Abstract
The evaluation of pig hematology and biochemistry parameters is rarely done largely due to the costs associated with laboratory testing and labor, and the limited availability of reference intervals needed for interpretation. Within-herd and between-herd biological variation of these values also make it difficult to establish reference intervals. Regardless, baseline reference intervals are important to aid veterinarians in the interpretation of blood parameters for the diagnosis and treatment of diseased swine. The objective of this research was to provide reference intervals for hematology and biochemistry parameters of 3-week-old commercial nursing piglets in Ontario. A total of 1032 pigs lacking clinical signs of disease from 20 swine farms were sampled for hematology and iron panel evaluation, with biochemistry analysis performed on a subset of 189 randomly selected pigs. The 95% reference interval, mean, median, range, and 90% confidence intervals were calculated for each parameter.
Résumé
Intervalles de références de l’hématologie et de la biochimie pour des porcelets à l’allaitement vers le moment du sevrage dans des élevages commerciaux de l’Ontario. L’évaluation des paramètres hématologiques et biochimiques des porcs est rarement réalisée surtout en raison des coûts associés aux tests de laboratoire ainsi qu’à la main-d’œuvre et à la disponibilité limitée d’intervalles de référence requis pour l’interprétation. La variation de ces valeurs au sein de troupeaux et entre les troupeaux complique l’établissement des intervalles de référence. Néanmoins, des intervalles de référence de base sont importants pour appuyer les vétérinaires dans l’interprétation des paramètres sanguins pour le diagnostic et le traitement des porcs malades. Cette recherche avait pour objectif de fournir des intervalles de référence pour les paramètres hématologiques et biochimiques des porcelets commerciaux à l’allaitement âgés de 3 semaines en Ontario. Des échantillons ont été prélevés pour un total de 1032 porcs ne présentant pas de signes cliniques de maladie provenant de 20 fermes porcines pour une évaluation du profil d’hématologie et du fer et une analyse biochimique a été réalisée pour un sous-groupe de 189 porcs choisis au hasard. L’intervalle de référence de 95 %, la moyenne, la médiane, l’étendue de référence et les intervalles de confiance de 90 % ont été calculés pour chaque paramètre.
(Traduit par Isabelle Vallières)
Introduction
There are many important reasons for performing blood analyses in pigs. Firstly, the assessment of these parameters can be used as a component of determining the health status of a herd (1). Hematology and biochemistry reference intervals can also contribute to the early identification of disease or poor growth performance, aiding clinicians and researchers in interpreting the results of tests on blood samples (2–5). However, despite the importance of analyzing these parameters, hematology and biochemistry assessments are rarely used in the swine industry. This may be due to the dearth of information on reference intervals for commercial pigs, the costs associated with labor and laboratory testing, especially in comparison to the low economic value of an individual pig (1), or the perception that hematology and blood biochemistry will provide little useful information. Additionally, it is possible for the results to be biased by factors such as improper blood collection and handling techniques (i.e., not properly mixing the blood with the anticoagulant), which may result in poor sample quality, or the effects of animal stress or excitement from the handling and collection process (1).
Many within-herd variables can influence hematology and biochemistry parameters. These include environmental and physiological factors such as age, breed, gender, diet, and housing, as well as pathogen challenge, and stress (1,3). Between-herd differences can include the same reasons mentioned, as well as variables associated with management practices, biosecurity, and overall health status of the herd. Hematology and biochemistry parameters from a particular animal or herd are typically compared to reference intervals that have been previously determined from a similar group of animals using similar laboratory techniques. Additionally, some previously published studies that developed reference intervals have used small sample sizes, breeds that are uncommon, or have combined data from different age categories of pig or different stages of production (6–8). However, there are limited studies that have published such reference intervals for nursing commercial piglets. Reference intervals are influenced by analytical factors such as instrumentation and technology, time or temperature of a chemical reaction, or the substrate used (5). Many laboratories have been updated with advanced automated and computerized systems to improve the overall accuracy and precision of analytical measurements. Thus, it is important to report updated reference intervals that reflect current analytical methods/automation, and advancements in genetics and pig production practices. The objective of this study was to develop reference intervals for hematology and biochemistry parameters for Ontario commercial nursing piglets close to the time of weaning at approximately 21 d of age.
