Abstract
Research findings over the past several decades have shown that inflammation is a prominent feature of many chronic diseases, with poor diet being one likely inflammatory stimulus. Specifically, a single high-fat meal (HFM) has been suggested to increase inflammation, although there is currently no consensus with regard to the specific changes in many of the proinflammatory markers that are frequently assessed after an HFM. The aim of this systematic review was to objectively describe the postprandial timing and magnitude of changes in 5 common inflammatory markers: interleukin (IL) 6, C-reactive protein (CRP), tumor necrosis factor (TNF) α, IL-1β, and IL-8. Ten relevant databases were searched, yielding 494 results, of which 47 articles met the pre-established inclusion criteria: 1) healthy men and women aged 18–60 y, 2) consuming a single HFM (≥30% fat, ≥500 kcal), and 3) assessing relevant inflammatory markers postmeal for ≥2 h. The only marker found to consistently change in the postprandial period was IL-6: on average, from a baseline of ∼1.4 pg/mL, it peaked at ∼2.9 pg/mL ∼6 h post-HFM (an average relative change of ∼100%). CRP, TNF-α, IL-1β, and IL-8 did not change significantly in 79% (23 of 29), 68% (19 of 28), 67% (2 of 3), and 75% (3 of 4) of included studies, respectively. We conclude that there is strong evidence that CRP and TNF-α are not responsive at the usual time scale observed in postprandial studies in healthy humans younger than age 60 y. However, future research should further investigate the role of IL-6 in the postprandial period, because it routinely increases even in healthy participants. We assert that the findings of this systematic review on markers of inflammation in the postprandial period will considerably aid in informing future research and advancing clinical knowledge.
Keywords: cytokine, interleukin, C-reactive protein, postmeal, tumor necrosis factor
Introduction
Cardiovascular disease (CVD)8 is widely recognized to be the leading cause of death in the United States and throughout Western society (1). Lifestyle factors that appear to increase the risk of CVD include insufficient physical activity (2), obesity (3), and poor dietary habits (4). Although the causal factors leading to the manifestation of CVD are certainly complex and numerous, it has become clear that a common feature of heart and vascular diseases is inflammation (5). Atherosclerotic lesions, a prominent feature of CVD, are a hotbed of inflammatory activity. Briefly, immune cells such as T cells, macrophages, and mast cells will infiltrate into an atheromatous lesion, where they can perform the following: 1) promote prothrombotic factors, 2) cause the release of metalloproteinases and cysteine proteases that can reduce the stability of the atherosclerotic plaque, and 3) promote the release of proinflammatory cytokines, such as IL-6 and TNF-α (6). These acute markers of inflammation then travel to the liver, whereby they stimulate the increased release of chronic, low-grade markers of inflammation, such as C-reactive protein (CRP). Excessive inflammation is positively associated with type 2 diabetes, obesity, and coronary artery disease (7, 8).
But what are the stimuli that jumpstart the deleterious inflammatory cascade? A commonly suggested inflammatory stimulus is a chronic high-fat diet. Indeed, when rodents are fed a high-fat diet, there is an increase in markers of inflammation both in adipose tissue and in the systemic vasculature (9–11). Thus, a diet high in fat and overall energy may partly cause the elevated systemic inflammation that underpins CVD, as well as insulin resistance, and is associated with obesity (12). However, the effect of dietary consumption on inflammation may not be limited to chronic intake but may be evident after the consumption of a single meal.
To this end, numerous studies have been undertaken to investigate the effects of a single high-fat meal (HFM) on postprandial inflammation (see Table 1, Supplemental Tables 1–4). Many studies have found a significant increase in markers of systemic inflammation after an HFM, whereas others have found no changes. Study design variables that could potentially affect relevant findings and consequently precipitate inter-investigation differences include meal size, meal composition, subject characteristics, previous acute exercise, postprandial period assessment length, and method of drawing blood. As a result, we are currently far from consensus with regard to the response features (i.e., timing and magnitude) after an HFM of even the most commonly assessed markers of inflammation. A synthesis of previous research that investigated postprandial inflammation, with particular attention to the specific features of the response, would inform future research and advance clinical understanding.
TABLE 1.
Details of studies that assessed pre- and post-HFM IL-6 in healthy participants1
| Study (reference) | Meal | Blood draw method | S or P | MO | Fat,2 %E | Energy, kcal | n | M/ F, n/n | Age, y | BMI, kg/m2 | PPP, h | HFM response | TTP, h | Fasting3 IL-6, pg/ml | Peak4 IL-6, pg/mL | QA |
| Arjunan et al., 2013–South Asian (13) | White bread, butter, cheese, milkshake | Cannula | P | N | 57 | 14.3/kg | 10 | 10 M/0 F | 22.3 ± 1.3 | 25.4 ± 2.5 | 9 | ↑ | 9 | 1.31 ± 1.11 | 3.47 ± 1.43 | 6 |
| Arjunan et al., 2013–European (13) | White bread, butter, cheese, milkshake | Cannula | P | N | 57 | 14.3/kg | 10 | 10 M/0 F | 23.2 ± 2.0 | 25.2 ± 1.6 | 9 | ↑ | 6 | 1.25 ± 0.80 | 2.65 ± 1.40 | 6 |
| Arjunan et al., 2015–South Asian (14) | White bread, butter, cheese, milkshake | Cannula | P | N | 57 | 14.3/kg | 15 | 15 M/0 F | 24.0 ± 3.0 | 25.4 ± 3.3 | 9 | ↑ | 9 | 0.80 (0.50–1.28) | 6.39 ± 6.20 | 6 |
| Arjunan et al., 2015–European (14) | White bread, butter, cheese, milkshake | Cannula | P | N | 57 | 14.3/kg | 14 | 14 M/0 F | 22.0 ± 1.0 | 22.7 ± 2.2 | 9 | ↑ | 9 | 0.34 (0.21–0.55) | 4.48 ± 4.90 | 6 |
| Bidwell et al., 2014 (15) | Eggs, muffin, butter, sugary drink | Cannula | P | N | 40 | 600 | 22 | 11 M/11 F | M: 20.8 ± 0.7; F: 21.5 ± 0.9 | M: 23.9 ± 0.9; F: 21.1 ± 0.5 | 6 | ↑ | NS | NS | NS | 6 |
| Brandauer et al., 2013 (16) | Sugar, heavy cream, chocolate syrup, powdered milk | Cannula | P | Y | 84 | 1310 ± 34.1 | 10 | 10 M/0 F | 27 ± 1 | 24.6 ± 0.7 | 4 | ↑ | 4 | 0.64 ± 0.39 | 0.97 ± 0.64 | 4.5 |
| Burton-Freeman et al., 2012 (17) | Bagel, cream cheese, potato, milk, apple juice | Cannula | P | N | 46 | 852 | 25 | 13 M/12 F | 27 ± 8 | 22 ± 2 | 6 | ↑ | 6 | NS | NS | 5 |
| Caixàs et al., 2008–lean (18) | Liquid test meal | Cannula | P | Y | 30 | 750 | 7 | 6 M/1 F | 23.0 (21.0–26.0) | 20.3 (18.9–25.1) | 6 | ↑ | 6 | 0.61 (0.56–2.37) | 3.69 ± 1.13 | 4 |
| Caixàs et al., 2008–obese (18) | Liquid test meal | Cannula | P | Y | 30 | 750 | 7 | 6 M/1 F | 26.0 (23.0–27.0) | 43.9 (32.8–48.0) | 6 | ↑ | 6 | 1.75 (1.09–2.68) | 5.04 ± 0.71 | 4 |
| Campbell et al., 2006 (19) | Apple muffins, milkshake | Venipuncture | S | N | 41 | 976 | 15 | 15 M/0 F | 28 ± 9 | NS | 6 | ↓ | 4 | 1.6 ± 0.3 | 1.3 ± 0.3 | 6.5 |
