Abstract
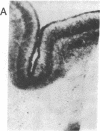
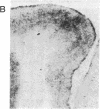
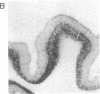
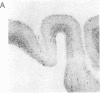
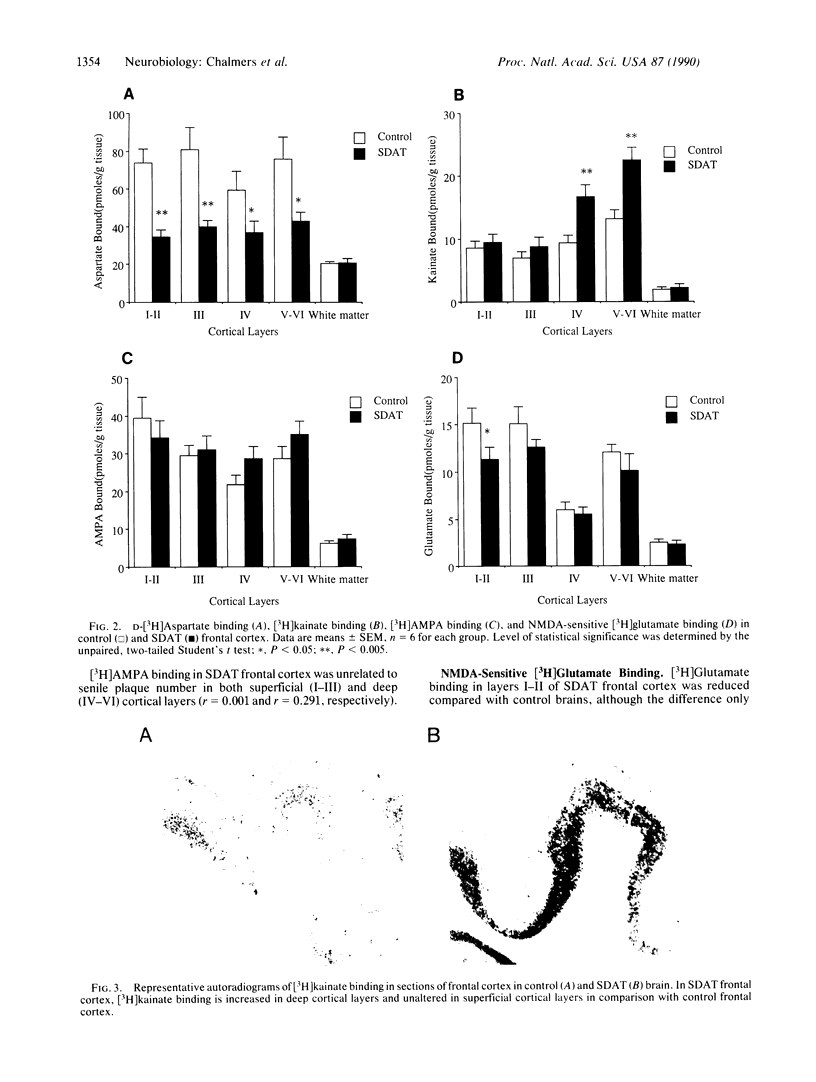
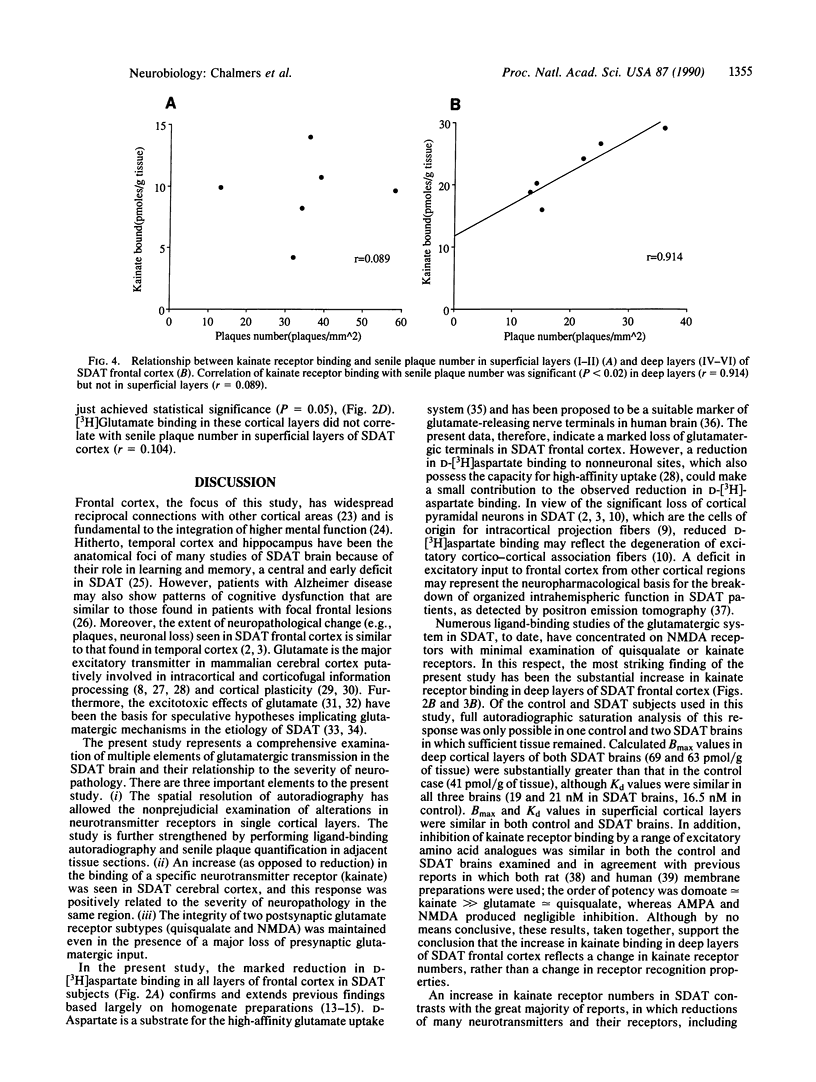
Involvement of cortical glutamatergic mechanisms in senile dementia of the Alzheimer type (SDAT) has been investigated with quantitative ligand-binding autoradiography. The distribution and density of Na(+)-dependent glutamate uptake sites and glutamate receptor subtypes--kainate, quisqualate, and N-methyl-D-aspartate--were measured in adjacent sections of frontal cortex obtained postmortem from six patients with SDAT and six age-matched controls. The number of senile plaques was determined in the same brain region. Binding of D-[3H]aspartate to Na(+)-dependent uptake sites was reduced by approximately 40% throughout SDAT frontal cortex relative to controls, indicating a general loss of glutamatergic presynaptic terminals. [3H]Kainate receptor binding was significantly increased by approximately 70% in deep layers of SDAT frontal cortex compared with controls, whereas this binding was unaltered in superficial laminae. There was a positive correlation (r = 0.914) between kainate binding and senile plaque number in deep cortical layers. Quisqualate receptors, as assessed by 2-amino-3-hydroxy-5-[3H]methylisoxazole-4-propionic acid binding, were unaltered in SDAT frontal cortex compared with controls. There was a small reduction (25%) in N-methyl-D-aspartate-sensitive [3H]glutamate binding only in superficial cortical layers of SDAT brains relative to control subjects. [3H]Glutamate binding in SDAT subjects was unrelated to senile plaque number in superficial cortical layers (r = 0.104). These results indicate that in the presence of cortical glutamatergic terminal loss in SDAT plastic alterations occur in some glutamate receptor subtypes but not in others.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcar V. J., Johnston G. A. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972 Nov;19(11):2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Mazurek M. F., Tran V. T., Chattha G., Bird E. D., Martin J. B. Reduced numbers of somatostatin receptors in the cerebral cortex in Alzheimer's disease. Science. 1985 Jul 19;229(4710):289–291. doi: 10.1126/science.2861661. [DOI] [PubMed] [Google Scholar]
- Chu D. C., Penney J. B., Jr, Young A. B. Cortical GABAB and GABAA receptors in Alzheimer's disease: a quantitative autoradiographic study. Neurology. 1987 Sep;37(9):1454–1459. doi: 10.1212/wnl.37.9.1454. [DOI] [PubMed] [Google Scholar]
- Cowburn R. F., Hardy J. A., Briggs R. S., Roberts P. J. Characterisation, density, and distribution of kainate receptors in normal and Alzheimer's diseased human brain. J Neurochem. 1989 Jan;52(1):140–147. doi: 10.1111/j.1471-4159.1989.tb10908.x. [DOI] [PubMed] [Google Scholar]