Materials and methods
The animal care committee at the University of Guelph, following the guidelines of the Canadian Council for Animal Care, reviewed and approved this study. The blood samples were taken from pigs from 20 southern Ontario swine farms and had been used in a previous study (9). Briefly, the farms sampled varied in production type, management practice, and sow-herd size, and were representative of the variation and types of herds operating in Ontario at the time. A questionnaire was administered to each producer from the participating farms to collect reference population information including age of piglets at weaning, management practices, and the size of the sow herd. All animals used in this study were raised according to the current Canadian Code of Practice for the Care and Handling of Pigs (10). Hence, all pigs received a 200-mg IM iron injection, either gleptoferron or iron dextran, within the first 7 d of life (9). Litters were not used if they were treated with any pharmacological agents (i.e., antibiotics). Thus, all participating farms were considered to have healthy pigs without any current health challenges or disease outbreaks. Creep feed was given to suckling pigs on some of the farms; however, the number of farms and contents of the feed were not recorded. Each farm was visited 1 to 2 d before the routine weaning day, and litters were systematically selected at this time by beginning with the initial crate in the farrowing room until a maximum of 20 litters were sampled. From each litter, a small, medium, and large piglet were purposely selected to account for size variation across litters. All male piglets enrolled in this study were barrows. Pigs were excluded if they had any health concerns such as the visible presence of an abscess, hernia, thin body condition, or lameness (9).
A technician and a veterinarian took blood samples from the selected piglets by the orbital sinus bleeding technique (11,12) using a Monoject Standard Hypodermic needle 16 G × 1″ (Covidien; Mansfield, Massachusetts, USA). Blood for serum biochemistry and iron profile was collected in 8.5-mL plain tubes containing no additive (BD Vacutainer; BD, Franklin Lakes, New Jersey, USA). Blood for hematology was collected in 6-mL tubes containing ethylenediamine tetraacetic acid (EDTA) (BD Vacutainer; BD). The EDTA tubes were immediately inverted 5 to 10 times after the sample was collected to mix the anticoagulant with the blood. The blood samples were immediately placed on ice packs in a cooler and transported from the farm to the laboratory.
Hematology evaluation
The blood samples (n = 1095) collected by Perri et al (9) were used for hematology analysis of individual piglets just prior to weaning at approximately 21 d of age. Within approximately 3 h of sampling on-farm, the whole blood samples were analyzed at the Animal Health Laboratory (AHL), University of Guelph, Guelph, Ontario. Standard hematology techniques using the ADVIA 2120/2120i Hematology system (Siemens Healthcare Diagnostics, Deerfield, Illinois, USA) were used to determine the red blood cell (RBC) count, white blood cell (WBC) count, hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width (RDW), platelets, and mean platelet volume (MPV). Platelet counts were measured photometrically. For further details on the hematology analytical methods and automation procedures, please contact the corresponding author.
Biochemistry evaluation
Blood samples (n = 1095) were centrifuged at the AHL, the serum was removed and then stored at −20°C until the time of analysis. Due to the cost associated with the biochemical analysis a subset of 200 samples was randomly chosen by using a random generator in STATA 12.0 (Stata 12 Statacorp LP, College Station, Texas, USA). A total of 10 serum samples, representing 10 individual pigs, were randomly selected from each of the participating farms and were analyzed using a Roche Cobas 6000 c501 biochemistry analyzer (Roche Diagnostics USA, Indianapolis, USA), as per standard protocols at the AHL. For further details on the biochemistry analytical methods and automation, please contact the corresponding author.
