Abstract
Background
Reducing the nicotine content in cigarettes could improve public health by reducing smoking and toxicant exposure, but may also have unintended consequences on alcohol use. The primary objective of this study was to examine the effect of reducing the nicotine content in cigarettes on alcohol outcomes. The secondary aim was to examine whether the effects of these cigarettes on alcohol outcomes were mediated by changes in nicotine exposure, smoking behavior, or withdrawal.
Methods
Between June 2013 and July 2014, we conducted a 7-arm, double-blind, randomized clinical trial at 10 U.S.-based sites. Daily smokers not currently interested in quitting (n = 839) were assigned to equally sized groups to smoke for 6 weeks cigarettes containing either normal nicotine content (NNC; 15.8 mg/g, 9 mg tar), moderate nicotine content (5.2 mg/g nicotine, 9 mg tar), or very low nicotine content (VLNC; 0.4 to 2.4 mg/g, 9 to 13 mg tar). This investigation focused on a subsample of current drinkers (n = 403). Each reduced nicotine content cigarette condition was compared to the NNC control condition with respect to trajectories over the 6-week period of average daily alcohol use and occurrence of binge drinking. Moderating variables were considered. Mediation analyses tested potential explanatory processes including changes in nicotine exposure, cigarettes per day, and withdrawal.
Results
Over time, reduced nicotine exposure and smoking rate mediated effects of VLNC cigarette use on reduced alcohol use. There was no evidence of compensatory drinking in response to nicotine reduction or nicotine withdrawal, even among subgroups expected to be at greater risk (e.g., relatively heavier drinkers, highly nicotine-dependent individuals).
Conclusions
The findings suggest that compensatory drinking is unlikely to occur in response to switching to VLNC cigarettes. In contrast, reducing the nicotine content of cigarettes may reduce alcohol use (clinicalTrials.gov number, NCT01681875).
Keywords: Nicotine, Very Low Nicotine Content Cigarettes, Alcohol Use, Binge Drinking
Cigarette smoking is the leading preventable cause of death in the United States, contributing annually to at least 480,000 deaths (U.S. Department of Health and Human Services, 2014). Nicotine is the primary addictive substance in tobacco that sustains smoking (Corrigall, 1999; Harvey et al., 2004; USDHSS, 1988), and the U.S. Food and Drug Administration has the authority to implement product standards that drastically reduce, although not eliminate, the nicotine content of cigarettes (Congress, 2009). Mandating nicotine levels in cigarettes at or below a level that results in minimal dependence may reduce the negative public health impact of cigarettes (Benowitz and Henningfield, 1994; Zeller and Hatsukami, 2009). In line with this hypothesis, randomized trials of very low nicotine content (VLNC) cigarettes, which contain substantially reduced levels of nicotine in the tobacco compared to conventional cigarettes (e.g., 16 mg/g; Kozlowski et al., 1998; Malson et al., 2001), have been shown to reduce nicotine exposure, nicotine dependence, and smoking (Benowitz et al., 2012; Donny et al., 2007; Hatsukami et al., 2010, 2013a) and promote abstinence (Hatsukami et al., 2010; Walker et al., 2012).
To fully describe the public health impact of VLNC cigarettes, it is critical to identify unintended consequences of this strategy on nonsmoking behaviors (Henningfield et al., 1998). One such behavior is alcohol use, a leading contributor to morbidity and death in the United States (Danaei et al., 2009; Mokdad et al., 2004). Alcohol and tobacco co-use is widespread (Falk et al., 2006), and the use of both substances may be causally linked (Dermody and Donny, 2014; McKee and Weinberger, 2013; Shiffman et al., 1995). Only 1 published study has reported the effect of VLNC cigarette use on drinking (Barrett et al., 2006). Among college-aged men (n = 15), VLNC cigarettes decreased alcohol use during a laboratory session relative to normal nicotine content (NNC) cigarettes. The results suggest that reduced nicotine exposure in the presence of smoking-related sensorimotor cues from VLNC cigarettes may decrease alcohol intake.
Laboratory studies of nicotine patches also support a link between nicotine and alcohol consumption; however, the findings are inconsistent. Relative to a 14-mg nicotine patch, placebo patch decreased drinking among male, but increased drinking among female social drinkers who were light smokers (1 to 10 cigarettes per day [CPD]; n = 34; Acheson et al., 2006). A subsequent study in heavy drinkers (n = 19) who also smoked more heavily (15 to 25 CPD) found that placebo patch (relative to nicotine patch) increased alcohol craving and decreased latency to drink among males and females (McKee et al., 2008). Thus, acute changes in nicotine impact drinking, but the direction of the effect may depend on sex, level of nicotine dependence, or hazardous drinking.
Extended reductions in nicotine intake via smoking VLNC cigarettes may introduce processes that would further impact drinking, such as nicotine withdrawal (Dermody and Donny, 2014). On average, withdrawal after switching to VLNC cigarettes increases within 1 day (Buchhalter et al., 2005) and returns to baseline levels within 2 weeks (Hatsukami et al., 2010, 2013b). While withdrawal tends to be less severe when smoking VLNC cigarettes compared to smoking abstinence (Buchhalter et al., 2001, 2005; Donny et al., 2007; Hatsukami et al., 2010), smokers who do not experience complete withdrawal relief may drink alcohol to cope with withdrawal-related negative mood (Dermody and Donny, 2014). For instance, in laboratory studies, smoking abstinence has increased drinking among heavy-drinking smokers relative to wearing a nicotine patch (McKee et al., 2008) or smoking an NNC cigarette (n = 56; Palfai et al., 2000); however, this effect has not been consistently replicated (n = 25; Perkins et al., 2000). Moreover, given the transient nature of withdrawal, any compensatory drinking would likely be short-lived.
Another process that may mediate the effect of extended VLNC cigarette use on alcohol intake is reduced smoking behavior (Dermody and Donny, 2014). Repeated cigarette and alcohol co-use may condition cigarettes as a cue for drinking (Shiffman et al., 1995). As VLNC cigarette use reduces cigarettes smoked per day relative to NNC cigarettes (Donny et al., 2007, 2015; Hatsukami et al., 2010, 2013a,b), this may impact alcohol use to the extent that cigarettes cue drinking (Dermody and Donny, 2014). Specifically, smoking cues (e.g., smoking-related images, holding a lit cigarette) have been shown to increase drinking urges among alcohol-dependent smokers, but less so for social drinkers (Drobes, 2002; Palfai et al., 2000). Thus, particularly among alcohol-dependent or heavy-drinking smokers, VLNC cigarettes could limit pharmacologic or cue-based primes for drinking, thereby reducing alcohol use.
