Abstract
Nitric oxide (NO)-derived products may modify tissue constituents, forming S- and N-nitroso adducts and metal nitrosyls implicated in NO signaling. Nitrovasodilator drugs have been in widespread use for more than a century, yet their biotransformation pathways to NO and their effects as NO donors across tissues remain ill defined. By using a metabonomics approach (termed “NObonomics”) for detailing the global NO-related metabolism of the cornerstone nitrovasodilator, glyceryl trinitrate (GTN; 0.1–100 mg/kg), in the rat in vivo, we find that GTN biotransformation elicits extensive tissue nitros(yl)ation throughout all major organ systems. The corresponding reaction products remained detectable hours after administration, and vascular tissue was not a major nitros(yl)ation site. Extensive heart and liver modifications involved both S- and N-nitrosation, and RBC S-nitrosothiol formation emerged as a sensitive indicator of organic nitrate metabolism. The dynamics of GTN-derived oxidative NO metabolites in blood did not reflect the nitros(yl)ation patterns in the circulation or in tissues, casting doubt on the usefulness of plasma nitrite/nitrate as an index of NO/NO-donor biodynamics. Target-tissue NO metabolites varied in amount and type with GTN dose, suggesting a dose-sensitive shift in the prevailing routes of GTN biotransformation (“metabolic shunting”) from thiol nitrosation to heme nitrosylation. We further demonstrate that GTN-induced nitros(yl)ation is modulated by a complex, tissue-selective interplay of enzyme-catalyzed pathways. These findings provide insight into the global in vivo metabolism of GTN at pharmacologically relevant doses and offer an additional experimental paradigm for the NObonomic analysis of NO-donor metabolism and signaling.
Keywords: nitric oxide, nitrosylheme, nitrosothiols, metabonomics
Nitric oxide (NO) regulates diverse aspects of mammalian physiology by enhancing cGMP production through soluble guanylyl cyclase activation (1). This classic signal transduction mechanism notwithstanding, NO's highly reactive, diffusible nature fosters an extensive chemical biology in vivo that influences gene expression, mitochondrial respiration, and drug metabolism; induces posttranslational protein modification; and generates numerous NO-derived metabolites (2, 3) (collectively referred to herein as the NO metabonome or “NObonome”).¶ Some of these metabolites have been implicated in NO's beneficial and pathological effects (2, 3). Recent demonstration that constitutive thiol, amine, and heme nitros(yl)ation in mammalian tissues generates, respectively, S-nitrosothiols (RSNOs), N-nitroso compounds (RNNOs), and nitrosylheme (NO-heme) species suggests new signaling pathways through which nitrogen oxides might influence cell physiology by covalently modifying tissue biomolecules (3, 5). Given the mounting evidence that thiol nitrosation modulates cell function (6), it is a matter of great interest to identify the biological targets of NO-dependent modification, the mechanism(s) responsible, and the distribution, dynamics, and activities of the resultant nitros(yl)ation∥ products in health and disease.
The therapeutic potential of exogenous NO sources in many disease states invites the metabolic profiling of NO donors (7). For more than a century, glyceryl trinitrate (GTN), nitroglycerin, has remained the foremost NO-donor drug and exemplary nitrovasodilator (8). Although GTN's antianginal efficacy is mainly a consequence of regional coronary vasodilation mediated by NO produced during GTN biotransformation (8), nitrosative tissue modifications not involving guanylyl cyclase may further modulate NO bioactivity. For instance, tissue RSNOs may extend NO generation from GTN and endow it with antiplatelet activity (9). Enzyme tyrosine nitration has been hypothesized to contribute to the vascular dysfunction in GTN tolerance (10). Despite GTN's prominent therapeutic niche, the mechanism of NO generation from GTN and the global metabolic consequences of GTN biotransformation remain largely undefined (11).
The pharmacodynamics of GTN have been characterized mainly by measurement of GTN disappearance (12) or assessed from circulating GTN catabolites (particularly, nitrite and glycerol 1,2- and 1,3-dinitrate as well as glycerol 1- and 2-mononitrate) or nitrosyl-Hb (13, 14). Largely because of analytical limitations, little information is available on the formation and dynamics of NO-related products in target tissues after GTN administration or the metabolic pathway(s) involved. The paucity of quantitative data on the GTN NObonome largely reflects the lack of techniques suitable for tracking NO's fate in vivo. Bryan et al. (15) recently used a validated methodology to demonstrate that group-specific chemical derivatization, coupled to subsequent denitrosation and NO detection by gas-phase chemiluminescence, can be used to quantify endogenous cellular nitros(yl)ation in the rat. Through application of this technology in combination with ion chromatography, we here report metabonomic characterization of GTN biotransformation by mapping the global in vivo oxidative and nitrosative events after GTN administration and identifying the metabolic pathways involved. We demonstrate that GTN biotransformation induces extensive nitrosative modifications of tissue constituents that are organ-specific and distinct from the production of the oxidative NO metabolites, nitrite and nitrate. Our data provide a quantitative, kinetic, and tissue-specific characterization of global GTN metabolism in vivo and demonstrate the utility of a metabonomic approach for quantitative NO-donor profiling.
Materials and Methods
Animals. Male Wistar rats (250–350 g) from Harlan Breeders (Indianapolis) were allowed food (2018 rodent diet, Harlan) and water ad libitum and were kept on a 12/12 light/dark cycle with at least 10 d of local vivarium acclimatization before experimental use.
Blood and Tissue Sampling. Biological specimens for quantitative analyses of nitrosative and oxidative NO metabolites were obtained as detailed in refs. 15–17.
Global NO Metabolite Profiling. Tissue nitroso/nitrosyl compounds were quantified by group-specific derivatization, denitrosation, and gas-phase chemiluminescence (5, 15). GTN itself is transparent to the detection system (5) and thus would not produce a signal even if present at significant tissue concentration. “RSNO” signifies mercury-labile S-nitroso species, whereas “RNNO” signifies mercury-resistant N-nitroso adducts and may include nitrosamines and metal nitrosyls other than NO-heme species (5). Nitrite and nitrate were quantified by ion chromatography (ENO20 Analyzer; Eicom, Kyoto) (3).
