cell population (101). Observed changes in cell density, reelin expression, and neurogenesis in the hippocampus further suggest that learning and memory may be particularly sensitive to maternal immune events (92, 102). Indeed, these morphologic changes are accompanied by a series of learning and memory-specific behavioral deficits, including impaired performance on the Morris Water Maze and novel object recognition task (92).
Maternal inflammation may impair offspring cognition by disrupting processes that mediate cell survival and neurogenesis. Evidence suggests that maternal cytokines may induce fetal microglia activation and cytokine production. A recent study demonstrated that a perinatal HFD increased Notch1/Hes family BHLH transcription factor 5 (Hes5) signaling in the hippocampus, suggesting inflammation-induced suppression of neuronal differentiation and neurogenesis (103). Furthermore, TNFα and IL-1b have been shown to serve cytotoxic roles in the brain. In vitro studies have shown that TNFα promotes neuronal death via activation of reactive oxygen species and the jun amino-terminal kinase pathway, as well as through higher glutamate production (104–108). Similarly, IL-1b can cause cell death by inducing the release of toxic concentrations of glutamate (107), and can prevent BDNF-dependent survival through the mechanistic target of rapamycin autophagy pathway (109, 110). In addition to neurons, oligodendrocytes are negatively affected by immune activation (111–113). With reduced glial support, the neuronal function may be even further compromised. Maternal inflammation changes apoptotic and proliferative processes in brain development and results in structural and functional abnormalities, as well as pervasive behavioral impairments.
Epigenetic Mechanisms
Epigenetics can be defined as factors that alter gene expression without changing primary DNA sequence. Epigenetic marks may fall into 1 of 3 categories: DNA methylation, histone modification, and noncoding RNA. DNA methylation is the process in which DNA methyltransferases (DNMTs) add methyl groups to DNA, typically to the five-carbon in a cytosine-guanine (CpG) sequence. Increased methylation, especially in the promoter region, recruits methyl CpG binding domain proteins and other repressive factors that close the chromatin and prevent the RNA polymerase II complex from initiating transcription. Transcription is also influenced by chromatin state, which is mediated in part by histone modifications. Methylation, acetylation, phosphorylation, or ubiquitination modifications at different positions on the histone tail may either activate or deactivate transcription by changing the way DNA wraps around the nucleosome. Finally, noncoding RNAs may interact with chromatin modifiers or act as molecular scaffolding to regulate large-scale epigenetic control of transcription and translation (114). MicroRNAs, for example, bind to the 3′ UTR of mRNA and recruit the RNA-induced silencing complex, which degrades the mRNA and prevents translation.
Unlike primary genetic sequence, epigenetic modifications are more labile and can be altered by environmental cues. DNA sequence only undergoes de novo mutations such as deletions, single-nucleotide polymorphisms, and copy number variations arising from extreme conditions such as cancer or environmental radiation. Epigenetic modifications, however, are much more sensitive to subtle environmental stimuli, having been shown to respond to conditions such as psychological stress and poor diet. However, epigenetic modifications attained during development become more solidified once the critical period ends. Thus, early-life nutritional disruptions can impair learning and memory across the lifespan.
Maternal programming refers to the impact of in utero environment on epigenetics and offspring health. The epigenome is easily shaped by environmental factors during the perinatal period. Large-scale epigenetic remodeling occurs after fertilization when a majority of the genome undergoes active and passive demethylation followed by de novo methylation (115). Because of the extensive crosstalk between histone marks and DNA methylation, there is evidence that histone modifications can recruit DNMTs or change chromatin accessibility in order to influence methylation state (116). This erasure and resetting of epigenetic marks is an opportune time for disruptions in the fetal environment to influence the epigenetic landscape.
To date, most studies have focused on the effects of perinatal nutrition on energy homeostasis and hypothalamic development (117, 118). For example, maternal malnutrition was shown to decrease methylation of the POMC and glucocorticoid receptor (GR) promoters and alter histone marks in the sheep hypothalamus (119, 120). Similarly, perinatal overnutrition altered hypothalamic feeding circuitry through hypermethylation of the POMC promoter (121). Moreover, these effects appear to be long-lasting, with altered methylation status not being reversed by nutritional intervention later in life (122). Outside the hypothalamus, a prenatal HFD is associated with altered reward circuitry via global and gene-specific DNA hypomethylation in the nucleus accumbens and prefrontal cortex (123). This includes increased expression of the dopamine transporter and preproenkephalin, accompanied by decreased methylation in their respective promoter regions. DNA methylation of the μ opioid receptor promoter, a gene that participates in food and drug reward, displayed increased promoter methylation, higher recruitment of methyl CpG binding protein 2 (MECP2), and a more inactive chromatin state in response to a perinatal HFD (124).