Taken together, changes in nicotine exposure, cigarette use, and nicotine withdrawal symptoms each may independently impact alcohol consumption. While some processes, such as withdrawal, may increase alcohol use, other processes may decrease use. The simultaneous contribution of these processes could very likely correspond with a small or null net effect of VLNC cigarette use on drinking at the population level. As a result, examining each of these processes is critical for determining whether a nicotine reduction standard could impact alcohol outcomes among subsets of individuals affected by specific processes.
To address gaps in the existing literature, the first aim of this study was to determine the effect of cigarettes varying in nicotine content on drinking over time. It was hypothesized that VLNC cigarette use would reduce alcohol use. The second study aim was to explore the mechanisms hypothesized to mediate the effects of VLNC cigarette use on drinking. Reduced nicotine exposure and smoking behavior were hypothesized to correspond with reduced alcohol use. Increased withdrawal was expected to increase drinking. A double-blind, multisite clinical trial of daily smokers not interested in quitting smoking was completed in which participants were randomly assigned to smoke cigarettes of varying nicotine content for 6 weeks. In the total study sample, those assigned to VLNC cigarettes (with 2.4 mg/g nicotine or less) reduced their nicotine exposure and CPD, but did not have increased withdrawal relative to those assigned to NNC cigarettes (Donny et al., 2015). Alcohol use data were collected, but have not yet been reported.
MATERIALS AND METHODS
Participants
From 2013 to 2014, adult daily smokers were recruited using flyers, direct mailings, television/radio, and other advertisements from 10 sites (University of Pittsburgh, Brown University, Johns Hopkins University, University of Minnesota Twin Cities, University of Minnesota Duluth, Duke University, MD Anderson Cancer Center, University of California, San Francisco, Moffitt Cancer Center, and University of Pennsylvania). Inclusion criteria included the following: ≥age 18; smoking ≥5 CPD; expired carbon monoxide (CO) >8 ppm; or urine cotinine >100 ng/ml. Exclusion criteria included the following: intention to quit smoking in next 30 days; regular use of other tobacco products or frequent binge drinking (i.e., >9 of past 30 days)1; significant or unstable medical/psychiatric conditions; positive illicit drug toxicology screen other than cannabis; pregnancy/breast-feeding; and exclusively using “roll-your-own” cigarettes.
A total of 1,270 individuals were eligible via phone screen and provided written informed consent. In-person screening resulted in 839 eligible and enrolled participants. This study examined 403 participants (48%) who were randomized to a study cigarette condition (described below) and reported at the screening visit any alcohol use during the past month or reported any alcohol use during the 2-week baseline period.
Study Design
The 7-arm, double-blind, randomized trial included a 2-week baseline period and 6-week experimental period (for additional details, see Donny et al., 2015). During the baseline period, participants smoked their usual brand of cigarettes. Participants were then randomly assigned in equal numbers to smoke their preferred choice of menthol or nonmenthol research cigarettes that varied in nicotine content: 0.4 mg/g; 0.4 mg/g high tar (HT); 1.3 mg/g; 2.4 mg/g; 5.2 mg/g; 15.8 mg/g (defined a priori as the primary control). Average tar yields were 8 to 10 mg with the exception of 1 of the 0.4 mg/g cigarette conditions that had a higher tar yield of 13 mg to test the effects of HT cigarettes on smoking outcomes, but this manipulation was not addressed in this study. A usual cigarette brand condition was included in the trial, but was not examined in this study as participants who were assigned to these cigarettes were not blind to nicotine content. Study cigarettes were supplied by the National Institute on Drug Abuse (NOT-DA-14-004). At each weekly visit during the experimental period, participants were provided a free 14-day supply of cigarettes (based on CPD at baseline ×14). Additional cigarettes were provided as needed between visits to prevent constraints on cigarette access. During the experimental period, participants were instructed not to use other cigarettes, received brief weekly counseling aimed to increase compliance, and completed weekly laboratory assessments. The study was approved by the institutional review board at each study site.
Measures
Nicotine Dependence
Nicotine dependence was assessed at the baseline visit as the sum of responses on the 6-item Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991).
Alcohol Dependence Symptoms
Drinking problems were assessed at the baseline visit with the Michigan Alcohol Screening Test—Short Form (SMAST; Selzer et al., 1975), which is scored as the sum of responses to 13 yes/no questions about one’s drinking difficulties.
Alcohol Outcomes
Standard drinks (each containing about 14 g of pure alcohol such as one 12 oz beer, 5 oz glass of wine, or 1.5 oz shot of distilled spirits) consumed daily during the 2 weeks prior to the baseline visit or since the last experimental visit were reported using an interviewer-administered timeline follow-back (Sobell and Sobell, 1992). Alcohol use was computed as a weekly average of drinks per day (i.e., first 7 days of baseline period; days between each weekly visit). Occurrence of any binge drinking was defined as consuming ≥5 drinks (men) or ≥4 drinks (women) within 2 hours.
Smoking Behavior
An interactive voice response (IVR) system automatically called participants daily to query about the number of cigarettes smoked the previous day. Participants separately reported study and nonstudy cigarettes smoked. Weekly averages of daily total CPD (study + nonstudy) were computed (i.e., last 7 days of baseline period; days between each weekly visit).
Nicotine Exposure
Nicotine exposure biomarkers were assessed at the baseline visit and at postrandomization weeks 2 and 6 using first void urine samples. Total nicotine equivalents (TNE), adjusted for creatinine, were computed as the sum of nicotine and 6 metabolites, which included total nicotine, total cotinine, total trans 3′-hydroxycotinine (sum of the analyte and respective glucuronide conjugate), and nicotine-N-oxide.
Nicotine Withdrawal
Withdrawal symptoms during the past week were measured at each visit using the Minnesota Nicotine Withdrawal Scale (Hughes and Hatsukami, 1986). Ratings (none to severe) of symptoms (i.e., irritability, anxiety, depressed mood, craving, increased appetite, restlessness, insomnia, difficulty concentrating) were reliable (Cronbach’s αs ≥ 0.80) and thus were averaged. During the last week of the baseline period and first week of the experimental period, participants reported withdrawal daily using the IVR system. Averages of daily total withdrawal were computed corresponding to the baseline period and first experimental week.