GTN Administration. Aqueous GTN solutions (0.1%, by weight, in 5% glucose) were used in the dose–response studies, except for the highest dose, whereas ethanolic GTN solutions (5%, by weight) (Cerilliant, Round Rock, TX) were used in all other experiments. Animals received either GTN or an equivalent volume of vehicle by i.p. injection, after which blood and tissues were sampled at designated times. Preliminary results revealed a qualitatively similar pattern of NO metabolites when GTN was administered s.c., although absorption kinetics, and thus NO metabolite concentrations, differed with route of administration (data not shown). Dose-ranging experiments were conducted from 0.1 to 100 mg/kg GTN with sample harvest 15 min after GTN administration.
Biotransformation Studies. In addition to plasma and RBCs, heart, aorta, and liver were profiled because of their relevance to GTN's cardiovascular pharmacology and first-pass metabolism (10, 13, 18–20). Glutathione S-transferase (EC 2.5.1.18; GST) activity was blocked by combined glutathione depletion with diethylmaleate (DEM; 1 g/kg) (18) and GST inhibition with ethacrynic acid (100 mg/kg) (19). Cytochrome P450 monooxygenases (EC 1.14.14.1) were inhibited by aminobenzotriazole (1 g/kg) (20) plus proadifen (SKF-525A) (100 mg/kg) (21). Aldehyde dehydrogenase (EC 1.2.1.3) was inhibited by cyanamide (25 mg/kg) (22), and xanthine oxidase (EC 1.2.32) by allopurinol (100 mg/kg) (23). Inhibitors were from Sigma, and the doses and route of administration (i.p) were selected from the citations. Vehicles alone had no effect in the studies reported. Except for aminobenzotriazole, which was administered 6 h before GTN, inhibitors were administered 60 min before GTN. In most cases, the GTN dose was 1 mg/kg, i.p. Because of nitroxyl production from cyanamide (24) and a resultant increase in tissue NO-heme levels (data not shown), a 10 mg/kg GTN dose was used to allow for reliable subtraction of any cyanamide-related background. Consequently, the effect of inhibition of the other oxidoreductase, xanthine oxidase, also was assessed at the 10 mg/kg GTN dose.
Data Presentation. Data are means ± SEM from n individual experiments. Enzyme inhibitor effects are expressed as mean percent change relative to the corresponding net GTN signal without inhibitor pretreatment, with standard errors representing the averages of the SEMs of the individual groups.
Results
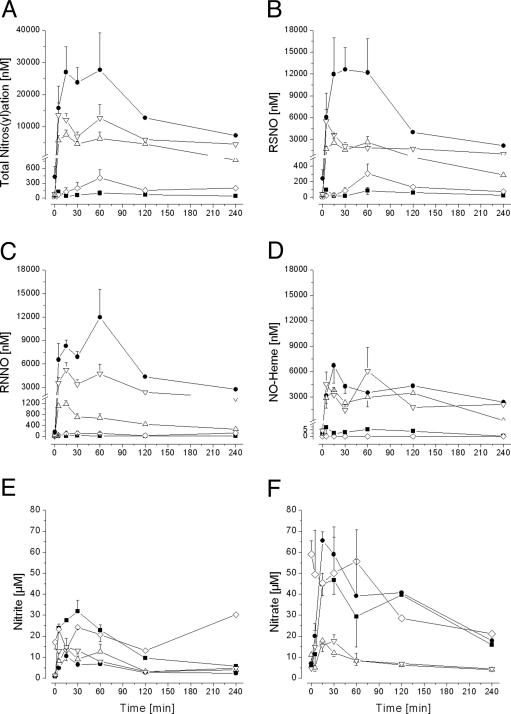
GTN-Induced Tissue Nitros(yl)ation in Vivo. We first determined the nitros(yl)ation kinetics in blood (plasma and RBCs), heart, liver, and thoracic aorta during in vivo GTN biotransformation to define the global dynamics of these processes and the lifetimes of the species formed as guidance for subsequent selection of sampling times. The i.p. dose of 10 mg/kg GTN is representative of that used in prior metabolic (14) and pharmacological (25, 26) rat studies. Biotransformation of GTN induced tissue nitros(yl)ation within minutes after administration (Fig. 1A). Net formation of total nitros(yl)ation products after GTN administration quickly approached micromolar concentrations in all compartments examined, far exceeding their low nanomolar steady-state concentrations (ref. 15 and data not shown). The vehicles alone were without effect. Liver and heart attained a virtually equivalent net nitros(yl)ation level, intermediate between the RBC and either aorta or plasma. Tissue nitros(yl)ation products remained markedly elevated up to 4 h after GTN administration.
Fig. 1.
GTN-induced nitros(yl)ation and nitrite/nitrate formation in vivo after i.p. GTN administration (10 mg/kg). Filled symbols denote blood components (▪, plasma; •, RBC); open symbols denote tissues (▵, heart; ▿, liver; ⋄, aorta). (A) Concentration of total NO-related nitros(yl)ation products, i.e., the sum of tissue RSNO, RNNO, and NO-heme compounds. (B) RSNO concentration. (C) RNNO concentration. (D) NO-heme concentration. (E) Nitrite concentration. (F) Nitrate concentration. (n = 3–4 at 0, 5, 15, 30, and 60 min; average of 2 animals at 120 and 240 min.)
Kinetic and tissue-specific differences were uncovered in the formation of distinct chemical classes of nitros(yl)ation products (Fig. 1 B–D). Net RSNO formation peaked almost immediately in the liver, later in RBCs and heart, and later still (at 60 min) in aorta and plasma (Fig. 1B). The RBC was the predominant site of GTN-induced S-nitrosation. Net RNNO formation was greatest in the RBC and liver (Fig. 1C). NO-heme concentrations increased to comparably high levels in RBCs, liver, and heart, with negligible NO-heme formation in plasma and aorta (Fig. 1D). Comparable levels of all three classes of nitros(yl)ation products were generated in liver, whereas plasma nitros(yl)ation mainly reflected RSNO formation.