Less is known about the role of perinatal nutrition in the epigenetic programming of cognitive outcomes. One of the few studies that has attempted to combine diet, epigenetics, and cognition found that IUGR rats showed lower global DNA methylation, altered histone 3 (H3) acetylation, and decreased hippocampal expression of DNMT1, histone deacetylase (HDAC) 1, and MECP2 at birth than did controls (125). IUGR was also shown to target specific pathways in the rat hippocampus, including the PPAR, wingless-type MMTV integration site family (Wnt) signaling, and glucocorticoid pathways (126). Changes included reduced global H4K20 methylation in the hippocampus and downregulation of peroxisome proliferator–activated receptor γ (Pparg), SET domain lysine methyl transferase 8 (Setd8), and wingless type 3a (Wnt3a), genes that are crucial in proper neurodevelopment. Furthermore, IUGR and maternal undernutrition induced higher hippocampal GR expression and altered histone methylation and acetylation in the GR promoter (127, 128). Long-term glucocorticoid exposure has been linked to hippocampal damage and is hypothesized to render the brain more susceptible to injury (129, 130). Together, the evidence suggests that perinatal undernutrition may affect cognitive function via epigenetic mechanisms. In the remainder of the review, we will survey the direct nutrient mechanisms, as well as the systemic immunologic and hormonal mechanisms by which the epigenetic landscape is regulated.
Direct regulation: micro and macronutrients as cofactors and methyl donors.
Specific nutrients are structurally and functionally implicated in the epigenetic machinery. Because of their role as cofactors and methyl donors, specific micronutrients may be responsible for mediating epigenetic processes in response to the maternal diet. For instance, iron and zinc are 2 minerals that are critical to proper epigenetic modifier activity. Iron serves as a cofactor in jumonji C–mediated demethylation of various H3 lysine residues, whereas zinc is required for the catalytic function of the majority of HDACs and DNMT1 (131–133). In addition, zinc is a catalytic ion for enzymes that synthesize the methyl donors needed for DNA and histone methylation (134, 135). In an iron-deficient perinatal environment, rats had lower concentrations of BDNF and reduced expression of H3 demethylases in the hippocampus (136). Fetal iron deficiency was also shown to increase HDAC activity, alter histone methylation, and decrease DNA methylation at exon IV of Bdnf, resulting in lower transcription of hippocampal Bdnf (137). These effects were measured well into adulthood. Similarly, zinc deficiency results in decreased histone modification and DNA methylation in the murine liver (138).
In addition to serving as cofactors for epigenetic modifiers, micronutrients can themselves act as methyl donors in DNA and histone methylation processes. The roles of choline and folate in methylation events are well studied. Both are crucial components of one-carbon metabolism, a pathway involved in such functions as nucleic acid synthesis and repair, as well as the production of methyl donors (139). In this pathway, choline is oxidized to betaine, which may be used to generate methionine and the universal methyl donor S-adenosylmethionine (140). Folate, as with choline, also participates in the methionine cycle. Methylenetetrahydrofolate reductase reduces folate to 5-methyltetrahydrofolate, after which methionine synthase transfers its methyl group to homocysteine to form methionine and tetrahydrofolate and, in turn, S-adenosylmethionine (141). Padmanabhan et al. (142) demonstrated the importance of one-carbon metabolism and the methionine cycle in epigenetic regulation. When methionine synthase reductase, an activator of methionine synthase, is mutated, mice show aberrant DNA methylation, which can be inherited across generations. These results point to the necessity of adequate dietary methyl donors in maintaining a healthy epigenome.
In animal models, prenatal availability of nutrients involved in one-carbon metabolism are critical for methylation events later in life. When rats were fed either a choline deficient, supplemented, or control diet during embryonic days 11–17, fetuses of the rats on the choline-supplemented diet showed increased H3 lysine residue 9 dimethylation (H3K9Me2) and increased protein expression of histone lysine methyltransferases euchromatic histone lysine methyltransferasse 2 (G9A) and suppressor of variegation 3-9 homolog 1 (SUV39H1) in the cerebral cortex (143). Choline-deficient fetal mice had lower global H3 methylation, lower calbindin 1 mRNA expression, and lower hippocampal neuronal proliferation (144). In addition, different concentrations of prenatal choline perturbed the expression of DNMT1 and DNMT3 in the cortex (145). Choline also affected global and Igf2 gene DNA methylation, suggesting possible differences in neuronal proliferation and survival, as well as synaptic development (146–148).
Along with choline, maternal folate has been shown to alter DNA methylation and histone modifications. Children subjected to maternal folate supplementation had altered DNA methylation of the Igf2 gene (149). Severe maternal folate deficiency also results in neural tube defects (NTDs) in which the brain and spinal cord fail to develop normally. In one study, brain tissue was collected from fetuses that were diagnosed with NTDs from low maternal folate intake (150). Folate deficiency resulted in lower concentrations of H3 lysine residue 79 dimethylation and reduced H3 lysine residue 79 dimethylation binding at NTD genes. When given a diet deficient in the methyl donors folate, choline, and methionine, rats showed increased anxiety behavior and altered methylation of neurontin, a gene important for neonatal brain development (151).
