Abstract
After the 1956 radiation scare to stop weapons testing, studies focused on cancer induction by low-level radiation. Concern has shifted to protecting “radiation-sensitive individuals.” Since longevity is a measure of health impact, this analysis reexamined data to compare the effect of dose rate on the lifespans of short-lived (5% and 10% mortality) dogs and on the lifespans of dogs at 50% mortality. The data came from 2 large-scale studies. One exposed 10 groups to different γ dose rates; the other exposed 8 groups to different lung burdens of plutonium. Reexamination indicated that normalized lifespans increased more for short-lived dogs than for average dogs, when radiation was moderately above background. This was apparent by interpolating between the lifespans of nonirradiated dogs and exposed dogs. The optimum lifespan increase appeared at 50 mGy/y. The threshold for harm (decreased lifespan) was 700 mGy/y for 50% mortality dogs and 1100 mGy/y for short-lived dogs. For inhaled α-emitting particulates, longevity was remarkably increased for short-lived dogs below the threshold for harm. Short-lived dogs seem more radiosensitive than average dogs and they benefit more from low radiation. If dogs model humans, this evidence would support a change to radiation protection policy. Maintaining exposures “as low as reasonably achievable” (ALARA) appears questionable.
Keywords: ionizing radiation, beagle dogs, individual sensitivity, longevity benefit, harmful thresholds, adaptive protection
Introduction
Many studies have been carried out on the effects of ionizing radiation on organisms over the past 120 years. The overall effects are well known at high doses. At high and low doses, the detailed cell response mechanisms are complicated and may involve all levels of biological organization. About 75% of the human body is water, and a principal effect of radiation is the creation of reactive oxygen species (ROS), including hydrogen peroxide. They are a double-edged sword. Depending on their concentrations, they may cause damage or signaling in terms of stress responses.1 Moreover, ROS are produced abundantly and constantly by aerobic metabolism.2 Most studies focus on harmful effects, mainly risks of cancer, because of the low-level radiation scare that was introduced in 1956 to stop nuclear weapon testing and proliferation.3,4 The government regulators, worldwide, accepted the recommendation of the US National Academy of Sciences in 1956 that the risk of radiation-induced genetic mutations can be assessed using a linear no-threshold (LNT) model.5 “Radiation exposure has never been demonstrated to cause hereditary effects in human populations”6; however, there is evidence for X-rays and nuclear radiations to cause mutations in cells, which may contribute to the risk of cancer.
Studies on experimental living systems and on humans have shown, depending on the individual genome, that low doses of radiation upregulate many biological protective mechanisms, which also operate against nonradiogenic toxins and produce beneficial effects, including a lower risk of cancer.7 Still, most regulators uniquely employ the LNT model to estimate the risk of radiation-induced cancer deaths. After considering the health consequences of the precautionary evacuations following the 2011 nuclear accident in Japan and the impacts of the radiation scare on the economy, it has become obvious that the society is paying a very high price because of public fear of low-dose radiation.8
For more than a century, extensive studies have been carried out on the effects of radiation, which demonstrate that harmful effects, such as radiation illness, may arise after exposures above known threshold dose levels, whereas a range of beneficial effects may be observed following low-dose exposures.7,9,10,11 Although there appears to be an awareness among the prominent leaders of the radiation protection establishment that radiation protection policy contradicts this biological evidence; there is a very broad consensus among them that it is impossible to attribute health effects to low radiation exposures, namely to exposures similar to the wide spectrum of background levels.12 This opinion does not consider the recent progress in biological research on the mechanisms that underlay the fact that living organisms are “complex adaptive systems.”13
In radiation protection, the words “health effects” imply radiation-induced fatal cancer incidence that is calculated using the LNT model. The “health effects” of background radiation are small when compared to the average incidence of cancer deaths (less than 1 in 40 deaths) and, therefore, cannot be demonstrated due to large statistical uncertainties.