Nitrite and Nitrate Dynamics. Inorganic nitrite and nitrate are end products of oxidative NO decomposition. After administration of a single 10 mg/kg i.p. GTN bolus, nitrite concentrations increased in all tissues sampled, reaching maxima by 15 min after dosing (Fig. 1E). During this interval, nitrate concentrations in RBCs, plasma, heart, and liver also increased, whereas nitrate concentrations in aorta remained high up to 60 min after dosing and decreased thereafter (Fig. 1F). Aorta aside, maximal increases above endogenous tissue nitrite or nitrate concentrations (15) were only severalfold at best after GTN administration and far less than the several-log peak increases of nitros(yl)ation products. Neither the magnitude nor the kinetics of net nitrite and nitrate formation in any target tissue examined after GTN administration mirrored that tissue's nitros(yl)ation pattern (cf. Fig. 1 A–D). The substantial increases in plasma nitrite and nitrate were not indicative of the rather modest GTN-induced plasma nitros(yl)ation.
Aortic and Venous Tissue Differences. Because of the preferential venodilator action of GTN (8), we compared the concentrations of NO-related products generated during GTN metabolism in thoracic aorta and vena cava. No significant differences in either nitrite/nitrate or total nitroso products were observed 15 min after i.p. application of 10 mg/kg GTN (4.5 ± 0.9 and 4.0 ± 1.0 nmol of nitrite per g of wet-weight tissue, 14.4 ± 4.5 and 17.5 ± 5.6 nmol of nitrate per g, 122 ± 40 and 88 ± 38 pmol of total nitroso per g in aorta and vein, respectively; n = 5). However, in venous tissue, ≈90% of the nitroso products were in the form of RSNOs, whereas in the aorta, only 30% consisted of RSNOs, the remaining 70% being RNNOs.
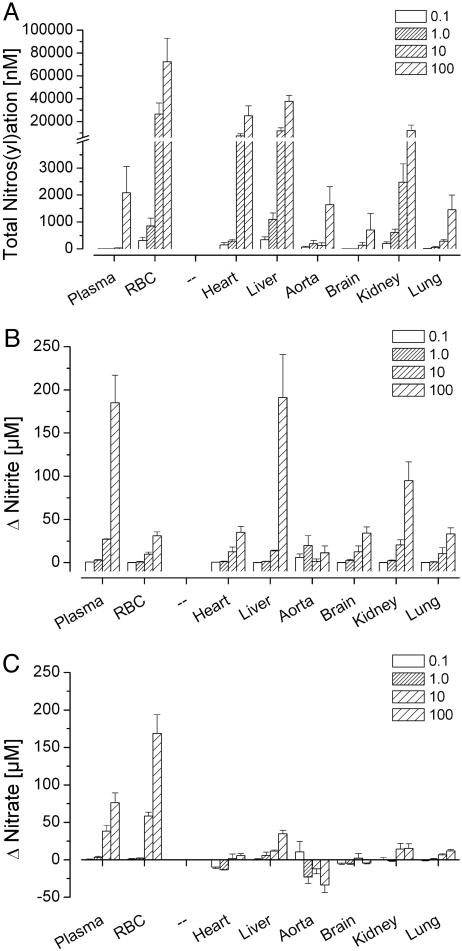
Dose–Response Effects. We next investigated the influence of dose on GTN-induced nitrosative and oxidative events 15 min after i.p. administration, when nitros(yl)ation and nitrite/nitrate formation were near maximal in most compartments (Fig. 1). Escalation of the GTN dose over a 1,000-fold range led to a dose-dependent increase in the concentrations of all nitros(yl)ation products (Fig. 2A). Nitros(yl)ation was limited in plasma and aorta relative to RBCs, heart, and liver, even at the highest GTN dose examined. The nitros(yl)ation dose-response in kidney resembled that of heart and liver, whereas brain and lung resembled aorta. Increases in tissue nitros(yl)ation were not dose-proportional. The 10-fold dose increase from 0.1 to 1.0 mg/kg GTN elicited a relatively modest potentiation of nitros(yl)ation, whereas further dose escalation from 1.0 to 10.0 mg/kg markedly and disproportionally elevated RBC, heart, liver, and kidney nitros(yl)ation products. The substantial further elevation of plasma, brain, lung, and aorta nitros(yl)ation products observed at the 100 mg/kg GTN dose was not evident in the other tissues examined.
Fig. 2.
Influence of GTN dose (mg/kg) on total nitros(yl)ation (i.e., the sum of tissue RSNOs, RNNOs, and NO-heme compounds) (A), nitrite (B), and nitrate (C) in rat blood (plasma, RBCs) and select tissues 15 min after i.p. GTN administration. Endogenous tissue levels of each metabolite class in animals not administered GTN have been subtracted from the values shown. (n = 3.)
At the highest GTN dose, nitrite concentrations increased in most compartments, especially in plasma, liver, and kidney (Fig. 2B). A marked nitrate dose–response relationship was evident only in blood (Fig. 2C). Aortic nitrite concentrations did not increase with GTN dose escalation, and aortic nitrate levels actually decreased. The prominent increases in plasma nitrite and nitrate were not reflected in the very modest plasma nitros(yl)ation capacity.
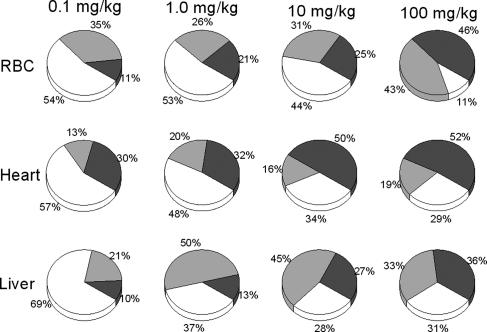
Importantly, the proportions of different classes of nitros(yl)ation metabolites within a given tissue changed with increasing GTN dose. A consistent pattern emerged whereby NO-heme formation increased 2- to 5-fold, i.e., markedly relative to tissue RSNOs (Fig. 3).
