Abstract
High levels of HDL cholesterol (HDL-C) have traditionally been linked to lower incidence of cardiovascular disease, prompting the search for effective and safe HDL-C raising pharmaceutical agents. Although drugs such as niacin and fibrates represent established therapeutic approaches, HDL-C response to such therapies is variable and heritable, suggesting a role for pharmacogenomic determinants. Multiple genetic polymorphisms, located primarily in genes encoding lipoproteins, cholesteryl ester transfer protein, transporters and CYP450 genes have been shown to associate with HDL-C drug response in vitro and in epidemiologic studies. However, few of the pharmacogenomic findings have been independently validated, precluding the development of clinical tools that can be used to predict HDL-C response and leaving the goal of personalized medicine to future efforts.
Keywords: dyslipidemia, fibrates, HDL, niacin, pharmacogenomics, statins
Numerous epidemiologic studies have documented the inverse association between HDL cholesterol (HDL-C) and cardiovascular risk [1]. Notably, that paradigm was recently challenged by a large-scale Mendelian randomization analysis [2] that failed to find evidence of a causal link between HDL-C levels and the risk of myocardial infarction. However, because of decades of conflicting evidence and concerns surrounding the validity of the Mendelian randomization approach, current clinical practice continues advocating for increasing HDL-C levels, prompting the search for safe and effective interventions [1].
Although several classes of HDL-raising therapies exist, individual response to interventions aimed at increasing HDL-C is highly variable. In addition, the magnitude of change in HDL-C levels resulting from an intervention is moderately heritable, suggesting a role for genetic determinants. Data from the Health, Risk Factors, Exercise Training, and Genetics Family Study show that HDL-C response to lifestyle interventions such as exercise training clusters in families with heritability estimates ranging from 0.25 to 0.64 [3]. For pharmacological agents, heritability of the HDL-C response has not been explicitly estimated, but polymorphisms in several genes have been shown to contribute to the variability of treatment success [4]. This article provides a comprehensive review of the genetic mediators of HDL-C response to currently prescribed therapies, and delineates ways in which pharmacogenomics could inform future treatment approaches.
Dynamics of HDL metabolism
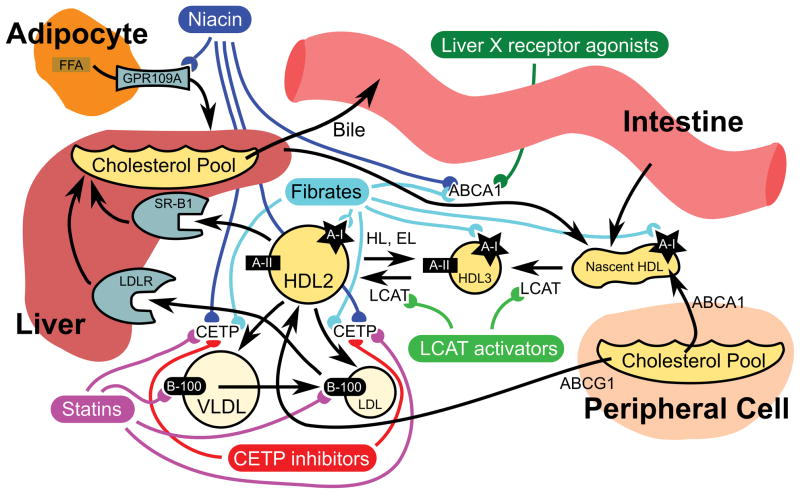
HDL participates in the reverse flux of cholesterol from peripheral tissues to the liver for eventual excretion from the body (Figure 1). The first steps of the process involve the addition of phospholipids to lipid-poor apolipoprotein A-I (apoA-I), synthesized in the liver and intestine, to form the HDL precursor. Addition of free cholesterol from peripheral cells to the HDL precursor, mediated by ABCA1 and LCAT forms mature HDL. Mature HDL particles are heterogeneous in size, density, composition and atherogenicity, and can be classified into two HDL2 subfractions (HDL2a and HDL2b) and three HDL3 subfractions (HDL3a, HDL3b and HDL3c) [5,6]. Modification of mature HDL is multifaceted and involves the addition of lipids via ABCG1 and SR-BI1. CETP exchanges cholesteryl esters acquired from HDL for triglycerides with VLDL or LDL cholesterol, effecting depletion in cholesteryl esters and enrichment in triglycerides. The resulting HDL 3 fraction undergoes continuous conversion into HDL2 and vice versa depending on the metabolic context [7]. The particles are then either taken up by the liver via SR-BI or modified by hepatic lipase and endothelial lipase. Metabolism by the latter releases lipid-poor apoA-I (LpA-I), completing the cycle. Most HDL-C directed interventions target these complex pathways, making several biological considerations germane to the pharmacogenomics of HDL-C raising therapies.
Figure 1.
HDL metabolism with known and possible targets of therapeutic intervention.
Black arrows indicate routes of transport and/or transformation. Colored lines indicate targets of HDL-directed pharmacotherapy.
apoB100: Apolipoprotein B100; EL: Endothelial lipase; FFA: Free fatty acid; HL: Hepatic lipase; LCAT: Lecithan-cholesterol acyltransferase; LDLR: LDL receptor; SR-B1: Scavenger receptor class B, type I.
It is important to note that HDL2 and HDL3 subfractions vary in their association with cardiovascular disease risk, and most notably, their response to HDL-C-raising interventions [3,8,9]. In addition to classifying subfractions by size and density, HDL particles can be distinguished by composition: LpA-I particles, which only contain apoA-I; and LpA-I:A-II particles, which contain both apoA-I and apoA-II [8]. However, it is still unclear if the most promising approach to improving lipid metabolism involves targeting the overall levels of HDL-C, the interconversion of the subfractions or HDL particle size [10]. Therefore, the complex and dynamic nature of HDL metabolism requires a nuanced approach to evaluating potential genetic predictors of therapeutic efficacy.
Genetic modifiers of HDL-C response to drug treatment
Several classes of drugs have been evaluated in clinical trials as means to raise HDL-C levels. Currently, the major pharmaceutical agents include niacin (nicotinic acid and vitamin B3), fibrates, statins and different forms of combination therapies. In addition, several other drugs prescribed for conditions other than dyslipidemia (e.g., thiazolidinedions) affect HDL-C levels, but their pharmacogenomic determinants have not been explicitly evaluated for lipid outcomes; therefore, the authors have excluded them from this review. Other approaches - that is, apoA1 mimetics, CETP inhibitors or recombinant HDL infusion have been proposed and are subjects of ongoing trials discussed in the following sections [10]. The evidence in support of genetic modifiers of drug efficacy and toxicity varies widely by class and is summarized in Table 1.
Table 1.