Statistical Analyses
Analyses were conducted in the structural equation modeling framework using Mplus 7.2 (Muthén and Muthén, 1998–2012). Missing data were accounted for using maximum-likelihood estimation. Cutoff of p < 0.05 was used for statistical significance.
Latent growth curve models of weekly alcohol use and binge drinking were modeled with intercept factor loadings fixed to 1. Slope factor loadings were fixed to reflect equally spaced visits and intercept at baseline. Conventional cutoff criteria for fit was used, including root mean square error of approximation (RMSEA) ≤ 0.05 and comparative fit index (CFI) ≥ 0.95 (Browne et al., 1993; Hu and Bentler, 1999). Chi-square test was not used due to sensitivity to large samples. Linear, quadratic, and piecewise2 trajectories were compared using relative fit (better fit with smaller RMSEA, Akaike information criterion (AIC), and Bayesian information criterion (BIC), and CFI/Tucker Lewis index (TLI) closer to 1) or chi-square testing for nested models.
The effect of nicotine content on drinking was evaluated by regressing the slope(s) of each alcohol outcome on a dummy-coded predictor comparing the NNC control (coded as “0”) to each reduced nicotine condition (coded as “1”). Non normal alcohol use distribution was accounted for using bootstrapped corrected confidence intervals (CIs; 500 draws; Hoyle, 2014; Yung and Bentler, 1996). Covariates included sex, age, minority status, and baseline CPD and FTND (without CPD item).
Moderation analyses, using product terms, examined whether the effects of VLNC cigarette use on drinking differed based on sex, baseline levels of alcohol outcome (latent intercept variable), nicotine dependence, and alcohol problems. Continuous moderators were mean-centered. Only significant interactions are reported in the results and were probed using simple slope analyses.
Next, mediation analyses examined the effect of combined VLNC cigarette conditions (0.4 to 2.4 mg/g) on intermediate processes (i.e., nicotine exposure, withdrawal, CPD) and, in turn, the effect of intermediate processes on drinking. Statistical significance was determined using bootstrapping (MacKinnon et al., 2004).
TNE were examined as a mediator at weeks 2 and 6, controlling for baseline TNE. Changes in withdrawal and CPD were tested as mediators using parallel process models, which estimated relations between latent growth curves of the mediator and outcome (Cheong et al., 2003). If model fit was acceptable, the same trajectory shape was chosen for the mediator as the dependent variable. An additional mediation model was estimated for withdrawal, examining whether IVR-assessed week 1 withdrawal mediated the association between VLNC cigarette use and week 1 alcohol outcomes (controlling baseline period IVR-assessed withdrawal and aforementioned covariates). Sex and baseline nicotine dependence and alcohol problems were tested as moderators using product terms for both pathways in the mediated effect. Baseline level of latent alcohol outcome was not examined as a moderator due to estimation challenges associated with the complexity of the model (i.e., testing 3 latent variable interactions alongside up to 6 latent variables for each model). Only significant moderators are reported.
RESULTS
Participant Characteristics
On average, at the baseline visit, participants (n = 403) were 39.24 years of age, smoked 14.98 CPD, and were moderately nicotine dependent (Table 1). Approximately 57.9% were male, 57.8% were non-Hispanic White, 65.3% completed at least some college, and 52.9% were menthol smokers. Participants averaged 0.98 standard drinks per day, and 11.7% reported at least 1 binge drinking episode in the past week. Prevalence of high-risk drinking (i.e., consumed above NIAAA guidelines of 14 drinks per week for men and 7 for women) was 18.5% for men and 21.2% for women. Nearly 25% reported a history of problem drinking (SMAST > 4). Relative to the nondrinkers (who were excluded from this study), current drinkers smoked fewer CPD and were less nicotine dependent, younger, less likely to smoke menthol cigarettes, and less likely to be of minority race or ethnicity (ps < 0.05). No gender differences on drinking status were detected. Retention at week 6 was 92% (n = 370). From weeks 1 to 5, IVR and in-person visit completion rates were 92 to 99 and 87 to 98%, respectively.
Table 1.
Demographics and Baseline Smoking and Drinking Characteristics
| Variable | Level/unit | Overall | 15.8 mg/g | 5.2 mg/g | 2.4 mg/g | 1.3 mg/g | 0.04 mg/g | 0.04 mg/g HT |
|---|---|---|---|---|---|---|---|---|
| n | 403 | 64 | 61 | 67 | 71 | 60 | 80 | |
| Age | Years | 39.24 (13.75) | 39.93 (14.20) | 39.39 (13.70) | 40.16 (12.63) | 38.77 (14.63) | 40.38 (14.16) | 37.48 (13.42) |
| Sex | Female | 42.2 | 39.1 | 39.3 | 37.3 | 45.1 | 45.0 | 46.3 |
| Race | Non-Hispanic White | 57.8 | 57.8 | 50.8 | 59.7 | 62.0 | 45.0 | 60.0 |
| Black | 33.5 | 37.5 | 41.0 | 32.8 | 28.2 | 36.7 | 27.5 | |
| Education | High school or less | 34.7 | 32.8 | 42.6 | 31.3 | 36.6 | 33.3 | 322.5 |
| Some college or more | 65.3 | 67.2 | 57.4 | 68.7 | 63.4 | 66.7 | 67.5 | |
| Menthol | Menthol smoker | 52.9 | 54.7 | 50.8 | 59.7 | 49.3 | 48.3 | 53.8 |
| Cigarettes per day | 14.98 (7.23) | 14.81 (7.50) | 14.97 (7.21) | 14.75 (7.05) | 14.98 (7.43) | 14.52 (6.80) | 15.66 (7.51) | |
| Fagerström Test for Nicotine Dependence | Total score | 4.66 (2.27) | 4.50 (2.50) | 4.87 (2.14) | 4.61 (2.31) | 4.52 (2.40) | 4.48 (2.14) | 4.93 (2.13) |
| Alcohol | Drinks per day | 0.98 (1.57) | 1.11 (2.02) | 1.12 (1.65) | 1.09 (1.82) | 0.80 (1.27) | 1.05 (1.51) | 0.78 (1.12) |
| Binge | Any binge | 11.7 | 9.4 | 11.5 | 11.9 | 11.0 | 16.7 | 11.9 |
| Michigan Alcohol Screening Test—Short Form | Total score | 3.56 (2.01) | 3.44 (1.91) | 3.51 (1.89) | 3.57 (2.03) | 3.17 (2.00) | 4.13 (2.05) | 3.60 (2.08) |
HT, high tar.