| Delgado-Lista et al., 2011 (20) | NS | NS | P | N | 60 | NS | 45 | 45 M/0 F | NS | NS | 4 | NS | NS | NS | NS | 3.5 |
| Drew et al., 2014 (21) | Turkey burger, white bread | Cannula | P | N | 50 | 600 | 16 | 16 M/0 F | 45 ± 11 | 27.6 ± 5.3 | 6 | ↑ | 6 | 1.96 ± 1.70 | 3.35 ± 2.29 | 3.5 |
| Ehlers et al., 2014 (22) | Hamburger, French fries | Cannula | P | N | 39 | 1106 | 6 | 6 M/0 F | 44.3 ± 5.2 | 24.8 ± 2.5 | 8 | ↑ | 6 | NS | NS | 5 |
| Esser et al., 2013 (23) | Milkshake (cream, sugar, water) | Cannula | P | Y | 85 | 954 | 20 | 20 M/0 F | 22 ± 2 | 22.7 ± 2.4 | 6 | ↑ | 6 | 0.74 ± 0.24 | 1.38 ± 0.73 | 5 |
| Gill et al., 2003 (24) | Whipping cream, fruit, cereal, nuts, chocolate | Cannula | P | N | 67 | 1075 | 8 | 8 M/0 F | 27.8 ± 12.1 | 23.6 ± 1.0 | 6 | ↑ | 6 | 1.34 ± 1.16 | 6.93 ± 5.77 | 5 |
| Gregersen et al., 2012 (25) | Cheese, eggs, oil, cream, white bread | Cannula | P | Y | 77 | 928 | 15 | 4 M/11 F | 44 ± 3 | 26.3 ± 2.0 | 3 | ↑ | 3 | 0.87 ± 0.12 | 1.13 ± 0.1 | 3 |
| Harrison et al., 2009 (26) | Croissants, butter, ice cream, chocolate, potato crisps | Cannula | S | N | 60 | 1450 | 8 | 8 M/0 F | 26.9 ± 4.1 | 26.0 ± 3.6 | 6 | ↑ | 4 | 0.74 ± 0.45 | 3.13 ± 2.87 | 5.5 |
| Jiménez-Gómez et al., 2009 (27) | Butter, whole-meal bread, hard-boiled egg, whole milk | Venipuncture | P | Y | 60 | NS | 20 | 20 M/0 F | NS | NS | 9 | ↔ | — | NS | — | 6 |
| Johnson et al., 2016 (28) | Ice cream, whipping cream | Cannula | P | N | 45 | 1360–2160 | 12 | 12 M/0 F | 23.0 ± 3.2 | 24.5 ± 2.7 | 4 | ↔ | — | 29.8 ± 38.0 | — | 4 |
| Kiecolt-Glaser et al., 2015 (29) | Eggs, turkey sausage, biscuits, gravy | Cannula | S | N | 60 | 930 | 86 | 43 M/43 F | 38.22 ± 8.18 | 32.07 ± 5.83 | 7 | ↑ | 6.5 | 1.76 ± 4.03 | 4.34 ± 3.03 | 4.5 |
| Kračmerová et al., 2014 (30) | Pork meat, egg, French fries, hazelnut spread, croissant | Cannula | P | Y | 47 | 1470 | 10 | 10 M/0 F | 26.3 ± 1.04 | 23.11 ± 0.59 | 4 | ↑ | 4 | 0.899 ± 0.509 | 2.168 ± 0.44 | 3.5 |
| Lundman et al., 2007 (31) | Pasta, chicken, peas, mayonnaise | Venipuncture | P | Y | 60 | 1000 | 26 | 26 M/0 F | 51 ± 3 | 26.4 ± 3.3 | 6 | ↑ | 4 | 3.81 ± 3.49 | NS | 6.5 |
| Madec et al., 2011 (32) | Butter, bread, ham | NS | P | Y | 52 | 730 | 16 | NS | NS | NS | 6 | ↔ | — | 0.43 ± 0.27 | — | 4 |
| Mariotti et al., 2015 (33) | Milk cream, sucrose, whey protein | Cannula | P | N | 70 | 1200 | 10 | 10 M/0 F | 34 ± 9 | 30.2 ± 1.5 | 6 | ↔ | — | 3.66 ± 1.46 | — | 3.5 |
| Miglio et al., 2013 (34) | Fried potatoes, eggs, cheese, bread rolls | Cannula | P | Y | 52 | 1416 | 15 | 13 M/2 F | 45 ± 8 | 26.7 ± 1.9 | 8 | ↑ | 8 | 0.3 ± 0.3 | 0.97 ± 0.52 | 4 |
| Nappo et al., 2002 (35) | Sausage, bread, egg, butter, olive oil | Venipuncture | P | N | 59 | 760 | 20 | 10 M/10 F | 44 ± 5 | 26.8 ± 1.2 | 4 | ↑ | 2 | 1.9 ± 1.0 | 3.1 ± 1.0 | 5 |
| Payette et al., 2009–men (36) | Cheese, eggs, toast, butter, cream, milk, peanut butter | Cannula | P | Y | 64 | 1600–2200 | 39 | 39 M/0 F | 44.0 ± 9.1 | 28.9 ± 4.3 | 8 | ↑ | 8 | 2.40 ± 1.36 | 4.38 ± 2.35 | 5 |
| Payette et al., 2009–women (36) | Cheese, eggs, toast, butter, cream, milk, peanut butter | Cannula | P | Y | 64 | 1600–2200 | 41 | 0 M/41 F | 43.7 ± 9.4 | 26.5 ± 5.7 | 8 | ↑ | 8 | 2.77 ± 1.81 | 5.83 ± 3.49 | 5 |
| Peluso et al., 2012 (37) | Fried potatoes, eggs, cheese, bread | Cannula | P | N | 55 | 1344 | 14 | 12 M/2 F | 45.1 ± 8.6 | 26.8 ± 2.2 | 8 | ↑ | 8 | 0.39 ± 0.27 | 1.09 ± 0.20 | 2.5 |
| Phillips et al., 2013–lean (38) | Bacon, egg, muffin, hash browns, milk | Cannula | P | N | 52 | 989 | 10 | 10 M/0 F | 43.4 ± 11.3 | 22.8 ± 1.5 | 6 | ↑ | 6 | 0.9 ± 0.3 | NS | 6 |
| Phillips et al., 2013–obese (38) | Bacon, egg, muffin, hash browns, milk | Cannula | P | N | 52 | 989 | 10 | 10 M/0 F | 40.9 ± 9.8 | 38.2 ± 6.7 | 6 | ↑ | 6 | 2.0 ± 1.3 | NS | 6 |
| Poppitt et al., 2008 (39) | Blueberry muffin | Cannula | S | Y | 71 | 748 | 18 | 18 M/0 F | 23 ± 4 | 22.9 ± 2.0 | 6 | ↑ | 6 | 29.3 ± 16.8 | 33.4 ± 16.4 | 6.5 |
| Rankin et al., 2008 (40) | Eggs, sausage, biscuit, pancake, jelly candy | Venipuncture | S | Y | 53 | 900 | 17 | 8 M/9 F | 26.5 ± 7.6 | 33.5 ± 6.7 | 4 | ↔ | — | 1.5 ± 1.1 | — | 5.5 |
| Sanders et al., 2011 (41) | Muffin, milkshake | Cannula | P | N | 53 | 846 | 50 | 25 M/25 F | M: 25.4 ± 4.2; F: 24.2 ± 6.3 | M: 23.3 ± 2.1; F: 23.7 ± 3.4 | 8 | ↑ | 8 | 0.4 ± 0.1 | 0.9 ± 0.2 | 5 |
| Schmid et al., 2015 (42) | Bread, salami, palm fat, boiled eggs | Cannula | P | Y | 61 | 1005 | 21 | 21 M/0 F | 41.8 ± 9.0 | 27.1 ± 8.2 | 6 | ↑ | 6 | 3.0 ± 1.1 | 5.1 ± 1.9 | 4.5 |
| Schwander et al., 2014–NW (43) | Bread, salami, palm fat, boiled eggs | Cannula | S | Y | 61 | 1000 | 19 | 19 M/0 F | 40.6 ± 9.2 | 23.6 ± 1.4 | 6 | ↔ | — | 20.1 ± 1.7 | — | 4.5 |
| Schwander et al., 2014–obese (43) | Bread, salami, palm fat, boiled eggs | Cannula | S | Y | 61 | 1000 | 17 | 17 M/0 F | 44.1 ± 8.0 | 38.8 ± 4.9 | 6 | ↔ | — | 17.9 ± 1.7 | — | 4.5 |
| Schwander et al., 2014–NW (43) | Bread, salami, palm fat, boiled eggs | Cannula | S | Y | 61 | 1500 | 19 | 19 M/0 F | 40.6 ± 9.2 | 23.6 ± 1.4 | 6 | ↔ | — | 19.8 ± 1.9 | — | 4.5 |
| Schwander et al., 2014–obese (43) | Bread, salami, palm fat, boiled eggs | Cannula | S | Y | 61 | 1500 | 17 | 17 M/0 F | 44.1 ± 8.0 | 38.8 ± 4.9 | 6 | ↑ | 4 | 16.7 ± 1.5 | 21.7 ± 1.9 | 4.5 |
| Strohacker et al., 2012 (44) | Sausage, egg, cheese, biscuit, hash browns | Cannula | P | N | 59 | 1070 | 8 | 4 M/4 F | 21 ± 3 | 23.1 ± 3.9 | 3 | ↔ | — | 1.7 ± 0.9 | — | 5 |
| Teng et al., 2011 (45) | Mashed potatoes, baked beans, milk, orange juice, lard | NS | S | N | 60 | 683 | 10 | 10 M/0 F | 21.9 ± 0.7 | 21.0 ± 1.6 | 4 | ↔ | — | 14.5 ± 1.0 | — | 5.5 |
| Tholstrup et al., 2011 (46) | Mashed potatoes with fat powder | NS | S | Y | 76 | 620 | 10 | 0 M/10 F | 38.2 ± 10.7 | 20.9 ± 1.3 | 6 | ↓ | 4 | 0.81 ± 0.57 | 0.6 ± 0.3 | 5 |
| Twickler et al., 2003 (47) | Liquid cream meal | NS | P | Y | 40 | NS | 10 | 6 M/4 F | 48.6 ± 7.7 | 25.4 ± 1.6 | 24 | ↑ | 10 | 0.9 ± 0.7 | 3.4 ± 2.3 | 4 |
| Volek et al., 2008 (48) | Whipping cream, pudding, macadamia nuts | Cannula | P | Y | 84 | 908 | 30 | 16 M/14 F | 30 ± 8 | 24.1 ± 4.3 | 6 | ↑ | 3 | 0.85 ± 0.84 | 1.42 ± 1.36 | 6 |
| Wood et al., 2011 (49) | Fast-food burger, hash browns | Venipuncture | P | N | 49 | 919 | 21 | 9 M/12 F | 49.6 ± 4.6 | 24.0 ± 0.7 | 4 | ↑ | 4 | 0.8 ± 0.2 | 1.1 ± 4.4 | 4.5 |
Values are means ± SDs or means (ranges) unless otherwise indicated. Forty-five studies met the inclusion criteria and assessed IL-6 before and after the consumption of an HFM. If studies separately assessed different groups, those specific subsets are specified alongside the study. HFM, high-fat meal; MO, main outcome (whether or not IL-6 was the main outcome being studied); N, no; NS, not stated; NW, normal weight; P, plasma; PPP, length of postprandial period assessment; QA, quality appraisal; S, serum; TTP, time to peak or maximal observed concentration if a significant change was detected; Y, yes; ↑, significant increase; ↓, significant decrease; ↔, no significant change; —, not applicable; %E, percentage of energy.