DNA alterations (damage) occur at a very high rate due to endogenous causes.2 To stay alive in a hard-to-avoid environment of multiple toxic impacts, all organisms have powerful protective mechanisms that prevent, repair, or remove damage in and to cells. Surviving cells continue to accumulate endogenous and exogenous mutations and may become cancer cells. These may be detected and destroyed by the immune system to prevent the development and spread of cancer. A weakened or impaired immune system is usually a precondition for cancer mortality.14 Since low doses of radiation stimulate many protective systems, including the immune system, it is very unlikely that low-level radiation causes more damage than benefit. Indeed, as damage propagation to molecules and cells from low doses can hardly be observed, protective mechanisms can be seen readily and be quantified.
Regulatory disregard of the biological evidence of beneficial health effects leaves lingering fear and uncertainty about cancer risks that sustain the risk assessment community. It restricts many medical applications of X-rays in diagnostic imaging and low-dose therapy. It blocks social acceptance of the nuclear energy option through fear of exposure to radioactive materials from power plants and waste management sites. When people increasingly question whether low levels or low doses of radiation are really harmful, protection practitioners argue that “radiation-sensitive individuals” exist who are more vulnerable than average people to potential “health effects” and must be protected.15 This concern about protecting sensitive individuals and the suggestion that longevity may be the most appropriate measure of the effect of radiation on health16,17 led to this examination of the effect of dose rate on the lifespans of dogs.
Analysis of 2 Studies on Beagle Dogs
To assess the effect of radiation level on more sensitive individuals, the authors reexamined data on the health effects of long-term irradiations in 2 large-scale studies on groups of beagle dogs. One exposed the dogs to whole-body cobalt-60 γ-radiation. The other evaluated dogs whose lungs were exposed to α-particle radiation from plutonium. Each group of dogs received a different dose rate. Beagle dogs are assumed to model humans well and have been the preferred choice for many studies by the US Department of Energy and its predecessor agencies since the 1950s.18
These studies had been reviewed previously to determine the dependence of the lifespan of 50% mortality dogs on dose rate9,19 Analysis of the data of the first study suggested an increase in the lifespan of dogs exposed to 50 mGy of γ-radiation per year, compared to the control dogs. Analysis of the data of the second study suggested an increase in longevity for dogs with an initial plutonium lung burden of 0.1 kBq/kg, compared to the control dogs.
These are very credible studies, carefully carried out by qualified and experienced scientists who bred the dogs and controlled all confounding factors. Particular attention was given to dosimetry. In the cobalt 60 study, all factors contributing to the dose rate and total dose were normalized in the irradiation field by migrating the dogs through all positions and orientations with respect to the irradiation source.18,20
Chronic γ-Irradiation
The methodology of this study is well described by Grahn and Fritz21 and by Fritz et al22. Using the data in Figure 1,23 the lifespans of dogs at 5%, 10%, and 50% mortality in the control group (background dose rate) were compared with the lifespans of the 5%, 10%, and 50% mortality dogs in each dose rate group. The intersection lifespans are tabulated against the dose rate presented in Table 1, and the normalized lifespans are plotted against the dose rate represented in Figure 2.
Figure 1.
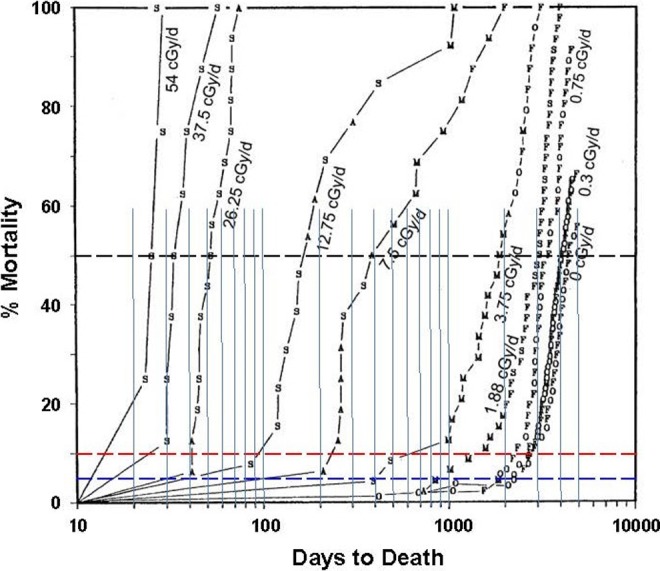
Mortality curves of dogs subjected to cobalt-60 γ-irradiation at different dose rates (Figure 3).23 The vertical lines were added to facilitate reading of the lifespan at the intersection of each mortality level (50%, 10%, and 5%) with the mortality curve of each group of dogs.