Means (standard deviations) are reported for continuous outcomes, and proportions are reported for dichotomous/categorical outcomes. None of the baseline characteristics significantly differed between the study conditions, which were determined using omnibus chi-square tests for dichotomous outcomes (ps > 0.59) and 1-way ANOVAs for continuous outcomes (ps > 0.39).
The Impact of Reduced Nicotine Content Cigarettes on Alcohol Use
Alcohol Use Trajectories
The best-fitting model was piecewise, χ2 = 66.99, p < 0.001; RMSEA = 0.07 (0.05, 0.09); CFI = 0.97; TLI = 0.98; AIC = 8,115; BIC = 8,167.3 On average, alcohol use increased from baseline to week 2 (Sl1 = 0.08, 99% CI: 0.02, 0.16, p < 0.001), with no significant variability (Sl1 variance equaled zero); thereafter, it did not change (i.e., weeks 2 to 6; Sl2 = 0.002, 95% CI: −0.03, 0.03, p > 0.10), but demonstrated significant variability (SL2 variance = 0.04, 99%CI: 0.004, 0.09).
The Effect of Nicotine Reduction on Alcohol Use Trajectories
During the first 2 weeks, the moderate nicotine condition (5.2 mg/g) exhibited a significantly smaller increase in drinking relative to the NNC cigarette condition (Table 2).4 The 0.4 mg/g condition demonstrated a qualitatively smaller, but nonsignificant, increase in alcohol use relative to the NNC control condition. During the last 4 weeks, no conditions differed from NNC control, regardless of covariates (ps > 0.10). When the intercept for the alcohol use trajectory was centered at week 6, there were no significant differences in week 6 alcohol use between the NNC cigarette and reduced conditions (ps > 0.10; results not shown). The combined VLNC conditions (0.4 to 2.4 mg/g) showed a qualitatively smaller increase during the first 2 weeks compared to the NNC condition (p < 0.10) and no difference during the last 4 weeks, regardless of covariates.5 There were no significant moderators (ps > 0.10).
Table 2.
Effect of Smoking Cigarettes with Reduced Nicotine Content on Alcohol Outcomes
| 5.2 mg/g | 2.4 mg/g | 1.3 mg/g | 0.4 mg/g | 0.4 mg/g HT | VLNCa | |
|---|---|---|---|---|---|---|
| Unadjusted estimates | ||||||
| Alcohol use | ||||||
| Slope 1 | −0.18* (−0.36, −0.04) | −0.08 (−0.28, 0.15) | −0.14 (−0.32, 0.02) | −0.16† (−0.34, 0.04) | −0.11 (−0.27, 0.07) | −0.12† (−0.28, 0.02) |
| Slope 2 | 0.09 (−0.02, 0.21) | −0.03 (−0.14, 0.08) | 0.03 (−0.08, 0.14) | 0.03 (−0.07, 0.14) | 0.01 (−0.09, 0.11) | 0.01 (−0.08, 0.10) |
| Binge drinking | ||||||
| Slope 1 | 0.12 (−0.09, 0.34) | −0.13 (−0.34, 0.08) | −0.004 (−0.19, 0.18) | 0.13 (−0.06, 0.38) | 0.04 (−0.15, 0.23) | 0.02 (−0.26, 0.31) |
| Covariate-adjusted estimates | ||||||
| Alcohol use | ||||||
| Slope 1 | −0.19* (−0.35, −0.03) | −0.08 (−0.30, 0.12) | −0.13 (−0.32, 0.02) | −0.16† (−0.39, 0.03) | −0.11 (−0.28, 0.06) | −0.12† (−0.28, 0.02) |
| Slope 2 | 0.089 (−0.02, 0.21) | −0.04 (−0.15, 0.08) | 0.03 (−0.08, 0.14) | 0.04 (−0.06, 0.15) | 0.01 (−0.09, 0.12) | 0.01 (−0.07, 0.10) |
| Binge drinking | ||||||
| Slope 1 | 0.13 (−0.08, 0.33) | −0.12 (−0.33, 0.08) | 0.04 (−0.16, 0.20) | 0.16† (−0.02, 0.35) | 0.03 (−0.15, 0.20) | 0.02 (−0.13, 0.18) |
Results are for combined very low nicotine content (VLNC) cigarette conditions (0.4 to 2.4 mg/g normal and high tar [HT]) versus normal nicotine content condition.
p < 0.10,
p < 0.05.
Unstandardized path coefficients (95% confidence interval) are reported. Confidence intervals for alcohol use outcome are bias-corrected using bootstrapping procedure. Slope 1 corresponds with change during the first 2 weeks of the study; slope 2 corresponds with change during the last 4 weeks of the study. The binge drinking slope represented linear change throughout the duration of the study. No covariates were included in the unadjusted analyses. Adjusted analyses control for gender, age, minority race/ethnicity, alcohol trajectory intercept, and baseline cigarettes per day and Fagerström Test for Nicotine Dependence scores (without cigarettes per day item).
The Impact of Reduced Nicotine Content Cigarettes on Binge Drinking
Modeling Binge Drinking Trajectories
The best-fitting model was linear, χ2 = 125.76, p = 0.37; AIC = 1,921; BIC = 1,941.6 On average, binge drinking did not change (slope = 0.10, 95% CI: −0.03, 0.23), but the significant individual variability in the slope suggested that some individuals increased and others decreased their binge drinking.
The Effect of Nicotine Reduction on Binge Drinking Trajectories
Reduced nicotine content cigarettes were unrelated to binge drinking slope, regardless of covariates (Table 2). One nonsignificant trend in the 0.4 mg/g condition suggested increased binge drinking (p = 0.08), which is suspected to be spurious.7 There were no significant differences at week 6 (ps: 0.19 to 0.97). The combined VLNC conditions did not differ in binge drinking slope from NNC condition (p = 0.76). There were no significant moderators (ps: 0.14 to 0.92).
