Abstract
Plasma membrane resealing is a Ca2+-dependent process that involves the exocytosis of intracellular vesicles next to the wound site. Recent studies revealed that conventional lysosomes behave as Ca2+-regulated secretory compartments and play a central role in membrane resealing. These findings raised the possibility that the complex pathology of lysosomal diseases might also include defects in plasma membrane repair. Here, we investigated the capacity for lysosomal exocytosis and membrane resealing of fibroblasts derived from Chediak–Higashi syndrome (CHS) patients, or from beige-J mice. By using a sensitive electroporation/fluorescence-activated cell sorter-based assay, we show that lysosomal exocytosis triggered by membrane wounding is impaired in both human Chediak–Higashi and mouse beige-J fibroblasts. Lysosomal exocytosis increased when the normal size of lysosomes was restored in beige-J cells by expression of the CHS/Beige protein. A similar effect was seen when the lysosomal enlargement in beige-J cells was reversed by treatment with E64d. In addition, the survival of Chediak–Higashi and beige-J fibroblasts after wounding was reduced, indicating that impaired lysosomal exocytosis inhibits membrane resealing in these mutant cells. Thus, the severe symptoms exhibited by CHS patients may also include defects in the ability of cells to repair plasma membrane lesions.
Keywords: lysosome, injury, resealing
The Chediak–Higashi syndrome (CHS) is an autosomal, recessive disorder of humans that is caused by mutations in CHS, a cytosolic protein of ≈430 kDa. The same gene is responsible for the mouse beige-J mutation. Cells from CHS patients and beige-J mice contain abnormally large lysosomes, melanosomes, and lysosome-related secretory granules (1, 2). Although the function of the CHS/Beige protein is still poorly understood, its expression in beige-J cells restores normal lysosomal morphology (3). CHS symptoms are very severe, with high lethality resulting from infections or from a lymphocytic infiltrate known as the accelerated phase. The accelerated phase has been linked to defects in cytotoxic lymphocyte function (4, 5) because it can be alleviated by replacement of the hematopoietic system through bone marrow transplantation (6). However, patients who have undergone transplantation show a progressive neuropathy (7), indicating that the pathology of this disease is complex, and may result from dysfunction in many different cell types.
Recently, it became apparent that Ca2+-regulated exocytosis is not an exclusive property of the specialized secretory lysosomes of hematopoietic cells (8). Conventional lysosomes in many cells types respond to elevations in cytosolic Ca2+ by fusing with the plasma membrane (9), in a process mediated by specific soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins (10), and regulated by the Ca2+-binding protein synaptotagmin VII (11, 12). Modulation of synaptotagmin VII function through dominant-negative or gene knock-out approaches revealed a role for lysosomal exocytosis in the repair of plasma membrane lesions (12, 13). Given that most cells from CHS patients and beige-J mice have enlarged lysosomes (2), these findings suggested that lysosome-mediated plasma membrane repair might also be compromised in cells carrying this mutation. In this study, we present evidence in support of this view by demonstrating that lysosomal exocytosis and survival after wounding are impaired in CHS and beige-J fibroblasts.
Materials and Methods
Cells. Fibroblasts from clinically normal (GM08148) or CHS syndrome (GM02075A) human patients were obtained from the NIGMS Human Genetic Cell Repository (Bethesda) and used at passages 23 (GM08148) and 22 (GM02075A). Fibroblasts from beige-J mice complemented with a yeast artificial chromosome (YAC) carrying the Beige gene (YAC-bgJ/bgJ/Big24) or not carrying the Beige gene (bgJ/bgJ/MCHS) were generated in J.K.'s laboratory at the University of Utah (14).
Immunofluorescence. Immunofluorescence with mAbs against rat (LY1C6, provided by I. Mellman, Yale University, New Haven, CT), human (H4A3), or mouse (Protein Data Bank ID code 1D4B) lysosomal-associated membrane protein 1 (Lamp1) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City) was performed as described for attached cells (13). Electroporated cells in suspension were incubated on ice for 30 min with anti-Lamp1 mAbs, washed with ice-cold PBS, fixed in 2% paraformaldehyde, stained with Alexa Fluor 488 secondary Abs (Molecular Probes) and 4′,6-diamidino-2-phenylindole (Sigma), and viewed with a Zeiss Axiovert 135 microscope equipped with a Hamamatsu Orca II camera controlled by metamorph software (Universal Imaging, Downingtown, PA).