Statistical analysis
Reference intervals were statistically evaluated using a computer program (Analyze-It Version 3.0; Analyze-It Software, Leeds, UK). This software program works within Microsoft Excel. Data were examined using non-parametric methods as recommended by the American Society for Veterinary Clinical Pathology (ASVCP). Non-parametric methods are recommended when using ≥ 120 samples for the determination of reference intervals and when the data do not follow a normal distribution (13).
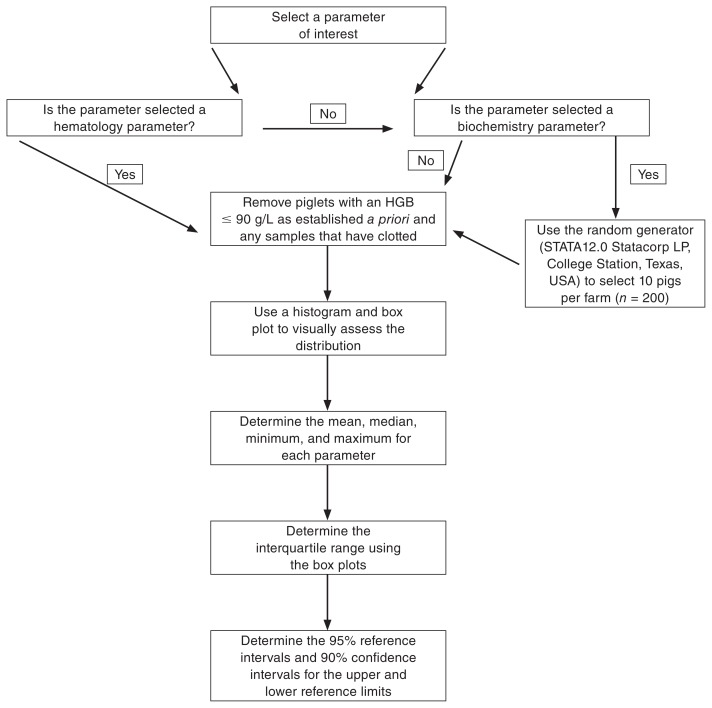
Five blood samples were omitted from the datasets due to small clots. Additionally, the authors decided a priori to exclude any pigs from the study if they were considered anemic. Anemia was defined as a pig having a HGB value ≤ 90 g/L. The decision tree indicating whether a pig should be included or excluded in the statistical analysis to define reference intervals for the hematology and biochemistry parameters is displayed in Figure 1.
Figure 1.
Decision tree used for the inclusion and exclusion criteria of piglet data for statistical analyses of hematology and biochemistry parameters in commercial nursing piglets at the time of weaning.
After the removal of anemic pigs, 1032 pigs (from the original 1095) were used for hematology reference interval determination. From the original 1095 pigs, 200 were randomly selected for biochemical analysis. However, of these 200 pigs selected 11 were removed because they were anemic, leaving a total of 189 pigs available for biochemistry reference interval determination. Each hematology and biochemistry parameter was initially screened visually using histograms and boxplots to assess the distribution. Box plots illustrate a sample distribution using the 25th, 50th, and 75th percentiles, which are also referred to as the lower quartile (Q1), median (m or Q2), and upper quartile (Q3), respectively (14). The interquartile range (IQR) is equal to Q3 – Q1 and this range covers the central 50% of the data (14). The 95% reference intervals, mean, median, minimum, maximum, and 90% CI’s for the lower and upper reference limits were calculated for each hematology and biochemistry parameter. The statistical examination of outliers is not recommended for reference interval analysis since large values from a skewed population (non-Gaussian) may be mislabeled as an outlier(s) (15). Since nonparametric methods establish reference limits by trimming the most extreme values, outliers have less of an effect on the reference intervals than with parametric methods (13). Thus, outlier detection was not performed.
Results
The mean age of piglets sampled was 21.8 ± 4.2 d. The 95% reference intervals, mean, median, minimum, maximum, and 90% CI’s for the lower and upper reference limits for the hematology parameters are presented in Table 1. The 95% reference intervals, mean, median, minimum, maximum, and 90% CI’s for the lower and upper reference limits for the biochemistry parameters are displayed in Table 2.