Summary of plausible genetic modifiers of HDL-cholesterol raising therapies.
| Gene (SNPS if identified) | OMIM number | Lines of evidence | Ref |
|---|---|---|---|
| Niacin | |||
| ABCA1 | 600046 | Cell experiments | [17,18] |
| CETP | 118470 | Animal models | [21] |
| HCAR2/GPR109A/HM74A | 609163 | Animal models | [13] |
| NR1H3/LXRA | 602423 | Cell experiments | [18] |
| PPARG | 601487 | Cell experiments | [18] |
| Fibrates | |||
| ABCA1 | 600046 | Candidate gene study | [35] |
| APOA5 (rs3135506) | 606368 | Candidate gene study Cell experiments |
[29–31] [28] |
| APOE (rs429358, rs7412) | 107741 | Candidate gene study | [32–34] |
| CETP | 118470 | Animal models Gene expression study |
[24] [26] |
| CYP7A1 | 118455 | Candidate gene study | [39] |
| PPARA (rs1800206) | 170998 | Candidate gene study | [33,36–38] |
| LPL (rs28935469) | 609708 | Candidate gene study | [33,40,41] |
| Statins | |||
| APOA1 (rs670) | 107680 | Candidate gene study | [50,56,57] |
| APOB | 107730 | Candidate gene study | [58] |
| CETP (rs708272) | 118470 | Candidate gene study | [50,63–66] |
| CYP3A4 (rs4986907, | 124010 | Candidate gene study | [50–54] |
| rs2740574, rs2242480) | [50,55] | ||
| CYP7A1 (rs8192871, rs3808607) | 118455 | [58] [60–62] |
|
| LPL | 609708 | Candidate gene study | |
| SLCO1B1 (rs4149015, rs4149056) | 604843 | Candidate gene study Candidate gene study |
|
| CETP Inhibitors | |||
| ABCA1 | 600046 | Cell experiments | [72] |
| ABCG1 | 603076 | Cell experiments | [72] |
| APOE | 107741 | Cell experiments | [72] |
| CETP | 118470 | Animal models | [71] |
| LCAT | 609967 | Cell experiments | [72] |
CETP, cholesterylester transfer protein; HDL-C, high-density lipoprotein cholesterol; LCAT, lecithin-cholesterol acyltransferase; LPL, lipoprotein lipase.
Niacin
To date, niacin represents the most effective pharmaceutical approach to treat patients exhibiting low HDL-C levels [11,12]. Niacin improves the overall lipid profile primarily by activating GPR109A, the G-protein-coupled receptor expressed on adipocytes that reduces the release of free fatty acids to the liver leadsing to reduced triglyceride synthesis and circulating VLDL [13]. In turn, the reduction in VLDL-C inhibits CETP activity, resulting in increased HDL-C [14]. In addition to treating dyslipidemia, the activation of GPR109A is also responsible for the most common side effect of niacin therapy: the cutaneous vasodilatory response, or flushing [14]. However, niacin has also been shown to raise circulating HDL-C levels through several non-GPR109A dependent mechanisms. In hepatocytes, niacin downregulates cell surface expression of [beta]-chain adenosine triphosphate synthase, an enzyme that mediates endocytosis of HDL particles, which inhibits HDL liver uptake and catabolism [15]. The anticatabolic effect of niacin is specific to LpA-I particles [16] and enhances the reverse cholesterol transport function. Furthermore, it facilitates the conversion of smaller, denser HDL3 particles to the larger, less dense HDL2 particles as the smaller particles are allowed time to grow via uptake of peripheral tissue cholesterol [15]. Finally, niacin stimulates cholesterol efflux through activation of the PPAR[gamma]-liver X receptor (LXR)[alpha]-ABCA1 pathway, which further increases apoA-I lipidation and HDL biogenesis in the liver [17,18].
Although there is evidence demonstrating that response to niacin, in particular the side effect of flushing, clusters in families [19], no pharmacogenomic determinants of niacin efficacy or toxicity have been evaluated in humans [20]. However, evidence from animal and cell culture studies points to several plausible candidate genes. The mouse homolog of CETP has been identified as a potential mediator of niacin-induced HDL-C increase [21]. Based on the current understanding of niacin pharmacodynamics, it is possible that variation at the CETP locus affects the HDL-C response independently or via epistatic interactions with HCAR2, the gene encoding the GPR109A receptor in humans. In addition to considering genetic variation in HCAR2 itself, novel pharmacogenomic targets could also emerge from the evidence implicating PPAR[gamma] (encoded in humans by PPARG), LXR[alpha] (encoded by NR1H3) and ATP-binding cassette transporters, encoded by genes such as ABCA1, in niacin-mediated HDL efflux [17,18]. Overall, several candidate genes merit a closer evaluation as potential mediators of niacin response in humans, presenting fruitful opportunities for future studies.
Fibrates
In contrast to niacin, several of the pharmacogenomic determinants of HDL-C response to fibrates have been identified and characterized. Fibrates activate the intrahepatic nuclear receptor (PPAR[alpha]) by forming a heterodimer with retinoid X receptor, which in turn, binds to the peroxisome proliferator response element, thus modulating protein transcription of key drug target genes. Activation of PPAR ultimately increases plasma lipoprotein lipase (LPL) activity and raises A-I and A-II levels by stimulating the promoter region of APOA1 while reducing apoCIII, resulting in the observed changes in lipid fractions [22]. Similar to niacin, fibrates also increase the transcription of ABCA1, leading to increased synthesis of HDL in the liver in a PPAR-dependent manner [23]. In animal studies, fenofibrate also inhibited CETP expression, thus reducing cholesteryl ester transfer from HDL to VLDL [24]. It is important to note that human and rodent HDL-C responses to fibrate therapy vary substantially, which limits pharmacogenomic inference from animal models and increases the relative importance of epidemiologic evidence and/or human clinical studies [25]. However, in the case of CETP, animal model, data have been indirectly corroborated by a gene expression study in human subjects, although the effect of CETP variation on HDL-C response to fenofibrate has not been explicitly evaluated [26].
