Abstract
Coxiella burnetii is a zoonotic pathogen that causes Q fever in humans. Serological and questionnaire data on C. burnetii were obtained from 32 small ruminant veterinarians and veterinary students in Ontario, Canada, in February 2012. Overall, 59% of participants were seropositive; advanced stage of career and increased age were associated with seropositivity.
Résumé
Prévalence et facteurs de risques pour la séropositivité à Coxiella burnetii chez les vétérinaires des petits ruminants et les étudiants en médecine vétérinaire en Ontario, au Canada. Coxiella burnetii est un agent pathogène zoonotique qui cause la fièvre Q chez les humains. Des données sérologiques et provenant de réponses à un questionnaire portant sur C. burnetii ont été obtenues auprès de 32 vétérinaires et étudiants en médecine vétérinaire en Ontario, au Canada, en février 2012. Globalement, 59 % des participants étaient séropositifs; un stade de carrière avancé et un âge supérieur étaient associés à la séropositivité.
(Traduit par Isabelle Vallières)
Coxiella burnetii is a zoonotic bacterium that causes Q fever in humans (1). Human infection has most frequently been attributed to indirect or direct contact with infected ruminants, primarily sheep, goats, and cattle (1). Therefore, by the nature of their occupation, veterinarians have been identified as having a higher risk of C. burnetii exposure than the general population (2,3).
The presence of C. burnetii specific antibodies in serum is used to indicate past exposure to the bacterium. Immunofluorescence assay (IFA) is the reference serological test used to diagnose human Q fever (4). The IFA detects the immunoglobulin G (IgG) antibody response to phase I and phase II C. burnetii antigens (5). A phase II titer > phase I titer is suggestive of recent exposure, while phase I titer ≥ phase II titer is suggestive of past exposure (5).
The objectives of this study were to: i) determine the prevalence of C. burnetii seropositivity in Ontario small ruminant veterinarians and veterinary students; and ii) investigate demographic, hygiene, biosecurity and lifestyle factors for association with seropositivity. A convenience sampling procedure was used; all attendees at the Small Ruminant Veterinarians of Ontario (SRVO) Annual General Meeting (AGM), which took place in Orangeville, Ontario, on February 24, 2012, were invited to participate. The SRVO is a voluntary professional organization of veterinarians and veterinary students with an interest in the health of ovine, caprine, and camelid species. Informed written consent was obtained from all 32 participants (18 males, 14 females). The University of Toronto Research Ethics Board (Certification of Ethical Acceptability of Research Involving Human Participants: Reference 27340) approved the study.
Blood samples were collected on-site by a certified phlebotomist via venipuncture into 10-mL red top serum BD vacutainer tubes (Becton, Dickson and Company, Franklin Lakes, New Jersey, USA). Serological analysis was performed at the Public Health Ontario Laboratory in Toronto using the Focus Diagnostic IFA (Cypress, California, USA), according to manufacturer instructions. Serum samples were stored at 4°C if tested within 48 h, or stored at −20°C if tested beyond 48 h. Samples were considered seropositive when either the phase I or phase II IgG titer was ≥ 1:16, as per manufacturer’s guidelines (5).
Univariable exact logistic models were constructed in Stata Intercooled Version 10.1 (StataCorp, 2007; College Station, Texas, USA) to assess putative risk factor associations with the outcome of seropositivity. Univariable exact logistic regression modelling was used in place of standard asymptotic logistic regression, as the former is ideal for analyzing small, skewed, or sparse datasets (6). Associations were considered significant at confidence level of α < 0.05. If a covariate predicted seropositivity perfectly, an estimate of the coefficient was calculated using a median unbiased estimates procedure to give a reasonable estimate of the covariate of interest (6). The dataset was considered too small for the development of a multivariable model.
At the time of sampling, 5 participants indicated in the questionnaire that they suspected they had had Q fever at some point in the past. Two of these individuals sought medical attention but had negative serological tests for C. burnetii at that time. The reported symptoms attributed to Q fever by the 5 individuals were: fever (n = 5), headache and muscle ache (n = 3), fatigue (n = 2), cough (n = 1), and sore throat (n = 1). All 5 of these individuals were seropositive for C. burnetii at the time of sampling for the current study. None of the 14 female participants were pregnant at the time of sampling. Of the 4 women who reported having been pregnant in the previous 2 y, all tested seropositive; none reported an adverse pregnancy outcome.