Fig. 3.
Dose-dependent changes in the distribution of nitros(yl)ated products in three exemplary tissue compartments 15 min after i.p. GTN administration over the 1,000-fold GTN dose range designated. □, RSNO;  , RNNO; ▪, NO-heme. [Average mean percentages of net total nitros(yl)ation products formed; n = 3.]
, RNNO; ▪, NO-heme. [Average mean percentages of net total nitros(yl)ation products formed; n = 3.]
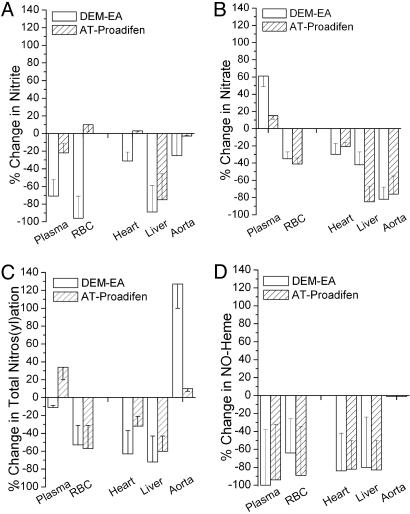
Enzymatic GTN Biotransformation Pathways. GST and cytochrome P450 are the two enzyme systems most frequently implicated in organic nitrate catabolism (11). Inhibiting GST or cytochrome P450 markedly attenuated the GTN-induced formation of NO-related oxidative and nitrosative products in most compartments examined (Fig. 4). In both instances, either inhibitor alone had less of an effect than the combination (data not shown). Despite marked reductions in aortic nitrate levels, neither GST nor cytochrome P450 inhibition attenuated GTN-induced nitros(yl)ation in the aorta. Indeed, GST inhibition doubled nitros(yl)ation product formation in this compartment.
Fig. 4.
Effect of selective inhibitors of GST [diethyl maleate (1 g/kg, i.p.) plus ethacrynic acid (100 mg/kg, i.p.)] (DEM-EA) or cytochrome P450 [aminotriazole (1 g/kg, i.p.) plus proadifen (100 mg/kg, i.p.)] (AT-Proadifen) on nitrite, nitrate, total nitrosation products, and NO-heme formation after GTN administration (1 mg/kg, i.p.). Data are expressed as the percent change relative to metabolite levels from GTN administration alone, i.e., without inhibitor pretreatment. (n = 3.0.)
More recently, mitochondrial aldehyde dehydrogenase and xanthine oxidase have been implicated in GTN biotransformation (27, 28). The involvement of these two oxidoreductases in GTN biotransformation was tested by pretreatment of rats with the inhibitors cyanamide (22) and allopurinol (23), respectively (Table 1). For this study, a GTN dose of 10 mg/kg i.p. was used to increase the GTN-induced signal, affording reliable background subtraction of any NO-heme formed from exposure of animals to cyanamide alone. The profound inhibition of both nitros(yl)ation and nitrite/nitrate formation by cyanamide in most tissues examined implicates aldehyde dehydrogenase in GTN biotransformation, except in the aorta, where GTN-induced nitroso/nitrosyl levels were markedly enhanced. In select compartments (blood and liver), xanthine oxidase inhibition suppressed GTN-induced nitros(yl)ation with relatively less attenuation of nitrite/nitrate formation. Unexpectedly, xanthine oxidase inhibition actually increased the content of nitros(yl)ation products in heart and aorta, suggesting that this enzyme mainly represents an inactivation pathway in these compartments.
Table 1. Effect of aldehyde dehydrogenase or xanthine oxidase inhibition on the GTN NObonome.
| Inhibitor | Compartment | NOx, % change | Total nitros(yl)ation, % change |
|---|---|---|---|
| Cyanamide | Plasma | -84 ± 22 | -100 ± 40 |
| RBC | -72 ± 36 | -100 ± 44 | |
| Heart | -100 ± 22 | -71 ± 40 | |
| Liver | -79 ± 24 | -68 ± 33 | |
| Aorta | -97 ± 40 | 97 ± 42 | |
| Allopurinol | Plasma | -12 ± 4 | -74 ± 42 |
| RBC | -26 ± 8 | -25 ± 16 | |
| Heart | 14 ± 4 | 103 ± 47 | |
| Liver | -22 ± 6 | -27 ± 9 | |
| Aorta | -16 ± 4 | 32 ± 14 |
Animals were pretreated with the aldehyde dehydrogenase inhibitor cyanamide (25 mg/kg, i.p.) or the xanthine oxidase inhibitor allopurinol (100 mg/kg, i.p.) for 1 h before i.p. administration of either 10 mg/kg GTN or vehicle. Separate groups of animals were administered one inhibitor or GTN alone. After 15 min, the tissues indicated were processed and analyzed for their contents of nitrite plus nitrate (NOx) and total nitros(yl)ation products (3, 5, 15). The net GTN-induced metabolite signal in the presence of inhibitor was expressed as a mean percent change relative to the net GTN-induced signal in the absence of inhibitor (means ± SEM of three animals).