Percentage of energy in the test meal from fat.
Baseline or fasting concentration of the marker.
Peak or maximal observed concentration of the marker.
Therefore, the purpose of this systematic review was to characterize the postprandial inflammatory response, in terms of magnitude and timing, to an HFM in healthy men and women (aged 18–60 y) on the basis of the consolidated findings of previous relevant investigations. The markers of inflammation included in the present review include IL-6, IL-1β, IL-8, TNF-α, and CRP, because these are frequently assessed inflammatory markers in the postprandial period.
Methods
Inclusion criteria.
To be incorporated into the present systematic review, there were multiple inclusion criteria that each study was required to meet. Individuals being assessed had to be men or women aged 18–60 y. Participants had to be healthy and not diagnosed with any chronic disease. Studies featuring overweight and obese participants were included in the analyses as long as they did not present with any other chronic disease. The study must have included an HFM challenge that provided ≥500 kcal of energy with ≥30% of the energy from fat. The study needed to feature a single meal or studies with serial meals provided in the postprandial period were included if there were data included for time points before the second meal. Any data reported after a second meal were excluded from the present analyses. If a study contained multiple meal trials or subsets of participants, each meal or participant group that met the inclusion criteria was considered separately. If a study included an exercise session, only the control (no exercise) condition was included. To be included, each study must have assessed ≥1 of the previously stated markers of inflammation (IL-6, IL-1β, IL-8, TNF-α, and CRP), both at baseline (fasting) and in the postprandial period for ≥2 h. Only data from full-text, peer-reviewed, and published articles were included (i.e., data from conference proceedings, abstracts, and textbooks were not included). There were no restrictions on year of publication, but only English-language articles were included. If a study did not satisfy all of the aforementioned criteria, it was excluded from the present systematic review.
Search strategy.
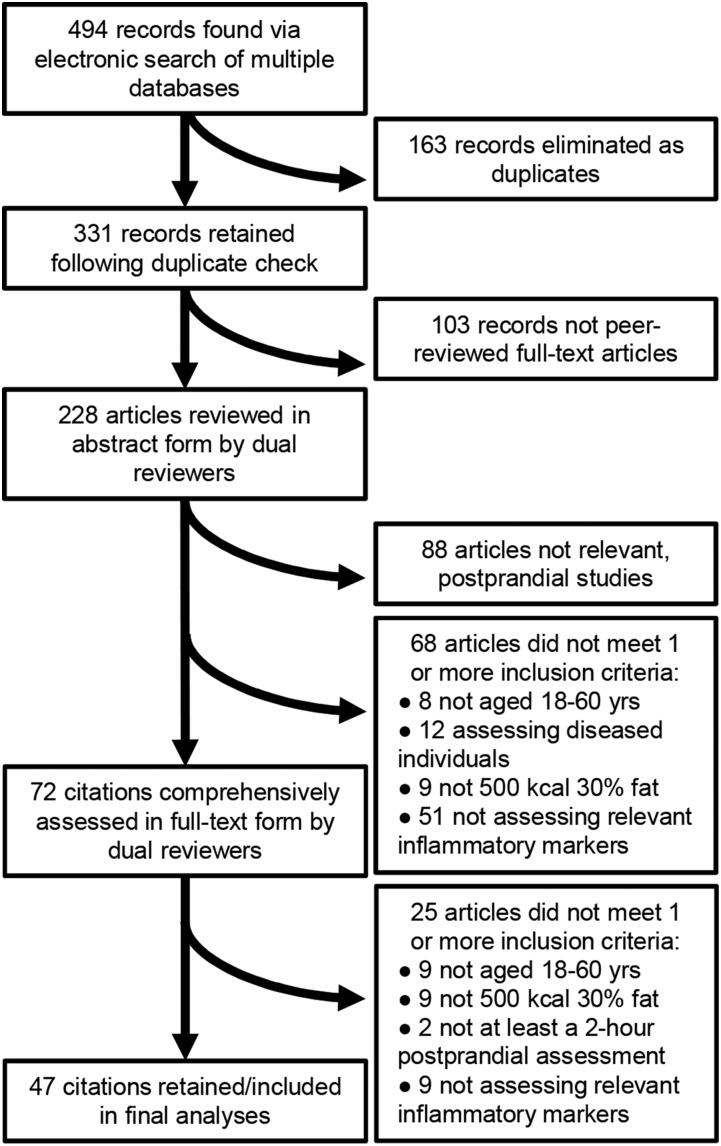
Article searches occurred in January and February of 2016. Automatic searches recurred weekly throughout manuscript preparation in order to capture very recent publications. However, recurring automatic searches produced no new articles. Databases that were searched include the following: NCBI Pubmed, Scopus, Proquest Nursing and Allied Health, Web of Science, Cochrane Library, SpringerLink, SPORTdiscus, Health and Wellness Resource Center, Health Reference Center Academic, and PsycINFO. Key search terms were as follows: “postprandial” or “post prandial,” “high fat meal,” “inflammat*” or “cytokine or interleukin,” “healthy” or “normal,” and “humans” or “men” or “women.” Appropriate search modifiers were used to exclude “children,” “elder*,” “rodents,” “rats,” and “mice.” Articles retrieved during searching were imported into and cataloged by using RefWorks reference management software (ProQuest LLC). The process and results of the systematic search are shown in Figure 1. There were 494 total citation hits from all databases combined. Of these, 163 citations were eliminated as duplicates (75 duplicates were retained). Of the 331 citations that passed the duplicate check, 103 citations were eliminated on the grounds of not being full-text, peer-reviewed research articles. The remaining 228 articles were assessed on the basis of the aforementioned criteria, in abstract-form only, by 2 independent reviewers (SRE and SPK). The reviewers then met to discuss the inclusion or exclusion of each abstract, and 156 articles were eliminated. Of the eliminated articles, 88 articles were eliminated due to not being postprandial studies assessing responses to a meal. The other 68 abstracts that were postprandial studies were eliminated due to not testing humans aged 18–60 y (8 articles), participants presenting with a chronic disease (12 articles), not using test meals that were ≥500 kcal and 30% fat (9 articles), and/or not testing ≥1 of the relevant inflammatory markers (51 articles). The remaining 72 articles were retrieved in full-text form and thoroughly assessed by both reviewers. Finally, 25 articles were eliminated after full-text assessment, leaving 47 articles to be included in the final analyses. The reasons for the eliminated 25 full-text articles are detailed in Figure 1. Some abstracts and full-text articles were eliminated for not complying with multiple inclusion criteria.
FIGURE 1.
Flowchart of article search and selection process. Ten relevant databases were searched, yielding 494 total citations. The final number of citations included in the present study was 47. See Methods section for more details.
Data extraction.
Information with regard to the test meal (composition and fat and energy content), participants (number of participants, male-to-female ratio, and age and BMI of participants), blood draw method (cannula or repeated venipuncture), and length of postprandial assessment was extracted from each study. In addition, for each inflammatory marker of interest (IL-6, IL-8, IL-1β, TNF-α, and CRP), the following information was extracted: whether or not the marker significantly changed from baseline, the fasting value, the time to peak or nadir (if applicable), and the peak or nadir value (if applicable). For many studies, all of the necessary information was not explicitly included in the article. In these cases, authors were directly contacted to obtain the missing information. Many authors provided the missing information (see Acknowledgments), although some did not; thus, some data are missing from the present analyses.
Assessing risk of bias.