Wounding by Electroporation, Surface Staining for Lamp1, and Flow Cytometry. Monolayers were trypsinized and resuspended in Hanks' balanced salt solution containing 2% FBS and 10 mM Hepes, pH 7.0, at 106 cells per ml. Four hundred microliters of this cell suspension was added to an 0.2-cm electrode gene pulser cuvette (Bio-Rad), subjected to electroporation at 200 V and variable levels of capacitance, and incubated for 1 min at 37°C. Cells were then washed with cold PBS, incubated with 100 μl anti-Lamp1 hybridoma supernatant on ice for 30 min, washed, fixed, quenched with PBS containing 10 mM NH4Cl and 10 mg/ml BSA, and stained with Alexa Fluor 488 secondary Abs. Flow cytometry on at least 10,000 cells per sample was performed with a FACS (FACSCalibur, Becton Dickinson) and data were analyzed by using cellquest software (Becton Dickinson). Windows were drawn on plots encompassing the region outside of the background fluorescence, which was determined by incubation with the secondary Ab alone. The total Lamp1 content of cells was measured in the same manner after permeabilization with 0.1% saponin.
Cell Viability Assays. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction: Immediately after electroporation, cell suspensions were resuspended in DMEM containing 10% FBS, plated in serial dilutions into 96-well tissue culture dishes (100 μl per well), incubated for 12 h at 37°C in a humidified 5% CO2 incubator, followed by medium replacement by100 μl of phenol red-free DMEM containing 10% FBS, and 10 μl of 12 mM MTT (Sigma). After 4 h at 37°C, 100 μl of 10% SDS/10 mM HCl was added to each well, plates were incubated overnight at 37°C, and the concentration of MTT reduced to formazan was determined colorimetrically at 570 nM (15). [35S]Methionine incorporation: 12 h after electroporation and replating, cells were incubated with 100 μCi/ml (1 Ci = 37 GBq) [35S]methionine (ICN) in methionine-free DMEM and 10% dialyzed FBS for 1 h. After precipitation with 10% trichloroacetic acid, the cell-associated radioactivity was measured in a scintillation counter (Beckman). For assessment of viability by direct microscopic counting, electroporated cell suspensions were cultured for 12 h, trypsinized, and counted in a hemocytometer.
Results
Exocytosis of Lysosomes Is Proportional to the Degree of Membrane Injury. Ca2+ influx through mechanically induced lesions on the plasma membrane of fibroblasts triggers a resealing response that involves the exocytosis of lysosomes (12, 13). It was not previously determined, however, whether lysosomal exocytosis was proportional to the degree of cell injury. Thus, to more quantitatively assess the membrane repair capacity of CHS/Beige cells, we first sought to develop an assay that would allow us to quantify injury induced lysosomal exocytosis in a dose-dependent fashion.
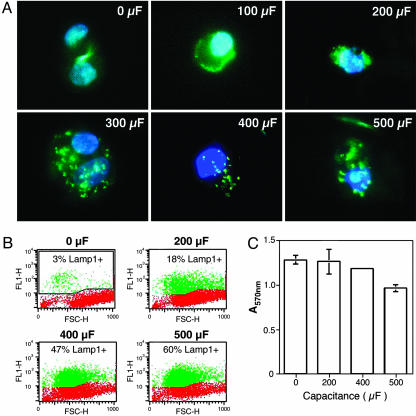
Several studies showed that hydrophilic pores large enough to allow the entry of macromolecules can be generated in the plasma membrane of mammalian cells by externally applied electric fields. This method is widely used to introduce DNA or protein into cells, a process that depends on the capacity of mammalian cells to reseal their plasma membrane after electroporation (16, 17). Increases in capacitance, which lengthens the electric pulse duration, increases pore formation and membrane disruption (18). This ability to modulate the degree of membrane permeabilization provided us with a tool to determine directly whether the lysosomal exocytic response corresponded to the amount of membrane damage. This methodology was initially tested by using normal rat kidney epithelial (NRK) cells, a cell line with a well characterized lysosomal secretory response to injury and Ca2+ influx (9, 13). Cells were electroporated under conditions of increasing capacitance, and lysosomal exocytosis was immediately assayed by detection of a luminal epitope of the abundant lysosomal glycoprotein Lamp1 on the cell surface (9, 13). Analysis by fluorescence microscopy revealed a gradual increase in surface-exposed Lamp1, proportional to the duration of the electric pulse applied to the cells. The surface fluorescence pattern was punctate, similar to what was previously observed in cells wounded by scraping (13) (Fig. 1A). This surface-staining pattern was clearly different from the pattern detected when NRK cells are permeabilized to reveal intracellular Lamp1 (10). Quantification of Lamp1 exposed on the cell surface by flow cytometry revealed a positive signal on only ≈3% of nonelectroporated cells (Fig. 1B). In contrast, when cells were electroporated at 200, 400, and 500 μF, Lamp1 was detected on the surface of 18%, 47%, and 60% of the cells, respectively (Fig. 1B). Thus, this assay allowed us to demonstrate for the first time, to our knowledge, that there is a direct correlation between the degree of membrane damage and the amount of lysosomal exocytosis.