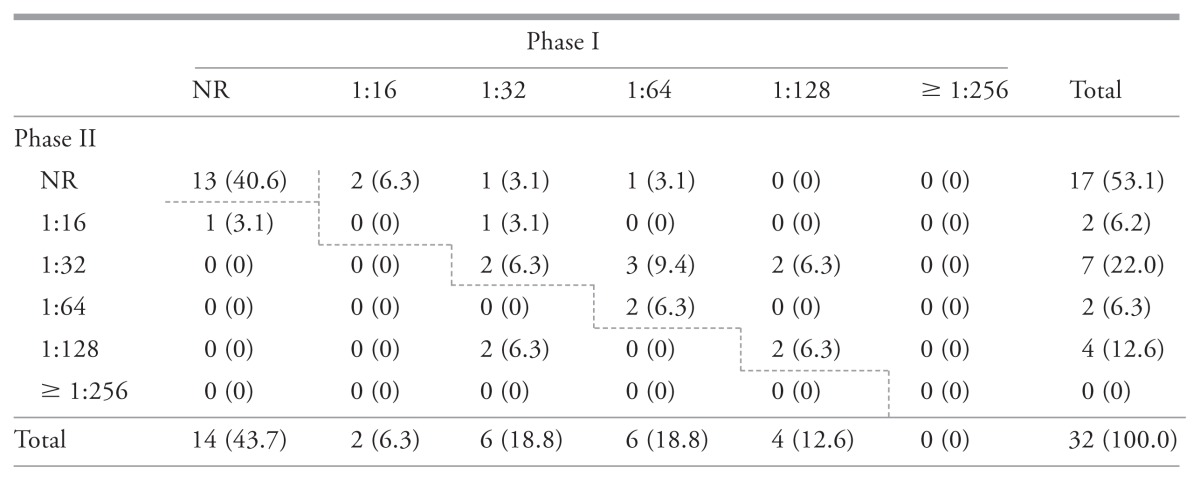
Serosurvey results indicated that 59.4% [19/32, 95% confidence interval (CI): 40.6% to 76.3%] of participating veterinarians and veterinary students were seropositive to C. burnetii. Practicing veterinarians had a seroprevalence of 76.2% (16/21, 95% CI: 52.8% to 91.8%) compared to 50.0% (2/4, 95% CI: 6.8% to 93.2%) in veterinarians not active in clinical practice at the time of sampling, and 14.3% (1/7, 95% CI: 0.3% to 57.9%) in veterinary students. Table 1 demonstrates the distribution of phase I and phase II antibodies to C. burnetii antigens among the study population; 9.4% (3/32) and 50.0% (16/32) were considered to have recent and past exposures, respectively.
Table 1.
Number of individuals (percentage of total samples) with specific serum titers for immunoglobulin G to Phase I and Phase II Coxiella burnetii antigens, among 32 small ruminant veterinarians and veterinary students as determined by the immunofluorescence assay (Focus Diagnostics) (February 24, 2012, Ontario, Canada)
With the exception of non-reactive titers, titers above the dashed line are suggestive of a chronic infection and those below, suggestive of an acute infection.
NR — Not reactive.
40.6% (13/32) Unexposed (phase I and phase II IgG not reactive).
9.4% (3/32) Titers suggestive of recent exposure (phase II titer > phase I titer).
50.0% (16/32) Titers suggestive of past exposure (phase I titer ≥ phase II titer).
The exact logistic univariable analysis identified 2 covariates significantly associated with seropositivity. Being a veterinary student had a sparing effect, as students had 0.06 times (95% CI: 0.0011 to 0.65) the odds of seropositivity compared to practicing veterinarians; the odds of seropositivity among veterinarians not active in clinical practice were not significantly different from the odds for either practicing veterinarians or veterinary students. In addition, participants aged 30 to 39 y and 40 to 49 y had 15 times (95% CI: 1.01 to 1059.64) and 13 times (95% CI: 1.26 to +Infinity) the odds of seropositivity, respectively, compared to those aged 18 to 29 y.