A quality-appraisal or risk-of-bias assessment was conducted for all 47 articles included in the analyses. All of the included studies used the same general study design; therefore, traditional quality-appraisal assessment tools were not applicable to the present systematic review. Consequently, we developed an internal validity checklist to assess the strength of each study (Supplemental Appendix A). This tool included 9 criteria based on different components of postprandial inflammation study design: control of diet, fasting quality control, control of exercise, sample size adequacy, postprandial period length, blood draw frequency, blood draw method, normalization of test meal, and proper processing of inflammatory marker analyses. Each study was assessed against the above criteria and given a score ranging from 0 to 9. Studies were scored separately by 2 independent reviewers (SRE and SPK), after which the reviewers met to confirm a final score for each study.
Statistical analyses.
Data analyses were performed by using GraphPad Prism (version 6.05; GraphPad Software) and SPSS Statistics software (version 22; IBM Corporation). The primary outcome measures in this review were mean baseline and peak values and the time-to-peak value for each marker of inflammation (in some studies, certain inflammatory markers were found to decrease, in which case the nadir and time-to-nadir values were used). Fasting and peak values for each inflammatory marker were assessed for objective statistical outliers by using the robust regression and outlier removal (ROUT) method (50), which utilizes nonlinear regression, in GraphPad Prism. The Q value (or maximum false-discovery rate) was set at 1%. The ROUT method involves 3 steps. First, a robust nonlinear regression is used to fit a curve that is not affected by outliers. Second, the residuals of the robust regression are assessed to determine whether or not there are any outliers. The third step of the ROUT method is simply removing the outliers from the data set. Nine outliers (7 fasting values and 2 peak values) were removed from the IL-6 data set, 1 outlier (a fasting value) was removed from the CRP data set, and 7 outliers (4 fasting values and 3 peak values) were removed from the TNF-α data set. The data sets for IL-8 and IL-1β were too small for outlier analyses; thus, all values were retained. For analyses of changes and differences between baseline and peak values, if there was no significant change postmeal for a given marker in a study, the baseline value was used as both the fasting value and the peak value in order to have a complete data set and not bias the findings toward a significant change. Many studies did not report a post-HFM value in the event of no significant change. Thus, in the original analyses, in all studies that did not find a significant change after the meal, the baseline value was used as the postmeal value. However, a secondary analysis that included post-HFM values of studies that did not find a significant change (when available) did not appreciably change the results. In addition, the studies that did report a nonsignificant postmeal value generally did not find a biologically significant change either. A paired t test was used to assess differences between mean baseline and peak IL-6 values from each study. This analysis was only performed for IL-6 because most studies found no significant change in the other markers postmeal. Spearman rank correlations were used to assess relations between percentage change in IL-6 (from fasting to peak) and mean participant BMI, mean participant age, energy content (in kilocalories) of the test meal, and percentage of energy content from fat in the test meal. Because BMI and percentage change in IL-6 were not normally distributed, they were transformed by using the Log-10 method. Data are presented as means ± SDs. For all relevant analyses, the P value was set at <0.05.
Results
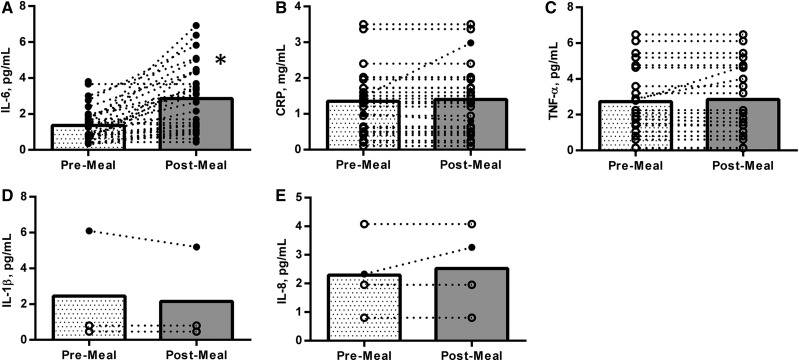
Table 1 and Supplemental Tables 1–4 show the extracted information from each study separated by the respective marker of inflammation. Some studies are represented in multiple tables, because they assessed >1 of the relevant markers of inflammation. For IL-6, 32 of 45 studies (∼71%) found a significant increase in the marker after HFM consumption. Ten studies (∼22%) found no change in IL-6 post-HFM, 2 studies (∼4%) found a significant decrease, and 1 study did not report whether or not the marker changed. Of the 32 studies that found a significant increase, 2 used repeated venipuncture and 30 used a cannula. In studies that found a significant change post-HFM, the time to peak was 5.9 ± 2.0 h. For 22 of the 34 studies that found a significant postprandial IL-6 change, the mean peak value occurred at the final time point assessed in the study protocol. Before the removal of outliers, the baseline (fasting) and peak IL-6 values for all studies combined were 4.83 ± 8.02 and 4.76 ± 6.87 pg/mL, respectively. After outlier removal, the mean baseline and peak values were 1.37 ± 0.93 and 2.85 ± 1.85 pg/mL, respectively. The mean percentage change from baseline to peak (after outlier removal) was 153% ± 256%. After the removal of outliers, but including studies that found no change or a decrease in IL-6 post-HFM, there was a significant increase (P < 0.0001) in IL-6 from baseline to peak value. Figure 2A shows the change in IL-6 from fasting to peak values for each individual study (after outlier removal) and the mean response.
FIGURE 2.
Post-HFM responses for the 5 assessed cytokines in healthy participants. The panels represent the change from fasting or baseline concentrations to the peak or maximally observed concentrations for IL-6 (A), CRP (B), TNF-α (C), IL-1β (D), and IL-8 (E). Open circles represent individual studies, and bars represent mean values. For markers other than IL-6, filled circles represent studies that found a significant change from pre- to postmeal, and open circles represent studies that found no significant postprandial change (difficult to differentiate with IL-6). For IL-6, CRP, and TNF-α, the data presented are after the removal of formal outliers. *Significant increase in IL-6 from fasting to peak value, P < 0.05. CRP, C-reactive protein; HFM, high-fat meal.
Twenty-nine studies met the inclusion criteria and measured CRP in the postprandial period. Of these, 23 studies (∼79%) found no change in CRP in the assessed postprandial period. Four studies (∼14%) found an increase and 2 studies (∼7%) found a decrease in CRP after the meal. Of the studies that found an increase, the peak occurred at 4.7 ± 2.3 h post-HFM. The fasting values for CRP were 1.50 ± 1.15 g/L before outlier removal and 1.35 ± 0.86 g/L after outlier removal. Three of the 4 studies that found a significant increase in CRP post-HFM did not report the peak value; thus, a mean peak value was not calculated. Figure 2B shows the mean and individual responses of CRP to an HFM for all studies.
TNF-α was assessed in 28 of the included studies. Five studies (∼18%) found an increase, 4 studies (∼14%) found a decrease, and 19 studies (∼68%) found no significant change in TNF-α from baseline during the postprandial period. Before the removal of outliers, fasting and peak TNF-α values were 33.5 ± 143.5 and 102.0 ± 242.0 pg/mL, respectively. After the removal of outliers, the fasting and peak values were 2.76 ± 1.95 and 2.85 ± 2.02 pg/mL, respectively. In the studies that found a significant postmeal TNF-α increase, the peak occurred at 5.5 ± 3.0 h. In the studies that found a decrease, the nadir occurred at 7.5 ± 1.0 h. Figure 2C provides a visual representation of change or stagnation of TNF-α after HFM intake for the studies that passed the outlier check.
With regard to IL-1β, 3 studies assessed this marker in the postprandial period. Two studies found no significant change from baseline during the postprandial period, whereas 1 study found a significant decrease. In this study, the mean nadir value occurred at 4 h postmeal. Figure 2D shows fasting and postprandial values for the 3 studies that measured IL-1β.
Four studies measured IL-8 in the postprandial period. Of these, 1 study found a significant increase in IL-8 from baseline to peak and 3 studies found no change. The baseline value for IL-8 was 2.29 ± 0.36 pg/mL. In the 1 study that found a significant increase, a peak value of 3.26 ± 2.56 pg/mL was found 4 h after the HFM. Figure 2E shows the mean and individuals values for IL-8 at baseline and postmeal for the 4 respective studies.