Mediators of Effect of VLNC Cigarettes on Alcohol Use
Nicotine Exposure
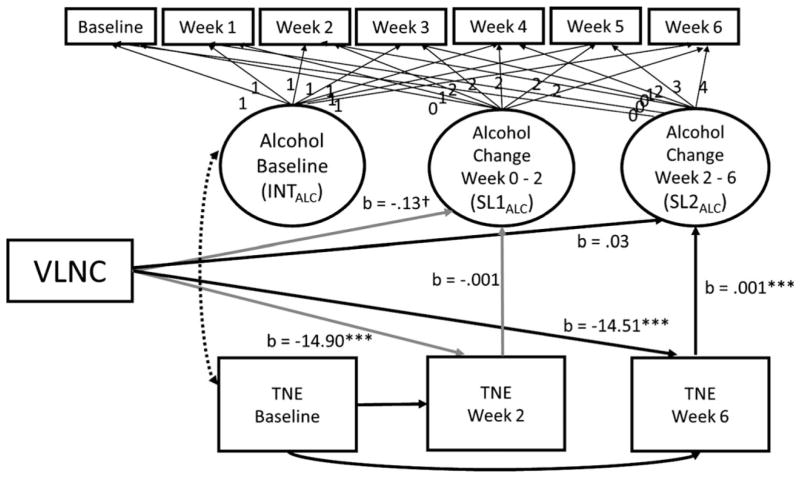
VLNC cigarette use was associated with lower TNE at week 2 than NNC cigarette use (Fig. 1).8 TNE were not associated with changes in alcohol use. Thus, mediation was not supported. VLNC cigarette use was associated with significantly lower week 6 TNE, which, in turn, was associated with relative reductions in alcohol use from weeks 2 to 6. The significant pathways were consistent with mediation, in which reduced nicotine exposure corresponded with reduced alcohol use (αβ = −0.02, p < 0.05, 95% CI: −0.04, −0.001).
Fig. 1.
The mediating role of nicotine exposure in the effect of very low nicotine content (VLNC) cigarette use on alcohol use. INTALC is the intercept of the alcohol use trajectory; SL1ALC is the slope for the first 2 weeks of the alcohol use trajectory; SL2ALC is the slope for the last 4 weeks of the the alcohol use trajectory. TNE (total nicotine equivalents) were observed values at baseline, week 2, and week 6. The fit of the mediation model was acceptable (χ2 = 116.66, p < 0.001; RMSEA = 0.045, 90% CI: 0.03, 0.06, CFI = 0.98, TLI = 0.98). Significance testing was completed using bias-corrected confidence intervals. The following pathways were estimated but are not included in the figure to improve readability: correlation between baseline levels of alcohol use with alcohol use slope terms, correlations between biomarkers at weeks 2 and 6, and the effect of nicotine biomarkers at baseline on the 2 alcohol slopes.
Sex moderated the association with VLNC cigarette use on TNE at week 2 (p < 0.01) and week 6 (p < 0.05). The VLNC cigarette effect on TNE was strongest for females. History of problem drinking moderated the association between week 6 TNE and alcohol use during the last 4 weeks (p < 0.05). Individuals endorsing a history of more drinking problems demonstrated a stronger association between lower TNE and drinking (1 SD above mean: αβ = −0.03, 95%CI: −0.08, −0.004) than those who endorsed fewer problems (1 SD below: αβ = −0.01, 95%CI: −0.03, 0.01).
Nicotine Withdrawal
The best-fitting latent growth curve model for withdrawal was piecewise function with a freely estimated week 1 factor loading, χ2 = 35.52, p = 0.01; RMSEA = 0.05; 90% CI: 0.03 to 0.08; CFI = 0.98; TFI = 0.97. There was a significant increase in withdrawal symptoms during the first 2 weeks (slope 1 = 0.07, SE = 0.02, p < 0.001); thereafter, withdrawal decreased (slope 2 = −0.04, SE = 0.01, p < 0.001).
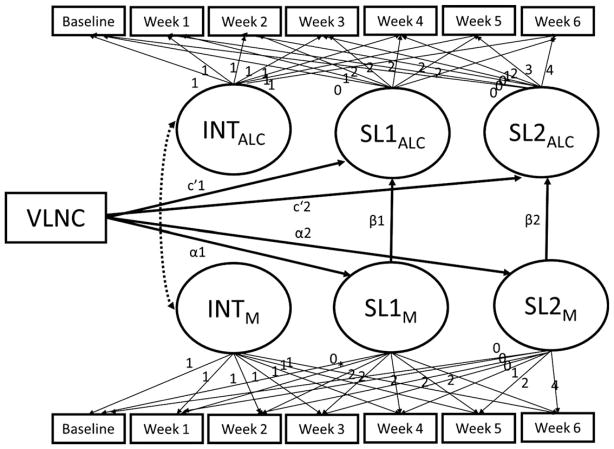
The parallel process model used to test mediation is depicted in Fig. 2. VLNC cigarette use was not associated with withdrawal during the first 2 weeks (α1 = −0.01, p > 0.10), nor was withdrawal associated with changes in alcohol use (β1 = 0.51, p > 0.10). During the last 4 weeks, VLNC cigarette use did not predict withdrawal (α2 = −0.002, p > 0.10), and withdrawal was not associated with alcohol use (β2 = 0.51, p > 0.10). Thus, withdrawal was not a mediator. There were no significant moderators (ps > 0.10).
Fig. 2.
Parallel process model testing the mediating pathways between very low nicotine content (VLNC) cigarette use and alcohol use. INTM is the intercept of the mediator trajectory; SLM is the slope of the mediator trajectory; INTALC is the intercept of the alcohol outcome trajectory; SLALC is the slope of the alcohol outcome trajectory. Some estimated paths are not depicted to simplify the model, including SL1M and SL2M regressed on INTALC, and SL1ALC and SL2ALC regressed on INTM.
Week 1 IVR-assessed withdrawal was also not a mediator as it was not predicted by VLNC cigarette use, and in turn, it was unrelated to week 1 alcohol use (β = 0.01, p > 0.10).
Cigarettes Per Day
The best-fitting latent growth curve model for CPD was piecewise with a freely estimated week 1 factor loading, χ2 = 40.64, p = 0.001; RMSEA = 0.06; 90% CI: 0.04 to 0.09; CFI = 0.98; TFI = 0.98. On average, CPD increased during the first 2 weeks (b = 0.26, SE = 0.06, p < 0.001) and then did not change (b = −0.08, SE = 0.07, p = 0.22).