As with micronutrients, macronutrients may serve as methyl donors and can interact with histone modifiers. For instance, amino acids play a large role in one-carbon metabolism. Methionine, an essential amino acid, acts as a substrate that is converted into S-adenosylmethionine. Thus protein deficiency may reduce methyl availability and subsequent DNA methylation. Fats can also act directly on epigenetic modifiers and methylation processes. For instance, SCFAs such as butyrate and acetate have been shown to inhibit HDACs and alter DNA methylation (152, 153). Furthermore, acetyl-CoA, the end-product of β oxidation, is a rate-limiting cofactor in histone acetylation (154, 155). ω-3 PUFAs are perhaps the most well-studied lipid mediators of brain development (156). Although they serve a variety of functions, reports have suggested that ω-3 FAs may modulate epigenetic events. In cells, ω-3 FA treatment suppresses the expression of enhancer of zeste 2 (Ezh2), an enzyme responsible for H3 lysine residue 27 trimethylation (157). DNA methylation also appears to be affected by ω-3 PUFA supplementation; however, it is unclear whether ω-3 FAs act primarily through their role as mediators of inflammation, structural components of membranes, or epigenetic modulators (158, 159). Because of the bidirectional recruitment and interaction between histone modifications and DNA methylation, fats have the potential to perturb chromatin state and severely affect transcription. Further study is necessary to discern the direct impact of macronutrients on epigenetic processes and how these affect learning and memory.
Systemic regulation: inflammation and leptin.
Another possibility is that macronutrient deficiency or overabundance may induce systemic changes that interfere with epigenetic processes. Extended periods of protein deficiency or excessive HFD consumption are often accompanied by altered hormonal and inflammatory states, as discussed previously. These factors can themselves induce epigenetic changes. In the case of inflammation, evidence suggests that cytokines exert epigenetic control by upregulating DNMT1 expression and inducing subsequent DNA hypermethylation (160). Perinatal models of inflammation have corroborated these findings in the mouse brain. Offspring that were subjected to inflammation-inducing polyinosinic-polycytidylic acid in utero displayed hypomethylation of long interspersed nuclear element 1 (LINE1) elements and the MECP2 promoter in the striatum and hypothalamus (161). In a similar experiment, maternal cytokine activation was shown to disrupt histone modification. Exposure to polyinosinic-polycytidylic acid late in gestation subtly altered concentrations of H3 lysine residue 4 trimethylation (H3K4Me3) in the cerebral cortex, resulting in impaired performance in a T-maze working memory task (162). Thus, maternal dietary perturbations that affect immune status may induce epigenetic changes in the brain that directly affect cognitive processes.
Similarly, early life diet can influence endocrine production. For instance, obesity and HFD intake is associated with hyperleptinemia. Neonatal leptin supplementation in a rat model decreased Pomc promoter methylation in the hypothalamus (163). Changes in methylation were not observed around the leptin receptor (Lepr) and suppressor of cytokine signaling 3 (Socs3) genes. Thus, it is unknown whether leptin would have affected DNA methylation of specific genes in the hippocampus important for learning and memory. In addition to DNA methylation, histone acetylation is also mediated by leptin. Leptin has been shown to act in a signal transducer and activator of transcription 3–dependent matter. One study showed that leptin-activated signal transducer and activator of transcription 3 binds the cyclin D1 promoter and recruits histone acetyltransferase steroid receptor coactivator 1 to increase acetylation at the locus (164). Additionally, leptin activates the HDAC sirtuin 1. In a leptin-deficient ob/ob mouse model, sirtuin 1 was shown to be necessary for leptin’s anorectic effects (165). In a human neuroblastoma cell line, increased sirtuin 1 activity was accompanied by lower histone acetylation in the NF-κB promoter and reduced amyloid B protein accumulation (166). This mechanism is particularly interesting, because it may account for the epidemiological data linking low circulating leptin, low hippocampal gray matter volume, and higher incidence of Alzheimer disease (167, 168).
Conclusions and Future Directions
Maternal diet is a major determinant of offspring health. Most studies have focused on metabolic consequences of perinatal nutrition; however, an expanding body of literature has recently begun to address the maternal programming of cognition and psychiatric disease. Both nutritional deficit and excess result in poor cognitive performance later in life. We propose that inadequate nutrition creates a dangerous in utero environment lacking adequate building blocks for neuronal function. Furthermore, systemic inflammation and hormonal imbalance add to this volatile milieu that can disrupt neurodevelopment and change the fetal epigenome. Consequently, the brain is equipped with insufficient anatomical and genetic machinery to carry out normal cognitive processes.
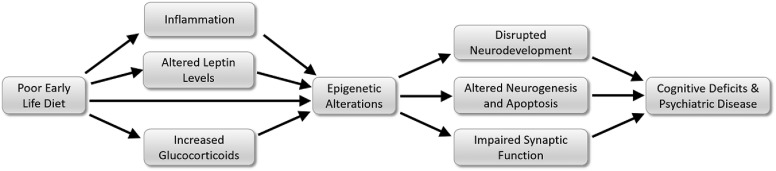
Diet influences every organ and body system, which can, in turn, affect brain health. However, although nutrition induces comprehensive changes, many of these effects, especially those involving immune and endocrine function, converge to alter epigenetic processes (Figure 1). Thus, epigenetics is an enticing area of future study. Future investigation should focus on understanding the route by which maternal diet alters the epigenome within specific brain regions and should track how such changes impact long-term cognitive outcomes and psychiatric health. Just as nutritional status is a systemic state, researchers should treat the epigenome as an integrated network. Rather than focus on epigenetic modifications of individual genes, future work should aim to understand how diet epigenetically regulates the entire learning and memory process. This includes examining the role of epigenetics in neuronal differentiation and survival, dendritic morphology, synaptic scaffolding, and neurotransmitter release and receptor function. By better understanding how early-life diet programs long-term cognitive health, effective steps may be taken to prevent and treat psychiatric disorders.
FIGURE 1.