Fig. 1.
Electroporation-mediated wounding triggers dose-dependent lysosomal exocytosis and cell resealing. (A) Immunofluorescence of Lamp1 on the surface of NRK cells after electroporation at increasing capacitance. Anti-Lamp1 mAb is green and 4′,6-diamidino-2-phenylindole DNA stain is blue. (B) Quantification of surface Lamp1 in electroporated NRK cells by FACS. Red indicates background fluorescence and green indicates the amounts of surface-exposed Lamp1. FL1-H, fluorescence intensity; FSC-H, forward scatter. (C) Viable NRK cells 12 h after electroporation at increasing capacitance levels were detected by using a MTT reduction colorimetric assay. The data correspond to the mean and SD of triplicate samples.
The ability of cells to reseal their plasma membrane was assessed by determining the number of viable cells 12 h after electroporation. Under the more stringent electric field intensity condition (500 μF), there was a small loss in the viability of NRK cells (of ≈24%), whereas under all other conditions, the majority of the cells survived the wounding procedure (Fig. 1C). These results show that electroporation induces plasma membrane lesions in NRK cells, and that these lesions, similar to what was shown previously for mechanical wounds (13), trigger lysosomal exocytosis as a resealing mechanism.
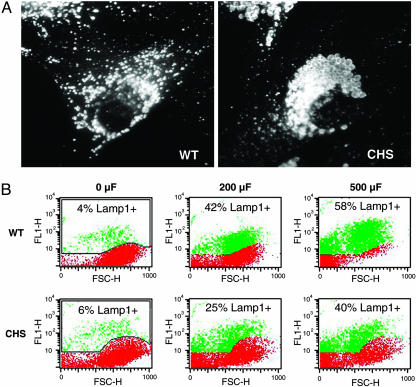
Lysosomal Exocytosis Induced by Membrane Injury Is Reduced in Fibroblasts from CHS Patients. Taking advantage of the dose-dependent wounding assay described above, we investigated whether the enlarged lysosomes of cells from Chediak–Higashi patients (19) were capable of a normal exocytic response in response to membrane injury. Control (WT) and CHS primary fibroblasts showed the expected lysosomal morphology after immunofluorescence with anti-Lamp1: normal for WT, and markedly enlarged for CHS (Fig. 2A). The fibroblasts were subjected or not subjected to electroporation, and lysosomal exocytosis was assessed by FACS detection of surface-exposed Lamp1. In nonelectroporated cells, only ≈4–6% of both cell types showed detectable surface Lamp1 (Fig. 2B, 0 μF). After electroporation at 200 μF, ≈42% of WT cells became positive for surface Lamp1. In contrast, under this condition, only 25% of CHS fibroblasts showed detectable surface Lamp1 (Fig. 2B, 200 μF). When the capacitance was increased to 500 μF, 58% of WT fibroblasts exposed Lamp1 on the surface, in contrast to 40% of CHS fibroblasts under the same conditions (Fig. 2B, 500 μF). Thus, lysosomal exocytosis in response to identical wounding conditions appeared to be reduced by 30–40% in CHS fibroblasts, when compared with normal cells.
Fig. 2.
CHS fibroblasts show reduced lysosomal exocytosis after wounding. (A) Immunofluorescence of normal human fibroblasts (WT) or CHS patient fibroblasts (CHS) with anti-Lamp1 mAbs. Enlarged lysosomes in the perinuclear area are evident in CHS cells. (B) Quantification of surface Lamp1 in electroporated WT or CHS fibroblasts by FACS. Red indicates background fluorescence and green indicates the amounts of surface-exposed Lamp1 triggered by increasing capacitance. FL1-H, fluorescence intensity; FSC-H, forward scatter.