To date, most pharmacoepidemiologic studies of fibrates have focused on the lipoprotein genes, because upregulation of apoA-I (encoded by APOA1) is believed to be the central mechanism of action [10]. The APOA1/C3/A4 genes are located on human chromosome 11q23, extending to approximately 17 kb. The fourth member of that genetic cluster, APOA5, lies approximately 30 kb distal to APOA1/C3/A4 [27]. The focus on lipoproteins is justified by experimental evidence identifying APOA5 as an important PPAR[alpha] target gene and a potential mediator of fibrate effects on plasma lipids [28]. This evidence is further corroborated by epidemiologic studies. Data from the GOLDN study revealed that carriers of the minor G allele at the functional 56C→G (rs3135506) locus in APOA5 experienced a significantly larger increase in HDL-C following 3 weeks of daily 160 mg fenofibrate therapy when compared with noncarriers [29,30]. Another study, conducted in individuals with mixed dyslipidemia, linked common variants in the APOA5 region with response to combination therapy with fenofibrate and statins [31]. Studies investigating polymorphisms in APOE as potential mediators of fenofibrate response have yielded mixed results. In GOLDN, rs7412 was associated with changes in HDL-C among healthy subjects but not among participants with metabolic syndrome [32]. In the context of hypertriglyceridemia, the [epsilon]2 (T at rs429358 + T at rs7412) APOE genotype was associated with a more favorable lipid profile change than the [epsilon]3 (T at rs429358 + C at rs7412) or the [epsilon]4 (C at rs429358 + C at rs7412) genotypes [33]. However, in another study of dyslipidemic individuals, the association of the APOE genotypes with HDL-C response to fenofibrate was not significant, probably because of insufficient statistical power [34].
Fibrate therapy has been shown to increase the overall number of HDL particles and small HDL subclass particles (HDL3), the latter due to the increase in cholesterol efflux triggered by enhanced ABCA1 expression [9,35]. Consistent with that hypothesis, GOLDN investigators reported a synergistic effect between ABCA1 variants and fenofibrate on HDL particle concentrations and small HDL [35]. The effect of fibrates on HDL subfraction profile also appears to be modulated by variation in PPARA itself, as evidenced by data from a small clinical trial of gemfibrozil conducted among abdominally obese men [36,37]. In the gemfibrozil trial, participants who were carriers of the variant allele of the functional L162V (rs1800206) polymorphism exhibited only a 6% increase in HDL2 particles, compared with a 50% increase in wild-type participants [36]. The same polymorphism, however, was not a statistically significant predictor of HDL-C response to gemfibrozil in the Veterans Affairs HDL Intervention Trial, possibly because of baseline differences between populations [38]. On the other hand, the associations between common variation in CYP7A1, which encodes cholesterol 7[alpha]-hydroxylase and is crucial to bile acid synthesis, and the HDL-C response to fenofibrate were not specific to any subfraction but showed an overall gene-drug interaction [39].
Several other biologically plausible candidate genes, including LPL and PPARG, were investigated as potential mediators of fibrate response but were not found to be independently significant predictors of therapeutic outcomes [32,40]. Similarly, a recent analysis of the GOLDN data investigated whether previously validated genetic predictors of HDL particle number and size were associated with fenofibrate response, but did not yield any statistically significant results [41]. However, evidence suggests that the genetic determinants of fenofibrate response are nonlinear. A study conducted in hypertriglyceridemic subjects showed that the beneficial effect of [epsilon]2 APOE genotype on fenofibrate response is stronger among individuals with missense PPAR[alpha] −L162V (rs1800206) and LPL −P207L (rs28935469) variants [33]. Such synergy has clear biological plausibility, because all three gene products are in the fenofibrate pathway and have been shown to interact with each other. Specifically, the LPL enzyme, encoded by LPL, anchors to the endothelial matrix via an APOE -dependent mechanism and also interacts with apo E at the cell surface to recognize specific lipoproteins; Brisson et al. postulate that fenofibrate improves this interaction, resulting in a more favorable change in blood lipids [33]. As for the LPL/PPAR[alpha] interaction, LPL has been shown to induce generation of PPAR[alpha] ligands from circulating lipoproteins [42]. In individuals with the P207L (rs28935469) mutation, that process is compromised, but fenofibrate therapy can compensate by providing an exogenous mechanism of PPAR[alpha] activation, resulting in greater improvements in the lipid profile. These data show that such gene-gene interactions, although not extensively investigated, are critical to understanding complex traits such as lipid responses to fibrate therapy.
An additional avenue of pharmacogenomic investigations involves metabolic pathways responsible for the elimination or inactivation of the active therapeutic moiety of interest. In the case of fenofibrate, the elimination of fenofibric acid—the active moiety of the drug—occurs primarily through the glucuronidation pathway [43]. Assuming that access to the PPAR[alpha] site of action is indeed determined in part by circulating levels of fenofibric acid, as in vitro evidence suggests [43], genetic variation in uridine 5′-diphospho-glucuronosyltransferases emerges as a potentially important predictor of lipid response to fenofibrate. Data from the GOLDN study [44] have pursued associations between glucuronidation activity and fenofibrate response and established that uridine 5′-diphospho-glucuronosyltransferases are indeed important determinants of lipid changes, identifying a new class of markers that independently or in concert with other genes could eventually be used to predict HDL-C response to fenofibrate therapy.
HMG-CoA reductase inhibitors (statins)
Although the effect of statin therapy on HDL-C levels is subtle in comparison with the concomitant changes in LDL-C, it nevertheless contributes to the observed reduction in overall cardiovascular risk. Statins affect HDL profile via two complementary mechanisms: the downregulation of CETP and the reduction in particles containing apoB that can accept cholesterol esters [10]. Consequently, cholesterol transfer from HDL is impeded, resulting in an increase in the relative concentration of larger (HDL2) particles and a more favorable HDL subfraction profile [45]. As expected, statin effects on HDL-C vary by dose and formulation, with pitavastatin showing the strongest HDL-C elevations in a meta-analysis of published trials [46]. Of note, the HDL-C-raising response observed from certain statins may not parallel their dose-dependent LDL-C-lowering response. For example, atorvastatin’s HDL-C-raising response appears to decline with higher doses that are typically associated with further LDL-C lowering; by contrast, rosuvastatin exhibits a more consistent dose response relationship for both LDL-C lowering and HDL-C raising [47]. Although this observation may bear clinical significance, the mechanisms underlying differential response remain poorly understood.
As with fibrates, lipid response to statin therapy is highly heterogeneous and in part mediated by genetic variation at multiple loci [48]. However, the modest increase in HDL-C attributed to statin therapy limits the ability to detect pharmacogenomic effects. The largest genome-wide association study of statin-mediated HDL-C changes to date, which combined three clinical trial populations involving almost 4000 individuals, did not yield any genome-wide Candidate gene studies, focusing primarily on CYP450 (CYP -) and drug transporter variants because of their importance in statin pharmacokinetics have yielded conflicting results. A pharmacogenetic study in newly diagnosed patients with coronary artery disease has linked common variation in CYP3A4 (CYP3A4*17, rs4986907) with significant alterations of HDL-C response to atorvastatin therapy [50]. The association with CYP3A4*17 (rs4986907) was evaluated in other atorvastatin-treated cohorts but no carriers of the variant allele were found among the studied subjects; as a result, this finding remains to be replicated [51]. The association with CYP3A4 was also not observed in analyses of other mutations in the same gene, CYP3A4*1G (rs2242480) and CYP3A4*1B (rs2740574), in hyperlipidemic patients treated with either atorvastatin or simvastatin [52,53]. The CYP3A4*1B (rs2740574) polymorphism, located in the promoter region was also associated with higher HDL-C variation in a study of Chilean hypercholesterolemic individuals [54]. Although insufficient statistical power remains a plausible explanation for the inconsistency, it is notable that CYP3A4 expression is affected by other coadministered therapeutic agents that could confound the genotype-drug response association; nevertheless, sensitivity analyses excluding patients who were administered other CYP3A4 substrates or inhibitors reported similar results [53].