Discussion
After almost 125 years of GTN's clinical use as a nitrovasodilator (8), the present study at last constitutes a global metabolic profiling of GTN. A systems biology approach (“NObonomics”) was predicated on recently developed methods for quantifying NO-related oxidative and nitrosative events in vivo (5, 15). Our approach has yielded several insights into the biotransformation and metabolism of GTN as a NO-donor drug. We have uncovered a hitherto unknown consequence of GTN biotransformation, extensive tissue nitros(yl)ation. The RSNO, RNNO, and NO-heme adducts generated can serve as sensitive biomarkers of NO donor biotransformation in vivo that enable the tracking of GTN metabolism at pharmacologically relevant doses. Moreover, we have documented tissue- and dose-dependent differences in the formation and chemical nature of the NO-related products of GTN biotransformation. Our data further demonstrate that enzyme systems previously implicated in NO formation from GTN also support GTN-induced tissue nitros(yl)ation. Containing the entire complement of the metabolic machinery appropriately distributed and organized throughout all organs, the whole-animal approach we have developed should reflect with fidelity the myriad of as yet ill defined factors underlying organic nitrate biotransformation. As illustrated by the controversy surrounding rat data on S-nitroso-Hb as an allosteric mediator of NO tissue delivery (29), results obtained with rodent models should be extrapolated to other species with appropriate caution.
Extensive, if inconclusive, in vitro experiments on the GTN denitration mechanism (9, 30, 31) and routine biochemical (e.g., GTN disappearance from the circulation) or functional (e.g., cGMP production) indices of GTN pharmacodynamics (12, 13) do not afford the spatial and temporal detail and global scope of our in vivo NObonomics approach. Although partial denitration products of GTN have some pharmacological interest in their own right (for example, as vasodilators and surrogate markers of “GTN activation” in the form of the dinitrate ratio) and may act in vivo as “secondary” NO donors, their contribution to the pattern of NO metabolites we have documented, if any, is currently unknown. Similarly, the relationships between common pharmacological indices of GTN action (e.g., smooth muscle relaxation, platelet aggregation) and GTN's NObonome await elucidation. The acute response of GTN's NObonome after dosing is consistent with the rapid (within 1–2 min) onset of GTN action (13, 32). Yet, pharmacological characterization of GTN as a “short-acting” vasodilator with a plasma t1/2 of <1 h at clinically relevant doses (13) does not begin to reflect its long-lasting effects upon target tissues with regard to tissue nitros(yl)ation (Fig. 1 A–D). The discordance between tissue nitros(yl)ation and nitrite/nitrate formation (Fig. 1) renders NOx measurements grossly inadequate, if not misleading, for describing and tracking the GTN NObonome, perhaps because of compartment-specific differences in elimination kinetics and further metabolism and, consequently, the half-lives of nitrite, nitrate, and the various nitros(yl)ation products. This discordance also brings into question the general use of plasma nitrite and nitrate to index the pharmacodynamics and metabolism of NO-donor drugs in laboratory animals and humans (33).
The tissue-specific nature of both the extent and kinetics of in vivo GTN-induced nitros(yl)ation (Fig. 1 A–D) is compatible with reports that rabbit (34–36) and rat (37) organs differ in their capacity to generate NO from GTN. These spin-trapping studies indicate that NO formation during acute GTN infusion (34, 35, 37) or more prolonged transdermal administration (36) is greatest in kidney, liver, and lung, less in heart and vena cava, and marginal in aorta and RBCs. Our data show that, during GTN biotransformation, heart and liver also are major nitros(yl)ation sites, especially compared with vascular tissue. Differential tissue nitros(yl)ation likely reflects a number of factors in addition to GTN denitration, e.g., the tissue content of endogenous nitros(yl)ation targets (5, 15) and variation among specialized cell types in their handling of nitrite/nitrate and the redistribution/metabolism of nitros(yl)ation products (31, 38). Local redox poise might be another factor. Tissue antioxidant content has been shown to influence constitutive nitroso/nitrosyl levels (15), and GTN biotransformation can stimulate the production of superoxide anions, whose avid reactivity with NO is believed to contribute to nitrovasodilator tolerance and pathological NO insufficiency (39). Evaluation of the extent to which these and other factors govern the tissue-specific dynamics of the GTN NObonome awaits further study. However, preliminary results indicate that neither circulating nor tissue glutathione or ascorbate concentrations are affected 15 min after i.p. administration of any GTN dose used in this study, with the exception of significant plasma ascorbate oxidation at the 100 mg/kg dose (data not shown).
Blood vessels are important sites of GTN's therapeutic vasodilator action and biotransformation (8,9,). Yet, aortic tissue was not a main locus of nitros(yl)ation after GTN administration (Fig. 1 A–D). The modest nitros(yl)ation in aorta could theoretically have reflected a low overall NO generation from GTN in arterial conductance vessels, compared with venous capacitance vessels (35, 36), which would be consistent with GTN's preferential venodilator action (8). However, our results indicate that the molecular nature of the nitros(yl)ated adducts in vascular tissue may be more critical for GTN's vasoactivity than the total amount of NO adducts generated. We have observed similar extents of total nitros(yl)ation in rat aorta and vena cava 15 min after i.p. administration of 10 mg/kg GTN. At that time, RSNOs accounted for the majority of the total nitroso species in the vena cava but not in the aorta, suggesting an important role for selective nitrosation of blood vessel constituents (i.e., RSNO formation) in GTN's preferential venodilator effect.
Hb has long been implicated in GTN biotransformation (40). Of the tissues examined, the RBC was the predominant site of nitros(yl)ation, with plasma representing the quantitatively least significant biological compartment (Fig. 1A). RBC RSNO formation emerged as a particularly sensitive indicator of GTN metabolism in vivo (Fig. 1B). The marked increase in RBC RSNO after GTN administration may partly reflect the relatively high thiol reactivity of rodent Hb that may favor nonenzymatic reaction to form S-nitroso-Hb (41). Our data, however, do not preclude the potential formation of other RBC S-nitrosoproteins or lower-molecular-weight thionitrites. Hb may likewise be involved in the RBC NO-heme formation observed after GTN administration (Fig. 1D). Reversible NO binding to deoxy-Hb to form nitrosyl-Hb is a fast thermodynamically favored reaction that would increase RBC NO-heme content (42). GTN biotransformation also was accompanied by significant RBC N-nitrosation, leading to RNNO levels that approached those of RSNOs (Fig. 1 B and C). RNNOs have recently been identified as endogenous RBC constituents in the rat, and in human plasma high-molecular-weight RNNOs are at steady-state levels some 5-fold above those of RSNOs (3, 15). Whatever the role of the endogenous and GTN-induced RNNOs in these blood compartments may be, RBCs emerge as a prime study object for molecular resolution of GTN's nitros(yl)ation targets. It is tempting to speculate that some of the GTN-induced RBC nitros(yl)ation products might serve as NO congeners that extend or transduce GTN's bioactivity, a view compatible with the recent hypothesis that increased RBC nitrosation may play a causative role in sepsis-associated hypotension (43).