This is the first investigation examining C. burnetii seropositivity among veterinarians and veterinary students in the province of Ontario, and the second in Canada (2). The seroprevalence (59.4%, 19/32) indicates that exposure to C. burnetii was common. Coxiella burnetii has been identified as an occupational hazard for veterinarians and veterinary students elsewhere (3,7,8). For the present research, an IFA IgG titer cut-off of ≥ 1:16 was used, as this is the cut point recommended by manufacturers, and the low cut-point maximizes case capture of those who have been previously exposed to C. burnetii (6). Although an IFA titer cut-point analysis using past C. burnetii exposure as the outcome has not been published, manufacturers reported a high specificity (100%) using the cut-off of ≥ 1:16 (5). Due to the high specificity, false positives are not anticipated, though cross-reactivity to non-specific antibodies cannot be excluded (9).
Within our study group, there was an increased risk of C. burnetii seropositivity among practicing veterinarians compared to veterinary students. While veterinary students perform similar activities as veterinarians, particularly in upper years of study (7), they typically do not have as much opportunity for exposure to potentially infected animals as do veterinarians. Other risk factors for seropositivity previously identified and consistent with dose-response relationships between degree of animal exposure and human seropositivity include age, number of hours with animal contact per week, number of years graduated as a veterinarian, and number of years lived on a farm (3,7,8). Young participants (aged 18 to 29 y) had decreased odds of seropositivity compared to those who were 30 to 39 y and 40 to 49 y; however, all 7 of the participating veterinary students, and 3 of the practicing veterinarians, were between 18 and 29 y of age. When students were excluded from the analysis, age was no longer associated with seropositivity. Age may therefore be an explanatory antecedent of position (practicing veterinarian/student) (6), since age can largely explain the participants’ stage of career. Due to the small sample size of our dataset, we were unable to determine whether age confounded the relationship between stage of career and seropositivity.
We hypothesized that the percent of a veterinarian’s practice dedicated to sheep, goats, or to a lesser extent cattle, would have had an association with seropositivity, as up to 1 × 109 organisms may be shed in the sheep/goat placenta (10) and these animal species have been linked to human cases of Q fever (1); however, this study failed to demonstrate any relationship. The potential animal source of C. burnetii exposure among seropositive veterinarians and veterinary students remains unclear. While sheep and goats have high seroprevalences in Ontario (11,12), C. burnetii could have been transmitted to participants from cows, cats, or other animals.
By the nature of their occupations, small ruminant veterinarians and veterinary students may be exposed to C. burnetii frequently throughout their career. The animal Q fever vaccine (Coxevac; CEVA Animal Health, Libourne, France) is now available for use in Ontario in sheep and goats. However, since veterinarians potentially have contact with animals on many farms, use of the vaccine in sheep and goats may not infer veterinarian protection unless use was widespread. Therefore, vaccinating unexposed veterinary students and veterinarians, particularly those at high risk of developing chronic Q fever (e.g., those with pre-existing heart disease), with a human C. burnetii vaccine (Q-Vax; CSL Biotherapies, Melbourne, Australia), merits future consideration. The Q-Vax vaccine is currently used in Australia, and research has demonstrated that it induces a long-lived immune response to C. burnetii (13).
Several limitations of the study should be noted. While 1/3 of all SRVO members were sampled for this study, the sample size was nevertheless small. The exact logistic regression models served to limit the bias of the coefficients and P-values obtained from small sample sizes (6); however, our sample size, and an expected proportion C. burnetii exposure of 59% (as observed here), means the statistical power is low (calculated at 16.5%). Larger sample sizes may be required to further elucidate the relationship between risk factors and veterinarian seropositivity in Ontario, and to increase the precision of identified associations. This study may also have been subject to selection bias. Our sampling frame of SRVO members is not an exhaustive list of all veterinarians and veterinary students who work with small ruminants in Ontario. As previously noted, SRVO is a voluntary organization and is involved in the continuing education of veterinarians in the health and welfare of small ruminants. The SRVO members may therefore be more engaged in learning about small ruminants and consider small ruminants as a more important part of their caseload or anticipated caseload, than non-members. In addition, research has identified a number of other barriers to participation in continuing education events among veterinarians including: ownership of a solo practice, stage of career, and family demands (14). These factors may have influenced attendance at the AGM, and thus, subsequent participation. Most participants had not been tested for C. burnetii exposure before participating in our study and most did not suspect that they have had Q fever.