The mediating role of CPD was investigated using the model depicted in Fig. 2. VLNC cigarettes reduced CPD during the first 2 weeks relative to NNC cigarettes (α1 = −1.32, p < 0.01, 95% CI: −2.07, −0.60), but CPD did not affect alcohol use (β1 = 0.02, p > 0.10, 95% CI: −0.006, 0.06), which does not support mediation. During the last 4 weeks, VLNC cigarette use was associated with reduced CPD (α2 = −0.66, p < 0.01, 95% CI: −0.99, −0.32), which, in turn, was associated with reductions in alcohol use (β2 = 0.07, p < 0.05, 95% CI: 0.001, 0.30). This corresponded with a significant mediating pathway in which reduced CPD in response to VLNC cigarette use was associated with reduced alcohol use (α2β2 = −0.05, p < 0.05, 95% CI: −0.24, −0.002). No significant moderators were identified (ps > 0.05).
Unique Mediating Pathways
As both TNE and CPD mediated the effects of VLNC cigarettes on alcohol use, they were examined simultaneously using the model in Fig. 3 to determine unique mediating effects. Unique mediating effects were observed for both TNE and CPD. VLNC cigarette use was associated with reduced TNE levels at week 6, which, in turn, was associated with reductions in alcohol use from week 2 to week 6 (αβ = −0.02, 95%CI: −0.05, −0.003). Similarly, VLNC cigarette use was associated with significant reductions in CPD between weeks 2 and 6 relative to the NNC control condition, which, in turn, was associated with concurrent reductions in alcohol use (αβ = −0.05, 95% CI: −0.18, −0.01).
Fig. 3.
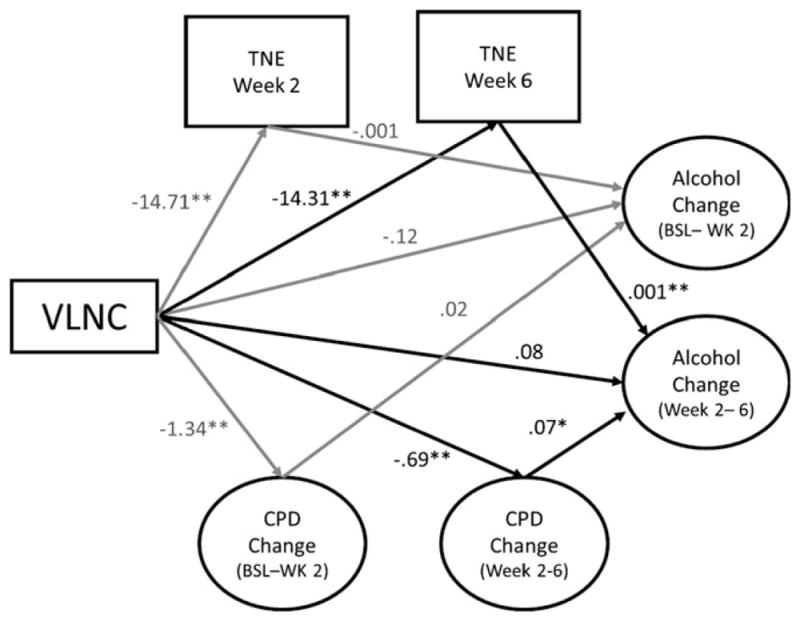
The unique mediating effects of nicotine exposure and changes in cigarettes per day on alcohol use. Abbreviations include TNE (total nicotine equivalents), CPD (cigarettes per day), BSL (baseline), WK (week), and VLNC (very low nicotine content cigarettes). The change in alcohol use and CPD from baseline to week 2 and weeks 2 to 6 (represented by corresponding circles in the figure) were estimated using piecewise latent growth curve models. The resulting model demonstrated adequate fit (χ2 = 478.80, p < 0.001; RMSEA = 0.07, 90% CI: 0.06, 0.08, CFI = 0.95, TFI = 0.94). Several variables and pathways were excluded from the diagram to simplify the figure, including the following: Intercepts of CPD and alcohol use were allowed to correlate with each other and predicted the slope terms of the parallel process; baseline TNE was included as a covariate for effects on weeks 2 and 6 TNE and change in alcohol use, and the relations of all other covariates with TNE, changes in CPD, and alcohol use including gender, FTND score (minus CPD), minority status, and age.
Mediators of Effect of VLNC Cigarettes on Binge Drinking
Nicotine Exposure
Week 6 TNE was not significantly associated with change in binge drinking (β = 0.0001, p > 0.10); therefore, mediation was not supported. No moderators were identified (ps > 0.08).
Nicotine Withdrawal
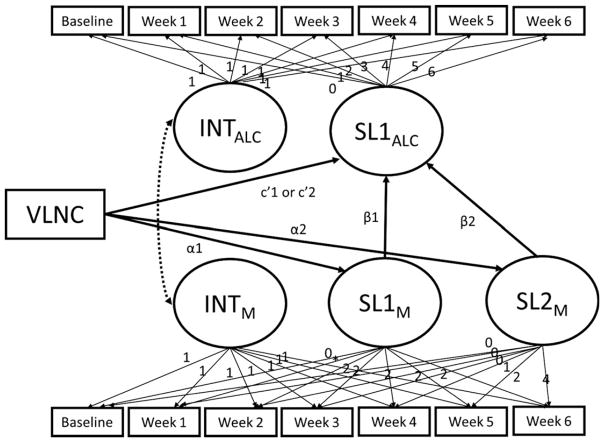
Mediation was not supported using the parallel process model depicted in Fig. 4 because VLNC cigarettes were not associated with changes in withdrawal (ps > 0.10) and changes in withdrawal were unrelated to changes in binge drinking (β1 = −0.59, p = 0.37; β2 = −0.04, p = 0.98). There were no significant moderators (ps > 0.10).
Fig. 4.
Parallel process model testing the mediating pathways between very low nicotine content (VLNC) cigarette use and binge drinking. INTM is the intercept of themediator trajectory; SLM is the slope of themediator trajectory; INTALC is the intercept of the alcohol outcome trajectory; SLALC is the slope for the alcohol outcome trajectory. Some estimated paths are not depicted to simplify the model, including SL1M and SL2M regressed on INTALC, and SL1ALC regressed on INTM.
Similarly, VLNC cigarette used was unrelated to week 1 withdrawal (α = 0.45, p > 0.10), which, in turn, was unrelated to week 1 binge drinking (β = 0.01, p > 0.10).
