Abstract
Successful therapy for many inherited disorders could be improved if the intervention were initiated early. This is especially true for lysosomal storage disorders. Earlier intervention may allow metabolic correction to occur before lipid buildup has irreversible consequences and/or before the immune system mounts limiting responses. We have been developing gene therapy to treat lysosomal storage disorders, especially Fabry disease. We describe studies directed toward metabolic correction in neonatal animals mediated by recombinant lentiviral vectors. To develop this method, we first injected a marking lentiviral vector that engineers expression of luciferase into the temporal vein of recipient neonatal animals. The use of a cooled charged-coupled device camera allowed us to track transgene expression over time in live animals. We observed intense luciferase expression in many tissues, including the brain, that did not diminish over 24 weeks. Next, we injected neonatal Fabry mice a single time with a therapeutic lentiviral vector engineered to express human α-galactosidase A. The injection procedure was well tolerated. We observed increased plasma levels of α-galactosidase A activity starting at our first plasma collection point (4 weeks). Levels of α-galactosidase A activity were found to be significantly elevated in many tissues even after 28 weeks. No immune response was observed against the corrective transgene product. Increased levels of enzyme activity also led to significant reduction of globotriaosylceramide in the liver, spleen, and heart. This approach provides a method to treat lysosomal storage disorders and other disorders before destructive manifestations occur.
Keywords: gene therapy, lentivirus, luciferase marking, lysosomal storage disorder
Fabry disease is an X-linked lysosomal storage disorder (LSD) caused by a deficiency in α-galactosidase A (α-Gal A) (EC 3.2.1.22) (1). This deficiency causes systemic accumulation of galactosylsphingolipid moieties, especially globotriaosylceramide (Gb3) (2). Currently, enzyme replacement therapy is available for Fabry disease and can lead to improvement of some manifestations (3, 4); however, frequent enzyme infusions are required throughout life. Long-term outcomes in clinically important tissues have not been fully established, and immune responses are encountered (3, 4). Most problematic, however, is that enzyme replacement therapy often is started after the damage to most organs may be irreversible.
We have been developing retroviral-mediated gene therapy for Fabry disease, focusing on directing overexpression of α-Gal A in the hematopoietic system to provide local intracellular and systemic correction (5–8). Systemic correction is enhanced by metabolic cooperativity wherein α-Gal A-overexpressing cells contribute to improvement of unmodified cells through secretion of enzyme that can be taken up and used. Although we have observed highly effective correction from this therapeutic approach (7, 8), the efficiency of engraftment of transduced hematopoietic cells without some degree of myeloablative conditioning remains low. This aspect is especially true in most disorders wherein a selective growth advantage is not conferred to transduced cells, such as seems to be the case for Fabry disease (9).
Like most other LSDs, Fabry disease is progressive; however, there is little evidence of disease manifestation at birth (2). Therefore, early therapeutic intervention may be more effective than delayed treatment, even with higher dosing. Neonatal therapy can decrease the terminal galactosyllipid load before irreversible damage occurs to the cardiovascular, renal, and nervous systems, which are the main organ systems clinically affected in Fabry disease. Neonatal therapy also provides the potential to tolerize affected individuals to the corrective product, as the recipient's immune system is not yet fully developed (10).
We used recombinant lentiviral vectors (LVs) (family Retroviridae) to effect neonatal therapy. We injected these integrating vectors a single time into recipient animals at day 1 or 2 after birth. The LV system we used provides vesicular stomatitis virus glycoprotein-pseudotyped virions that can be concentrated to a high titer. This system also offers the advantage of stable transgene expression in many different recipient tissues mediated by heterologous promoters (11). To track transgene expression, we first used tracking vectors that engineer overexpression of luciferase (12, 13). LVs were injected a single time into the temporal veins of newborn mice. The injection procedure was well tolerated in all recipients. The expression of luciferase allowed us to track functional transgene expression throughout the recipient over time as detected by conversion of the luminescent substrate luciferin and imaging by a cooled charged-coupled device (CCCD) camera. Real-time in vivo imaging demonstrated stable and sustained expression of luciferase in many tissues mediated by direct injection of the marking LV into neonatal recipients.