A poor early-life diet creates a systemic state characterized by heightened inflammation, as well as leptin and glucocorticoid concentrations. These factors converge to induce epigenetic modifications that disrupt neuronal development and function, ultimately compromising cognitive performance.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; CpG, cytosine-guanine; DNMT, DNA methyltransferase; GABA, γ-aminobutyric acid;GR, glucocorticoid receptor; HDAC, histone deacetylase; HFD, high-fat diet; IGF, insulin-like growth factor; H3, histone 3; IUGR, intrauterine growth restriction; LTP, long-term potentiation; MECP2, methyl CpG binding protein 2; NMDA, N-methyl-D-aspartate; NR, N-methyl D-aspartate receptor; NTD, neural tube defect; PND, postnatal day; POMC, pro-opiomelanocortin.
References
- 1.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology 2010;35:147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García AP, Palou M, Priego T, Sanchez J, Palou A, Pico C. Moderate caloric restriction during gestation results in lower arcuate nucleus NPY- and alphaMSH-neurons and impairs hypothalamic response to fed/fasting conditions in weaned rats. Diabetes Obes Metab 2010;12:403–13. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 2000;1:41–50. [DOI] [PubMed] [Google Scholar]
- 4.Matos RJ, Orozco-Solis R, Lopes de Souza S, Manhaes-de-Castro R, Bolanos-Jimenez F. Nutrient restriction during early life reduces cell proliferation in the hippocampus at adulthood but does not impair the neuronal differentiation process of the new generated cells. Neuroscience 2011;196:16–24. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wei J, Yang Z. Perinatal undernutrition attenuates field excitatory postsynaptic potentials and influences dendritic spine density and morphology in hippocampus of male rat offspring. Neuroscience 2013;244:31–41. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Li N, Yang Z. Perinatal food restriction impaired spatial learning and memory behavior and decreased the density of nitric oxide synthase neurons in the hippocampus of adult male rat offspring. Toxicol Lett 2010;193:167–72. [DOI] [PubMed] [Google Scholar]
- 7.Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA 2011;108:3011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Xu RJ. The effects of perinatal protein malnutrition on spatial learning and memory behaviour and brain-derived neurotrophic factor concentration in the brain tissue in young rats. Asia Pac J Clin Nutr 2007;16(Suppl 1):467–72. [PubMed] [Google Scholar]
- 9.Lister JP, Tonkiss J, Blatt GJ, Kemper TL, DeBassio WA, Galler JR, Rosene DL. Asymmetry of neuron numbers in the hippocampal formation of prenatally malnourished and normally nourished rats: a stereological investigation. Hippocampus 2006;16:946–58. [DOI] [PubMed] [Google Scholar]
- 10.Austin KB, Bronzino J, Morgane PJ. Prenatal protein malnutrition affects synaptic potentiation in the dentate gyrus of rats in adulthood. Brain Res 1986;394:267–73. [DOI] [PubMed] [Google Scholar]
- 11.Bronzino JD, Austin La France RJ, Morgane PJ, Galler JR. Diet-induced alterations in the ontogeny of long-term potentiation. Hippocampus 1996;6:109–17. [DOI] [PubMed] [Google Scholar]
- 12.Bronzino JD, Austin-LaFrance RJ, Mokler D, Morgane PJ. Effects of prenatal protein malnutrition on hippocampal long-term potentiation in freely moving rats. Exp Neurol 1997;148(1):317–23. [DOI] [PubMed] [Google Scholar]
- 13.Chang YM, Galler JR, Luebke JI. Prenatal protein malnutrition results in increased frequency of miniature inhibitory postsynaptic currents in rat CA3 interneurons. Nutr Neurosci 2003;6:263–7. [DOI] [PubMed] [Google Scholar]
- 14.Luebke J, St John J, Galler JR. Prenatal protein malnutrition results in increased frequency of miniature inhibitory synaptic currents in rat CA1 pyramidal cells. Synapse 2000;37:23–31. [DOI] [PubMed] [Google Scholar]
- 15.Blatt GJ, Chen JC, Rosene DL, Volicer L, Galler JR. Prenatal protein malnutrition effects on the serotonergic system in the hippocampal formation: an immunocytochemical, ligand binding, and neurochemical study. Brain Res Bull 1994;34:507–18. [DOI] [PubMed] [Google Scholar]
- 16.Holmes-McNary MQ, Loy R, Mar MH, Albright CD, Zeisel SH. Apoptosis is induced by choline deficiency in fetal brain and in PC12 cells. Brain Res Dev Brain Res 1997;101:9–16. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy BC, Dimova JG, Siddappa AJ, Tran PV, Gewirtz JC, Georgieff MK. Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal-neonatal iron deficiency in rats. J Nutr 2014;144:1858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schober ME, McKnight RA, Yu X, Callaway CW, Ke X, Lane RH. Intrauterine growth restriction due to uteroplacental insufficiency decreased white matter and altered NMDAR subunit composition in juvenile rat hippocampi. Am J Physiol Regul Integr Comp Physiol 2009;296:R681–92. [DOI] [PubMed] [Google Scholar]
- 19.Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci 2004;24(40):8885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang H, Sun M, Lv J, Li J, Wu C, Chen N, Bo L, Wei X, Gu X, Liu Z, et al. Hippocampal apoptosis involved in learning deficits in the offspring exposed to maternal high sucrose diets. J Nutr Biochem 2014;25:985–90. [DOI] [PubMed] [Google Scholar]
- 21.Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, Wada K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int 2010;57:235–47. [DOI] [PubMed] [Google Scholar]
- 22.Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J 2009;23:1920–34. [DOI] [PubMed] [Google Scholar]