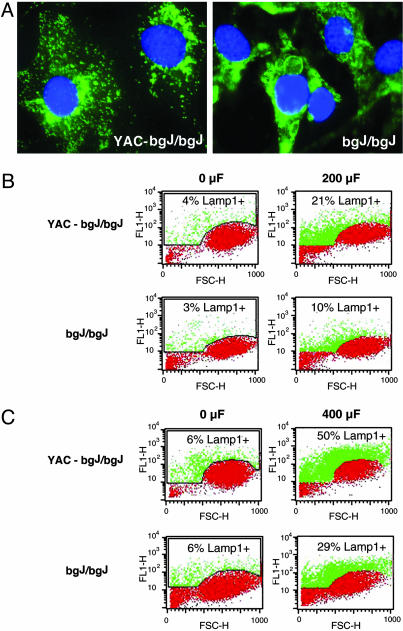
Lysosomal Exocytosis Induced by Membrane Injury Is Reduced in Fibroblasts from beige-J Mice. To be able to further investigate the apparent defect in lysosomal exocytosis of cells lacking the CHS/Beige protein in a homogeneous genetic background, we examined fibroblast cell lines derived from beige-J mice. Previous studies (20) showed that complementation with a YAC containing the full-length Beige gene restored the normal morphology of lysosomes in beige-J fibroblasts. Immunofluorescence with mAbs to Lamp1 confirmed that the size of lysosomes was reduced in beige-J fibroblasts carrying the YAC (Fig. 3A). In nonelectroporated cells, only 3–6% of the cells had detectable surface Lamp1 fluorescence on the cell surface (Fig. 3 B and C, 0 μF). After electroporation at 200 μF, 21% of the complemented cells (YAC-bgJ/bgJ) were Lamp1-positive. In contrast, only 10% of the noncomplemented beige-J cells (bgJ/bgJ) were positive for surface Lamp1 under the same conditions (Fig. 3B, 200 μF). With a capacitance increase to 400 μF, 50% of the YAC-bgJ/bgJ cells had detectable Lamp1 on the surface, in contrast to only 29% of bgJ/bgJ cells (Fig. 3C, 400 μF). Similar amounts of total Lamp1 were detected by FACS in detergent-permeabilized YAC-bgJ/bgJ and bgJ/bgJ fibroblasts, indicating that expression of the Beige protein does not affect the expression level of Lamp1 (see Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 3.
Beige-J fibroblasts show reduced lysosomal exocytosis after wounding. (A) Immunofluorescence of fibroblasts from beige-J mice complemented with a YAC carrying the Beige gene (YAC-bgJ/bgJ), or fibroblasts from beige-J mice (bgJ/bgJ) with anti-Lamp1 mAbs. Anti Lamp1 mAb is green and 4′,6-diamidino-2-phenylindole DNA stain is blue. Enlarged lysosomes in the perinuclear area are evident in beige-J cells. (B) Quantification by FACS of surface Lamp1 in YAC-bgJ/bgJ or bgJ/bgJ fibroblasts not electroporated (0 μF) or electroporated at 200 μF. (C) Quantification by FACS of surface Lamp1 in YAC-bgJ/bgJ or bgJ/bgJ fibroblasts not electroporated (0 μF) or electroporated at 400 μF. Red indicates background fluorescence and green indicates the amounts of surface-exposed Lamp1 triggered by increasing capacitance. FL1-H, fluorescence intensity; FSC-H, forward scatter.
These results show that the enlarged lysosomes of beige-J fibroblasts have a reduction of ≈40% in their capacity to fuse with the plasma membrane after injury, and that expression of the Beige protein rescues this phenotype. Taken together with the similar defect in lysosomal exocytosis observed in human CHS fibroblasts (Fig. 2), these findings strongly indicate that the CHS/Beige mutation has an adverse effect not only on lysosomal morphology but also on the capacity of lysosomes to respond to Ca2+ influx by fusing with the plasma membrane.