Table 1.
Hematology reference valuesa and reference intervals for Ontario piglets (n = 1032) sampled 1 to 2 days prior to weaningb on 20 commercial farms
| Variable | Mean | Median | Min–Max | IQR | 95% Reference interval | 90% CI Lower limit | 90% CI Upper limit |
|---|---|---|---|---|---|---|---|
| WBC (×109/L) | 11.2 | 10.1 | 4.3–53.9 | 5.0 | 6.0–21.7 | 5.8–6.2 | 20.7–22.8 |
| RBC (× 1012/L) | 6.0 | 5.9 | 3.9–8.0 | 0.9 | 4.8–7.3 | 4.6–4.9 | 7.2–7.4 |
| HGB (g/L) | 115.0 | 115.0 | 91.0–144.0 | 15.0 | 93.0–136.0 | 92.0–94.0 | 135.0–137.0 |
| HCT (L/L) | 0.4 | 0.4 | 0.3–0.5 | 0.05 | 0.3–0.5 | 0.32–0.33 | 0.45–0.46 |
| MCV (10−15 L) | 66.0 | 66.0 | 48.0–87.0 | 9.0 | 53.0–79.0 | 53.0–54.0 | 78.0–80.0 |
| MCH (10−12 g) | 19.5 | 20.0 | 13.0–27.0 | 3.0 | 15.0–23.0 | 15.0–15.0 | 23.0–24.0 |
| MCHC (g/L) | 295.0 | 294.0 | 262.0–397.0 | 14.0 | 275.0–317.2 | 274.0–276.0 | 316.0–319.0 |
| RDW (%) | 18.2 | 17.4 | 10.0–31.1 | 3.6 | 14.3–26.0 | 14.2–14.5 | 25.3–26.9 |
| Platelets (× 109/L) | 487.0 | 474.5 | 49–1498 | 201.0 | 171.8–833.2 | 156.0–196.0 | 795.0–857.0 |
| MPV | 10.5 | 10.0 | 6.6–22.7 | 2.8 | 7.4–16.5 | 7.4–7.5 | 15.7–16.8 |
Reference values were determined using Analyze-It Software (Analyze-It Version 3.0; Leeds, UK) and based on non-parametric analyses.
Average age of piglets (1 to 2 d prior to weaning) was 21.8 ± 4.2 d.
IQR — interquartile range, which is equal to the upper quartile (Q3) minus the lower quartile (Q1) (Q3–Q1) and this range covers the central 50% of the data; CI — confidence interval; WBC — white blood cells; RBC — red blood cells; HGB — hemoglobin; HCT — hematocrit; MCV — mean corpuscular volume; MCH — mean corpuscular hemoglobin; MCHC — mean corpuscular hemoglobin concentration; RDW — red blood cell distribution width; MPV — mean platelet volume.
Table 2.