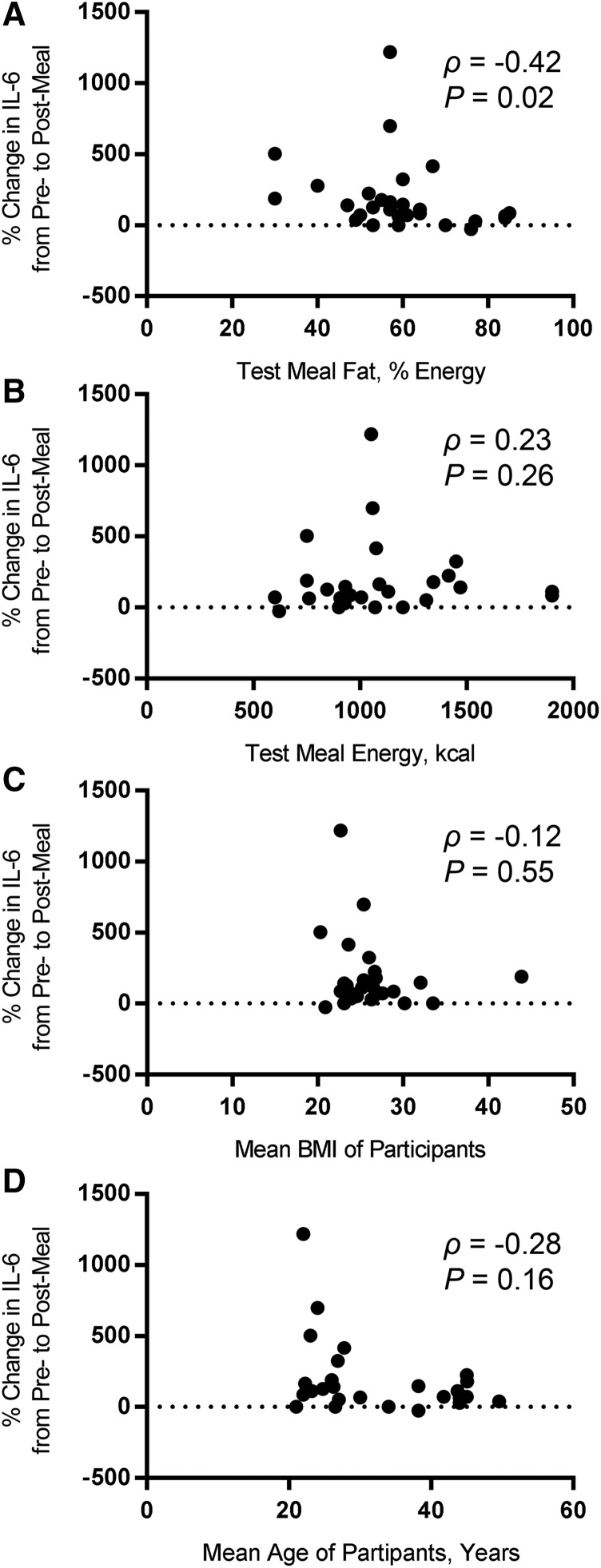
Figure 3 shows correlations of the percentage change in IL-6 from fasting to postmeal with different independent variables that could potentially affect the IL-6 response. There was a significant negative correlation (ρ = −0.42, P = 0.02) between the percentage of energy from fat in the test meal and the percentage change in IL-6 (Figure 3A). There was not a significant correlation (ρ = 0.23, P = 0.26) between the energy content of the test meal and the percentage change in IL-6 (Figure 3B). Similarly, mean BMI showed no significant relation (ρ = −0.12, P = 0.55) with the percentage change in IL-6 (Figure 3C). Finally, there was not a significant negative correlation (ρ = −0.28, P = 0.16) between mean age of the participants and the percentage change in IL-6 (Figure 3D).
FIGURE 3.
Correlations of several study variables with percentage change in IL-6 from pre- to post-HFM in healthy participants. The percentage change in IL-6 from baseline to peak or maximal observed response concentration was assessed for potential correlations with the percentage of energy from fat in the test meal (A), the energy content of the test meal (B), the mean BMI of the study participants (C), and the mean age of the study participants (D). The only variable that was found to have a significant correlation with percentage change in IL-6 was the percentage of fat in the test meal, which exhibited a moderate negative correlation.
The results of the quality appraisal for each study are shown in Table 1 and Supplemental Tables 1–4. There was little variability in quality-assessment scores among studies, ranging from 2.5 to 6.5 points out of a possible 9 points. Generally, no differences were observed between studies with regard to appraisal score and whether or not a postprandial inflammatory change was detected. Because the quality-appraisal scores did not noticeably affect our primary outcomes, we did not adjust analyses to weight studies differently on the basis of appraisal score. Furthermore, because the research questions primarily involved calculation of baseline and peak means in inflammatory markers (as opposed to effect sizes of interventions, etc.), the present analyses did not fit well with traditional meta-analyses statistics conducted within systematic reviews.
Discussion
Main findings.
The purpose of this systematic review was to characterize the magnitude and timing of changes in markers of inflammation after HFM consumption with the use of methodically selected research articles that met pre-established criteria. The primary findings were as follows: 1) that, very often, there was a postprandial increase in IL-6; 2) IL-6 typically peaked at ∼2.9 pg/mL or, more relatively, exhibited an ∼100% increase from baseline that typically occurred ∼5.9 h after the HFM; 3) TNF-α and CRP were assessed many times and yet very infrequently showed an increase post-HFM; 4) IL-8 and IL-1β have only rarely been assessed post-HFM in studies meeting our criteria; and 5) in studies that did assess IL-8 and IL-1β, although equivocal, the data suggested that these markers of inflammation did not significantly change after the consumption of an HFM. We believe that these findings are likely to be instrumental in advancing our understanding of the immune and inflammatory status of healthy individuals before and after HFM intake and will have utility in designing and interpreting future research.
Importance of postprandial inflammation.
Why should we be concerned with the timing and magnitude of inflammatory cytokine responses after HFM ingestion? It is because substantial research points to the notion that persistent low-grade inflammation is an underlying factor in several high-mortality chronic diseases and that diet can contribute to, or attenuate, that inflammation (5). It was previously thought that atherosclerosis was a lipid-storage disease (7). However, we have come to realize the vital role of inflammation in the etiology of vascular diseases (5, 6, 51). Certainly, lipids play a role in the disease process, because subendothelial penetration and retention of lipoproteins can serve as an initiating event for the atherosclerotic cascade (52). However, once the lipoproteins are in the endothelium, oxidative stress and inflammation processes assume a prominent role (6, 7). The lipoproteins are oxidized by reactive oxygen species, forming oxidized LDL. Oxidized LDL particles have several proinflammatory effects, including the following: 1) increased expression of adhesion molecules [e.g., vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1)], which promote the adhesion and penetration of immune cells to the endothelium (53); 2) increased proinflammatory cytokine release (54); and 3) activation of smooth muscle and endothelial cells (55). In turn, the increased presence of immune cells, via the functions of adhesion molecules, further increases the oxidation of lipoproteins (7). Many oxidized LDL particles will be phagocytically ingested by macrophages. These macrophages, formerly monocytes, are present consequent to their recruitment by inflammatory mediators, such as cytokines, and the linkage and induction properties of adhesion molecules (6). When monocytes penetrate the endothelium, they differentiate into macrophages. Macrophages play a crucial role in the inflammatory processes that characterize atherosclerosis. Macrophages present cell surface receptors, which, when activated, result in the increased production of many proinflammatory cytokines and adhesion molecules (56). Furthermore, as macrophages become increasingly lipid-laden as a result of oxidized lipoprotein phagocytosis, they will be laid down in the endothelium as foam cells, which are the hallmark cells of the atherosclerotic process and promote the progression of intima media thickness (6).
The increased production of proinflammatory cytokines throughout the process outlined above is important considering their physiologic effects. First, cytokines act as intermediary messengers, recruiting immune cells such as monocytes, dendritic cells, and lymphocytes to the site of vascular damage and increasing the inflammatory tone of the endothelium (57). Furthermore, it appears that inflammatory cytokines promote the activation of vascular smooth muscle and increase vascular sympathetic tone. These effects are evidenced by increased systolic blood pressure, decreased flow-mediated dilation, reduced release of the vasodilator NO, and decreased nitroglycerin-induced vasodilation (58). There is also evidence that proinflammatory cytokines, such as IL-6 and TNF-α, directly increase insulin resistance in adipocytes (59). Finally, locally produced proinflammatory cytokines often travel to the liver and increase the production of acute-phase response proteins, such as CRP and serum amyloid A (SAA) (6, 12). CRP and SAA are strongly associated with chronic disease risk. Briefly, CRP concentrations have been shown to be predictive of, among other things, peripheral vascular disease (60), future cardiovascular events (61), and ischemic stroke and transient ischemic attack (62). Similarly, SAA concentrations have been shown to be associated with coronary artery disease and future cardiovascular events (63). However, evidence that implicates markers of inflammation with CVD is not limited to acute-phase response proteins. Elevated concentrations of IL-6 in circulation are associated with myocardial infarction (64), mortality (65), and type 2 diabetes (66). Evidence linking inflammatory marker concentrations to chronic disease also exists for TNF-α (67), IL-1β (68), and IL-8 (69). Clearly, there is a connection between elevated markers of systemic inflammation and the development of disease.
The connection between diet and systemic inflammatory tone has often been suggested (70, 71). Several studies have investigated potential single-meal effects on markers of inflammation, with most studies using HFMs. However, as evidenced by our findings, there is inconsistency and ambiguity between studies with regard to the “normal” inflammatory response to an HFM. A recent review (12) performed a similar systematic search (with slightly different inclusion and exclusion criteria) and compilation of findings, but the authors elected to not quantify the collective response characteristics across studies. In the context of the equivocal nature of the postprandial inflammation research findings to date, as well as considering the physiologically important implications of altered inflammatory marker concentrations, the present systematic review was conducted to better inform future research studies, as well as to advance our understanding with regard to which inflammatory markers are responsive in the transient window after HFM intake.
Findings for individual inflammatory markers.