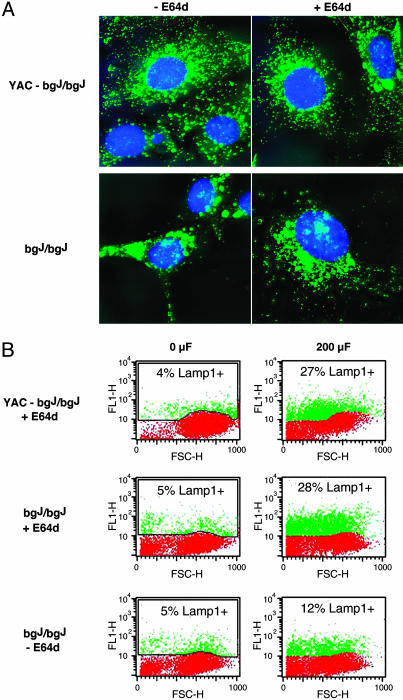
Lysosomal Exocytosis in Beige-J Fibroblasts Is Restored by Reducing Lysosome Size. The results above showed that complementation of the beige-J mutation restored both lysosome size and wounding-induced lysosomal exocytosis (Fig. 3 A and B). Next, we investigated whether the defect in exocytosis could be attributed directly to the abnormal size of lysosomes. For this investigation, we took advantage of previous studies (21), showing that treatment of beige-J cells with the protease inhibitor E-64d reduced the size of the giant lysosomes in these cells. A reduction in lysosome size after overnight treatment with E-64d was evident in bgJ/bgJ fibroblasts, after immunofluorescence with anti-Lamp1 mAbs. There was no apparent adverse effect of E-64d on the morphology of the complemented cell line, YAC-bgJ/bgJ (Fig. 4A).
Fig. 4.
Reduction in the size of lysosomes in beige-J fibroblasts restores exocytosis in response to wounding. (A) Immunofluorescence with anti-Lamp1 mAbs of fibroblasts from beige-J mice complemented with a YAC carrying the Beige gene (YAC-bgJ/bgJ), or fibroblasts from beige-J mice (bgJ/bgJ) treated or not treated with 1 μM E64d overnight. The enlarged lysosomes in the perinuclear area of beige-J fibroblasts are reduced after E64d treatment. (B) Quantification by FACS of surface Lamp1 in YAC-bgJ/bgJ or bgJ/bgJ fibroblasts treated or not treated with E64d, and not electroporated (0 μF) or electroporated at 200 μF. Red indicates background fluorescence and green indicates the amounts of surface-exposed Lamp1 triggered by increasing capacitance. FL1-H, fluorescence intensity; FSC-H, forward scatter.
Next, we determined whether reducing the size of the giant lysosomes of beige-J cells with E-64d affected the exocytic response of electroporated cells. Cells treated or not treated with E-64d were subjected to electroporation-mediated wounding, and surface exposure of Lamp1 was measured by FACS. Complemented YAC-bgJ/bgJ cells cultured in the presence of E-64d did not show any significant alteration in their normal lysosomal exocytosis pattern after electroporation at 200 μF (Fig. 4B Top, compare with Fig. 3B Upper). In bgJ/bgJ cells, however, significantly higher fluorescence levels were detected in the population pretreated with E-64d. Cells positive for surface-exposed Lamp1 rose from 12% (Fig. 4B Bottom) to 28% (Fig. 4B Middle), a level comparable to what is observed in the complemented cell line, YAC-bgJ/bgJ, after electroporation at 200 μF (Fig. 4B Top). These results indicate that the giant lysosomes in beige-J cells have a defective exocytic response to membrane wounding, and that reduction in lysosome size can correct this defect.