Biochemistry reference valuesa and reference intervals for Ontario piglets (n = 189) sampled 1 to 2 days prior to weaningb on 20 commercial farms
| Variable | Mean | Median | Min–Max | IQR | 95% Reference interval | 90% CI Lower limit | 90% CI Upper limit |
|---|---|---|---|---|---|---|---|
| Calcium (mmol/L) | 2.8 | 2.9 | 1.70–3.2 | 0.19 | 2.4–3.1 | 2.0–2.6 | 3.1–3.2 |
| Phosphorus (mmol/L) | 3.3 | 3.3 | 2.3–3.9 | 0.33 | 2.6–3.8 | 2.4–2.7 | 3.7–3.8 |
| Magnesium (mmol/L) | 1.2 | 1.1 | 0.9–1.5 | 0.1 | 0.9–1.5 | 0.9–0.9 | 1.4–1.5 |
| Sodium (mmol/L) | 138.3 | 140.0 | 94–150 | 6.0 | 122.0–145.3 | 107.0–128.0 | 144.0–147.0 |
| Potassium (mmol/L) | 5.0 | 5.0 | 3.6–7.2 | 0.6 | 3.9–6.2 | 3.8–4.2 | 6.0–6.4 |
| Chloride (mmol/L) | 97.2 | 98.0 | 64–106 | 4.0 | 86.8–103.3 | 75.0–88.0 | 102.0–104.0 |
| Serum carbon dioxide (mmol/L) | 25.3 | 26.0 | 18–32 | 3.0 | 19.0–31.0 | 18.0–20.0 | 30.0–32.0 |
| Anion gap (mmol/L) | 20.9 | 21.0 | 15–29 | 4.0 | 16.0–28.3 | 15.0–16.0 | 27.0–29.0 |
| Na/K ratio (mmol/L) | 27.8 | 28.0 | 18–36 | 4.0 | 22.8–33.3 | 22.0–23.0 | 33.0–36.0 |
| Serum iron (μmol/L) | 19.4 | 17.0 | 2–85 | 17.0 | 4.0–45.0 | 4.0–5.0 | 43.0–47.0 |
| UIBC (μmol/L) | 60.0 | 61.0 | 0–143 | 42.0 | 7.0–116.0 | 6.0–9.0 | 114.0–118.0 |
| TIBC (μmol/L) | 80.0 | 79.0 | 23–149 | 28.6 | 37.0–122.2 | 36.0–40.0 | 121.0–125.0 |
| Prop saturation (%) | 28.0 | 24.0 | 2–100 | 29.0 | 4.0–82.0 | 4.0–4.0 | 77.0–86.0 |
| Total protein (g/L) | 48.0 | 48.1 | 31.0–61.0 | 4.0 | 40.8–55.3 | 34.0–42.0 | 53.0–59.0 |
| Albumin (g/L) | 36.9 | 38.0 | 23–46 | 6.0 | 24.9–46.0 | 22.0–27.0 | 43.0–46.0 |
| Globulin (g/L) | 11.2 | 11.0 | 4–33 | 3.0 | 5.0–24.5 | 5.0–6.0 | 18.0–28.0 |
| A/G ratio | 3.8 | 3.5 | 0.7–10.5 | 1.8 | 1.2–8.4 | 1.0–1.5 | 7.0–9.2 |
| Urea | 2.5 | 2.2 | 0.7–9.3 | 1.2 | 0.9–4.9 | 0.7–1.1 | 4.5–6.2 |
| Creatinine (μmol/L) | 89.7 | 88.0 | 36.0–141.0 | 24.0 | 54.3–127.0 | 50.0–65.0 | 120.0–140.0 |
| Glucose (mmol/L) | 6.5 | 6.5 | 2.7–8.9 | 1.0 | 4.98–8.05 | 3.9–5.3 | 7.8–8.5 |
| Cholesterol (mmol/L) | 4.7 | 4.6 | 1.74–9.76 | 1.9 | 2.2–8.4 | 1.9–2.8 | 8.0–9.2 |
| Total bilirubin (μmol/L) | 6.0 | 5.0 | 1–22 | 3.0 | 2.0–18.0 | 1.0–2.0 | 11.0–16.0 |
| Conjugated bilirubin (μmol/L) | 2.9 | 3.0 | 0–10 | 1.0 | 1.0–6.0 | 1.0–1.0 | 5.0–7.0 |
| Free bilirubin (μmol/L) | 3.1 | 3.0 | 0–15 | 2.0 | 0.0–8.3 | 0.0–0.0 | 7.0–10.0 |
| Alkaline phosphatase (U/L) | 589.3 | 552.0 | 160–2119 | 326.7 | 233.0–1332.0 | 224.0–276.0 | 1077.0–1400.0 |
| GGT (U/L) | 35.0 | 35.0 | 0–75 | 20.0 | 14.0–64.0 | 6.0–15.0 | 61.0–68.0 |
| AST (U/L) | 38.3 | 35.0 | 3–130 | 14.0 | 18.0–83.5 | 13.0–20.0 | 73.0–98.0 |
| CK (U/L) | 365.0 | 302.0 | 111–4918.0 | 144.7 | 146.0–869.8 | 133.0–174.0 | 569.0–2885.0 |
| GLDH (U/L) | 5.1 | 2.0 | 0–65 | 3.0 | 0.0–9.0 | 0.0–0.0 | 11.0–61.0 |
| BHBA (μmol/L) | 3.2 | 0 | 0–58 | 0 | 0.0–34.0 | 0.0–0.0 | 36.0–37.0 |
| Haptoglobin (g/L) | 0.6 | 0.4 | 0.2–3.7 | 0.2 | 0.26–2.59 | 0.24–0.26 | 1.71–3.69 |
| Calculated osmolality (mmol/L) | 278.0 | 278.0 | 188–297 | 11.0 | 243.5–288.1 | 212.0–259.0 | 287.0–293.0 |
Reference values were determined using Analyze-It Software (Analyze-It Version 3.0, Leeds, United Kingdom) and based on non-parametric analyses.