We also examined the effects of long-term correction of α-Gal A-deficient animals mediated by a therapeutic LV. Our construct engineered the expression of human α-Gal A and a cell surface marker, human CD25 (huCD25) (8). We observed correction of enzyme activity up to 28 weeks that was accompanied by the reduction of Gb3 in the liver, spleen, and heart. No indication of an inhibitor formation against the transgene product was detected throughout the experiment. We also demonstrate that levels of soluble huCD25 in the plasma correlate directly with α-Gal A activity, providing a surrogate assay for correction mediated by this approach. Our positive results point to encouraging scenarios where lifetime cures of Fabry disease and other LSDs may be mediated by a single, minimally invasive therapeutic intervention using recombinant LVs early in life.
Materials and Methods
LV Production and Titration. A marking HIV-1-based recombinant LV was constructed by replacing the enhanced GFP (enGFP) cDNA in pHR′-EF-GW-SIN plasmid (provided by Robert Hawley, American Red Cross, Rockville, MD) with the firefly luciferase cDNA. This sequence was amplified from plasmid pGL3-control (Promega) with KOD Hot Start DNA polymerase (Toyobo, Osaka) and the cDNA-subcloned downstream of the elongation factor 1α (EF1α) promoter to yield plasmid pHR′-EF1α-luciferase/WPRE-SIN (LV/luciferase). Further modification was performed by introducing the 118-bp cPPT sequence from pol gene in the packaging plasmid pCMVΔR8.91 to facilitate viral translocation to the nucleus (14). The therapeutic LV plasmid, pHR′cPPT-EF1α-α-Gal A-IRES-huCD25-WPRE-SIN (LV/α-Gal A-huCD25), was constructed by exchanging the enGFP cDNA in pHR′-EF-GW-SIN with the α-Gal A-IRES-huCD25 fragment from pMFG-α-Gal A-IRES-huCD25 (8, 9) with the cPPT sequence added as above.
Vesicular stomatitis virus glycoprotein-pseudotyped LVs, including an enGFP marking vector (LV/enGFP), were generated by transient transfection of 293T cells (provided by Robert Pawliuk, Massachusetts Institute of Technology, Cambridge, MA) by using the three-plasmid system (LV plasmid constructs, the packaging plasmid pCMVΔR8.91, and the vesicular stomatitis virus glycoprotein envelope-coding plasmid pMD.G) (15). The transfections were performed with FuGENE6 (Roche). Viral supernatants were harvested 48 h later and concentrated at 50,000 × g for 2 h. The concentrated viral supernatants were serially diluted and titered on HeLa cells (ATCC). Flow cytometric analyses were performed 72 h later by using FACSCalibur (BD Biosciences, San Jose, CA) for enGFP expression or huCD25 expression after staining (8). Luciferase expression was measured in a luminometer (Lumat 9507; Berthold, Germany) by using the Luciferase Assay System (Promega). Functional LV titers ranged from 1–5 × 108 infectious particles (IP)/ml for the LV/enGFP and 1–4 × 107 IP/ml for the LV/α-Gal A-huCD25. LV/luciferase titers ranged from 4 × 107 to 8 × 107 relative light units per sec per μg of protein per ml. p24 antigen levels also were determined by an HIV-1 p24 ELISA (PerkinElmer) on supernatant stocks before neonatal injection. All recombinant LV stocks were determined to be replication incompetent by using a direct assay procedure (16).
Animal Procedures. α-Gal A-deficient Fabry mice (17) were bred at the University Health Network at the University of Toronto. Animal experimentation followed protocols approved by the University Health Network Animal Care Committee. Injections of a maximum of 100 μl of concentrated LVs (see above) were delivered under ×6.25 magnification by using a dissection microscope (Wild M3C, Leica) through a 30-gauge, half-inch-long needle attached to a 1-ml tuberculin syringe. Intravenous injections into newborn mice were performed unilaterally through the superficial temporal vein (18). Strain-matched C57BL/6 mice were purchased from The Jackson Laboratory and used as wild-type controls.