The pattern of mixed and generally insufficient evidence holds true for other genes encoding CYP450 proteins. The candidate gene analysis of atorvastatin response determinants in newly-diagnosed coronary artery disease patients by Poduri et al. also found significant associations between changes in HDL-C and the rs8192871 variant in CYP7A1, which tags a promoter variant known as −204A→C (rs3808607) [50]. However, that finding was not replicated in an independent analysis [55], probably because of either different linkage disequilibrium patterns between populations, insufficient power to detect an effect, or gene-gene/gene-environment interactions. It is also possible that variation in CYP7A1, a gene encoding the rate-limiting enzyme in the biosynthesis of bile acids from cholesterol, explains more of the variation in LDL-C response to statins [55] and any concurrent changes in HDL-C response are spurious.
Despite the relevance of apoA-I to HDL metabolism, data on APOA1 variation and the HDL-C raising effects of statin therapy are similarly inconsistent. Candidate gene studies identified an APOA1 promoter polymorphism (−75G→A, rs670) as a robust predictor of HDL-C response to pravastatin [56] and atorvastatin [50], but the association was not replicated in another atorvastatin-treated population [57]. However, variation in APOB was found to be strongly associated with fluvastatin-mediated HDL-C increases in the Malmo Diet and Cancer Cardiovascular Cohort [58]. In the same population, it was also found that genetic mediators of lipid response to statins may vary by gender, with an LPL variant predicting treatment outcomes in women but not men [58]. Haplotypes in LPL were also associated with changes in HDL-C following lovastatin treatment in individuals who underwent coronary artery bypass surgery, providing indirect validation for the Malmo findings and supporting the hypothesis of pleiotropic effects of LPL [4].
Among drug transporters, OATP1B1 (encoded by SLCO1B1) has received considerable attention as it enables statins to enter hepatocytes, a required step for both action and subsequent clearance via bile [59]. In children diagnosed with familial hypercholesterolemia, the −11187G→A (rs4149015) mutation in SLCO1B1 was associated with lower peak plasma pravastatin Cmax and a greater increase in HDL-C following 2-month statin therapy [60]. In another pediatric population described in the study by Hedman et al. that included heart transplant recipients treated with statins, similar increases were associated with the C allele at the 521T→C (rs4149056) locus, also in SLCO1B1 [60]. Fitting the pattern of inconsistency in pharmacogenetic studies of statins, a candidate gene study of the SLCO1B1 521T→C (rs4149056) mutation in adults failed to replicate the differential changes in HDL-C [61]. In addition, the rs4149056 variant was shown to be unrelated to pitavastatin-mediated HDL-C changes in a Chinese population [62]. It is likely that SLCO1B1 polymorphisms are less relevant to HDL-C response than to the risk of adverse events (i.e., statin-induced myopathy), where the observed associations are more consistent [48].
Because of the postulated CETP-dependent mechanism of statin action on HDL-C levels [10], variation in CETP has been considered as a potential predictor of treatment efficacy. Both CETP haplotypes [63,64] and individual variants [48,64,65], including the well-studied TaqIB polymorphism (rs708272) [50], have been shown to mediate HDL-C response to statin therapy. However, a large-scale meta-analysis of patients treated with pravastatin failed to detect any pharmacogenetic interactions with the TaqIB (rs708272) genotype and HDL-C changes, although the null findings may, in part, have been explained by the lack of statistical power as two of the three trials in the meta-analysis were aimed at secondary prevention [66].
On balance, the evidence of pharmacogenomic determinants of HDL-C response to statins is mixed. As most studies are designed to detect statin-mediated changes in LDL-C that are considerably greater than in HDL-C, lack of statistical power remains a pivotal concern. Future insights into the genetic predictors of statin-induced HDL-C changes are likely to come from the newly formed Genomic Investigation of Statin Therapy consortium, which includes genome-wide association study data on approximately 20,000 statin-treated individuals and plans to collect exome sequencing and epigenetic data on their subset [48].
Emerging HDL-C-raising therapies
The process of CETP inhibition, which promotes cholesterol efflux and raises the levels of large HDL particles, is the common mechanism underlying the HDL-C effects of niacin, fibrates and statins [67]. The new emerging class of HDL-C-raising therapies, currently undergoing trials for safety and efficacy, targets the CETP molecule directly. Although torcetrapib—the first CETP inhibitor assessed in clinical trials—showed an association with increased cardiovascular morbidity and mortality in statin-treated patients, largely attributed to poorly understood off-target effects on blood pressure, new agents such as dalcetrapib and anacetrapib do not seem to confer the same hazard [68]. Unfortunately, a Phase III study of dalcetrapib was recently stopped due to the lack of effectiveness in reducing adverse coronary events, echoing the fate of the AIM-HIGH trial [69], which similarly failed to demonstrate that addition of an HDL-raising component (niacin) to LDL-lowering therapy (simvastatin) can further the risk of myocardial infarction. However, in an on-treatment analysis of AIM-HIGH data, the difference in median HDL-C between groups was hardly clinically meaningful (4 mg/dl), suggesting that these results should not be extrapolated to other HDL-raising agents and indicating possible drug interactions [70].
Until the burden of proof to break the causal link between HDL-C and cardiovascular disease is met, however, the search for HDL-C raising agents continues. In addition to CETP, promising HDL-raising candidates identified by cell culture studies include ABCA1 and ABCG1, which encode transporters, LCAT, which encodes the lecithin-cholesterol acyltransferase enzyme, and APOE [71,72]. Direct LCAT activators and LXR agonists represent other potential venues of raising HDL-C by augmenting reverse cholesterol transport, but both classes of drugs and agents such as apoA-I mimetics, are in early stages of development and have not undergone pharmacogenomic testing [73].
From bench to bedside: challenges to clinical application of pharmacogenomic findings
Despite the plethora of replicated genetic loci that have been shown to be associated with HDL-C levels in cross-sectional studies, few such predictors have been validated for longitudinal changes in HDL-C following treatment. Reasons for failure to replicate positive findings are manifold and include the challenges of finding independent study populations with comparable drug interventions and phenotype ascertainment, small effect sizes, inadequate statistical power, low frequency of functional alleles and chance [74–76]. As a result, some investigators have pursued alternative validation approaches, for example, functional assays in cell lines and/or animal knockout models. However, the vast majority of pharmacogenomic findings in general, and of HDL-C raising therapies in particular, are yet to be validated.