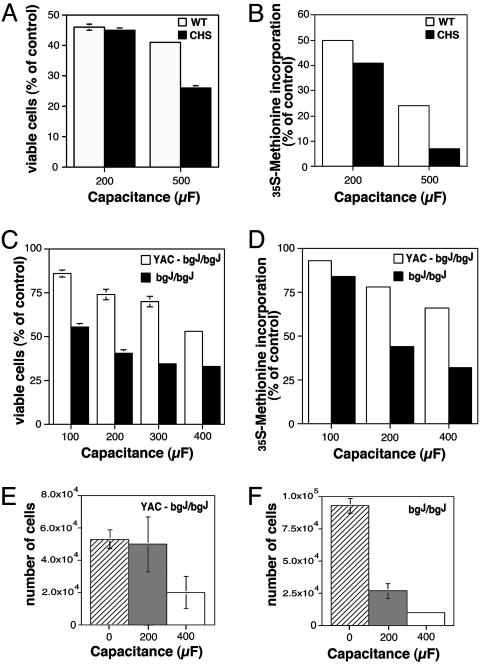
CHS and beige-J Fibroblasts Have Reduced Viability After Wounding. To determine whether the defective lysosomal exocytosis in response to wounding observed in CHS and beige-J fibroblasts affected their ability to reseal the damaged plasma membrane, we performed viability assays. WT and CHS fibroblasts were electroporated at 200 μF and 500 μF, plated out in serial dilutions, and allowed to recover overnight before measuring the numbers of viable cells by two methods: MTT reduction and [35S]methionine incorporation. A 40–65% reduction in viability/protein synthesis capacity was detected between WT and CHS cells after electroporation at 500 μF. A smaller difference was also observed after electroporation at 200 μF (Fig. 5 A and B). Similar to what was observed with the human fibroblasts, the viability of the beige-J mouse cell line bgJ/bgJ, when compared with the complemented cell line YAC-bgJ/bgJ, decreased more significantly as the duration of the electric pulse increased (Fig. 5 C and D). This result is consistent with the reduced number of adherent fibroblasts recovered from bgJ/bgJ cultures 12 h after electroporation, when compared with YAC-bgJ/bgJ cells (Fig. 5 E and F). Both YAC/bgJ/bgJ and bgJ/bgJ cells that survived electroporation replicated at normal rates in the subsequent 96 h, and in subsequent passages (data not shown), indicating that beige cells do not have an intrinsic survival/growth defect. Thus, these findings demonstrate that there is a direct correlation between the reduced levels of lysosomal exocytosis in response to wounding and the capacity of CHS/Beige mutant cells to repair their plasma membrane.
Fig. 5.
CHS and beige-J fibroblasts show reduced viability after wounding. Viable WT or CHS human fibroblasts (A) or mouse YAC-bgJ/bgJ or bgJ/bgJ fibroblasts (B) 12 h after electroporation at increasing capacitance levels were detected with an MTT reduction assay. Data are expressed as a percentage of viable cells in nonelectroporated samples and correspond to the mean and SD of triplicate samples. Incorporation of [35S]methionine by WT or CHS human fibroblasts (C) or mouse YAC-bgJ/bgJ or bgJ/bgJ fibroblasts (D) 12 h after electroporation at increasing capacitance. The data are expressed as a percentage of the total amount of [35S]methionine incorporated in nonelectroporated cells and it is representative of two independent experiments (the variation between the independent experiments was <10–12%). The number of YAC-bgJ/bgJ (E) or bgJ/bgJ (F) mouse fibroblasts recovered by trysinization 12 h after plating, with no electroporation (0 μF) or electroporation at 200 μF and 400 μF. The data correspond to the mean and SD of triplicate samples.
Discussion
In this study, we developed an assay that allows the quantification of lysosomal exocytosis in whole populations of cells, after simultaneous wounding of the plasma membrane in a dosedependent manner. This electroporation-based assay provided us with a very useful tool to investigate the role of lysosomal exocytosis in plasma membrane repair and revealed that fibroblasts from CHS human patients and from beige-J mice are defective in this process.
Although the exact mechanism by which eukaryotic cells reseal plasma membrane lesions is still unknown, the process depends on the exocytosis of a population of intracellular vesicles, triggered by Ca2+ influx (22). Recent studies (23) showed that lysosomes are the only intracellular vesicles detected fusing with the plasma membrane in response to Ca2+ in fibroblasts. The involvement of lysosomes in membrane repair was initially demonstrated through inhibition of lysosomal exocytosis in fibroblasts lacking functional synaptotagmin VII, a member of the synaptotagmin family of transmembrane Ca2+ sensors (12, 13). The present study further emphasizes the important role that lysosomes play in plasma membrane repair because lysosomal enlargement is a characteristic feature of all CHS/Beige cell types (2). Our results point directly to the abnormal enlargement of CHS/Beige lysosomes as the factor interfering with normal lysosomal exocytosis. Reduction in lysosome size by E-64d (21) allowed normal levels of surface Lamp1 to be exposed on the cell surface after wounding (Fig. 4). Although the exact mechanism involved in this size-dependent defect remains to be determined, it is an intriguing possibility that lysosome enlargement may deplete the peripherally located lysosomal population, which is preferentially involved in Ca2+-triggered exocytosis in fibroblasts (23, 24).