In Vivo Bioluminescent Imaging (BLI) and Fluorescent Imaging. In vivo BLI was performed with an IVIS ImagingSytem (Xenogen, Alameda, CA) composed of a highly sensitive CCCD camera mounted in a light-tight camera box at the Advanced Optical Microscopy Facility at the University Health Network. Images and measurements of bioluminescent signals were acquired and analyzed by using living image (Xenogen). For in vivo imaging, mice were anesthetized and shaved before receiving d-luciferin (Promega) at 100 mg/kg in Dulbecco's PBS (D-PBS) by i.p. injection 10 min before imaging. For organ imaging, organs from killed mice were collected and washed with D-PBS 2 min after d-luciferin injection. All images were acquired with a 5-min exposure. Fluorescent organ imaging was performed with a stereomicroscope (MZ FLIII, Leica) with a fluorescence filter system equipped with a CCCD camera (DC 300 F, Leica) at the Advanced Optical Microscopy Facility. Images were acquired and analyzed by using media cybernetics image pro express (Meyer Instruments, Houston).
Detection of Human CD25 Expression in Tissues by Immunohistochemistry. Frozen tissue sections were stained with 1:100 affinity-purified rabbit polyclonal anti-IL-2Rα (N-19) antibody (sc-665, Santa Cruz Biotechnology) for 1 h, followed by incubation with goat anti-rabbit IgG-Alexa 488 (Molecular Probes) at a dilution of 1:500 for 1 h. Nuclei were counterstained with propidium iodide (Sigma). Organs from D-PBS-injected mice were used as negative controls. Images were acquired by using a confocal laser scanning microscope (model LSM-510, Zeiss) at the Department of Anatomy, University of Toronto.
α-Gal A Assay and HPLC Quantitation of Gb3. α-Gal A activity was measured by a microtiter-plate-based fluorometric assay as described in ref. 8. Samples were added to a microtiter-plate reader (Dynex, Chantilly, VA), and the 4-methylumbelliferone product was determined quantitatively by comparison with known standards (Sigma). Frozen mouse tissue samples were homogenized with a tissue homogenizer for 10 sec twice in the assay buffer (28 mM citric acid/44 mM disodium phosphate, pH 4.4) with 5 mg/ml sodium taurocholate before sonication. HPLC analyses of Gb3 levels in mouse organ extracts were performed as described (19). The protein concentrations of assay samples were determined by the BCA Protein Assay Kit (Pierce). α-Gal A activity and Gb3 levels in each sample were measured in triplicate.
Soluble Human IL-2Rα (sCD25) Antigen ELISA and Anti-α-Gal A IgG Antibody Assay. Plasma was collected from experimental and control animals at various time points. Plasma levels of soluble human IL-2Rα antigen were measured by using the Human IL-2sRα BD OptEIA Set (BD Bioscience) according to the manufacturer's instructions. Antibody responses against the expressed human α-Gal A cDNA product were measured by ELISA as described (20) with minor modifications. Briefly, 96-well flat-bottom microtiter plates (Nalgene) were coated overnight at 4°C with 50 μl per well of 1 mg/ml recombinant human α-Gal A (Fabrazyme, Genzyme) in D-PBS with 0.02% sodium azide. After three washes in wash buffer (BD Bioscience), the plates were blocked with 100 μl per well of 10% rabbit serum (Sigma) for 2 h at room temperature (RT). Subsequently, 50 μl of 1:250 diluted murine plasma samples were added to the wells in triplicate and incubated for 2 h at RT. After washing, plates were blocked with 100 μl per well of 10% sheep serum (Sigma) for 2 h at RT. Plates were washed, and 50 μl of a goat anti-mouse IgG (whole molecule) alkaline phosphatase-conjugated antibody (Sigma), diluted to 1:500 in 10% sheep serum, was added and incubated for 1 h at RT. After five washes, 50 μl per well of Alkaline Yellow Liquid Substrate System (Sigma) was added and incubated for 30 min. The reaction was terminated by addition of 50 μl of 0.5 M sodium hydroxide. Absorbance was measured at 405 nm by using a microplate reader (Softmax, Molecular Devices). Titers were estimated from a standard curve obtained by serial dilutions of a monoclonal antirecombinant human-α-Gal A antibody (clone no. 6F5.11.5.1, kindly supplied by Genzyme). The lower limit of antibody detection corresponded to 100 ng/ml (data not shown). Sera from mice immunized by injecting 25 μg of recombinant human α-Gal A (Genzyme) with complete Freund's adjuvant (Sigma) once and incomplete Freund's adjuvant twice 2–4 weeks apart acted as a positive control. For negative control, sera were collected from mice immunized against human prostate-specific antigen (J.A.M., unpublished data).