Average age of piglets at (1 to 2 d prior to weaning) was 21.8 ± 4.2 days.
IQR — interquartile range, which is equal to the upper quartile (Q3) minus the lower quartile (Q1) (Q3–Q1) and this range covers the central 50% of the data. CI — confidence interval; UIBC — unsaturated iron-binding capacity; TIBC — total iron-binding capacity; GGT — gamma-glutamyl transferase; AST — aspartate transaminase, CK — creatine kinase; GLDH — glutamate dehydrogenase; BHBA — blood β-hydroxybutyrate.
Discussion
The establishment of hematology and biochemistry reference intervals in nursing piglets is important for veterinarians and researchers. Reference intervals can be used to interpret laboratory results in order to better understand the health status of either an individual pig or the entire herd. The interpretation of these parameters is often difficult due to the variation within individual pigs and herds, as well as between herds. There are many animal-related pre-analytical factors such as age, gender, breed, growth rate, nutritional and health status, season, physical activity and stress that can affect both hematology and biochemistry parameters (16).
Nursing piglets undergo rapid growth and immune system changes, which may result in variation in hematology and biochemistry parameters. It is therefore essential to develop reference intervals for this age group to aid in the diagnosis of disease and to enable timely intervention to prevent further health-challenge issues in subsequent stages of production. There are only a few published manuscripts containing reference intervals for hematology and biochemistry parameters for 3-week-old nursing piglets. Thus, contributions to the literature on reference intervals for these parameters are needed.
All pigs selected for sampling lacked any visible outward clinical signs or physical abnormalities. However, subclinical disease and/or marginal nutritional deficiencies may have been present in some of the pigs included in this study. This further highlights the importance of assessing hematology and biochemistry parameters. The decision to omit anemic pigs from the study was based on both the authors’ previous work on pig anemia and iron deficiency (9) as well as on other studies that have published accepted values for pig hemoglobin (HGB) concentration (17,18). The work conducted by Perri et al (9) established that iron deficiency and anemia are present in a proportion of Ontario pigs at weaning, despite their healthy physical appearance. Hence, anemic pigs were identified and removed from the current study in order to establish hematology and biochemistry parameters for healthy pigs. It was hypothesized that the timing of iron administration would contribute to the number of anemic pigs found in the herds. However, Perri et al (9) found no statistically significant difference in the number of anemic pigs when comparing pigs administered an iron injection at ≤ 1 d of age, 2 to 4 d, or 5 to 7 d of age.