Cigarettes Per Day
Changes in CPD during the first 2 weeks (β1 = 0.002, p = 0.90) or last 4 weeks (β2 = 0.02, p = 0.67) were unrelated to changes in binge drinking. Thus, mediation was not supported. No moderators were identified (ps > 0.10).
DISCUSSION
Enacting a low nicotine product standard for cigarettes is expected to improve public health by reducing exposure to nicotine and harmful smoke constituents (Benowitz and Henningfield, 1994; Zeller and Hatsukami, 2009). We found no evidence of compensatory alcohol use or binge drinking in response to nicotine reduction in cigarettes, even among subgroups expected to be at higher risk (e.g., heavier smokers and drinkers). In fact, during the first 2 weeks, there was a smaller initial increase in alcohol use in the moderate nicotine condition and a similar qualitative trend for the VLNC cigarette condition; no significant differences were seen after 6 weeks.
This investigation also suggests that a nicotine reduction standard could further improve public health by reducing alcohol use among individuals who reduce their nicotine exposure and smoking rate. Specifically, VLNC cigarette use significantly reduced nicotine exposure, which, in turn, was associated with reduced alcohol use. These findings are consistent with and extend prior laboratory research in which smoking VLNC cigarettes reduced alcohol use (Barrett et al., 2006). The current findings extend this work by indicating that this relation is due, at least in part, to pharmacological effects of nicotine. Similarly, VLNC cigarette use reduced CPD compared to NNC cigarette use, which, in turn, was also associated with reduced alcohol use. The mediating role of CPD was independent of the effect of nicotine exposure on drinking and was expected as prior work implicates cigarettes as a cue for alcohol craving (Drobes, 2002). Thus, similar to prior legislations targeting smoking that have reduced drinking (McKee and Weinberger, 2013), regulation reducing the nicotine content of cigarettes may reduce alcohol use specifically through its effects on nicotine and cigarette exposure.
VLNC cigarettes did not impact binge drinking. The differential relations between CPD and nicotine with alcohol use and binge drinking were not predicted, and prior research supporting an effect of VLNC cigarettes on alcohol self-administration has not reported effects for binge drinking (Barrett et al., 2006). Our findings suggest that for smokers whose typical drinking patterns resemble our sample (i.e., not frequent binge drinkers), changes in nicotine exposure due to VLNC cigarette use may reduce alcohol use, but may not impact binge drinking.
This investigation had several methodological strengths. The use of VLNC cigarettes, in which participants receive sensorimotor cues associated with smoking but not pharmacological effects of nicotine, enabled us to examine independent contributions of nicotine and smoking behavior in explaining effects on drinking. Additional investigations of VLNC cigarettes could provide insight into the effects of nicotine, when obtained alongside other tobacco constituents, on alcohol metabolism, reinforcement, and subjective effects. Furthermore, the impact of VLNC cigarettes on drinking was examined longitudinally. Finally, the large multisite design, including a diverse population of daily smokers, allowed for examining individual differences.
This study also had limitations. Self-report measures of alcohol use are susceptible to factors that may have led to the initial increase in alcohol use seen in all study conditions.9 These factors may include reporting bias (such as from social desirability), chance fluctuations in drinking during the 1-week baseline period, and having more disposable income during the experiment (due to study payments and obtaining free cigarettes) to buy alcohol. Compliance with study cigarettes could not be guaranteed as usual brand cigarettes were freely available in participants’ natural environments. While all participants reported the use of study cigarettes, on average, participants also self-reported smoking <2 nonstudy CPD—which may be underreported (Benowitz et al., 2015). The use of nonstudy cigarettes may have led to underestimated associations between VLNC cigarette use, nicotine reduction, withdrawal, and alcohol outcomes. The present analyses partly addressed this limitation by demonstrating that reductions in nicotine exposure measured by biomarkers were associated with reduced alcohol use. Future research may benefit from efforts to reduce noncompliance to better estimate the effect of a nicotine reduction policy, under which conventional cigarettes would be unavailable.
Furthermore, causal inferences cannot be made based on the statistical mediation tests. The relations between the mediators and alcohol outcomes are likely bidirectional, as alcohol use also increases smoking behavior (Barrett et al., 2013; Mintz et al., 1985). For instance, the supported mediating roles of CPD and nicotine exposure may have in part been due to the effect of alcohol use on smoking and noncompliance; however, baseline drinking and alcohol problems did not impact the strength of intervention effects on drinking or intermediate processes. Thus, it is unlikely that the observed effects are entirely explained by these reverse pathways. Similarly, the effect of other constituents in the cigarettes on drinking cannot be ruled out, such as acetaldehyde—a constituent of cigarette smoke, as well as a metabolite of alcohol (Salaspuro and Salaspuro, 2004). Additional research is needed to disaggregate the effects of cigarettes as a cue to drink from other aspects of smoking.
Finally, 6 frequent binge drinkers (>9 times per month) were excluded from the study at screening, which may have partly contributed to the lack of association between study variables and binge drinking. Our subgroup analyses suggest individuals with a history of problem drinking show greater reductions in alcohol use in response to VLNC cigarette use. Relatedly, defining binge drinking based on NIAAA Council recommendations (NIAAA, 2004) may underestimate heavy-drinking episodes (Corbin et al., 2014). Additional work examining the tolerability of VLNC cigarettes among heavier or problem drinkers is warranted, particularly beyond 6 weeks as the effects on alcohol use appear to increase over time.
In light of these strengths and limitations, this study demonstrated that a reduced nicotine product standard could positively impact related health risk behaviors like drinking. Among many smokers, it appears that reducing the nicotine content of cigarettes would not lead to compensatory drinking. Instead, over time through a combination of reduced nicotine exposure and smoking behavior, a broader positive impact on public health may be observed through corresponding reductions in alcohol use.
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659), and National Institute on Alcohol Abuse and Alcoholism (F31AA022291). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. The authors have no conflicts of interest to report.
Footnotes
Six individuals were excluded at screening because they exceeded 9 binge drinking episodes in the past 30 days.
For the piecewise model, the inflection point was chosen a priori at week 2 (factor loadings: 0, 1, 2, 2, 2, 2, 2 & 0, 0, 0, 1, 2, 3, 4) due to the hypothesized time frame of withdrawal and nicotine reduction, instances when mediators were measured (i.e., nicotine exposure assessed at baseline, weeks 2 and 6), and model identification (i.e., latent linear slope factors need minimum of 3 indicators for identification purposes).