- 23.Yu M, Jiang M, Yang C, Wu Y, Liu Y, Cui Y, Huang G. Maternal high-fat diet affects Msi/Notch/Hes signaling in neural stem cells of offspring mice. J Nutr Biochem 2014;25:227–31. [DOI] [PubMed] [Google Scholar]
- 24.Page KC, Jones EK, Anday EK. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am J Physiol Regul Integr Comp Physiol 2014;306:R527–37. [DOI] [PubMed] [Google Scholar]
- 25.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis 2009;35:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci 2009;27:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res 2012;233:398–404. [DOI] [PubMed] [Google Scholar]
- 28.Ergaz Z, Avgil M, Ornoy A. Intrauterine growth restriction-etiology and consequences: what do we know about the human situation and experimental animal models? Reprod Toxicol 2005;20(3):301–22. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol 2003;110(Suppl 1):S99–107. [DOI] [PubMed] [Google Scholar]
- 30.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 2001;50:2279–86. [DOI] [PubMed] [Google Scholar]
- 31.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 2003;41:457–62. [DOI] [PubMed] [Google Scholar]
- 32.Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU. Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am J Physiol Regul Integr Comp Physiol 2001;280:R183–90. [DOI] [PubMed] [Google Scholar]
- 33.Lodygensky GA, Seghier ML, Warfield SK, Tolsa CB, Sizonenko S, Lazeyras F, Huppi PS. Intrauterine growth restriction affects the preterm infant’s hippocampus. Pediatr Res 2008;63:438–43. [DOI] [PubMed] [Google Scholar]
- 34.Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, Hanquinet S, Pfizenmaier M, Huppi PS. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 2004;56:132–8. [DOI] [PubMed] [Google Scholar]
- 35.Leitner Y, Heldman D, Harel S, Pick CG. Deficits in spatial orientation of children with intrauterine growth retardation. Brain Res Bull 2005;67(1–2):13–8. [DOI] [PubMed] [Google Scholar]
- 36.Paz I, Gale R, Laor A, Danon YL, Stevenson DK, Seidman DS. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet Gynecol 1995;85(3):452–6. [DOI] [PubMed] [Google Scholar]
- 37.Leitner Y, Fattal-Valevski A, Geva R, Eshel R, Toledano-Alhadef H, Rotstein M, Bassan H, Radianu B, Bitchonsky O, Jaffa AJ, et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol 2007;22(5):580–7. [DOI] [PubMed] [Google Scholar]
- 38.Wigglesworth JS. Morphological variations in the insufficient placenta. J Obstet Gynaecol Br Commonw 1964;71:871–84. [DOI] [PubMed] [Google Scholar]
- 39.Mallard C, Loeliger M, Copolov D, Rees S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience 2000;100(2):327–33. [DOI] [PubMed] [Google Scholar]
- 40.Fung C, Ke X, Brown AS, Yu X, McKnight RA, Lane RH. Uteroplacental insufficiency alters rat hippocampal cellular phenotype in conjunction with ErbB receptor expression. Pediatr Res 2012;72:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caprau D, Schober ME, Bass K, O’Grady S, Ke X, Block B, Callaway CW, Hale M, Yu X, McKnight RA, et al. Altered expression and chromatin structure of the hippocampal IGF1r gene is associated with impaired hippocampal function in the adult IUGR male rat. J Dev Orig Health Dis 2012;3:83–91. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds LK, Ho HP, Taper LJ. Effect of caloric restriction during pregnancy on maternal and fetal body composition in the obese Sprague-Dawley rat. J Nutr 1984;114:2247–55. [DOI] [PubMed] [Google Scholar]
- 43.Mackay H, Khazall R, Patterson ZR, Wellman M, Abizaid A. Rats perinatally exposed to food restriction and high-fat diet show differences in adipose tissue gene expression under chronic caloric restriction. Adipocyte 2013;2:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boothe RG, Greenough WT, Lund JS, Wrege K. A quantitative investigation of spine and dendrite development of neurons in visual cortex (area 17) of Macaca nemestrina monkeys. J Comp Neurol 1979;186:473–89. [DOI] [PubMed] [Google Scholar]
- 45.Galvez R, Gopal AR, Greenough WT. Somatosensory cortical barrel dendritic abnormalities in a mouse model of the fragile X mental retardation syndrome. Brain Res 2003;971:83–9. [DOI] [PubMed] [Google Scholar]
- 46.Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014;83:1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 2012;22:496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 1994;368:144–7. [DOI] [PubMed] [Google Scholar]
- 49.Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 2001;29(1):157–69. [DOI] [PubMed] [Google Scholar]
- 50.Cai Z, Rhodes PG. Intrauterine hypoxia-ischemia alters expression of the NMDA receptor in the young rat brain. Neurochem Res 2001;26:487–95. [DOI] [PubMed] [Google Scholar]
- 51.Halasy K, Miettinen R, Szabat E, Freund TF. GABAergic interneurons are the major postsynaptic targets of median raphe afferents in the rat dentate gyrus. Eur J Neurosci 1992;4(2):144–53. [DOI] [PubMed] [Google Scholar]
- 52.Hinkle SN, Schieve LA, Stein AD, Swan DW, Ramakrishnan U, Sharma AJ. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int J Obes (Lond) 2012;36:1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanda R, Salsberry PJ, Reagan PB, Fang MZ. The impact of prepregnancy obesity on children’s cognitive test scores. Matern Child Health J 2013;17:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012;129:e1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brion MJ, Zeegers M, Jaddoe V, Verhulst F, Tiemeier H, Lawlor DA, Smith GD. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011;127:e202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One 2012;7:e37758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui Z, Feng R, Jacobs S, Duan Y, Wang H, Cao X, Tsien JZ. Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci Rep 2013;3:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 2011;59:279–89. [DOI] [PubMed] [Google Scholar]