The values reported in the current study are similar to values reported in 2 previous publications (19,20). Gong et al (19) evaluated hematology values for 368 piglets that were 20 d of age and the values were similar to the hematology values from the current study. Egeli et al (20) conducted a study on biochemistry and hematology values of pigs at 1 d, 21 d, and 35 d of age. The study compared values from pigs (n = 60) that were defined as having a normal hemoglobin concentration (HGB > 80 g/L) to pigs (n = 42) that were anemic (HGB ≤ 80 g/L). When comparing our hematology parameters to those of Egeli et al (20), the current study reports higher HGB and HCT values. The minor variation in these parameters may be due to differences in the iron supplementation protocols, such as timing of injection and dosage. Egeli et al (20) administered 180 mg of iron dextran at 1 d of age, compared to 200 mg of either iron dextran or gleptoferron at 1 to 7 d of age from the current study. Egeli et al (20) reported slightly lower reference intervals for AST, magnesium, cholesterol, and similar reference intervals for total protein and albumin compared with the current study. The larger sample size herein and different analytical methods may explain the dissimilarities in these parameters. The disparities in cholesterol and total iron may be a result of nutritional variations due to access to milk and creep feed. Also, variations in the amount of protein in milk and creep feed might also affect urea and creatinine concentrations (21).
It is inappropriate to compare hematology and biochemistry reference intervals of nursing piglets to those of other age groups because nursing pigs undergo significant physiological changes such as bone development which will lead to differences in parameters (i.e., elevated phosphorus concentration and alkaline phosphatase activity) compared to older growing pigs (22). There are also nutritional variances between pigs from different age groups, which can affect values such as phosphorus, cholesterol, total bilirubin, conjugated bilirubin, and free bilirubin (21).
A general limitation for assessing reference intervals for hematology and biochemistry parameters is the large variation in biological features found within pigs on a single farm as well as an even larger variation when comparing pigs from different farms. However, a strength of this study was the large sample size for both hematology evaluation (n = 1032) and biochemistry evaluation (n = 189).
The results from this study fill an important gap in the literature by providing reference intervals for hematology and biochemistry parameters in nursing piglets from Ontario commercial herds. The herds selected appeared to be in good health and the pigs chosen lacked clinical signs or physical abnormalities on physical examination, thus the variation in parameters may reflect what is expected in Ontario commercial pigs at weaning. Since the nursing period is a critical stage of development for pigs, it is important to continue to assess and publish hematological and biochemical blood parameters for these animals, as well as for other stages of production, to aid veterinarians and researchers in the identification of clinical or subclinical disease or metabolic and nutritional problems.
Acknowledgments
This study was funded by the Saskatchewan Ministry of Agriculture and Food, Saskatchewan Pork Development Board, the Canadian Swine Health Board, and the Ontario Ministry of Agriculture Food and Rural Affairs-University of Guelph Research Partnership. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Evans RJ. Porcine haematology: Reference ranges and the clinical value of the haematological examination in the pig. Pig J. 1994;32:52–57. [Google Scholar]
- 2.Klem TB, Bleken E, Morberg H, Thoresen SI, Framstad T. Hematologic and biochemical reference intervals for Norwegian crossbreed grower pigs. Vet Clin Path. 2010;39:221–226. doi: 10.1111/j.1939-165X.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 3.Friendship R, Lumsden JH, McMillan I, Wilson MR. Hematology and biochemistry reference values for Ontario swine. Can J Com Med. 1984;48:390–393. [PMC free article] [PubMed] [Google Scholar]