Although the overall net amount of NO-related metabolites after GTN administration increased dose-dependently in some tissues (particularly RBCs, heart, liver, and kidney), the magnitude of this increase was not directly proportional to the dose escalation imposed (Fig. 2). These data imply a metabolic shunting of GTN (and/or GTN-derived NO or a NO congener) from a “pharmacological” route at lower GTN doses to alternative, “spillover” routes at higher doses. Such shunting may occur as GTN-metabolizing enzymes reach saturation and/or cofactor availability becomes limiting. As a result of a shift to another metabolic route, NO metabolite ratios change accordingly. In this regard, two distinct pathways capable of generating NO from GTN are present in vascular smooth muscle cells (44) and have been invoked to explain the biphasic concentration–response curve for GTN-induced vasorelaxation (45). Alternatively, the lack of dose proportionality and the changes in composition of NO-related metabolites with increasing GTN dose may reflect gradual saturation of hepatic first-pass metabolism during GTN uptake from the peritoneal cavity. However, a qualitatively similar spectrum of tissue metabolites was observed after s.c. application of GTN (data not shown). Whatever the mechanism, the shift in profile of nitros(yl)ation products with increasing GTN dose away from RSNOs toward RNNOs and NO-heme species (Fig. 3) affords direct in vivo evidence for the dynamic nature of tissue nitros(yl)ation product classes.
Somewhat controversially (11, 27), four candidate enzymes have been implicated in NO formation from GTN. Our pharmacological studies are consistent with the active involvement of all four enzyme systems (GST, the cytochrome P450 superfamily, aldehyde dehydrogenase, and xanthine oxidase) in GTN biotransformation in vivo. Although inhibition of each individual enzyme class suppressed formation of NO oxidation products (nitrite/nitrate) and nitros(yl)ated tissue adducts in most compartments examined, the degree to which any one enzyme contributed to GTN metabolism varied among tissues as well as with the NO metabolite class used to index the response (Fig. 4 and Table 1). These characteristics and the vast differences in specificity among the different inhibitors used exclude the possibility advocated elsewhere (27) that one enzyme alone is responsible for GTN biotransformation. Nor do our results support the view that the involvement of xanthine oxidase in organic nitrate biotransformation is limited to hypoxic conditions (28). Rather, GTN's bioactivity appears to reflect a complex interplay of different enzyme pathways, all metabolically competent to support nitros(yl)ation in a tissue-selective manner. The involvement of any of these enzymes in the biological effects of GTN (venodilation, blood pressure reduction, etc.) or the importance of proposed nonenzymatic routes of GTN catabolism (11, 44) remains to be investigated.
The relative role of each enzymatic GTN biotransformation pathway in a given tissue or specialized cell type may be influenced by factors such as its prevailing abundance, isozyme pattern, and substrate specificity. Limited data exist to show that mammalian liver is enriched in GST and cytochrome P450 activities, compared with heart (46, 47). Results from spin-trapping studies in rabbits suggest that acute NO formation during GTN infusion is greatest in cytochrome P450-rich tissues such as liver (34, 36), and correlation has been made between hepatic glutathione-dependent “organic nitrate reductase” activity and liver GTN turnover (48). These results are consistent with the marked suppression of GTN-induced formation of NO-related metabolites in the liver of rats treated with either GST or cytochrome P450 inhibitors (Fig. 4). The observed increases in aortic nitros(yl)ation in response to inhibition of GST, aldehyde dehydrogenase, or xanthine oxidase may imply that these enzymes are preferentially involved in vascular GTN inactivation pathways. Care must be taken, however, in attempting to correlate the apparent activity of an enzyme as assayed biochemically in a disrupted tissue with the effect of the in vivo inhibition of that enzyme. For instance, the low cardiac activity of glutathione-dependent “organic nitrate reductase” relative to that in liver (48) was not reflected in the comparable generation of nitros(yl)ation products in heart and liver after GTN administration (Fig. 1). The possibility also must be entertained that the inhibitors used may have additional indirect influence on the GTN NObonome. For example, nitroxyl generated from cyanamide could conceivably affect the biotransformation of GTN through inhibition of cytochrome P450 catalysis (49). The almost complete suppression of GTN-dependent heme nitrosylation by the combination of aminobenzotriazole and proadifen, in light of their marginal effect on nitrite/nitrate formation (Fig. 4), further suggests that some of the inhibitors could also modify the fate of NO at the level of the nitros(yl)ation substrates.
In summary, this detailed examination of GTN's NObonome has uncovered a rich in vivo nitrosative chemistry involving specific enzyme systems capable of metabolizing GTN to NO. It is conceivable that developmental, pathological, and other factors (rat strain, route of dosing, animal species) might influence GTN's global metabolic profile as a NO donor. The targets of tissue nitros(yl)ation, the molecular identities and function of the nitros(yl)ation products formed after GTN administration (e.g., do all or part of the NO-heme signals represent the activated form of soluble guanylyl cyclase?), and the contribution of endogenous vs. GTN-derived NO to GTN-induced nitros(yl)ation await further elucidation. The extent to which the analytical methods used herein can be used to discriminate among various nitrovasodilator medicines also is unknown. For the moment, our data show how a metabonomics approach can be successfully applied in a whole-animal system to map quantitatively the systems biology of GTN's NO-related tissue modifications and the influence of perturbations thereon. Given the increasing number of disease states associated with NO insufficiency and the impetus to develop effective NO-supplementation therapies (7, 50), NObonomics could represent a valuable experimental paradigm to help decode the metabolic network underlying NO signaling and the metabolism of extant and candidate NO-donor therapeutics, leading to the identification of NO-related molecular signatures (“NObonomic fingerprints”) and predictive biomarkers for health and disease.