In addition to the challenge of replication, it is important to consider potential gene-environment interactions between pharmacogenetic variants and patients’ dietary and exercise habits. Although evidence on the effectiveness of lifestyle changes on HDL-C levels is mixed [77,78], it is plausible that some putative genetic predictors on HDL-C response synergistically interact with nutritional factors, as was shown in the context of xenobiotic-metabolizing enzymes [79]. That could lead to the main effects of the genetic variant to be obscured unless evaluated in a stratified manner. Although investigations to detect such interactions will require a large number of participants, future studies should consider possible interactions between pharmaceutical agents, diet/exercise and genetic variants in determining success of HDL-C-raising therapies.
Considering that most variants explain only a small fraction of response variability, another challenge to the concept of personalized treatment of low HDL-C levels involves the cost-effectiveness of genetic tests. Even in the case of the lipid response to statins, for which pharmacogenomic determinants have been thoroughly investigated, routine genetic testing is not currently recommended [80]. In addition, the field is yet to reach agreement on the level of evidence required to justify clinical utility of such testing [81]. However, rapid advancements in next-generation sequencing assays stand to improve the cost-effectiveness of screening for pharmacogenomic markers by making the availability of sequence data possible for each patient [82]. The realization of comprehensive DNA sequence-based pharmacogenomic studies holds much promise to identify higher impact variants and validate novel predictors of drug response for a wide range of therapies. In summary, discovery of pharmacogenomic determinants of HDL-C raising therapies has been limited, but previous studies will ultimately guide ongoing efforts with the fundamental goal to advance personalization of lipid normalizing agents.
Expert commentary
The pursuit of pharmacogenomic determinants of HDL-C-raising therapies has the dual purpose of advancing the understanding of systems biology underlying the variability of response xenobiotics, and of guiding clinicians in making treatment decisions on the basis of individual likelihood of drug effectiveness and/or adverse reactions.
Knowledge gained through pharmacogenomic studies must ultimately bring us closer to distinguishing superior or inferior candidates to receive a given drug therapy on the basis of genomic markers. To date, advances in genomic techniques have made possible the systematic pursuit and discovery of numerous genetic markers. Nonetheless, the translational value of the data forthcoming from such pursuits to personalized medicine has been limited.
For currently used therapies targeting HDL-C, there is either a paucity of data regarding pharmacogenomic sources of variability in response (as is the case for niacin), or, where such data are available (as is the case for fibrates), currently identified genomic markers explain only a small percentage of drug response variability. Such modest impact can be attributed to either small absolute effect of the polymorphism and/or modest effect allele frequency in the relevant population. At this point, it appears that actionable pharmacogenomics-based guidance for clinicians seeking to raise HDL-C will have to include a broader array of both genomic and nongenomic factors contributing to drug response variability, particularly in the case of the emerging HDL-C-raising methods such as CETP inhibitors.
Finally, to realize the translational value of genetic determinants of HDL-C response, prospective clinical trials must test the practical utility and cost-effectiveness of population-scale pharmacogenomics. Although much has been learned to date, further work is clearly needed before genetic tools are widely used to optimize HDL-C raising therapy regimens and thus clinical outcomes.
Five-year view
The search for pharmacogenomic predictors of HDL-C response to therapy will continue and expand beyond candidate gene studies. Studies such as GOLDN and the GIST consortium are currently investigating lipid response to fenofibrate and statins, respectively, via genome-wide association scans, exome sequencing and epigenetic approaches. In addition, studies that follow currently ongoing trials of novel HDL-C-raising therapies will yield new information on genetic predictors of response heterogeneity for several other classes of drugs. These findings will augment the initial set of putative genetic determinants of HDL-C response, which should subsequently be validated in independent cohorts. Upon successful replication, future studies could develop algorithms that would enable clinicians to use DNA testing to predict response to HDL-C-raising therapies, leading to truly personalized prescription approaches. Concurrently, studies will continue re-evaluating the causal link between HDL-C and heart disease, which could ultimately alter the nature of clinical guidelines and therapeutic approaches, challenging the current priorities of pharmaceutical companies and pharmacogenomics researchers.
Key issues.
HDL metabolism is dynamic and multifaceted, requiring a nuanced evaluation of putative genetic determinants of response to HDL-cholesterol (HDL-C)-raising therapy.
HDL-C response to therapeutic interventions is characterized by high variability and moderate heritability, suggesting a role for pharmacogenomic factors.
Although niacin is currently the most effective HDL-C-raising agent, no pharmacogenomic determinants of niacin efficacy have been described in humans.
HDL-C response to fenofibrate is associated with genetic variation in the APOA1/C3/A4/A5 cluster, ABCA1, APOE, CETP, CYP7A1 and PPARa.
Potential genetic predictors of HDL-C response to statin therapy are dependent on the type of statin and include variants in genes encoding lipoproteins, CYP450 proteins, CETP, LPL and OATP1B1.
The effect of novel HDL-C-raising therapies, such as CETP inhibitors is currently under investigation and is likely to be modified by polymorphisms in relevant biologic pathways.
Most of the currently available pharmacogenomic findings regarding HDL-C response have not been replicated in independent cohorts, leaving open the question of their validity.
