Abstract
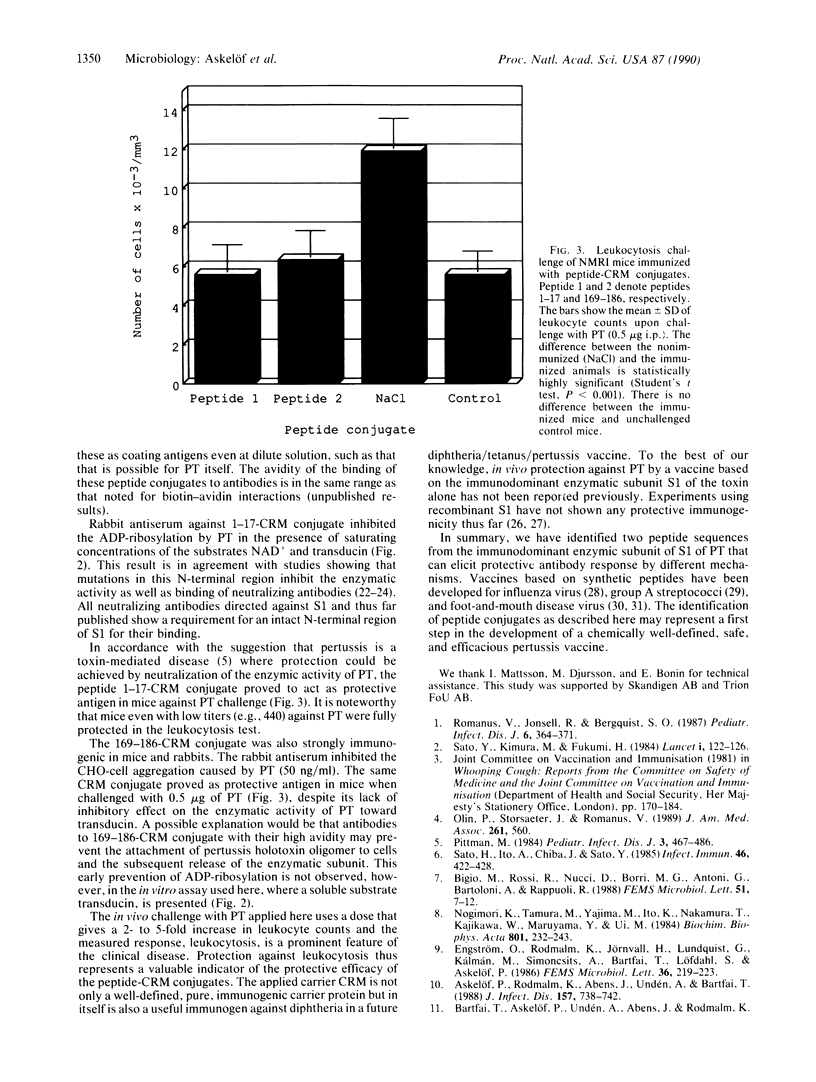
Two peptides, corresponding to amino acids 1-17 and 169-186 of the amino acid sequence of pertussis toxin (PT) subunit S1, were synthesized and coupled to the diphtheria toxin cross-reactive mutant protein CRM 197 and evaluated for immunogenicity and protective capacity against PT challenge in vivo. The peptide-CRM conjugates induced high antibody titers against native toxin in mice (BALB/c, C57/Black, and outbred NMRI) as measured by ELISA. Upon PT challenge (0.5 microgram of toxin) of the NMRI mice, the CRM conjugates of peptides 1-17 and 169-186 fully protected the mice from PT-induced leukocytosis. Immunization with the corresponding bovine serum albumin conjugates of these two peptides also fully protected mice. Rabbit antiserum to the peptide 1-17-CRM conjugate was highly efficient in inhibiting the ADP-ribosylating activity of PT but did not neutralize the clustering effect of PT on Chinese hamster ovary cells. In contrast, the rabbit antiserum raised against the peptide 169-186-CRM conjugate neutralized the clustering effect of PT on Chinese hamster ovary cells but did not inhibit the enzymatic activity of PT. Peptide 169-186-CRM conjugates mimic the immunoglobulin binding properties of PT and also cause clustering of Chinese hamster ovary cells. The CRM conjugates of these two peptides constitute a synthetic pertussis vaccine candidate with the ability to provide a chemically well-defined, safe, and efficient pertussis vaccine.