Linear trajectory model fit was χ2 = 89.10, p < 0.001; RMSEA = 0.08 (0.07, 0.10); CFI = 0.96; TLI = 0.97; AIC = 8,135; BIC = 8,181, and quadratic trajectory model fit was χ2 = 80.92, p < 0.001; RMSEA = 0.09 (0.07, 0.11); CFI = 0.96; TLI = 0.96; AIC = 8,135; BIC = 8,199.
Only 13.2% of the nondrinkers at baseline (who were excluded from these analyses) reported drinking any alcohol during the 6-week trial, and their drinking status during the trial was not predicted by VLNC cigarette use (logistic regression odds ratio = 0.97, p = 0.95).
Analyses were repeated with the outcome average number of drinks per drinking day, which was highly correlated with the concurrent values for average drinks per day used in the primary analyses (rs 0.75 to 0.82). The pattern of findings for the reduced nicotine content cigarette conditions and combined VLNC cigarette conditions relative to the control cigarette was replicated.
Quadratic trajectory model fit was χ2 = 119.95, p < 0.41; AIC = 1,923; BIC = 1,959, and piecewise was χ2 = 120.72, p = 0.39; AIC = 1,924; BIC = 1,960. Quadratic and piecewise slope and variance terms were nonsignificant.
The difference in slope between the 0.4 mg/g and NNC control condition was nonsignificant when only examining the first 5 weeks of the trial (p = 0.73).
The analyses excluded 5 individuals with missing or outlying values (i.e., baseline TNE <3), which is lower than that would be expected for a daily smoker.
Alcohol use also increased in the usual brand condition (not examined in this paper), and the change in alcohol use did not significantly differ between the usual brand and NNC cigarette control condition.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Campbell ML, Roach S, Stewart SH, Darredeau C. The effects of alcohol on responses to nicotine-containing and denicotinized cigarettes in dependent and nondaily smokers. Alcohol Clin Exp Res. 2013;37:1402–1409. doi: 10.1111/acer.12094. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomark Prev. 2012;21:761–769. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Nardone N, Hatsukami DK, Donny EC. Biochemical estimation of noncompliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomark Prev. 2015;24:331–335. doi: 10.1158/1055-9965.EPI-14-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R, Bollen KA, Long JS. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Sage Focus Editions. Vol. 154. Sage Publications; Newbury Park, CA: 1993. pp. 136–162. [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking- related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine Tob Res. 2001;3:111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using parallel process latent growth curve modeling. Struct Equ Model. 2003;10:238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congress. Family Smoking Prevention and Tobacco Control Act, in Series Family Smoking Prevention and Tobacco Control Act P.L. 111–31. U.S. Government Printing Office; Washington, DC: 2009. p. 123. [Google Scholar]
- Corbin WR, Zalewski S, Leeman RF, Toll BA, Fucito LM, O’Malley SS. In with the old and out with the new? A comparison of the old and new binge drinking standards. Alcohol Clin Exp Res. 2014;38:2657–2663. doi: 10.1111/acer.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody SS, Donny EC. The predicted impact of reducing the nicotine content in cigarettes on alcohol use. Nicotine Tob Res. 2014;16:1033– 1044. doi: 10.1093/ntr/ntu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey J, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le C, McClernon J, Montoya I, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK. Regulated reduction of nicotine in cigarettes: a randomized trial. N Engl J Med. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, Jensen J, Murphy SE, Thomas BF, Donny E. Dose–response effects of spectrum research cigarettes. Nicotine Tob Res. 2013a;15:1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, Carmella SG, al’Absi M, Joseph AM, Allen SS. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomark Prev. 2013b;22:1015–1024. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. This report was presented by the American Medical Association (AMA) Council on Scientific Affairs to the AMA House of Delegates at its 147th annual meeting in June 1998. The recommendations at the end of the report were adopted by the House as AMA policy. Tob Control. 1998;7:281–293. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle RH. Handbook of Structural Equation Modeling. Guilford Press; New York, NY: 2014. [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Mehta NY, Sweeney CT, Schwartz SS, Vogler GP, Jarvis MJ, West RJ. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7:369–375. doi: 10.1136/tc.7.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivar Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malson JL, Sims K, Murty R, Pickworth WB. Comparison of the nicotine content of tobacco used in bidis and conventional cigarettes. Tob Control. 2001;10:181–183. doi: 10.1136/tc.10.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S, O’Malley S, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology. 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH. How can we use our knowledge of alcohol- tobacco interactions to reduce alcohol use? Annu Rev Clin Psychol. 2013;9:649. doi: 10.1146/annurev-clinpsy-050212-185549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra V, Jarvik ME. Alcohol increases cigarette smoking: a laboratory demonstration. Addict Behav. 1985;10:203–207. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Muthén & Muthén; Los Angeles, CA: 1998–2012. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. [Accessed October 27, 2015];NIAAA Council approves definition of binge drinking. 2004 Available at: http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.htm.
- Palfai TP, Monti PM, Ostafin B, Hutchison K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychol. 2000;109:96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Perkins K, Fonte C, Grobe J. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behav Pharmacol. 2000;11:63–70. doi: 10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Salaspuro V, Salaspuro M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int J Cancer. 2004;111:480–483. doi: 10.1002/ijc.20293. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Rooijen LV. A self-administered short Michigan alcoholism screening test (SMAST) J Stud Alcohol Drugs. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M, Fertig J, Allen J. Associations between alcohol and tobacco. Alcohol and tobacco: from basic science to clinical practice. NIAAA Res Monogr. 1995;30:17–36. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Accessed June 1, 2015]. Available at: http://www.cdc.gov/tobacco/data_statistics/sgr/50thanniversary/index.htm. [Google Scholar]
- USDHSS. Nicotine addiction: a report of the surgeon general, in Series Nicotine Addiction: A Report of the Surgeon General. U.S. Department of Health and Human Services; Rockville, MD: 1988. [Google Scholar]
- Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, Laugesen M, Parag V, Whittaker R. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction. 2012;107:1857–1867. doi: 10.1111/j.1360-0443.2012.03906.x. [DOI] [PubMed] [Google Scholar]
- Yung Y-F, Bentler PM. Bootstrapping techniques in analysis of mean and covariance structures. In: Marcoulides GA, Schumacker RE, editors. Advanced Structural Equation Modeling: Issues and Techniques. Lawrence Erlbaum Associates; Mahwah, NJ: 1996. pp. 195–226. [Google Scholar]
- Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–332. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]