The present review found that the majority of studies detected an increase in IL-6 after the consumption of an HFM, which agrees with the findings of Herieka and Erridge (12). The increase is generally robust, because the average relative increase is ∼100% of the baseline value; thus, IL-6 is quite responsive to HFM intake. It has been suggested that detected increases in IL-6 in the postprandial period should be viewed with skepticism, because the process of cannulation has been shown to lead to increases in local IL-6 production (72). Indeed, it has been shown that cannulation without HFM consumption can lead to increases in IL-6 that are similar to those seen after HFM intake (73). In the present review, the vast majority of articles (30 of 32) that found a significant increase in IL-6 post-HFM used the insertion of a cannula for repeated blood sampling. Thus, it is possible that, in these studies, some or all of the increase in IL-6 could be an artifact of a local inflammatory response to the cannula, as opposed to a systemic response to the meal. However, 2 important points should be considered. First, 2 studies in our analysis used repeated venipuncture for blood sampling and found significant postprandial increases in IL-6 (35, 49). Second, one study (25) found both a significant increase in circulating IL-6 with the use of a forearm cannula and an increase in muscle expression of IL-6 with the use of a vastus lateralis biopsy, suggesting a systemic effect and not merely a local inflammatory response to the cannula. Collectively, these considerations suggest that at least some, if not all, of the IL-6 response to an HFM can be credited to the meal intake, instead of simply a local inflammatory response to cannulation. Regardless, because the majority of postprandial inflammation studies use cannulation, our findings nonetheless quantify the timing and magnitude of the collective IL-6 response to cannulation and an HFM. Because IL-6 is by far the most frequently assessed marker of inflammation in the postprandial period, and in consideration of its varied function in the progression of atherosclerosis, we assert that the findings of the present analyses have relevant clinical and research implications. Namely, elevated IL-6 concentrations have been linked to multiple clinical considerations. IL-6 concentrations have been found to be significantly associated with systolic and diastolic blood pressure, fasting insulin, and insulin sensitivity (74). A systematic review found that long-term elevated IL-6 concentrations are associated with coronary artery disease to a similar degree as most traditional risk factors (75). Finally, high IL-6 concentrations have been associated with mortality in a population-based study in older adults (65). Harris et al. (65) found that the individuals in the highest IL-6 quartile, and who therefore presented the highest mortality risk, showed IL-6 concentrations >3.19 pg/mL. Interestingly, the present review found that IL-6 starts at ∼1.4 pg/mL and peaks at ∼3 pg/mL after an HFM in healthy adults aged <60 y. Thus, a single HFM can induce a considerable postprandial increase in which circulating IL-6 concentrations can approach clinically high concentrations, even in young healthy individuals. Although more research is needed with regard to the clinical importance of acute IL-6 fluctuations, considering the established relations between IL-6 concentrations and adverse health outcomes, these acute IL-6 fluxes likely represent an important physiologic occurrence similar to other postprandial excursions (e.g., TGs, glucose) that have been shown to be associated with negative health outcomes.
On the basis of our search results, CRP was the second most frequently assessed (29 studies) marker of inflammation in the postprandial period after HFM intake. Because ∼80% of these studies found no significant change in CRP in the assessed postprandial period, and considering that the remaining 6 studies that found a significant change were divided in their findings (i.e., 4 found a significant increase and 2 found a significant decrease), the evidence strongly suggests that CRP is not a responsive marker of inflammation in the typically assessed 4- to 8-h postprandial period in healthy adults. This assertion is in agreement with our understanding of the physiologic pathway that results in an increase in CRP. The main drivers behind an increase in circulating CRP are proinflammatory cytokines produced locally at the site of damage (e.g., the inflamed endothelium). These proinflammatory cytokines, especially IL-6, then travel to the liver and stimulate increased production of acute-phase response proteins, such as CRP and SAA (6). The time course by which this pathway occurs is considerably slower than those of locally produced cytokines, because there is typically no detectable change in the first 5 h after a stimulus. Instead, CRP will slowly increase and peak at ∼24 h poststimulus. [Note: these responses are typically experimentally described by using an endotoxin model, not necessarily an HFM (12).] Thus, because CRP and other acute-phase response proteins reflect the cumulative inflammatory response (i.e., include the amplification and stimulation of many locally produced inflammatory molecules and their subsequent stimulation of acute-phase response proteins in the liver), as well as bearing in mind the delayed increase and decrease in acute-response phase proteins, CRP is a particularly advantageous marker of chronic inflammation to assess both clinically and in research. However, for these same reasons, CRP is not a viable inflammatory marker to assess in a prototypical postprandial assessment study. In consideration of the delayed response of CRP poststimulus, in combination with the findings of the present review indicating that CRP shows no change in the vast majority of postprandial inflammation studies, we recommend that CRP no longer be assessed for postprandial changes in response to an HFM in healthy adults. To be sure, CRP retains its utility in assessing overall or baseline inflammatory status; however, it is simply unlikely to change in the 4–8 h after the ingestion of an HFM in healthy individuals <60 y of age.
TNF-α has also been widely assessed in the postprandial period as an inflammatory marker that is thought to typically increase after HFM intake. Specifically, TNF-α, like IL-6, is believed to increase quickly in the poststimulus period, peaking at ∼2–3 h, then returning quickly to baseline (12). However, the findings of the current review disagree with this notion in the context of an HFM. We found that, of the studies that met the pre-established inclusion criteria, ∼70% (19 of 28 studies) found no significant change in TNF-α after an HFM. Similar to CRP, the remaining studies that did find a significant change were divided in terms of detecting a significant increase (5 studies) or decrease (4 studies). TNF-α is primarily produced by macrophages, such as those that populate inflamed regions of the vascular endothelium (6, 76). TNF-α is known to be an important mediator in both acute and sustained inflammation (76). Specifically, TNF-α can induce increased secretion of itself, as well as other proinflammatory cytokines, making it an important contributor to the amplifying nature of the inflammatory response (76). However, although it appears that TNF-α may be particularly responsive in an endotoxin model of inflammation, it is not very responsive to HFM intake. Because 23 of 28 studies assessing postprandial TNF-α in the present review found either no change or a significant decrease after an HFM, it appears that TNF-α is either not sufficiently responsive to an HFM stimulus or is too variable in its assessment to be deemed a reliable marker of inflammation in the hours after HFM intake.
Although the majority of included studies did not find a significant change in CRP or TNF-α, it is interesting that there was disagreement with regard to the directionality of the change in studies that did detect significant differences. This could possibly be driven by the composition of the test meal characteristics. With regard to meal composition, previous evidence suggests that type of fat (77), macronutrient distribution (35), and overall nutrient density (78) of the meal can alter the postprandial inflammatory response. Nevertheless, there were no clear, common differences between studies that found an increase compared with a decrease in CRP or TNF-α in the current review. Overall, due to the heterogeneity of study designs (especially test meal composition), this review is not well equipped to accurately identify the meal characteristics that induce inflammation. On the contrary, the goal of this review was to summarize the overall post-HFM inflammatory response. Although there are potentially certain nuances and influential factors that likely affect the response, the data synthesized in the present systematic review strongly suggest that CRP and TNF-α do not typically change in the acute hours after HFM consumption.
The remaining markers of inflammation assessed in the present study, IL-1β and IL-8, were rarely measured in the acute postprandial period in healthy individuals (IL-1β, 3 studies; IL-8, 4 studies). Because few studies analyzed these markers, we cannot make firm conclusions with regard to their activity in the hours after a meal. However, our findings do not suggest that these markers robustly change after the consumption of an HFM, because 2 studies found no change in IL-1β post-HFM and 1 found a significant decrease, and 3 studies found no change in IL-8 post-HFM and 1 found a significant increase. Despite being less frequently assessed, IL-1β and IL-8 are both considered proinflammatory cytokines that play adverse pathophysiologic roles in CVD development, recruiting immune cells to the site of vascular damage as well as promoting increased production of other proinflammatory cytokines (79). IL-8 is produced from a variety of cells, including monocytes, macrophages, T lymphocytes, and endothelial cells (80), whereas IL-1β is produced primarily by activated macrophages. Similar to TNF-α, although IL-1β and IL-8 are produced locally at the site of damage, they do not appear to transiently and/or robustly change in the postprandial period after the consumption of an HFM.
Strengths and limitations.
There are several strengths to the present systematic review. First, we used a robust systematic search of 10 relevant databases with a search strategy developed with the assistance of a librarian (CL). The relatively large number of citations found with the original search (494 citations), in addition to the number of duplicate citations found by multiple databases (164 duplicates eliminated), suggests that the search was comprehensive and that it is unlikely that many, if any, relevant articles were not captured with our systematic search. Next, our generally broad yet clearly defined inclusion criteria ensure that our findings are applicable to many people, namely healthy men and women between the ages of 18 and 60 y, independent of geographic region and body weight status. Finally, a strength of this study lies in its research and clinical utility. This systematic review represents the first attempt, to our knowledge, to clearly quantify the specific changes in commonly assessed markers of inflammation in response to an HFM.