Full text
PDF
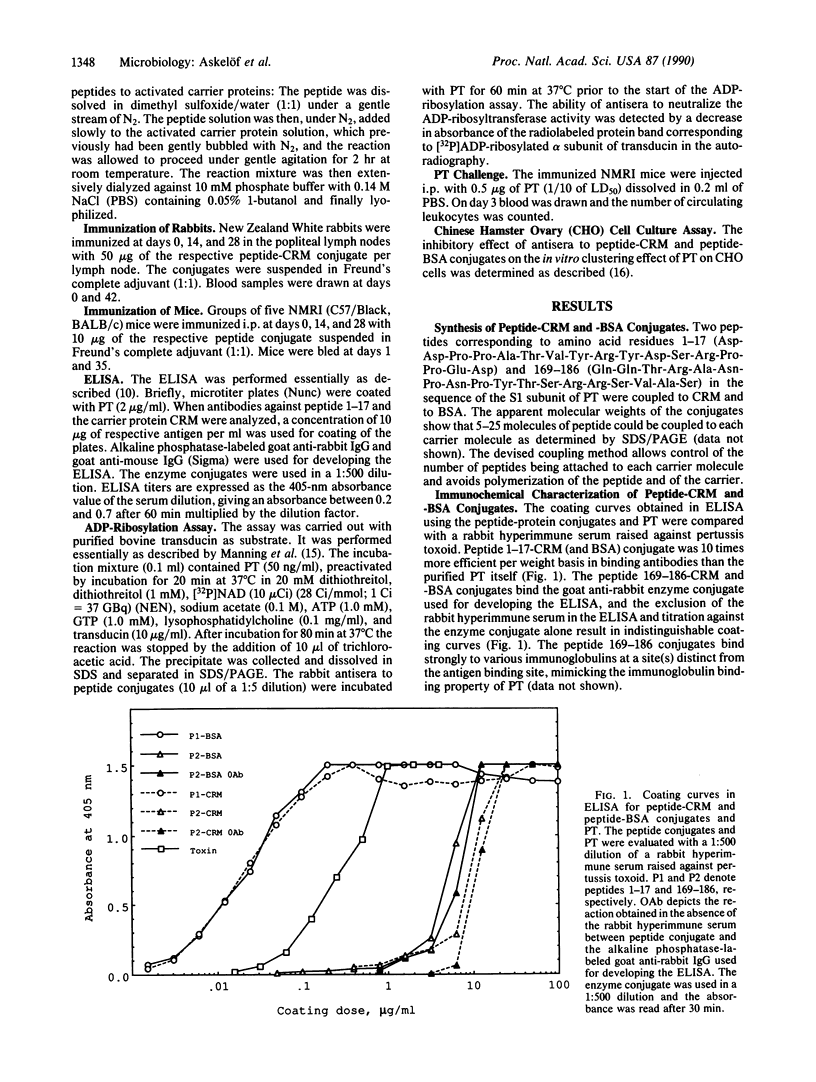


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askelöf P., Rodmalm K., Abens J., Undén A., Bartfai T. Use of synthetic peptides to map antigenic sites of Bordetella pertussis toxin subunit S1. J Infect Dis. 1988 Apr;157(4):738–742. doi: 10.1093/infdis/157.4.738. [DOI] [PubMed] [Google Scholar]
- Barbieri J. T., Cortina G. ADP-ribosyltransferase mutations in the catalytic S-1 subunit of pertussis toxin. Infect Immun. 1988 Aug;56(8):1934–1941. doi: 10.1128/iai.56.8.1934-1941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Tartar A., Seyer J. M., Chedid L. Epitope-specific protective immunogenicity of chemically synthesized 13-, 18-, and 23-residue peptide fragments of streptococcal M protein. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2203–2207. doi: 10.1073/pnas.81.7.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Munoz J. J., Peacock M. G., Schad P. A., Cowell J. L., Burchall J. J., Lim M., Kent A., Steinman L., Falkow S. ADP-ribosyltransferase activity of pertussis toxin and immunomodulation by Bordetella pertussis. Science. 1988 Apr 29;240(4852):656–659. doi: 10.1126/science.2896387. [DOI] [PubMed] [Google Scholar]
- Burnette W. N., Cieplak W., Mar V. L., Kaljot K. T., Sato H., Keith J. M. Pertussis toxin S1 mutant with reduced enzyme activity and a conserved protective epitope. Science. 1988 Oct 7;242(4875):72–74. doi: 10.1126/science.2459776. [DOI] [PubMed] [Google Scholar]
- Cieplak W., Burnette W. N., Mar V. L., Kaljot K. T., Morris C. F., Chen K. K., Sato H., Keith J. M. Identification of a region in the S1 subunit of pertussis toxin that is required for enzymatic activity and that contributes to the formation of a neutralizing antigenic determinant. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4667–4671. doi: 10.1073/pnas.85.13.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Barteling S. J., Meloen R. H. Small peptides induce antibodies with a sequence and structural requirement for binding antigen comparable to antibodies raised against the native protein. Proc Natl Acad Sci U S A. 1985 Jan;82(1):178–182. doi: 10.1073/pnas.82.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillenius P., Jätmaa E., Askelöf P., Granström M., Tiru M. The standardization of an assay for pertussis toxin and antitoxin in microplate culture of Chinese hamster ovary cells. J Biol Stand. 1985 Jan;13(1):61–66. doi: 10.1016/s0092-1157(85)80034-2. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Burnette W. N., Sublett R. D., Manclark C. R., Kenimer J. G. Epitopes on the S1 subunit of pertussis toxin recognized by monoclonal antibodies. Infect Immun. 1989 Mar;57(3):944–950. doi: 10.1128/iai.57.3.944-950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Capiau C., Feron C. Identification of amino acid residues essential for the enzymatic activities of pertussis toxin. Proc Natl Acad Sci U S A. 1989 May;86(9):3075–3079. doi: 10.1073/pnas.86.9.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D. R., Fraser B. A., Kahn R. A., Gilman A. G. ADP-ribosylation of transducin by islet-activation protein. Identification of asparagine as the site of ADP-ribosylation. J Biol Chem. 1984 Jan 25;259(2):749–756. [PubMed] [Google Scholar]