Our results showed lower recovery rates for normal human or mouse primary fibroblasts after electroporation, when compared with the NRK rat cell line. Whereas >90% of NRK cells survived electroporation up to 400 μF, wounding conditions from 100 to 400 μF caused larger losses, from 20% to 50%, in primary human and mouse fibroblasts (Figs. 1C and 5 A and B). Although the reason for this difference is not clear, a contributing factor may be the distribution of the transmembrane pores formed in primary cells after the electric pulse. It has been proposed that irreversibility can result, if pores at adjacent sites coalesce into larger lesions (25). Another intriguing observation was the higher amount of surface Lamp1 detected in human CHS fibroblasts both before and after electroporation (Fig. 2 B). This difference was not observed between mouse fibroblasts expressing or not expressing the Beige protein (Fig. 3 B and C), suggesting that it may be related to differences in genetic background between patient cells.
Most previous studies of the CHS/Beige mutation have focused on cells known to contain lysosome-related secretory granules, such as melanocytes, platelets, or cells of the immune system. Skin sections of CHS patients show abnormally large melanosomes clustered in the perinuclear region, a defect linked to the partial albinism of this syndrome (26). The same defect occurs with beige-J mice, which have a lighter coat color due to abnormal transfer of melanin from melanocytes to keratinocytes. A markedly increased bleeding time is another characteristic of this syndrome, and it has been attributed to abnormalities in platelet granules (27). Cytotoxic lymphocytes of CHS patients and beige-J mice are defective in target cell killing (4, 5), due to impaired secretion of their enlarged lytic granules (28). Defects in peptide loading and MHC class II-mediated antigen presentation have also been detected in B cells from CHS patients (29, 30). These abnormalities specific for hematopoietic cells are likely to be responsible for the immune dysfunction observed in CHS patients. However, several studies indicate that many additional tissues may be affected by the CHS/Beige mutation. An accumulation of glucoronidase-containing lysosomes and impaired lysosomal enzyme secretion into the urine occurs in kidney tubule cells of beige-J mice (31). Interestingly, muscle fiber pathology, which is a characteristic feature of synaptotagmin VII-deficient mice with defects in plasma membrane repair (12), has also been reported in CHS patients (32). Thus, a generalized impairment in plasma membrane repair, because of defects in the exocytosis of conventional lysosomes in several cell types, may contribute to the serious symptoms exhibited by these patients.
Supplementary Material
Acknowledgments
We thank Ira Mellman for kindly providing the LY1C6 anti-rat Lamp1 hybridoma. This work was supported by National Institutes of Health Grants GM64625 (to N.W.A.) and HL26922 (to J.K.).
Author contributions: C.H., D.M.W., J.K., and N.W.A. designed research; C.H. and D.R. performed research; C.H., D.R., and N.W.A. analyzed data; and C.H. and N.W.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CHS, Chediak–Higashi syndrome; YAC, yeast artificial chromosome; Lamp1, lysosomal-associated membrane protein 1; MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide); NRK, normal rat kidney epithelial.