Table 1.
Lifespans of Dogs Versus Radiation Dose Rate.a
| Dose Rate, cGy/d | Dose Rate, mGy/y | Lifespan, Days | Lifespan, Normalized | ||||
|---|---|---|---|---|---|---|---|
| 50% Mortality | 10% Mortality | 5% Mortality | 50% Mortality | 10% Mortality | 5% Mortality | ||
| Background | 2.4 × 100 | 4300 | 2700 | 2150 | 1.00 | 1.00 | 1.00 |
| 0.3 | 1.1 × 103 | 4050 | 2700 | 2150 | 0.94 | 1.00 | 1.00 |
| 0.75 | 2.7 × 103 | 3300 | 2200 | 1800 | 0.77 | 0.82 | 0.84 |
| 1.88 | 6.9 × 103 | 3000 | 1300 | 850 | 0.70 | 0.48 | 0.386 |
| 3.75 | 1.4 × 104 | 1900 | 600 | 400 | 0.44 | 0.222 | 0.182 |
| 7.5 | 2.7 × 104 | 400 | 220 | 95 | 0.093 | 0.081 | 0.043 |
| 12.75 | 4.7 × 104 | 150 | 91 | 40 | 0.035 | 0.034 | 0.0182 |
| 26.25 | 9.6 × 104 | 51 | 40 | 30 | 0.012 | 0.0148 | 0.0136 |
| 37.5 | 1.4 × 105 | 32 | 23 | 15 | 0.0074 | 0.0085 | 0.0068 |
| 54 | 2.0 × 105 | 24 | 13 | 11 | 0.0056 | 0.0048 | 0.0050 |
aAdapted from Data in Fliedner et al.23
Figure 2.
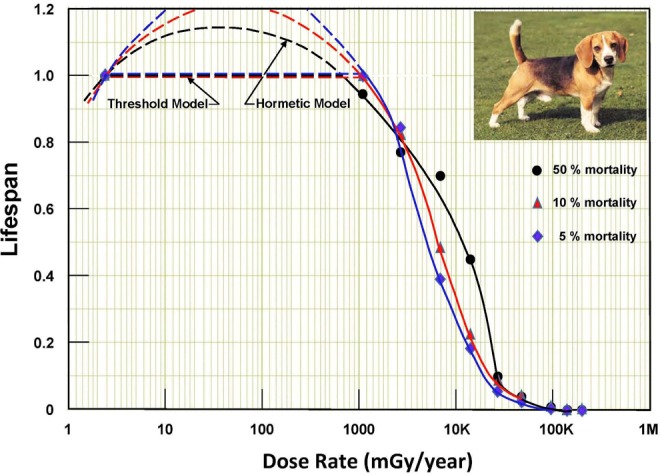
Lifespans of groups of dogs at different cobalt-60 γ-radiation dose rates. The black dot is the normalized lifespan of the 50% mortality dog in each group. The red triangle and the blue diamond are the normalized lifespans of 10% and 5% mortality dogs. Adapted data from Fliedner et al.23
Unfortunately, the design of this study did not include groups of dogs exposed to dose rates between background and 0.3 cGy/d. A group exposed to a dose rate of about 0.015 cGy/d (55 mGy/y) would have provided data to interpolate lifespans between the natural background level and the threshold dose rates at 700 to 1100 mGy/y. Lacking these data, interpolations based on both threshold and hormetic dose–response models are shown as dashed lines.