Until the findings are replicated and genetic risk prediction algorithms are developed and validated, the promise of personalized HDL-C therapy remains elusive.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
*of interest
**of considerable interest
- 1.Cannon CP. High-density lipoprotein cholesterol as the Holy Grail. JAMA. 2011;306(19):2153–2155. doi: 10.1001/jama.2011.1687. [DOI] [PubMed] [Google Scholar]
- 2*.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. A large-scale provocative study that suggests that the link between HDL-cholesterol (HDL-C) and heart disease may not be causal; however, some of the methodological assumptions of this approach remain untested. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice T, Després JP, Pérusse L, et al. Familial aggregation of blood lipid response to exercise training in the health, risk factors, exercise training, and genetics (HERITAGE) Family Study. Circulation. 2002;105(16):1904–1908. doi: 10.1161/01.cir.0000014969.85364.9f. [DOI] [PubMed] [Google Scholar]
- 4.Goodarzi MO, Taylor KD, Scheuner MT, et al. Haplotypes in the lipoprotein lipase gene influence high-density lipoprotein cholesterol response to statin therapy and progression of atherosclerosis in coronary artery bypass grafts. Pharmacogenomics J. 2007;7(1):66–73. doi: 10.1038/sj.tpj.6500402. [DOI] [PubMed] [Google Scholar]
- 5.Blanche PJ, Gong EL, Forte TM, Nichols AV. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981;665(3):408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(7):1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 7.Warnick GR. Measurement and clinical significance of high-density lipoprotein cholesterol subclasses. In: Rifai M, Warnick GR, Dominiczak MH, editors. Handbook of Lipoprotein Testing. AACC Press; Washington, DC, USA: 1997. pp. 251–266. [Google Scholar]
- 8.Duriez P, Fruchart JC. High-density lipoprotein subclasses and apolipoprotein A-I. Clin Chim Acta. 1999;286(1–2):97–114. doi: 10.1016/s0009-8981(99)00096-0. [DOI] [PubMed] [Google Scholar]
- 9.Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113(12):1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 10**.Natarajan P, Ray KK, Cannon CP. High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 2010;55(13):1283–1299. doi: 10.1016/j.jacc.2010.01.008. Recent and comprehensive review of available HDL-C raising therapies. [DOI] [PubMed] [Google Scholar]
- 11.Guyton JR, Blazing MA, Hagar J, et al. Extended-release niacin vs gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterol. Niaspan-Gemfibrozil Study Group. Arch Intern Med. 2000;160(8):1177–1184. doi: 10.1001/archinte.160.8.1177. [DOI] [PubMed] [Google Scholar]
- 12.Digby JE, Ruparelia N, Choudhury RP. Niacin in cardiovascular disease: recent preclinical and clinical developments. Arterioscler Thromb Vasc Biol. 2012;32(3):582–588. doi: 10.1161/ATVBAHA.111.236315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121(3):1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pike NB. Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest. 2005;115(12):3400–3403. doi: 10.1172/JCI27160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101(8A):20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Kamanna VS, Kashyap ML. Niacin, but not gemfibrozil, selectively increases LP-AI, a cardioprotective subfraction of HDL, in patients with low HDL cholesterol. Arterioscler Thromb Vasc Biol. 2001;21(11):1783–1789. doi: 10.1161/hq1001.096624. [DOI] [PubMed] [Google Scholar]
- 17.Zhang LH, Kamanna VS, Ganji SH, Xiong XM, Kashyap ML. Niacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein A-I in HepG2 cells. J Lipid Res. 2012;53(5):941–950. doi: 10.1194/jlr.M020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu ZH, Zhao SP. Niacin promotes cholesterol efflux through stimulation of the PPAR[gamma]-LXR[alpha]-ABCA1 pathway in 3T3-L1 adipocytes. Pharmacology. 2009;84(5):282–287. doi: 10.1159/000242999. [DOI] [PubMed] [Google Scholar]
- 19.Lin SH, Liu CM, Chang SS, et al. Familial aggregation in skin flush response to niacin patch among schizophrenic patients and their nonpsychotic relatives. Schizophr Bull. 2007;33(1):174–182. doi: 10.1093/schbul/sbl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katherisan S, Rader DJ. Lipoprotein disorders. In: Ginsburg GS, Willard H, editors. Essentials of Genomic and Personalized Medicine. Academic Press; Burlington, MA, USA: 2010. pp. 269–281. [Google Scholar]
- 21.van der Hoorn JW, de Haan W, Berbée JF, et al. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3 Leiden. CETP mice. Arterioscler Thromb Vasc Biol. 2008;28(11):2016–2022. doi: 10.1161/ATVBAHA.108.171363. [DOI] [PubMed] [Google Scholar]
- 22.Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem. 1995;270(22):13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 23.Hossain MA, Tsujita M, Gonzalez FJ, Yokoyama S. Effects of fibrate drugs on expression of ABCA1 and HDL biogenesis in hepatocytes. J Cardiovasc Pharmacol. 2008;51(3):258–266. doi: 10.1097/FJC.0b013e3181624b22. [DOI] [PubMed] [Google Scholar]
- 24.van der Hoogt CC, de Haan W, Westerterp M, et al. Fenofibrate increases HDL-cholesterol by reducing cholesteryl ester transfer protein expression. J Lipid Res. 2007;48(8):1763–1771. doi: 10.1194/jlr.M700108-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Berthou L, Duverger N, Emmanuel F, et al. Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest. 1996;97(11):2408–2416. doi: 10.1172/JCI118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guérin M, Bruckert E, Dolphin PJ, Turpin G, Chapman MJ. Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 1996;16(6):763–772. doi: 10.1161/01.atv.16.6.763. [DOI] [PubMed] [Google Scholar]
- 27.Lai CQ, Parnell LD, Ordovas JM. The APOA1/C3/A4/A5 gene cluster, lipid metabolism and cardiovascular disease risk. Curr Opin Lipidol. 2005;16(2):153–166. doi: 10.1097/01.mol.0000162320.54795.68. [DOI] [PubMed] [Google Scholar]
- 28.Vu-Dac N, Gervois P, Jakel H, et al. Apolipoprotein A5, a crucial determinant of plasma triglyceride levels, is highly responsive to peroxisome proliferator-activated receptor alpha activators. J Biol Chem. 2003;278(20):17982–17985. doi: 10.1074/jbc.M212191200. [DOI] [PubMed] [Google Scholar]