Acknowledgments
We thank Dr. G. Letts for support and discussion and S. Bauer and Dr. M. Garcia-Saura for skillful technical assistance. This work was supported in part by National Institutes of Health Grant R01 HL 69029 (to M.F.).
Author contributions: D.R.J., N.S.B., and M.F. designed research; N.S.B., F.S., V.D., D.J.S., and M.C.W. performed research; N.S.B., V.D., and M.F. analyzed data; and D.R.J., N.S.B., and M.F. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GTN, glyceryl trinitrate (nitroglycerin); RSNO, S-nitrosothiol; RNNO, N-nitroso compounds; NO-heme, nitrosylheme.
Footnotes
Emerging approaches for studying NO-donor metabolism in vivo and the cellular targets of NO-related modification invite specific terminology to distinguish this endeavor. A point of departure is suggested by the field of metabonomics, which classically denotes the quantification of endogenous (i.e., genomically determined) metabolites/metabolic processes at the whole-organism level and their response to perturbation (4). We wish to invite a broader approach to signify global NO metabolic profiling with the term “NObonomics,” which would encompass the quantitative in vivo mapping of NO-related metabolites of either endogenous or exogenous (i.e., extragenomic) origin and how they are affected by disease, drug exposure, or other (patho)physiological influences. The set of NO-related metabolites and any products of their interaction identified under the prevailing conditions of the organism would constitute a “NObonome.”
The hybrid “nitros(yl)ation” is used here to indicate the involvement of nitrosation and/or nitrosylation.
References
- 1.Ignarro, L. J. (2002) J. Physiol. Pharmacol. 53, 503-514. [PubMed] [Google Scholar]
- 2.Espey, M. G., Miranda, K. M., Thomas, D. D., Xavier, S., Citrin, D., Vitek, M. P. & Wink, D. A. (2002) Ann. N.Y. Acad. Sci. 962, 195-206. [DOI] [PubMed] [Google Scholar]
- 3.Rassaf, T., Bryan, N. S., Kelm, M. & Feelisch, M. (2002) Free Radical Biol. Med. 11, 1590-1596. [DOI] [PubMed] [Google Scholar]
- 4.Lindon, J. C., Holmes, E. & Nicholson, J. K. (2004) Expert Rev. Mol. Diagn. 4, 189-199. [DOI] [PubMed] [Google Scholar]
- 5.Feelisch, M., Rassaf, T., Mnaimneh, S., Singh, N., Bryan, N. S., Jourd'Heuil, D. & Kelm, M. (2002) FASEB J. 16, 1775-1785. [DOI] [PubMed] [Google Scholar]
- 6.Foster, M. W., McMahon, T. J. & Stamler, J. S. (2003) Trends Mol. Med. 9, 160-168. [DOI] [PubMed] [Google Scholar]
- 7.Janero, D.R. (2003) in Critical Reviews of Oxidative Stress and Aging, eds. Cutler, R. G. & Rodriguez, H. (World Scientific, River Edge, NJ), Vol. 2, pp. 1404-1417. [Google Scholar]
- 8.Ignarro, L. J., Napoli, C. & Loscalzo, J. (2002) Circ. Res. 90, 21-28. [DOI] [PubMed] [Google Scholar]
- 9.Wong, P. S.-Y. & Fukuto, J. M. (1999) Drug Metab. Dispos. 27, 502-509. [PubMed] [Google Scholar]
- 10.Hink, U., Oelze, M., Kolb, P., Bachschmid, M., Zou, M. H., Daiber, A., Mollnau, H., August, M., Baldus, S., Tsilimingas, N., et al. (2003) J. Am. Coll. Cardiol. 42, 1826-1834. [DOI] [PubMed] [Google Scholar]
- 11.Fung, H.-L. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 67-85. [DOI] [PubMed] [Google Scholar]
- 12.Torfgard, K., Ahlner, J., Axelsson, K. L., Norlander, B. & Bertler, A. (1991) Can. J. Physiol. Pharmacol. 69, 1257-1261. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto, S. & Kobayashi, A. (2003) Clin. Pharmacokinet. 42, 205-221. [DOI] [PubMed] [Google Scholar]
- 14.Kohno, M., Masumizu, T. & Mori, A. (1995) Free Radical Biol. Med. 18, 451-457. [DOI] [PubMed] [Google Scholar]
- 15.Bryan, N. S., Rassaf, T., Maloney, R. E., Rodriguez, C. M., Saijo, F., Rodriguez, J. R. & Feelisch, M. (2004) Proc. Natl. Acad. Sci. USA 101, 4308-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marley, R., Patel, R. P., Orie, N., Ceaser, E., Darley-Usmar, V. & Moore, K. (2001) Free Radical Biol. Med. 31, 688-696. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez, J. R., Maloney, R. E., Rassaf, T., Bryan, N. S. & Feelisch, M. (2003) Proc. Natl. Acad. Sci. USA 100, 336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deneke, S. M., Lynch, B. A. & Fanburg, B. L. (1985) J. Appl. Physiol. 58, 571-574. [DOI] [PubMed] [Google Scholar]
- 19.Mulder, G. J. & Ouwerkerk-Mahadevan, S. (1997) Chem. Biol. Interact. 105, 17-34. [DOI] [PubMed] [Google Scholar]