- 59.Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci USA 1997;94:11073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab 1997;82:4270–3. [DOI] [PubMed] [Google Scholar]
- 61.Blundell JE, Goodson S, Halford JC. Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes Relat Metab Disord 2001;25(Suppl 1):S29–34. [DOI] [PubMed] [Google Scholar]
- 62.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004;304:108–10. [DOI] [PubMed] [Google Scholar]
- 63.Devaskar SU, Ollesch C, Rajakumar RA, Rajakumar PA. Developmental changes in ob gene expression and circulating leptin peptide concentrations. Biochem Biophys Res Commun 1997;238:44–7. [DOI] [PubMed] [Google Scholar]
- 64.Rayner DV, Dalgliesh GD, Duncan JS, Hardie LJ, Hoggard N, Trayhurn P. Postnatal development of the ob gene system: elevated leptin levels in suckling fa/fa rats. Am J Physiol 1997;273:R446–50. [DOI] [PubMed] [Google Scholar]
- 65.Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology 2004;145:2621–6. [DOI] [PubMed] [Google Scholar]
- 66.Dhar M, Wayman GA, Zhu M, Lambert TJ, Davare MA, Appleyard SM. Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci 2014;34:10022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 1998;101:1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci 2001;21(24):RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci 2007;35(4):559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Udagawa J, Hashimoto R, Hioki K, Otani H. The role of leptin in the development of the cortical neuron in mouse embryos. Brain Res 2006;1120(1):74–82. [DOI] [PubMed] [Google Scholar]
- 71.Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, et al. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem 2006;281(18):12950–8. [DOI] [PubMed] [Google Scholar]
- 72.Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res (Phila) 2011;4:568–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 2008;149:470–5. [DOI] [PubMed] [Google Scholar]
- 74.Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 2010;151:702–13. [DOI] [PubMed] [Google Scholar]
- 75.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 2006;27(11):2738–49. [DOI] [PubMed] [Google Scholar]
- 76.Oomura Y, Aou S, Fukunaga K. Prandial increase of leptin in the brain activates spatial learning and memory. Pathophysiology 2010;17:119–27. [DOI] [PubMed] [Google Scholar]
- 77.Walker CD, Naef L, d’Asti E, Long H, Xu Z, Moreau A, Azeddine B. Perinatal maternal fat intake affects metabolism and hippocampal function in the offspring: a potential role for leptin. Ann N Y Acad Sci 2008;1144:189–202. [DOI] [PubMed] [Google Scholar]
- 78.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 2000;105:1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 1996;348(9021):159–61. [DOI] [PubMed] [Google Scholar]
- 80.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 1999;20(11):1341–5. [DOI] [PubMed] [Google Scholar]
- 81.Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes 2000;49:1219–23. [DOI] [PubMed] [Google Scholar]
- 82.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet 2009;18:1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med 2013;7:207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valladolid-Acebes I, Fole A, Martin M, Morales L, Cano MV, Ruiz-Gayo M, Del Olmo N. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol Learn Mem 2013;106:18–25. [DOI] [PubMed] [Google Scholar]
- 85.Udagawa J, Hatta T, Hashimoto R, Otani H. Roles of leptin in prenatal and perinatal brain development. Congenit Anom (Kyoto) 2007;47:77–83. [DOI] [PubMed] [Google Scholar]
- 86.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 2005;1:371–8. [DOI] [PubMed] [Google Scholar]
- 87.Walker CD, Long H, Williams S, Richard D. Long-lasting effects of elevated neonatal leptin on rat hippocampal function, synaptic proteins and NMDA receptor subunits. J Neurosci Res 2007;85:816–28. [DOI] [PubMed] [Google Scholar]
- 88.Visentin S, Lapolla A, Londero AP, Cosma C, Dalfra M, Camerin M, Faggian D, Plebani M, Cosmi E. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. Biomed Res Int 2014;2014:401595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007;55:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005;146:4192–9. [DOI] [PubMed] [Google Scholar]
- 91.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science 2002;295:2282–5. [DOI] [PubMed] [Google Scholar]
- 92.Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex 2004;14:97–105. [DOI] [PubMed] [Google Scholar]
- 93.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci USA 1998;95:7778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 2003;13:826–34. [DOI] [PubMed] [Google Scholar]
- 95.Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci 2015;35:4942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem 2003;278:19453–62. [DOI] [PubMed] [Google Scholar]
- 97.Tong L, Prieto GA, Kramar EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1beta via p38 mitogen-activated protein kinase. J Neurosci 2012;32:17714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oitzl MS, van Oers H, Schobitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res 1993;613:160–3. [DOI] [PubMed] [Google Scholar]