However, this review is not without limitations. As with any systematic review, it is possible that we may have missed ≥1 pertinent study. In addition, not all of the studies that met our inclusion criteria provided all of the information needed to help answer our research question. Although all of the authors whose articles had missing data were contacted in an effort to retrieve those data, and many authors complied and submitted their data to us (see Acknowledgments), not all responded, and consequently some studies are still missing important information such as peak and time to peak responses for an assessed cytokine. Next, the external validity of our findings are limited to healthy adults. Individuals with disease will typically present with a high systemic inflammatory tone; therefore, the postprandial inflammatory response may be more dramatic in these populations. In addition, it should be noted that most studies included in the present systematic review assessed postprandial inflammation for 4–8 h after HFM intake. Thus, our review is not equipped to describe any inflammatory marker changes that could potentially occur outside of that typically used window of time. Next, an additional analysis with regard to the relation between the type of fat or meal in determining the postprandial inflammatory response would have been informative. However, due to the heterogeneity of test meals and the manner in which they were reported, this point was not possible for the present systematic review to address in a qualitative analysis. Qualitatively, however, there do not appear to be any noticeable trends between studies that found an increase in a marker and those that found no change other than that most studies used meals reflective of the Western-type diet, which is high in animal (saturated) fats, simple carbohydrates, processed foods, and kilocalories and low in fruit, vegetables, whole grains, and fiber. Finally, a frequent consideration with postprandial metabolic and inflammatory response research is the use of test meals that are not necessarily representative of meals that individuals might consume during normal daily living. Consequently, this systematic review contained many studies with test meals that were quite large, energy-dense, and high in fat (Table 1, Supplemental Tables 1–4). This point should be considered when interpreting and drawing conclusions from the present data.
Conclusions and future directions.
This systematic review aimed to characterize the postprandial response of 5 commonly assessed markers of inflammation after the intake of an HFM. Our findings suggest that only 1 of those 5 markers, IL-6, consistently increases in the 4–8 h post-HFM. Specifically, IL-6 will, on average, start at a baseline of ∼1.4 pg/mL and peak at ∼2.9 pg/mL ∼6 h later. In relative terms, IL-6 will increase by ∼100% in response to an HFM. Of the potential independent variables considered, only the percentage of fat in the test meal showed a significant (negative) correlation with the percentage change in IL-6 post-HFM, although a linear regression model including age, BMI, percentage of fat in the test meal, and energy content of the test meal was found to significantly predict the percentage change in IL-6. With regard to CRP and TNF-α, these markers were found to be very commonly assessed in the postprandial period, although they rarely showed any change. IL-8 and IL-1β also infrequently changed after HFM consumption in healthy individuals, although these markers have only been assessed in a few studies. In light of these findings, we have the following several recommendations for future research: 1) we suggest that CRP and TNF-α no longer be assessed for postprandial changes in healthy individuals within the normal 6–8 h postprandial time course; 2) instead, there may be more merit in assessing other inflammatory markers, such as leukocyte-bound markers, in healthy individuals exposed to an HFM, because they may be more likely to show postprandial changes (12); 3) a similar review focusing on the postprandial inflammatory response of individuals with disease is warranted, because the results could very likely differ from the present review that focused on healthy individuals; and 4) further investigation into the specific role that IL-6 plays after HFM intake would be beneficial.
Acknowledgments
We thank the numerous researchers who were willing to send any necessary missing data: Jana Kracmerova, Lenka Rossmeislova, S Gregerson, LV Campbell, Heather K Bell, Richard J Bloomer, Josef Brandauer, Stephen J Prior, Jason MR Gill, Janice K Kiecolt-Glaser, Janice E Drew, Cristiana Miglio, Mauro Serafini, Lisa G Wood, Kelley Strohacker, Brian K McFarlin, David John Stensel, Alice Thackray, Assumpta Caixas, and Michael Harrison. All of the current authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; CVD, cardiovascular disease; HFM, high-fat meal; ICAM-1, intercellular adhesion molecule 1; ROUT, robust regression and outlier removal; SAA, serum amyloid A; VCAM-1, vascular cell adhesion molecule 1.
References
- 1.Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep 2013;62:1–96. [PubMed] [Google Scholar]
- 2.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc 2001;33:754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67:968–77. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 2000;72:912–21. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 7.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009;6:399–409. [DOI] [PubMed] [Google Scholar]
- 8.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol 2009;20:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madan M, Amar S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS One 2008;3:e3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheja L, Heese B, Zitzer H, Michael MD, Siesky AM, Pospisil H, Beisiegel U, Seedorf K. Acute-phase serum amyloid A as a marker of insulin resistance in mice. Exp Diabetes Res 2008;2008:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herieka M, Erridge C. High‐fat meal induced postprandial inflammation. Mol Nutr Food Res 2014;58:136–46. [DOI] [PubMed] [Google Scholar]
- 13.Arjunan SP, Bishop NC, Reischak-Oliveira A, Stensel DJ. Exercise and coronary heart disease risk markers in South Asian and European men. Med Sci Sports Exerc 2013;45:1261–8. [DOI] [PubMed] [Google Scholar]
- 14.Arjunan SP, Deighton K, Bishop NC, King J, Reischak-Oliveira A, Rogan A, Sedgwick M, Thackray AE, Webb D, Stensel DJ. The effect of prior walking on coronary heart disease risk markers in South Asian and European men. Eur J Appl Physiol 2015;115:2641–51. [DOI] [PubMed] [Google Scholar]
- 15.Bidwell AJ, Fairchild TJ, Redmond J, Wang L, Keslacy S, Kanaley JA. Physical activity offsets the negative effects of a high-fructose diet. Med Sci Sports Exerc 2014;46:2091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandauer J, Landers-Ramos RQ, Jenkins NT, Spangenburg EE, Hagberg JM, Prior SJ. Effects of prior acute exercise on circulating cytokine concentration responses to a high-fat meal. Physiol Rep 2013;1:e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton-Freeman B, Talbot J, Park E, Krishnankutty S, Edirisinghe I. Protective activity of processed tomato products on postprandial oxidation and inflammation: a clinical trial in healthy weight men and women. Mol Nutr Food Res 2012;56:622–31. [DOI] [PubMed] [Google Scholar]
- 18.Caixàs A, Giménez-Palop O, Broch M, Vilardell C, Megía A, Simón I, Giménez-Pérez G, Mauricio D, Vendrell J, Richart C. Adult subjects with Prader-Willi syndrome show more low-grade systemic inflammation than matched obese subjects. J Endocrinol Invest 2008;31:169–75. [DOI] [PubMed] [Google Scholar]
- 19.Campbell CG, Brown BD, Dufner D, Thorland WG. Effects of soy or milk protein during a high-fat feeding challenge on oxidative stress, inflammation, and lipids in healthy men. Lipids 2006;41:257–65. [DOI] [PubMed] [Google Scholar]
- 20.Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, Solivera J, Yubero-Serrano EM, Fuentes F, Parnell LD, Shen J, Gomez P, Jimenez-Gomez Y, et al. Interleukin 1B variant-1473G/C (rs1143623) influences triglyceride and interleukin 6 metabolism. J Clin Endocrinol Metab 2011;96:E816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drew JE, Farquharson AJ, Horgan GW, Duthie SJ, Duthie GG. Postprandial cell defense system responses to meal formulations: stratification through gene expression profiling. Mol Nutr Food Res 2014;58:2066–79. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers K, Brand T, Bangert A, Hauner H, Laumen H. Postprandial activation of metabolic and inflammatory signalling pathways in human peripheral mononuclear cells. Br J Nutr 2014;111:2167–75. [DOI] [PubMed] [Google Scholar]
- 23.Esser D, Oosterink E, op ’t Roodt J, Henry RM, Stehouwer CD, Müller M, Afman LA. Vascular and inflammatory high fat meal responses in young healthy men; a discriminative role of IL-8 observed in a randomized trial. PLoS One 2013;8:e53474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill JMR, Caslake MJ, McAllister C, Tsofliou F, Ferrell WR, Packard CJ, Malkova D. Effects of short-term detraining on postprandial metabolism, endothelial function, and inflammation in endurance-trained men: dissociation between changes in triglyceride metabolism and endothelial function. J Clin Endocrinol Metab 2003;88:4328–35. [DOI] [PubMed] [Google Scholar]