Overall, Coxiella burnetii seropositivity was common among SRVO members, particularly among veterinarians, presumably due to their contact with infectious animals. Veterinarians should be alert to the signs and symptoms of Q fever, and should ask their physician for the appropriate tests should these signs and symptoms appear. Hygiene and biosecurity practices, while not statistically associated with seropositivity here, are encouraged for their utility in preventing occupational exposure to not only C. burnetii, but also several other zoonotic disease agents (8).
Acknowledgments
The authors thank: the Ontario Ministry of Agriculture, Food and Rural Affairs-University of Guelph Agreement through the Animal Health Strategic Investment fund (AHSI) managed by the Animal Health Laboratory of the University of Guelph; the Ontario Ministry of Health and Long-Term Care; the Natural Sciences and Engineering Research Council of Canada; and Public Health Ontario. The authors also acknowledge the cooperation of the SRVO and the small ruminant veterinarians and veterinary student members who participated in this study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrie TJ, Fraser J. Prevalence of antibodies to Coxiella burnetii among veterinarians and slaughterhouse workers in Nova Scotia. Can Vet J. 1985;26:181–184. [PMC free article] [PubMed] [Google Scholar]
- 3.Whitney EAS, Massung RF, Candee AJ, et al. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin Infect Dis. 2009;48:550–557. doi: 10.1086/596705. [DOI] [PubMed] [Google Scholar]
- 4.Anderson A, Bijlmer H, Fournier PE, et al. Diagnosis and Management of Q Fever — United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR. 2013;62:1–30. [PubMed] [Google Scholar]
- 5.Q Fever IFA IgG [Internet] Focus Diagnostics; 2011. [Last accessed January 16, 2017]. pp. 2–5. Available from: www.focusdx.com/pdfs/pi/OUS/IF0200G.pdf. [Google Scholar]
- 6.Dohoo I, Martin W, Stryhn H. Veterinary epidemiologic research. Prince Edward Island, Canada: AVC Incorporated; 2003. [Google Scholar]
- 7.de Rooij MMT, Schimmer B, Versteeg B, et al. Risk factors of Coxiella burnetii (Q fever) seropositivity in veterinary medicine students. PLoS One. 2012;7:e32108. doi: 10.1371/journal.pone.0032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Brom R, Schimmer B, Schneeberger PM, Swart WA, van der Hoek W, Vellema P. Seroepidemiological survey for Coxiella burnetii antibodies and associated risk factors in Dutch livestock veterinarians. PLoS One. 2013;8:e54021. doi: 10.1371/journal.pone.0054021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol. 1997;4:208–212. doi: 10.1128/cdli.4.2.208-212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez J, Souriau A, Buendía AJ, et al. Experimental Coxiella burnetii infection in pregnant goats: A histopathological and immunohistochemical study. J Comp Pathol. 2006;135:108–115. doi: 10.1016/j.jcpa.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Meadows S, Jones-Bitton A, McEwen S, Jansen J, Menzies P. Coxiella burnetii seropositivity and associated risk factors in goats in Ontario, Canada. Prev Vet Med. 2015;121:199–205. doi: 10.1016/j.prevetmed.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Meadows S, Jones-Bitton A, McEwen S, Jansen J, Menzies P. Coxiella burnetii seropositivity and associated risk factors in sheep in Ontario, Canada. Prev Vet Med. 2015;122:129–134. doi: 10.1016/j.prevetmed.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Kersh GJ, Fitzpatrick KA, Self JS, Biggerstaff BJ, Massung RF. Long-term immune responses to Coxiella burnetii after vaccination. Clin Vaccine Immunol. 2013;20:129–133. doi: 10.1128/CVI.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore DA, Klingborg DJ, Brenner JS, Gotz AA. Perspectives in professional education in continuing veterinary medical education. J Am Vet Med Assoc. 1998;217:1001–1006. doi: 10.2460/javma.2000.217.1001. [DOI] [PubMed] [Google Scholar]