- Cowburn R., Hardy J., Roberts P., Briggs R. Presynaptic and postsynaptic glutamatergic function in Alzheimer's disease. Neurosci Lett. 1988 Mar 21;86(1):109–113. doi: 10.1016/0304-3940(88)90192-9. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Cross A. J., Skan W. J., Slater P. The association of [3H]D-aspartate binding and high-affinity glutamate uptake in the human brain. Neurosci Lett. 1986 Jan 16;63(2):121–124. doi: 10.1016/0304-3940(86)90047-9. [DOI] [PubMed] [Google Scholar]
- Deutsch S. I., Morihisa J. M. Glutamatergic abnormalities in Alzheimer's disease and a rationale for clinical trials with L-glutamate. Clin Neuropharmacol. 1988 Feb;11(1):18–35. doi: 10.1097/00002826-198802000-00002. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F., Søreide A., Kvale I., Walker J., Walaas I. Glutamate in cortical fibers. Adv Biochem Psychopharmacol. 1981;27:29–41. [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Geddes J. W., Chang-Chui H., Cooper S. M., Lott I. T., Cotman C. W. Density and distribution of NMDA receptors in the human hippocampus in Alzheimer's disease. Brain Res. 1986 Dec 3;399(1):156–161. doi: 10.1016/0006-8993(86)90611-6. [DOI] [PubMed] [Google Scholar]
- Geddes J. W., Monaghan D. T., Cotman C. W., Lott I. T., Kim R. C., Chui H. C. Plasticity of hippocampal circuitry in Alzheimer's disease. Science. 1985 Dec 6;230(4730):1179–1181. doi: 10.1126/science.4071042. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Penney J. B., D'Amato C. J., Young A. B. Dementia of the Alzheimer's type: changes in hippocampal L-[3H]glutamate binding. J Neurochem. 1987 Feb;48(2):543–551. doi: 10.1111/j.1471-4159.1987.tb04127.x. [DOI] [PubMed] [Google Scholar]
- Guttman M., Seeman P. L-dopa reverses the elevated density of D2 dopamine receptors in Parkinson's diseased striatum. J Neural Transm. 1985;64(2):93–103. doi: 10.1007/BF01245971. [DOI] [PubMed] [Google Scholar]
- Hart S., Smith C. M., Swash M. Word fluency in patients with early dementia of Alzheimer type. Br J Clin Psychol. 1988 May;27(Pt 2):115–124. doi: 10.1111/j.2044-8260.1988.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Honoré T., Drejer J. Chaotropic ions affect the conformation of quisqualate receptors in rat cortical membranes. J Neurochem. 1988 Aug;51(2):457–461. doi: 10.1111/j.1471-4159.1988.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Horwitz B., Grady C. L., Schlageter N. L., Duara R., Rapoport S. I. Intercorrelations of regional cerebral glucose metabolic rates in Alzheimer's disease. Brain Res. 1987 Mar 31;407(2):294–306. doi: 10.1016/0006-8993(87)91107-3. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Maragos W. F., Chu D. C., Young A. B., D'Amato C. J., Penney J. B., Jr Loss of hippocampal [3H]TCP binding in Alzheimer's disease. Neurosci Lett. 1987 Mar 9;74(3):371–376. doi: 10.1016/0304-3940(87)90326-0. [DOI] [PubMed] [Google Scholar]