- 99.Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun 1998;12:212–29. [DOI] [PubMed] [Google Scholar]
- 100.Fidel PL Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75. [DOI] [PubMed] [Google Scholar]
- 101.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res 2000;47:64–72. [DOI] [PubMed] [Google Scholar]
- 102.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 2006;26:4752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendes-da-Silva C, Lemes SF, Baliani Tda S, Versutti MD, Torsoni MA. Increased expression of Hes5 protein in Notch signaling pathway in the hippocampus of mice offspring of dams fed a high-fat diet during pregnancy and suckling. Int J Dev Neurosci 2015;40:35–42. [DOI] [PubMed] [Google Scholar]
- 104.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience 2010;166:464–75. [DOI] [PubMed] [Google Scholar]
- 105.Dvoriantchikova G, Ivanov D. Tumor necrosis factor-alpha mediates activation of NF-kappaB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways. Eur J Neurosci 2014;40:3171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005;120:649–61. [DOI] [PubMed] [Google Scholar]
- 107.Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem 2013;125:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 2006;281:21362–8. [DOI] [PubMed] [Google Scholar]
- 109.Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging 2008;29:1380–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith ED, Prieto GA, Tong L, Sears-Kraxberger I, Rice JD, Steward O, Cotman CW. Rapamycin and interleukin-1beta impair brain-derived neurotrophic factor-dependent neuron survival by modulating autophagy. J Biol Chem 2014;289:20615–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tanner DC, Campbell A, O’Banion KM, Noble M, Mayer-Proschel M. cFLIP is critical for oligodendrocyte protection from inflammation. Cell Death Differ 2015;22:1489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res 2002;70:570–9. [DOI] [PubMed] [Google Scholar]
- 113.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 1996;19:331–8. [DOI] [PubMed] [Google Scholar]
- 114.Magistri M, Faghihi MA, St Laurent G III, Wahlestedt C. Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends Genet 2012;28:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 2002;3:662–73. [DOI] [PubMed] [Google Scholar]
- 116.Rose NR, Klose RJ. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta 2014;1839:1362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond) 2015;39:633–41. [DOI] [PubMed] [Google Scholar]
- 118.Gali Ramamoorthy T, Begum G, Harno E, White A. Developmental programming of hypothalamic neuronal circuits: impact on energy balance control. Front Neurosci 2015;9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Begum G, Stevens A, Smith EB, Connor K, Challis JR, Bloomfield F, White A. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J 2012;26:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 2010;151:3652–64. [DOI] [PubMed] [Google Scholar]
- 121.Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, Ziska T, Schellong K, Rodekamp E, Melchior K, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol 2009;587:4963–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marco A, Kisliouk T, Tabachnik T, Meiri N, Weller A. Overweight and CpG methylation of the Pomc promoter in offspring of high-fat-diet-fed dams are not “reprogrammed” by regular chow diet in rats. FASEB J 2014;28:4148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010;151:4756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vucetic Z, Kimmel J, Reyes TM. Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology 2011;36:1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, et al. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics 2006;25:16–28. [DOI] [PubMed] [Google Scholar]
- 126.Ke X, Xing B, Yu B, Yu X, Majnik A, Cohen S, Lane R, Joss-Moore L. IUGR disrupts the PPARgamma-Setd8–H4K20me(1) and Wnt signaling pathways in the juvenile rat hippocampus. Int J Dev Neurosci 2014;38:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ke X, Schober ME, McKnight RA, O’Grady S, Caprau D, Yu X, Callaway CW, Lane RH. Intrauterine growth retardation affects expression and epigenetic characteristics of the rat hippocampal glucocorticoid receptor gene. Physiol Genomics 2010;42:177–89. [DOI] [PubMed] [Google Scholar]
- 128.Lesage J, Dufourny L, Laborie C, Bernet F, Blondeau B, Avril I, Breant B, Dupouy JP. Perinatal malnutrition programs sympathoadrenal and hypothalamic-pituitary-adrenal axis responsiveness to restraint stress in adult male rats. J Neuroendocrinol 2002;14:135–43. [DOI] [PubMed] [Google Scholar]
- 129.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 1990;10:2897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci 2008;19:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell 2007;25:1–14. [DOI] [PubMed] [Google Scholar]
- 132.Marks PA, Xu WS. Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 2009;107:600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fatemi M, Hermann A, Pradhan S, Jeltsch A. The activity of the murine DNA methyltransferase Dnmt1 is controlled by interaction of the catalytic domain with the N-terminal part of the enzyme leading to an allosteric activation of the enzyme after binding to methylated DNA. J Mol Biol 2001;309:1189–99. [DOI] [PubMed] [Google Scholar]
- 134.Evans JC, Huddler DP, Jiracek J, Castro C, Millian NS, Garrow TA, Ludwig ML. Betaine-homocysteine methyltransferase: zinc in a distorted barrel. Structure 2002;10:1159–71. [DOI] [PubMed] [Google Scholar]