- Nicosia A., Bartoloni A., Perugini M., Rappuoli R. Expression and immunological properties of the five subunits of pertussis toxin. Infect Immun. 1987 Apr;55(4):963–967. doi: 10.1128/iai.55.4.963-967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Olin P., Storsaeter J., Romanus V. The efficacy of acellular pertussis vaccine. JAMA. 1989 Jan 27;261(4):560–560. [PubMed] [Google Scholar]
- Pittman M. The concept of pertussis as a toxin-mediated disease. Pediatr Infect Dis. 1984 Sep-Oct;3(5):467–486. doi: 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Pizza M., Bartoloni A., Prugnola A., Silvestri S., Rappuoli R. Subunit S1 of pertussis toxin: mapping of the regions essential for ADP-ribosyltransferase activity. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7521–7525. doi: 10.1073/pnas.85.20.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro M., Saletti M., Nencioni L., Tagliaferri L., Marsili I. Immunogenic correlation between cross-reacting material (CRM197) produced by a mutant of Corynebacterium diphtheriae and diphtheria toxoid. J Infect Dis. 1980 Nov;142(5):716–724. doi: 10.1093/infdis/142.5.716. [DOI] [PubMed] [Google Scholar]
- Romanus V., Jonsell R., Bergquist S. O. Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr Infect Dis J. 1987 Apr;6(4):364–371. doi: 10.1097/00006454-198704000-00005. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Clarke B. E., Carroll A. R., Brown F., Nicholson B. H., Bittle J. L., Houghten R. A., Lerner R. A. Chemical basis of antigenic variation in foot-and-mouth disease virus. Nature. 1983 Dec 15;306(5944):694–697. doi: 10.1038/306694a0. [DOI] [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Shapira M., Jibson M., Muller G., Arnon R. Immunity and protection against influenza virus by synthetic peptide corresponding to antigenic sites of hemagglutinin. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2461–2465. doi: 10.1073/pnas.81.8.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P., Merrifield R. B. Solid phase synthesis of gastrin I. Comparison of methods utilizing strong acid for deprotection and cleavage. Int J Pept Protein Res. 1985 Sep;26(3):262–273. [PubMed] [Google Scholar]
- Yamamoto T., Suyama A., Mori N., Yokota T., Wada A. Gene expression in the polycistronic operons of Escherichia coli heat-labile toxin and cholera toxin: a new model of translational control. FEBS Lett. 1985 Feb 25;181(2):377–380. doi: 10.1016/0014-5793(85)80296-9. [DOI] [PubMed] [Google Scholar]