- McBean G. J., Roberts P. J. Neurotoxicity of L-glutamate and DL-threo-3-hydroxyaspartate in the rat striatum. J Neurochem. 1985 Jan;44(1):247–254. doi: 10.1111/j.1471-4159.1985.tb07137.x. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Geddes J. W., Yao D., Chung C., Cotman C. W. [3H]TCP binding sites in Alzheimer's disease. Neurosci Lett. 1987 Jan 14;73(2):197–200. doi: 10.1016/0304-3940(87)90017-6. [DOI] [PubMed] [Google Scholar]
- Mountjoy C. Q., Roth M., Evans N. J., Evans H. M. Cortical neuronal counts in normal elderly controls and demented patients. Neurobiol Aging. 1983 Spring;4(1):1–11. doi: 10.1016/0197-4580(83)90048-9. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Ho O. L., Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14(1):61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E. K. The cholinergic hypothesis--ten years on. Br Med Bull. 1986 Jan;42(1):63–69. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- Perry R. H. Recent advances in neuropathology. Br Med Bull. 1986 Jan;42(1):34–41. doi: 10.1093/oxfordjournals.bmb.a072096. [DOI] [PubMed] [Google Scholar]
- Procter A. W., Palmer A. M., Stratmann G. C., Bowen D. M. Glutamate/aspartate-releasing neurons in Alzheimer's disease. N Engl J Med. 1986 Jun 26;314(26):1711–1712. doi: 10.1056/nejm198606263142617. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Hahn S. Ketamine-xylazine anaesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987 Mar 12;326(6109):183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- Reynolds G. P., Arnold L., Rossor M. N., Iversen L. L., Mountjoy C. Q., Roth M. Reduced binding of [3H]ketanserin to cortical 5-ht2 receptors in senile dementia of the Alzheimer type. Neurosci Lett. 1984 Jan 27;44(1):47–51. doi: 10.1016/0304-3940(84)90219-2. [DOI] [PubMed] [Google Scholar]
- Roth M., Tym E., Mountjoy C. Q., Huppert F. A., Hendrie H., Verma S., Goddard R. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986 Dec;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- Simpson M. D., Royston M. C., Deakin J. F., Cross A. J., Mann D. M., Slater P. Regional changes in [3H]D-aspartate and [3H]TCP binding sites in Alzheimer's disease brains. Brain Res. 1988 Oct 11;462(1):76–82. doi: 10.1016/0006-8993(88)90587-2. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Pearson R. C. Degeneration of cholinergic neurons in the basal nucleus following kainic or N-methyl-D-aspartic acid application to the cerebral cortex in the rat. Brain Res. 1985 Jul 22;339(1):186–190. doi: 10.1016/0006-8993(85)90643-2. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Trifiletti R. R., Snowman A. M., Whitehouse P. J., Marcus K. A., Snyder S. H. Huntington's disease: increased number and altered regulation of benzodiazepine receptor complexes in frontal cerebral cortex. Neurology. 1987 Jun;37(6):916–922. doi: 10.1212/wnl.37.6.916. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]