- 29.Lai CQ, Arnett DK, Corella D, et al. Fenofibrate effect on triglyceride and postprandial response of apolipoprotein A5 variants: the GOLDN study. Arterioscler Thromb Vasc Biol. 2007;27(6):1417–1425. doi: 10.1161/ATVBAHA.107.140103. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Ordovas JM, Gao G, et al. Pharmacogenetic association of the APOA1/C3/A4/A5 gene cluster and lipid responses to fenofibrate: the genetics of lipid-lowering drugs and diet network study. Pharmacogenet Genomics. 2009;19(2):161–169. doi: 10.1097/FPC.0b013e32831e030e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brautbar A, Covarrubias D, Belmont J, et al. Variants in the APOA5 gene region and the response to combination therapy with statins and fenofibric acid in a randomized clinical trial of individuals with mixed dyslipidemia. Atherosclerosis. 2011;219(2):737–742. doi: 10.1016/j.atherosclerosis.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feitosa MF, An P, Ordovas JM, et al. Association of gene variants with lipid levels in response to fenofibrate is influenced by metabolic syndrome status. Atherosclerosis. 2011;215(2):435–439. doi: 10.1016/j.atherosclerosis.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Brisson D, Ledoux K, Bossé Y, et al. Effect of apolipoprotein E, peroxisome proliferator-activated receptor alpha and lipoprotein lipase gene mutations on the ability of fenofibrate to improve lipid profiles and reach clinical guideline targets among hypertriglyceridemic patients. Pharmacogenetics. 2002;12(4):313–320. doi: 10.1097/00008571-200206000-00007. The first pharmacogenetic study of fenofibrate efficacy in raising HDL-C. [DOI] [PubMed] [Google Scholar]
- 34.Christidis DS, Liberopoulos EN, Kakafika AI, et al. The effect of apolipoprotein E polymorphism on the response to lipid-lowering treatment with atorvastatin or fenofibrate. J Cardiovasc Pharmacol Ther. 2006;11(3):211–221. doi: 10.1177/1074248406293732. [DOI] [PubMed] [Google Scholar]
- 35.Tsai MY, Ordovas JM, Li N, et al. Effect of fenofibrate therapy and ABCA1 polymorphisms on high-density lipoprotein subclasses in the genetics of lipid lowering drugs and diet network. Mol Genet Metab. 2010;100(2):118–122. doi: 10.1016/j.ymgme.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossé Y, Pascot A, Dumont M, et al. Influences of the PPAR[alpha]-L162V polymorphism on plasma HDL(2)-cholesterol response of abdominally obese men treated with gemfibrozil. Genet Med. 2002;4(4):311–315. doi: 10.1097/00125817-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 37*.Cresci S. Pharmacogenetics of the PPAR genes and cardiovascular disease. Pharmacogenomics. 2007;8(11):1581–1595. doi: 10.2217/14622416.8.11.1581. A thorough review on the genetic factors underlying differential response to PPAR agonists; especially relevant for fibrates. [DOI] [PubMed] [Google Scholar]
- 38.Tai ES, Collins D, Robins SJ, et al. The L162V polymorphism at the peroxisome proliferator activated receptor alpha locus modulates the risk of cardiovascular events associated with insulin resistance and diabetes mellitus: the Veterans Affairs HDL Intervention Trial (VA-HIT) Atherosclerosis. 2006;187(1):153–160. doi: 10.1016/j.atherosclerosis.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Shen J, Arnett DK, Parnell LD, et al. The effect of CYP7A1 polymorphisms on lipid responses to fenofibrate. J Cardiovasc Pharmacol. 2012;59(3):254–259. doi: 10.1097/FJC.0b013e31823de86b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brousseau ME, Goldkamp AL, Collins D, et al. Polymorphisms in the gene encoding lipoprotein lipase in men with low HDL-C and coronary heart disease: the Veterans Affairs HDL Intervention Trial. J Lipid Res. 2004;45(10):1885–1891. doi: 10.1194/jlr.M400152-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Frazier-Wood AC, Aslibekyan S, Borecki IB, et al. Genome-wide association study indicates variants associated with insulin signaling and inflammation mediate lipoprotein responses to fenofibrate. Pharmacogenet Genomics. 2012;22(10):750–757. doi: 10.1097/FPC.0b013e328357f6af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziouzenkova O, Perrey S, Asatryan L, et al. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA. 2003;100(5):2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tojcic J, Benoit-Biancamano MO, Court MH, Straka RJ, Caron P, Guillemette C. In vitro glucuronidation of fenofibric acid by human UDP-glucuronosyltransferases and liver microsomes. Drug Metab Dispos. 2009;37(11):2236–2243. doi: 10.1124/dmd.109.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arafah A, Guillemette C, Tsai MY, et al. Evidence of UGT2B7 as a pharmacogenetic determinant of variability in serum fenofibric acid in human subjects. Presented at: Arteriosclerosis, Thrombosis, and Vascular Biology Scientific Sessions; Chicago, IL, USA. 18–20 April 2012. [Google Scholar]
- 45.Kostapanos MS, Milionis HJ, Filippatos TD, et al. Dose-dependent effect of rosuvastatin treatment on HDL-subfraction phenotype in patients with primary hyperlipidemia. J Cardiovasc Pharmacol Ther. 2009;14(1):5–13. doi: 10.1177/1074248408331031. [DOI] [PubMed] [Google Scholar]
- 46.Teramoto T. The clinical impact of pitavastatin: comparative studies with other statins on LDL-C and HDL-C. Expert Opin Pharmacother. 2012;13(6):859–865. doi: 10.1517/14656566.2012.660525. [DOI] [PubMed] [Google Scholar]
- 47.Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER Database. J Lipid Res. 2010;51(6):1546–1553. doi: 10.1194/jlr.P002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Postmus I, Verschuren JJ, de Craen AJ, et al. Pharmacogenetics of statins: achievements, whole-genome analyses and future perspectives. Pharmacogenomics. 2012;13(7):831–840. doi: 10.2217/pgs.12.25. [DOI] [PubMed] [Google Scholar]
- 49**.Barber MJ, Mangravite LM, Hyde CL, et al. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS ONE. 2010;5(3):e9763. doi: 10.1371/journal.pone.0009763. The largest genome-wide association study of lipid (including HDL-C) response to statin therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poduri A, Khullar M, Bahl A, Sehrawat BS, Sharma Y, Talwar KK. Common variants of HMGCR, CETP, APOAI, ABCB1, CYP3A4, and CYP7A1 genes as predictors of lipid-lowering response to atorvastatin therapy. DNA Cell Biol. 2010;29(10):629–637. doi: 10.1089/dna.2009.1008. [DOI] [PubMed] [Google Scholar]
- 51.Kajinami K, Brousseau ME, Ordovas JM, Schaefer EJ. CYP3A4 genotypes and plasma lipoprotein levels before and after treatment with atorvastatin in primary hypercholesterolemia. Am J Cardiol. 2004;93(1):104–107. doi: 10.1016/j.amjcard.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 52.Gao Y, Zhang LR, Fu Q. CYP3A4*1G polymorphism is associated with lipid-lowering efficacy of atorvastatin but not of simvastatin. Eur J Clin Pharmacol. 2008;64(9):877–882. doi: 10.1007/s00228-008-0502-x. [DOI] [PubMed] [Google Scholar]
- 53.Fiegenbaum M, da Silveira FR, Van der Sand CR, et al. The role of common variants of ABCB1, CYP3A4 and CYP3A5 genes in lipid-lowering efficacy and safety of simvastatin treatment. Clin Pharmacol Ther. 2005;78(5):551–558. doi: 10.1016/j.clpt.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Rosales A, Alvear M, Cuevas A, Saavedra N, Zambrano T, Salazar LA. Identification of pharmacogenetic predictors of lipid-lowering response to atorvastatin in Chilean subjects with hypercholesterolemia. Clin Chim Acta. 2012;413(3–4):495–501. doi: 10.1016/j.cca.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Wei KK, Zhang LR, Zhang Y, Hu XJ. Interactions between CYP7A1 A-204C and ABCG8 C1199A polymorphisms on lipid lowering with atorvastatin. J Clin Pharm Ther. 2011;36(6):725–733. doi: 10.1111/j.1365-2710.2010.01227.x. [DOI] [PubMed] [Google Scholar]