The dashed hormetic lines, drawn with the same curvature as the solid lines, very likely model the true response of longevity versus dose rate over this factor of 300 to 400 range. This judgment is based on the Calabrese and Baldwin17 review of many animal model studies on the effect on longevity of long-term, whole-body exposure to low dose rates of γ-rays. The magnitude of the increase in median lifespan ranged from 10% to 30%. In addition, there is extensive evidence and radiobiology endorsing the hormetic model for this interpolation.7,24,25,26
It was anticipated that the “short-lived” 5% and 10% mortality dogs would be more sensitive, adversely, to the effects of low dose-rate radiation—that their lives would be significantly shortened. On the contrary, Figure 2 suggests that their dose-rate thresholds for lifespan shortening are higher than those of the 50% mortality dogs. The lifespans of the 50% mortality dogs begin to decrease above a threshold of about 700 mGy per year, whereas the lifespans of the more radiation-sensitive (5% and 10% mortality) dogs begin to decrease above thresholds of about 1100 mGy per year. Their lifespans drop more steeply with increasing dose rate, indicating their greater sensitivity to radiation. The fitted lines are quite close to the data points.
The hormetic interpolations suggest that the optimum dose rate for longevity is about 50 mGy per year for all mortality levels. The lifespan increase is about 15% for 50% mortality dogs and much greater for the more radiation-sensitive dogs.
Chronic α-Irradiation of Lungs
The paper by Muggenburg et al27 describes in detail their study on 216 beagle dogs, which were exposed at 12 to 15 months of age by inhalation and pulmonary deposition of 7 graded activity levels of insoluble plutonium dioxide aerosols. The levels ranged from 0.16 to 29 kBq/kg initial lung burden. There were 36 control dogs, 18 male and 18 female (exposure level 0). The data on the observed carcinogenic and noncarcinogenic effects are analyzed and discussed.
Figure 3 below, from the article by Muggenburg et al27, presents the survival curves. They combined data from all 3 aerosol particle sizes within each exposure level. They also included survival curves for the 36 study controls and the 142 “other controls” from other lifespan studies on the same breed of dogs.
Figure 3.
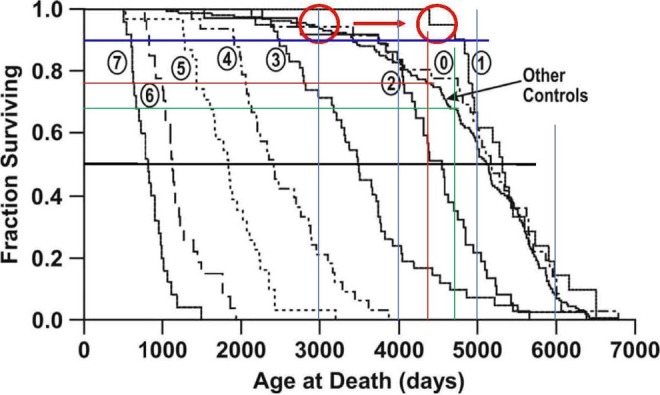
Fraction surviving curves of dogs with different lung burdens of inhaled plutonium-dioxide aerosols (Figure 4).27 The red circles indicate that the shorter lived control dogs (5% mortality level) have a lifespan of about 3000 days, whereas the dogs in group 1 (initial plutonium lung burden of 0.16 kBq/kg) have a lifespan of about 4500 days, 50% longer. Also shown are the 10% and 50% mortality levels.
This analysis determined the age at death (lifespan) of the dogs at 5%, 10%, and 50% mortality in the controls and in the 7 exposed groups from the intersections of the survival curves with the mortality levels, as shown in Figure 3. The results are shown in Table 2. Normalized lifespan versus plutonium lung burden (dose rate) are plotted in Figure 4. Lines were drawn that fit quite close to the data points.
Table 2.