- 25.Gregersen S, Samocha-Bonet D, Heilbronn LK, Campbell LV. Inflammatory and oxidative stress responses to high-carbohydrate and high-fat meals in healthy humans. J Nutr Metab 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison M, Murphy RP, O’Connor PL, O’Gorman DJ, McCaffrey N, Cummins PM, Moyna NM. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur J Appl Physiol 2009;106:555–62. [DOI] [PubMed] [Google Scholar]
- 27.Jiménez-Gómez Y, López-Miranda J, Blanco-Colio LM, Marín C, Pérez-Martínez P, Ruano J, Paniagua JA, Rodríguez F, Egido J, Pérez-Jiménez F. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis 2009;204:e70–6. [DOI] [PubMed] [Google Scholar]
- 28.Johnson AM, Kurti SP, Smith JR, Rosenkranz SK, Harms CA. Effects of an acute bout of moderate intensity exercise on postprandial lipemia and airway inflammation. Appl Physiol Nutr Metab 2016;41:284–91. [DOI] [PubMed] [Google Scholar]
- 29.Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA. Marital discord, past depression, and metabolic responses to high-fat meals: interpersonal pathways to obesity. Psychoneuroendocrinology 2015;52:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kračmerová J, Czudkova E, Koc M, Malisova L, Siklova M, Stich V, Rossmeislova L. Postprandial inflammation is not associated with endoplasmic reticulum stress in peripheral blood mononuclear cells from healthy lean men. Br J Nutr 2014;112:573–82. [DOI] [PubMed] [Google Scholar]
- 31.Lundman P, Boquist S, Samnegård A, Bennermo M, Held C, Ericsson CG, Silveira A, Hamsten A, Tornvall P. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutr Metab Cardiovasc Dis 2007;17:195–202. [DOI] [PubMed] [Google Scholar]
- 32.Madec S, Corretti V, Santini E, Ferrannini E, Solini A. Effect of a fatty meal on inflammatory markers in healthy volunteers with a family history of type 2 diabetes. Br J Nutr 2011;106:364–8. [DOI] [PubMed] [Google Scholar]
- 33.Mariotti F, Valette M, Lopez C, Fouillet H, Famelart M, Mathé V, Airinei G, Benamouzig R, Gaudichon C, Tome D, et al. Casein compared with whey proteins affects the organization of dietary fat during digestion and attenuates the postprandial triglyceride response to a mixed high-fat meal in healthy, overweight men. J Nutr 2015;145:2657–64. [DOI] [PubMed] [Google Scholar]
- 34.Miglio C, Peluso I, Raguzzini A, Villaño DV, Cesqui E, Catasta G, Toti E, Serafini M. Antioxidant and inflammatory response following high-fat meal consumption in overweight subjects. Eur J Nutr. 2013;52:1107–14. [DOI] [PubMed] [Google Scholar]
- 35.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 2002;39:1145–50. [DOI] [PubMed] [Google Scholar]
- 36.Payette C, Blackburn P, Lamarche B, Tremblay A, Bergeron J, Lemieux I, Després J, Couillard C. Sex differences in postprandial plasma tumor necrosis factor-α, interleukin-6, and C-reactive protein concentrations. Metabolism 2009;58:1593–601. [DOI] [PubMed] [Google Scholar]
- 37.Peluso I, Raguzzini A, Villano DV, Cesqui E, Toti E, Catasta G, Serafini M. High fat meal increase of IL-17 is prevented by ingestion of fruit juice drink in healthy overweight subjects. Curr Pharm Des 2012;18:85–90. [DOI] [PubMed] [Google Scholar]
- 38.Phillips LK, Peake JM, Zhang X, Hickman IJ, Briskey DR, Huang BE, Simpson P, Li SH, Whitehead JP, Martin JH, et al. Postprandial total and HMW adiponectin following a high-fat meal in lean, obese and diabetic men. Eur J Clin Nutr 2013;67:377–84. [DOI] [PubMed] [Google Scholar]
- 39.Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, Chan YK, McArdle BH, Cooper GJ. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein to a high-fat dietary load. Nutrition 2008;24:322–9. [DOI] [PubMed] [Google Scholar]
- 40.Rankin JW, Andreae MC, Oliver Chen CY, O’Keefe SF. Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes Obes Metab 2008;10:1086–96. [DOI] [PubMed] [Google Scholar]
- 41.Sanders TA, Filippou A, Berry SE, Baumgartner S, Mensink RP. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am J Clin Nutr 2011;94:1433–41. [DOI] [PubMed] [Google Scholar]
- 42.Schmid A, Petry N, Walther B, Bütikofer U, Luginbühl W, Gille D, Chollet M, McTernan PG, Gijs MA, Vionnet N, et al. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br J Nutr 2015;113:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwander F, Kopf-Bolanz KA, Buri C, Portmann R, Egger L, Chollet M, McTernan PG, Piya MK, Gijs MA, Vionnet N, et al. A dose-response strategy reveals differences between normal-weight and obese men in their metabolic and inflammatory responses to a high-fat meal. J Nutr 2014;144:1517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strohacker K, Breslin WL, Carpenter KC, Davidson TR, Agha NH, McFarlin BK. Moderate-intensity, premeal cycling blunts postprandial increases in monocyte cell surface CD18 and CD11a and endothelial microparticles following a high-fat meal in young adults. Appl Physiol Nutr Metab 2012;37:530–9. [DOI] [PubMed] [Google Scholar]
- 45.Teng KT, Nagapan G, Cheng HM, Nesaretnam K. Palm olein and olive oil cause a higher increase in postprandial lipemia compared with lard but had no effect on plasma glucose, insulin and adipocytokines. Lipids 2011;46:381–8. [DOI] [PubMed] [Google Scholar]
- 46.Tholstrup T, Teng KT, Raff M. Dietary cocoa butter or refined olive oil does not alter postprandial hsCRP and IL-6 concentrations in healthy women. Lipids 2011;46:365–70. [DOI] [PubMed] [Google Scholar]
- 47.Twickler TB, Dallinga-Thie GM, Visseren FLJ, de Vries WR, Erkelens DW, Koppeschaar HPF. Induction of postprandial inflammatory response in adult onset growth hormone deficiency is related to plasma remnant-like particle-cholesterol concentration. J Clin Endocrinol Metab 2003;88:1228–33. [DOI] [PubMed] [Google Scholar]
- 48.Volek JS, Judelson DA, Silvestre R, Yamamoto LM, Spiering BA, Hatfield DL, Vingren JL, Quann EE, Anderson JM, Maresh CM, et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am J Cardiol 2008;102:1413–7. [DOI] [PubMed] [Google Scholar]
- 49.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol 2011;127:1133–40. [DOI] [PubMed] [Google Scholar]
- 50.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 2006;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 52.Borén J, Matikainen N, Adiels M, Taskinen M. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta 2014;431:131–42. [DOI] [PubMed] [Google Scholar]
- 53.Kita T, Kume N, Ishii K, Horiuchi H, Arai H, Yokode M. Oxidized LDL and expression of monocyte adhesion molecules. Diabetes Res Clin Pract 1999;45:123–6. [DOI] [PubMed] [Google Scholar]
- 54.Hajjar DP, Haberland ME. Lipoprotein trafficking in vascular cells: molecular Trojan horses and cellular saboteurs. J Biol Chem 1997;272:22975–8. [DOI] [PubMed] [Google Scholar]
- 55.Maziere C, Auclair M, Djavaheri‐Mergny M, Packer L, Maziere J. Oxidized low density lipoprotein induces activation of the transcription factor NFκB in fibroblasts, endothelial and smooth muscle cells. Biochem Mol Biol Int 1996;39:1201–7. [DOI] [PubMed] [Google Scholar]
- 56.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol 2000;164:3471–5. [DOI] [PubMed] [Google Scholar]
- 57.Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. J Atheroscler Thromb 2004;11:313–21. [DOI] [PubMed] [Google Scholar]
- 58.Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr 2005;93:3–9. [DOI] [PubMed] [Google Scholar]
- 59.Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res 2009;50(Suppl):S395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 1998;97:425–8. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 62.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D’Agostino RB, Franzblau C, Wilson PW. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001;32:2575–9. [DOI] [PubMed] [Google Scholar]
- 63.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:726–32. [DOI] [PubMed] [Google Scholar]
- 64.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–72. [DOI] [PubMed] [Google Scholar]
- 65.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 1999;106:506–12. [DOI] [PubMed] [Google Scholar]
- 66.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- 67.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000;101:2149–53. [DOI] [PubMed] [Google Scholar]
- 68.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2003;23:656–60. [DOI] [PubMed] [Google Scholar]
- 69.Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res 2009;84:353–60. [DOI] [PubMed] [Google Scholar]
- 70.Galland L. Diet and inflammation. Nutr Clin Pract 2010;25:634–40. [DOI] [PubMed] [Google Scholar]
- 71.Esposito K, Ciotola M, Giugliano F, De Sio M, Giugliano G, D'armiento M, Giugliano D. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res 2006;18:405–10. [DOI] [PubMed] [Google Scholar]
- 72.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology 2002;27:921–31. [DOI] [PubMed] [Google Scholar]
- 73.Thompson D, Dixon N. Measurement of postprandial interleukin-6 via a catheter: what does it tell us? Eur J Appl Physiol 2009;107:621–2. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez-Real J, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab 2001;86:1154–9. [DOI] [PubMed] [Google Scholar]
- 75.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 2008;5:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chu WM. Tumor necrosis factor. Cancer Lett 2013;328:222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blum S, Aviram M, Ben-Amotz A, Levy Y. Effect of a Mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann Nutr Metab 2006;50:20–4. [DOI] [PubMed] [Google Scholar]
- 78.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal implications for insulin resistance. Diabetes Care 2009;32:2281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992;258:1798. [DOI] [PubMed] [Google Scholar]
- 80.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 1994;56:559–64. [PubMed] [Google Scholar]