References
- 1.Shiflett, S. L., Kaplan, J. & Ward, D. M. (2002) Pigm. Cell. Res. 15, 251–257. [DOI] [PubMed] [Google Scholar]
- 2.Ward, D. M., Griffiths, G. M., Stinchcombe, J. C. & Kaplan, J. (2000) Traffic 1, 816–822. [DOI] [PubMed] [Google Scholar]
- 3.Perou, C. M., Leslie, J. D., Green, W., Li, L., Ward, D. M. & Kaplan, J. (1997) J. Biol. Chem. 272, 29790–29794. [DOI] [PubMed] [Google Scholar]
- 4.Haliotis, T., Roder, J., Klein, M., Ortaldo, J., Fauci, A. S. & Herberman, R. B. (1980) J. Exp. Med. 151, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein, M., Roder, J., Haliotis, T., Korec, S., Jett, J. R., Herberman, R. B., Katz, P. & Fauci, A. S. (1980) J. Exp. Med. 151, 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad, E., Le Deist, F., Blanche, S., Benkerrou, M., Rohrlich, P., Vilmer, E., Griscelli, C. & Fischer, A. (1995) Blood 85, 3328–3333. [PubMed] [Google Scholar]
- 7.Misra, V. P., King, R. H., Harding, A. E., Muddle, J. R. & Thomas, P. K. (1991) Acta Neuropathol. 81, 354–358. [DOI] [PubMed] [Google Scholar]
- 8.Andrews, N. W. (2000) Trends Cell Biol. 10, 316–321. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez, A., Webster, P., Ortego, J. & Andrews, N. W. (1997) J. Cell Biol. 137, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao, S. K., Huynh, C., Proux-Gillardeaux, V., Galli, T. & Andrews, N. W. (2004) J. Biol. Chem. 279, 20471–20479. [DOI] [PubMed] [Google Scholar]
- 11.Martinez, I., Chakrabarti, S., Hellevik, T., Morehead, J., Fowler, K. & Andrews, N. W. (2000) J. Cell Biol. 148, 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti, S., Kobayashi, K. S., Flavell, R. A., Marks, C. B., Miyake, K., Liston, D. R., Fowler, K. T., Gorelick, F. S. & Andrews, N. W. (2003) J. Cell Biol. 162, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy, A., Caler, E. & Andrews, N. (2001) Cell 106, 157–169. [DOI] [PubMed] [Google Scholar]
- 14.Perou, C. M., Moore, K. J., Nagle, D. L., Misumi, D. J., Woolf, E. A., McGrail, S. H., Holmgren, L., Brody, T. H., Dussault, B. J., Jr., Monroe, C. A., et al. (1996) Nat. Genet. 13, 303–308. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann, T. (1983) J. Immunol. Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- 16.Graziadei, L., Burfeind, P. & Bar-Sagi, D. (1991) Anal. Biochem. 194, 198–203. [DOI] [PubMed] [Google Scholar]
- 17.Chu, G., Hayakawa, H. & Berg, P. (1987) Nucleic Acids Res. 15, 1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinosita, K., Jr., & Tsong, T. Y. (1977) Biochim. Biophys. Acta 471, 227–242. [DOI] [PubMed] [Google Scholar]
- 19.Perou, C. M. & Kaplan, J. (1993) Somatic Cell Mol. Genet. 19, 459–468. [DOI] [PubMed] [Google Scholar]
- 20.Ward, D. M., Shiflett, S. L., Huynh, D., Vaughn, M. B., Prestwich, G. & Kaplan, J. (2003) Traffic 4, 403–415. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe, F., Cui, S. H. & Ito, M. (2000) J. Leukocyte Biol. 67, 749–755. [DOI] [PubMed] [Google Scholar]
- 22.McNeil, P. L. & Steinhardt, R. A. (1997) J. Cell Biol. 137, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaiswal, J. K., Andrews, N. W. & Simon, S. M. (2002) J. Cell Biol. 159, 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal, J. K., Chakrabarti, S., Andrews, N. W. & Simon, S. M. (2004) PLoS Biol. 2, 1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi, R. P. & Schoenbach, K. H. (2002) Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 66, 052901/1–052901/4. [DOI] [PubMed] [Google Scholar]
- 26.Windhorst, D. B., Zelickson, A. S. & Good, R. A. (1968) J. Invest. Dermatol. 50, 9–18. [DOI] [PubMed] [Google Scholar]
- 27.Swank, R. T., Novak, E. K., McGarry, M. P., Rusiniak, M. E. & Feng, L. (1998) Pigm. Cell. Res. 11, 60–80. [DOI] [PubMed] [Google Scholar]
- 28.Baetz, K., Isaaz, S. & Griffiths, G. M. (1995) J. Immunol. 154, 6122–6131. [PubMed] [Google Scholar]
- 29.Faigle, W., Raposo, G., Tenza, D., Pinet, V., Vogt, A. B., Kropshofer, H., Fischer, A., de Saint-Basile, G. & Amigorena, S. (1998) J. Cell Biol. 141, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lem, L., Riethof, D. A., Scidmore-Carlson, M., Griffiths, G. M., Hackstadt, T. & Brodsky, F. M. (1999) J. Immunol. 162, 523–532. [PubMed] [Google Scholar]
- 31.Brandt, E. J., Elliott, R. W. & Swank, R. T. (1975) J. Cell Biol. 67, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchino, M., Uyama, E., Hirano, T., Nakamura, T., Fukushima, T. & Ando, M. (1993) Acta Neuropathol. 86, 521–524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.