Lifespans of Dogs Versus Initial Lung Burden (ILB) Inhaled at 12 to 15 Months.a,b
| Group | Initial Lung Burden, kBq/kg | Lifespan, Days | Lifespan, Normalized | ||||
|---|---|---|---|---|---|---|---|
| 50% Mortality | 10% Mortality | 5% Mortality | 50% Mortality | 10% Mortality | 5% Mortality | ||
| Control | 0 | 5150 | 3610 | 3000 | 1.00 | 1.00 | 1.00 |
| 1 | 0.16 | 5316 | 4760 | 4500 | 1.03 | 1.32 | 1.50 |
| 2 | 0.63 | 4526 | 3780 | 2910 | 0.88 | 1.05 | 0.97 |
| 3 | 1.6 | 3482 | 2500 | 2310 | 0.68 | 0.69 | 0.77 |
| 4 | 3.7 | 2421 | 1940 | 1500 | 0.47 | 0.54 | 0.50 |
| 5 | 6.4 | 1842 | 1280 | 1280 | 0.36 | 0.35 | 0.43 |
| 6 | 14 | 1122 | 840 | 810 | 0.22 | 0.23 | 0.27 |
| 7 | 29 | 807 | 625 | 530 | 0.16 | 0.17 | 0.18 |
aAdapted from data in Muggenburg et al.27
bCumulative lung dose for 10 kg dog (lung mass about 100 g) with ILB of 1 kBq at 1100 and 5000 days is about 0.5 and 1.2 Gy, respectively.
Figure 4.
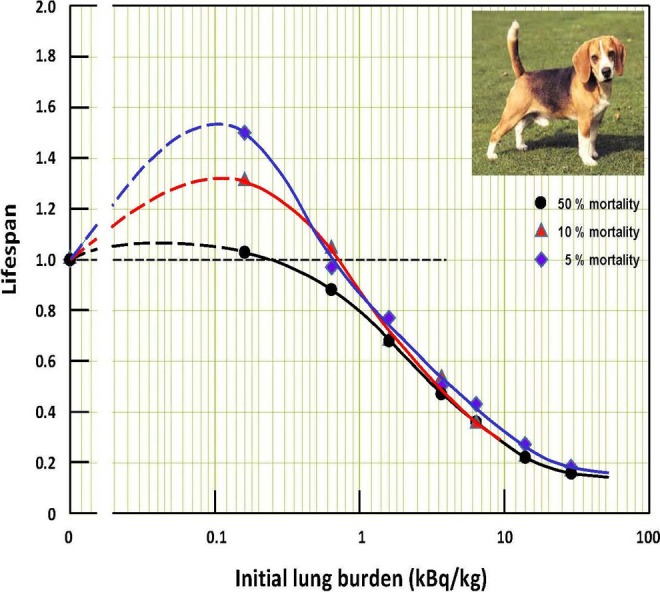
Lifespans of groups of dogs at different initial lung burdens of inhaled plutonium dioxide aerosols. The black dot is the lifespan of the 50% mortality dog of each group. The red triangle is the lifespan of the 10% mortality dog in each group, and the blue diamond is the lifespan of the 5% mortality dog in each group. Adapted from data in Muggenburg et al.27
It was anticipated that short-lived dogs at 5% and 10% mortality would be more sensitive, adversely, to the effects of α-radiation in their lungs—that their lives would be significantly shortened. On the contrary, Figure 4 suggests that their thresholds for lifespan reduction are significantly higher than those of the 50% mortality dogs, that is, about 0.65 kBq/kg versus 0.25 kBq/kg.
Dashed lines were drawn to interpolate the lifespans from the fitted lines to 0 plutonium burden. The lifespan lines suggest increased longevity when the lung burden is between 0 and the thresholds for harm (reduced lifespan). The more radiation-sensitive dogs experience a greater benefit than less sensitive dogs. The optimum lung burden appears to be about 0.1 kBq/kg, for all mortality levels.
These data again suggest that control dogs (no plutonium) at 5% mortality are much more sensitive to α-radiation in their lungs than are 50% mortality dogs. Figure 3 and Table 2 indicate that group 1 dogs at the 5% mortality level have a remarkably long lifespan. They live about 50% longer than the control dog at the 5% mortality level, 4500 days for group 1 versus 3000 days for the control dog.