- 135.Maret W, Sandstead HH. Possible roles of zinc nutriture in the fetal origins of disease. Exp Gerontol 2008;43:378–81. [DOI] [PubMed] [Google Scholar]
- 136.Blegen MB, Kennedy BC, Thibert KA, Gewirtz JC, Tran PV, Georgieff MK. Multigenerational effects of fetal-neonatal iron deficiency on hippocampal BDNF signaling. Physiol Rep 2013;1:e00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tran PV, Kennedy BC, Lien YC, Simmons RA, Georgieff MK. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am J Physiol Regul Integr Comp Physiol 2015;308:R276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kurita H, Ohsako S, Hashimoto S, Yoshinaga J, Tohyama C. Prenatal zinc deficiency-dependent epigenetic alterations of mouse metallothionein-2 gene. J Nutr Biochem 2013;24:256–66. [DOI] [PubMed] [Google Scholar]
- 139.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 2012;23:853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van den Veyver IB. Genetic effects of methylation diets. Annu Rev Nutr 2002;22:255–82. [DOI] [PubMed] [Google Scholar]
- 141.Guéant JL, Namour F, Gueant-Rodriguez RM, Daval JL. Folate and fetal programming: a play in epigenomics? Trends Endocrinol Metab 2013;24:279–89. [DOI] [PubMed] [Google Scholar]
- 142.Padmanabhan N, Jia D, Geary-Joo C, Wu X, Ferguson-Smith AC, Fung E, Bieda MC, Snyder FF, Gravel RA, Cross JC, et al. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 2013;155:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem 2009;284:1982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J 2010;24:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem 2007;282:31777–88. [DOI] [PubMed] [Google Scholar]
- 146.Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res 2008;1237:124–35. [DOI] [PubMed] [Google Scholar]
- 147.Knusel B, Hefti F. Trophic actions of IGF-I, IGF-II and insulin on cholinergic and dopaminergic brain neurons. Adv Exp Med Biol 1991;293:351–60. [DOI] [PubMed] [Google Scholar]
- 148.Konishi Y, Takahashi K, Chui DH, Rosenfeld RG, Himeno M, Tabira T. Insulin-like growth factor II promotes in vitro cholinergic development of mouse septal neurons: comparison with the effects of insulin-like growth factor I. Brain Res 1994;649:53–61. [DOI] [PubMed] [Google Scholar]
- 149.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One 2009;4:e7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Q, Xue P, Li H, Bao Y, Wu L, Chang S, Niu B, Yang F, Zhang T. Histone modification mapping in human brain reveals aberrant expression of histone H3 lysine 79 dimethylation in neural tube defects. Neurobiol Dis 2013;54:404–13. [DOI] [PubMed] [Google Scholar]
- 151.Konycheva G, Dziadek MA, Ferguson LR, Krageloh CU, Coolen MW, Davison M, Breier BH. Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr Res 2011;31:790–804. [DOI] [PubMed] [Google Scholar]
- 152.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem 2008;19:587–93. [DOI] [PubMed] [Google Scholar]
- 153.Boffa LC, Mariani MR, Parker MI. Selective hypermethylation of transcribed nucleosomal DNA by sodium butyrate. Exp Cell Res 1994;211:420–3. [DOI] [PubMed] [Google Scholar]
- 154.Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol Cell 2006;23:207–17. [DOI] [PubMed] [Google Scholar]
- 155.Taylor EM, Jones AD, Henagan TM. A review of mitochondrial-derived fatty acids in epigenetic regulation of obesity and type 2 diabetes. J Nutrit Health Food Sci 2014;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res 2008;1237:35–43. [DOI] [PubMed] [Google Scholar]
- 157.Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis 2010;31:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boddicker RL, Koltes JE, Fritz-Waters ER, Koesterke L, Weeks N, Yin T, Mani V, Nettleton D, Reecy JM, Baumgard LH, et al. Genome-wide methylation profile following prenatal and postnatal dietary omega-3 fatty acid supplementation in pigs. Anim Genet 2016;47:658–71. [DOI] [PubMed] [Google Scholar]
- 159.Huang Q, Wen J, Chen G, Ge M, Gao Y, Ye X, Liu C, Cai C. Omega-3 polyunsaturated fatty acids inhibited tumor growth via preventing the decrease of genomic DNA methylation in colorectal cancer rats. Nutr Cancer 2016;68:113–9. [DOI] [PubMed] [Google Scholar]
- 160.Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, Munroe DJ, Farrar WL. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res 2005;65:4673–82. [DOI] [PubMed] [Google Scholar]
- 161.Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC, McAlonan GM. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry 2014;4:e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res 2012;140:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Palou M, Pico C, McKay JA, Sanchez J, Priego T, Mathers JC, Palou A. Protective effects of leptin during the suckling period against later obesity may be associated with changes in promoter methylation of the hypothalamic pro-opiomelanocortin gene. Br J Nutr 2011;106:769–78. [DOI] [PubMed] [Google Scholar]
- 164.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem 2007;282:13316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 2010;12:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marwarha G, Raza S, Meiers C, Ghribi O. Leptin attenuates BACE1 expression and amyloid-beta genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta 2014;1842:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 2009;302:2565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Narita K, Kosaka H, Okazawa H, Murata T, Wada Y. Relationship between plasma leptin level and brain structure in elderly: a voxel-based morphometric study. Biol Psychiatry 2009;65:992–4. [DOI] [PubMed] [Google Scholar]