- 56.Lahoz C, Peña R, Mostaza JM, et al. RAP Study Group. Apo A-I promoter polymorphism influences basal HDL-cholesterol and its response to pravastatin therapy. Atherosclerosis. 2003;168(2):289–295. doi: 10.1016/s0021-9150(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 57.Sorkin SC, Forestiero FJ, Hirata MH, et al. APOA1 polymorphisms are associated with variations in serum triglyceride concentrations in hypercholesterolemic individuals. Clin Chem Lab Med. 2005;43(12):1339–1345. doi: 10.1515/CCLM.2005.229. [DOI] [PubMed] [Google Scholar]
- 58.Hamrefors V, Orho-Melander M, Krauss RM, et al. A gene score of nine LDL and HDL regulating genes is associated with fluvastatin-induced cholesterol changes in women. J Lipid Res. 2010;51(3):625–634. doi: 10.1194/jlr.P001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romaine SP, Bailey KM, Hall AS, Balmforth AJ. The influence of SLCO1B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J. 2010;10(1):1–11. doi: 10.1038/tpj.2009.54. [DOI] [PubMed] [Google Scholar]
- 60.Hedman M, Antikainen M, Holmberg C, et al. Pharmacokinetics and response to pravastatin in paediatric patients with familial hypercholesterolaemia and in paediatric cardiac transplant recipients in relation to polymorphisms of the SLCO1B1 and ABCB1 genes. Br J Clin Pharmacol. 2006;61(6):706–715. doi: 10.1111/j.1365-2125.2006.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin NG, Li KW, Murray H, Putt W, Packard CJ, Humphries SE. The effects of a single nucleotide polymorphism in SLCO1B1 on the pharmacodynamics of pravastatin. Br J Clin Pharmacol. 2012;73(2):303–306. doi: 10.1111/j.1365-2125.2011.04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang GP, Yuan H, Tang B, et al. Lack of effect of genetic polymorphisms of SLCO1B1 on the lipid-lowering response to pitavastatin in Chinese patients. Acta Pharmacol Sin. 2010;31(3):382–386. doi: 10.1038/aps.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winkelmann BR, Hoffmann MM, Nauck M, et al. Haplotypes of the cholesteryl ester transfer protein gene predict lipid-modifying response to statin therapy. Pharmacogenomics J. 2003;3(5):284–296. doi: 10.1038/sj.tpj.6500195. [DOI] [PubMed] [Google Scholar]
- 64.Bercovich D, Friedlander Y, Korem S, et al. The association of common SNPs and haplotypes in the CETP and MDR1 genes with lipids response to fluvastatin in familial hypercholesterolemia. Atherosclerosis. 2006;185(1):97–107. doi: 10.1016/j.atherosclerosis.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 65.Anagnostopoulou K, Kolovou G, Kostakou P, Mihas C, Mikhailidis D, Cokkinos DV. Pharmacogenetic study of cholesteryl ester transfer protein gene and simvastatin treatment in hypercholesterolaemic subjects. Expert Opin Pharmacother. 2007;8(15):2459–2463. doi: 10.1517/14656566.8.15.2459. [DOI] [PubMed] [Google Scholar]
- 66.Boekholdt SM, Sacks FM, Jukema JW, et al. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation. 2005;111(3):278–287. doi: 10.1161/01.CIR.0000153341.46271.40. [DOI] [PubMed] [Google Scholar]
- 67.Redondo S, Martínez-González J, Urraca C, Tejerina T. Emerging therapeutic strategies to enhance HDL function. Lipids Health Dis. 2011;10:175. doi: 10.1186/1476-511X-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barter PJ, Rye KA. Cholesteryl ester transfer protein (CETP) inhibition as a strategy to reduce cardiovascular risk. J Lipid Res. 2012;53(9):1755–1766. doi: 10.1194/jlr.R024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 70.Michos ED, Sibley CT, Baer JT, Blaha MJ, Blumenthal RS. Niacin and statin combination therapy for atherosclerosis regression and prevention of cardiovascular disease events: reconciling the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Sow HDL/High Triglycerides: Impact on Global Health Outcomes) trial with previous surrogate end point trials. J Am Coll Cardiol. 2012;59(23):2058–2064. doi: 10.1016/j.jacc.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 71.Hansen MK, McVey MJ, White RF, et al. Selective CETP inhibition and PPARalpha agonism increase HDL cholesterol and reduce LDL cholesterol in human ApoB100/human CETP transgenic mice. J Cardiovasc Pharmacol Ther. 2010;15(2):196–202. doi: 10.1177/1074248410362891. [DOI] [PubMed] [Google Scholar]
- 72.Yvan-Charvet L, Kling J, Pagler T, et al. Cholesterol efflux potential and anti-inflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30(7):1430–1438. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73**.Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol. 2011;8(5):266–277. doi: 10.1038/nrcardio.2010.200. A state-of-the-field update on emerging HDL-C raising therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.NCI-NHGRI Working Group on Replication in Association Studies. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 75.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29(3):306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 76.Daly AK. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11(4):241–246. doi: 10.1038/nrg2751. [DOI] [PubMed] [Google Scholar]
- 77.Couillard C, Després JP, Lamarche B, et al. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) family Study. Arterioscler Thromb Vasc Biol. 2001;21(7):1226–1232. doi: 10.1161/hq0701.092137. [DOI] [PubMed] [Google Scholar]
- 78.Siri-Tarino PW. Effects of diet on high-density lipoprotein cholesterol. Curr Atheroscler Rep. 2011;13(6):453–460. doi: 10.1007/s11883-011-0207-y. [DOI] [PubMed] [Google Scholar]
- 79.Northwood EL, Elliott F, Forman D, et al. Polymorphisms in xenobiotic metabolizing enzymes and diet influence colorectal adenoma risk. Pharmacogenet Genomics. 2010;20(5):315–326. doi: 10.1097/FPC.0b013e3283395c6a. [DOI] [PubMed] [Google Scholar]
- 80.Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice: why drugs work in some patients but not in others. Cleve Clin J Med. 2011;78(4):243–257. doi: 10.3949/ccjm.78a.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woodcock J. Assessing the clinical utility of diagnostics used in drug therapy. Clin Pharmacol Ther. 2010;88(6):765–773. doi: 10.1038/clpt.2010.230. [DOI] [PubMed] [Google Scholar]
- 82.Tzvetkov M, von Ahsen N. Pharmacogenetic screening for drug therapy: from single gene markers to decision making in the next generation sequencing era. Pathology. 2012;44(2):166–180. doi: 10.1097/PAT.0b013e32834f4d69. [DOI] [PubMed] [Google Scholar]