Assessing the statistical significance of the group 1 longevity data, it is apparent that the first of the group 1 dogs died at an age of about 4400 days. This means that none of these 21 dogs died before that age. Examining the survival curve of the large “Other Controls” group of 142 dogs, 4400 days correspond to a survival fraction of 0.77, as shown by the red lines in Figure 3. The probability that all of the 21 dogs will survive this long, each having a probability of 0.77, is 0.7721 = 0.004. The corresponding P value is 0.4%. That means that the probability of a statistical fluctuation leading to the actual result of all the group 1 dogs living longer than 4400 days is 0.4%. In medicine, a confidence level of 5% or 1% is generally accepted as significant, so a P value of 0.4% is very significant.
Figure 3 indicates that the longevity benefit from lung irradiation is smaller for the less sensitive dogs at the 10% mortality level. The longevity of the control dogs at the 10% mortality level is about 3600 days; the longevity of the group 1 dogs at the 10% mortality level is about 4800 days, an apparent increase of about 33%. Assessing the statistical significance, the second dog of the group 1 dogs died at an age of about 4720 days. In the Other Controls group, 4720 days correspond to a survival fraction of 0.68 or a death probability of 0.32. The probability that, out of 21 dogs, 0 or 1 will die (20 or 21 will survive), whereas individual survival is independent with a probability of 0.68, is given by the binomial distribution, and is equal to 0.6821 + 21 × 0.6820 × 0.32 = 0.0033. The corresponding P value is about 0.3%, which is also very significant.
The age at death of the Other Controls at a survival fraction of 0.5 (50% mortality) is about 5150 days. At this age, it is apparent from counting the steps that 8 of 21 group 1 dogs died. Assuming that individual survival is independent for each dog with P = 0.5, the probability of 13 or more dogs surviving is given by the binomial distribution as about 19%. Therefore, the indication in Figure 4 that the group 1 dogs at 50% mortality may live about 5% longer than the control dog at 50% mortality is not significant.
It is interesting to note that the median lifespan (50% mortality) of the control dogs in the plutonium inhalation study, about 5150 days, is longer than the median lifespan of the control dogs in the γ-irradiation study, about 4300 days. Different breeds of beagle dogs were apparently used in the 2 studies.
Studies on rats that inhaled plutonium dioxide aerosols have shown a threshold for lifespan reduction at a lung dose of about 10 Gy; the lifespan increase for short-lived rats with a low lung dose was not apparent.28
Discussion and Conclusions
This analysis of mortality/survival data in the 2 studies suggests that short-lived (5% and 10% mortality) dogs are more sensitive to radiation than are long-lived (50% mortality) dogs. These more radiation-sensitive dogs seem to receive the benefit of increased longevity from low-level radiation, instead of the presumed adverse health effect of decreased longevity. For the dogs which received low-level α-radiation in their lungs (group 1), the relative increases in the lifespans of the more radiation-sensitive dogs (about 50% and 33%) appear much greater than the relative increases in the lifespans of the less-sensitive dogs (about 5%).
This analysis suggests that the optimum γ-radiation level for beagle dogs is about 50 mGy per year, and the optimum initial plutonium lung burden is about 0.1 kBq/kg. A possible explanation for this observation is that more radiation-sensitive dogs are more receptive to low-dose, radiation-induced upregulation or stimulation of their adaptive protection systems than are the less sensitive, 50% mortality dogs.
The radiation threshold for the onset of lifespan reduction appears to be higher for more sensitive dogs. The threshold for γ-radiation is about 700 mGy per year (50% mortality dogs); about 1100 mGy per year for the more sensitive dogs. For inhaled plutonium aerosols, the threshold for decreased longevity is about 0.25 kBq/kg (50% mortality dogs); about 0.65 kBq/kg for the more sensitive dogs.
The longevity of the more sensitive dogs appears to decline more steeply with rising dose rate as the γ dose-rate increases above the threshold for harm. Inhaled plutonium aerosols remain in the lungs, and the short-range α-radiation damages only nearby cells. It is very important to note that this very local exposure seems to affect the lifespan of the entire dog. Low-level radiation induces a significant increase in the longevity of the more sensitive dogs.
If beagles model humans, then these conclusions would apply also to people, supporting the views of Siegel et al29 that regulatory application of the LNT hypothesis and ALARA to protect radiosensitive people is misguided.