- 20.Knickle, L. C. & Bend, J. R. (1992) Can. J. Physiol. Pharmacol. 70, 1610-1617. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez, G., Villarruel, M. C., Bernacchi, A., De Castro, C. R. & Castro, J. A. (1981) Toxicology 20, 185-193. [DOI] [PubMed] [Google Scholar]
- 22.Jamal, M., Ameno, K., Ameno, S., Okada, N. & Ijiri, I. (2003) Leg. Med. (Tokyo) 5, Suppl. 1, S79-S82. [DOI] [PubMed] [Google Scholar]
- 23.Giray, B., Gurbay, A. & Hincal, F. (2001) Toxicol. Lett. 118, 139-146. [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa, H. T., DeMaster, E. G., Redfern, B., Shirota, F. N. & Goon, D. J. W. (1990) J. Med. Chem. 33, 3122-3124. [DOI] [PubMed] [Google Scholar]
- 25.Zitting, A. & Savolainen, H. (1982) Res. Commun. Chem. Pathol. Pharmacol. 37, 113-121. [PubMed] [Google Scholar]
- 26.Khanna, A., Rossman, J., Caty, M. G. & Fung, H.-L. (2003) J. Surg. Res. 114, 15-24. [DOI] [PubMed] [Google Scholar]
- 27.Chen, Z., Zhang, J. & Stamler, J. S. (2002) Proc. Natl. Acad. Sci. USA 99, 8306-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, H., Samouilov, A., Liu, X. & Zweier, J. L. (2004) J. Biol. Chem. 279, 16939-16946. [DOI] [PubMed] [Google Scholar]
- 29.Gladwin, M. T. & Schechter, A. N. (2004) Circ. Res. 94, 851-855. [DOI] [PubMed] [Google Scholar]
- 30.Kojda, G., Patzner, M., Hacker, A. & Noack, E. (1998) Mol. Pharmacol. 53, 547-554. [DOI] [PubMed] [Google Scholar]
- 31.Kozlov, A.V., Dietrich, B. & Nohl, H. (2003) Br. J. Pharmacol. 139, 989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap, P. S. & Fung, H.-L. (1978) J. Pharm. Sci. 67, 584-586. [DOI] [PubMed] [Google Scholar]
- 33.Tarpey, M. M., Wink, D. A. & Grisham, M. B. (2004) Am. J. Physiol. 286, R431-R444. [DOI] [PubMed] [Google Scholar]
- 34.Mulsch, A., Mordvintcev, P., Bassenge, E., Jung, F., Clement, B. & Busse, R. (1995) Circulation 92, 1876-1882. [DOI] [PubMed] [Google Scholar]
- 35.Mulsch, A., Bara, A., Mordvintcev, P., Vanin, A. & Busse, R. (1995) Br. J. Pharmacol. 116, 2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clermot, C., Lecour, S., Vergely, C., Zeller, M., Perrin, C., Maupoil, V., Bouchot, O. & Rochette, L. (2003) Fund. Clin. Pharmacol. 17, 709-715. [DOI] [PubMed] [Google Scholar]
- 37.Laursen, J. B., Mulsch, A., Boesgaard, S., Mordvintcev, P., Trautner, S., Gruhn, N., Nielsen-Kudsk, J. E., Busse, R. & Aldershvile, J. (1996) Circulation 94, 2241-2247. [DOI] [PubMed] [Google Scholar]
- 38.Aulak, K. S., Koeck, T., Crabb, J. W. & Stuehr, D. J. (2004) Am. J. Physiol. 286, H30-H38. [DOI] [PubMed] [Google Scholar]
- 39.Dikalov, S., Fink, B., Skatchkov, M., Stalleicken, D. & Bassenge, E. (1998) J. Pharmacol. Exp. Ther. 286, 938-944. [PubMed] [Google Scholar]
- 40.Bennett, B. M., Brien, J. F., Nakatsu, K. & Marks, G. S. (1985) J. Pharmacol. Exp. Ther. 234, 228-232. [PubMed] [Google Scholar]
- 41.Rossi, R., Milzani, A., Dalle-Donne, I., Giannerini, F., Giustarini, D., Lusini, L., Colombo, R. & Di Simplicio, P. (2001) J. Biol. Chem. 276, 7004-7010. [DOI] [PubMed] [Google Scholar]
- 42.Tiravanti, E., Samouilov, A. & Zweier, J. L. (2004) J. Biol. Chem. 279, 11065-11073. [DOI] [PubMed] [Google Scholar]
- 43.Crawford, J. H., Chacko, B. K., Pruitt, H. M., Piknova, B., Hogg, N. & Patel, R. P. (2004) Blood 104, 1375-1382. [DOI] [PubMed] [Google Scholar]
- 44.Feelisch, M. & Kelm, M. (1991) Biochem. Biophys. Res. Commun. 180, 286-293. [DOI] [PubMed] [Google Scholar]
- 45.Kleschyov, A. L., Oelze, M., Daiber, A., Huang, Y., Mollnau, H., Schulz, E., Sydow, K., Fichtlscherer, B., Mulsch, A. & Munzel, T. (2003) Circ. Res. 93, E104-E112. [DOI] [PubMed] [Google Scholar]
- 46.Kraus, P. & Kloft, H.-D. (1980) Enzyme 25, 158-160. [DOI] [PubMed] [Google Scholar]
- 47.Morgenstern, R., Lundqvist, G., Andersson, G., Balk, L. & DePierre, J. W. (1984) Biochem. Pharmacol. 33, 3609-3614. [DOI] [PubMed] [Google Scholar]
- 48.Maier, G. A., Arena, C. & Fung, H.-L. (1980) Biochem. Pharmacol. 29, 646-648. [DOI] [PubMed] [Google Scholar]
- 49.Miranda, K. M., Nims, R. W., Thomas, D. D., Espey, M. G., Citrin, D., Bartberger, M. D., Paolocci, N., Fukuto, J. M., Feelisch, M. & Wink, D. A. (2003) J. Inorg. Biochem. 93, 52-60. [DOI] [PubMed] [Google Scholar]
- 50.Lin, C.-E., Garvey, D. S., Janero, D. R., Letts, L. G., Marek, P., Richardson, S. K., Serebryanik, D., Shumway, M. S., Tam, S. W., Trocha, A. M. & Young, D. V. (2004) J. Med. Chem. 47, 2276-2282. [DOI] [PubMed] [Google Scholar]