- 4.Lumsden JH, Mullen K. On establishing reference values. Can J Com Med. 1978;42:293–301. [PMC free article] [PubMed] [Google Scholar]
- 5.Lumsden JH, Mullen K, McSherry BJ. Canine hematology and biochemistry reference values. Can J Com Med. 1979;43:125–131. [PMC free article] [PubMed] [Google Scholar]
- 6.Elbers ARW, Geudeke MJ, van Rossem H, Kroon MC, Counotte GHM. Haematology and biochemistry reference values for sows kept under modern management conditions. Vet Q. 1994;16:127–130. doi: 10.1080/01652176.1994.9694433. [DOI] [PubMed] [Google Scholar]
- 7.Miller ER, Ullrey DE, Ackerman I, Schmidt DA, Luecke RW, Hoefer JA. Swine haematology from birth to maturity. II. Erythrocyte population, size and haemoglobin concentration. J Anim Sci. 1961;20:890–897. doi: 10.2527/jas1961.204890x. [DOI] [PubMed] [Google Scholar]
- 8.Ullrey DE, Miller ER, Brent BE, Bradley BL, Hoefer JA. Swine haematology from birth to maturity. IV. Serum calcium, magnesium, sodium, potassium, copper, zinc and inorganic phosphorus. J Anim Sci. 1967;26:1024–1029. doi: 10.2527/jas1967.2651024x. [DOI] [PubMed] [Google Scholar]
- 9.Perri AM, Friendship R, Harding J, O’Sullivan TL. An investigation of iron deficiency and anemia in piglets and the effect of iron status at weaning on post-weaning performance. J Swine Health Prod. 2016;24:10–20. [Google Scholar]
- 10.National Farm Animal Care Council. [Code of practice for the care and handling of pigs]. Ottawa, Canada: Canadian Pork Council; 2014. [Google Scholar]
- 11.Huhn RG, Osweiller GD, Switzer WP. Application of the orbital sinus bleeding technique to swine. Lab Animal Care. 1969;19:403–405. [PubMed] [Google Scholar]
- 12.Dove CR, Alworth LC. Blood collection from the orbital sinus of swine. Lab Animal. 2015;44:383–384. doi: 10.1038/laban.869. [DOI] [PubMed] [Google Scholar]
- 13.ASVCP Quality Assurance and Laboratory Standards Committee (QALS) Guidelines for the Determination of Reference Intervals in Veterinary Species and other related topics: SCOPE. American Society for Veterinary Clinical Pathology (ASVCP); [Last accessed August 28, 2015]. [18 October 2010]. Available from: https://www.asvcp.org/pubs/pdf/RI%20Guidelines%20For%20ASVCP%20website.pdf. [Google Scholar]
- 14.Krzywinski M, Altman N. Points of significance: Visualizing samples with box plots. Nature Methods. 2014;11:119–120. doi: 10.1038/nmeth.2813. [DOI] [PubMed] [Google Scholar]
- 15.Horn PS, Pesce AJ, Copeland BE. A robust approach to reference interval estimation and evaluation. Clinical Chemistry. 1998;44:622–631. [PubMed] [Google Scholar]
- 16.Humann-Ziehank E, Ganter M. Pre-analytical factors affecting the results of laboratory blood analyses in farm animal veterinary diagnostics. Animal. 2012;6:1115–1123. doi: 10.1017/S1751731111002679. [DOI] [PubMed] [Google Scholar]
- 17.Bhattarai S, Nielsen JP. Early indicators of iron deficiency in large piglets at weaning. J Swine Health Prod. 2015;23:10–17. [Google Scholar]
- 18.Nielsen JP, Busch ME, Friendship R, Martineau G-P, Framstad T. Herd diagnosis of iron deficiency in piglets. Proc European Symposium Porcine Health Management; Edinburgh. 2013. p. 168. [Google Scholar]
- 19.Gong YF, Lu X, Wang ZP, et al. Detection of quantitative trait loci affecting haematological traits in swine via genome scanning. BMC Genetics. 2010;11:56–81. doi: 10.1186/1471-2156-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egeli AK, Framstad T, Morberg H. Clinical biochemstry, haematology and body weight in piglets. Acta Vet Scand. 1998;39:281–393. doi: 10.1186/BF03547786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson GD, Harvey DG, Snook CR. A review of factors affecting blood biochemistry in the pig. Br Vet J. 1972;128:596–609. doi: 10.1016/s0007-1935(17)36632-0. [DOI] [PubMed] [Google Scholar]
- 22.Boyd RD, Hall D, Wu JF. Plasma alkaline phosphatase as a criterion for determining biologically available phosphorus for swine. J Anim Sci. 1982;55(Suppl 1):263. doi: 10.2527/jas1983.572396x. [DOI] [PubMed] [Google Scholar]