Recommendations
If dogs model humans, then one should expect that radiation-sensitive individuals would benefit more from exposures to low-level radiation than average humans. So protecting sensitive people from low-dose γ- or α-radiation would be inappropriate because it would deprive them of the health benefit of a longer life.
Protecting people against harm from high-level radiation is very important. Based on the results of this analysis, the threshold for increased mortality attributable to continuous exposure to γ-radiation appears to be about 700 mGy per year. Since beneficial health effects are likely below this level, the protection limit could be safely raised to at least 300 mGy per year, with no added risk.
Low-level exposure to inhaled α-emitters appears to bring health benefits, especially for more sensitive individuals. Efforts to eliminate residential radon appear to be misguided; Cuttler and Sanders30 have recommended a limit for radon concentration in homes that is about 7 times higher than the US Environmental Protection Agency’s radon action level.
The significant increase in the lifespan of short-lived dogs, chronically exposed to α-radiation from 1 inhalation of a small amount of plutonium aerosols, suggests the activation of very powerful signaling mechanisms. Studies should be carried out on mammals to understand this phenomenon. Such studies could lead to the discovery of important medical treatments for life-shortening diseases.
The results of this review suggest the need to change radiation protection policy. Obviously, maintaining exposures as low as reasonably achievable is very likely detrimental.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Human Exp Toxicol. 2002;21(2):85–90. [DOI] [PubMed] [Google Scholar]
- 2. Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Human Exp Toxicol. 2003;22(6):290–306. http://www.belleonline.com/newsletters/volume11/vol11-2.pdf. Accessed February 1, 2017. [DOI] [PubMed] [Google Scholar]
- 3. US National Academy of Science. BEAR I Committee. Genetic effects of atomic radiation. Science. 123(3209):1157–1164. doi:10.1126/science.123.3209.1157 [PubMed] [Google Scholar]
- 4. Jaworowski Z. Radiation hormesis—a remedy for fear. Human Exp Toxicol. 2010;29(4):263–270. http://het.sagepub.com/content/29/4/263.full.pdf. Accessed February 1, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Calabrese EJ. How the US National Academy of Sciences misled the world community on cancer risk assessment: new findings challenge historical foundations of the linear dose response. Arch Toxicol. 2013;87(12):2063–2081. [DOI] [PubMed] [Google Scholar]
- 6. United Nations Scientific Committee on the Effects of Atomic Radiation. Report to the General Assembly, with Scientific Annexes. Hereditary Effects of Radiation. New York, NY: United Nations; 2001. [Google Scholar]
- 7. Feinendegen LE, Pollycove M, Neumann RD. Hormesis by low dose radiation effects: low-dose cancer risk modeling must recognize up-regulation of protection In: Baum RP, ed. Therapeutic Nuclear Medicine. Berlin Heidelberg, Germany: Springer-Verlag; 2013. ISBN 973-3-540-36718-5. [Google Scholar]
- 8. Waltar AE, Brooks AL, Cuttler JM, Feinendegen LE, Gonzalez AJ, Morgan WL. The high price of public fear of low-dose radiation [published online June 6, 2016]. J Radiol Prot. 36(2):387. [DOI] [PubMed] [Google Scholar]
- 9. Cuttler JM. Commentary on Fukushima and beneficial effects of low radiation. Dose Response. 2013;11(4):432–443. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3834738/. Accessed February 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuttler JM. Remedy for radiation fear—discard the politicized science. Dose Response. 2014;12(2):170–184. www.ncbi.nlm.nih.gov/pmc/articles/PMC4036393/. Accessed February 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuttler JM. Urgent change needed to radiation protection policy. Health Phys. 2016;110(3):267–270. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez AJ. Unpublished letter; May 26, 2015.
- 13. Gell-Mann M. The Quark and the Jaguar. New York NY: St Martin’s Griffin, Macmillan; 1994. [Google Scholar]
- 14. Doss M. Changing the paradigm of cancer screening, prevention, and treatment. Dose Response. 2016;14(4):1–10. http://dos.sagepub.com/content/14/4/1559325816680539.full.pdf. Accessed February 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansson SO. Should we protect the most sensitive people? J Radiol Prot. 2009;29(2):211–218. [DOI] [PubMed] [Google Scholar]
- 16. Cameron JR. Longevity is the most appropriate measure of health effects of radiation. Radiology. 2003;229(1):14–15. http://pubs.rsna.org/doi/full/10.1148/radiol.2291030291. Accessed February 1, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Calabrese EJ, Baldwin LA. The effects of gamma rays on longevity. Biogerontology. 2000;1(4):309–319. [DOI] [PubMed] [Google Scholar]
- 18. Thompson RC. Life-span effects of ionizing radiation in the beagle dog: a summary account of four decades of research funded by the US Department of Energy and its predecessor agencies; 1989. PNL-6822 http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/20/071/20071717.pdf. Accessed February 1, 2017.
- 19. Cuttler JM, Feinendegen LE. Commentary on inhaled 239Pu02 in dogs a prophylaxis against lung cancer? Dose Response. 2015;13(1):1–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4674170/. Accessed February 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson RC, Mahaffey JA. Life-span radiation effects studies in animals: what can they tell us? In: Proceeding 22nd Hanford Life Sciences Symposium; September 27-29, 1983; Richland WA; 1986. OSTI USDOE CONF-830951 http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/20/029/20029409.pdf. Accessed February 1, 2017. [Google Scholar]
- 21. Grahn D, Fritz TE. Chronic radiation injury with mice and dogs exposed to external whole-body irradiation at the Argonne National Laboratory. In: Thompson RC, Mahaffey JA, eds. Richland, WA: Pacific Northwest Lab; 1986:14–31. [Google Scholar]
- 22. Fritz TE, Seed TM, Tolle DV, Lombard LS. Late effects of protracted whole-body irradiation of beagles by cobalt-60 gamma rays. In: Thompson RC, Mahaffey JA, eds. Richland, WA: Pacific Northwest Lab; 1986:116–141. [Google Scholar]
- 23. Fliedner TM, Graessle DH, Meineke V, Feinendegen LE. Hemopoietic response to low dose-rates of ionizing radiation shows stem cell tolerance and adaptation. Dose Response. 2012;10(4):644–663. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3526333/. Accessed February 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luckey TD. Hormesis with Ionizing Radiation. Boca Raton, FL: CRC Press; 1980. [Google Scholar]
- 25. Luckey TD. Radiation Hormesis. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 26. Calabrese EJ, Baldwin LA. Radiation hormesis: its historical foundations as a biological hypothesis. Human Exp Toxicol. 2000;19(1):41–75. http://www.radiation-hormesis.com/wp-content/uploads/2014/03/Hormesis-Historical-Investigations-Hum-Exp-Toxicol-2000-Calabrese-41-75.pdf. Accessed February 1, 2017. [DOI] [PubMed] [Google Scholar]
- 27. Muggenburg BA, Guilmette RA, Hahn FF, et al. Radiotoxicity of inhaled 239PuO2 in dogs. Radiat Res. 2008;170(6):736–757. [DOI] [PubMed] [Google Scholar]
- 28. Sanders CL, Lauhala KE, McDonald KE. Lifespan studies in rats exposed to 239PuO2 aerosol. III. Survival and lung tumours. Int J Radiat Biol. 1993;64(4):417–430. [DOI] [PubMed] [Google Scholar]
- 29. Siegel JA, Marcus CS, Welsh JS, Pennington CW, Stabin MG. Commentary: regulatory application of the LNT hypothesis and ALARA to protect radiosensitive people is misguided. Health Phys News. 2016;44(11):23–24. http://hps.org/hpspublications/newsletter_vol44no11.pdf. Accessed February 1, 2017. [Google Scholar]
- 30. Cuttler JM, Sanders CL. Threshold for radon-induced lung cancer from inhaled plutonium data. Dose Response. 2015;13(4):1–4. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679206/. [DOI] [PMC free article] [PubMed] [Google Scholar]