Statistics. Data are presented as SEM. Statistical comparisons were obtained by ANOVA with the Bonferroni multiple comparison test by using instat (GraphPad, San Diego). P values <0.05 were considered statistically significant. Regression analysis was performed by using excel (Microsoft).
Results
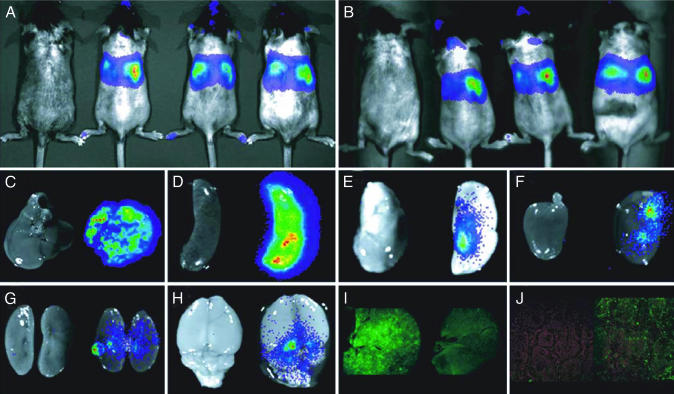
Real-Time in Vivo BLI of Expression and Persistence of the Marking LV. We first assessed the biodistribution of our marking LV as measured by functional expression of luciferase after a single unilateral injection of concentrated vector supernatant into the temporal veins of neonatal mice. Five animals in each group were injected with 100 μl of either LV/enGFP or LV/luciferase (0.8 μg/ml and 3.2 μg/ml p24 antigen, respectively). The injection procedure was well tolerated. Fig. 1 shows images of recipient mice and individual organs obtained using a CCCD camera. Fig. 1A shows whole-body images obtained from recipient mice at 12 weeks. The left-most mouse was injected with the LV/enGFP. No background bioluminescence was observed. Although the central body portion of the animal exhibits the strongest pseudocolor bioluminescence pattern, expression also can be detected in the head region, the hind limbs, and even the tail. Even though this was a unilateral injection, bilateral expression was observed. Because both the assay and the detection system are noninvasive, we were able to reimage the same animals as in Fig. 1A at 24 weeks after the single neonatal LV injection event (Fig. 1B). No significant diminution of signal strength was observed after 24 weeks, indicating that this LV-mediated procedure can provide long-term functional and systemic expression of transgenes.
Fig. 1.
Serial BLI of luciferase expression in living mice after LV injection into neonatal animals. (A) BLI at 12 weeks. (B) The same animals in A imaged at 24 weeks. Shown are the LV/enGFP-injected control mouse (far left) and three LV/luciferase-injected mice. The pseudocolor overlay represents the intensity of light emission and thus the level of luciferase expression. (C–H) BLI of collected organs from representative mice (left-most is LV/enGFP-injected) 24 weeks after injection. Shown are liver (C), spleen (D), lung (E), heart (F), kidney (G), and brain (H). (I) enGFP expression in the liver of LV/enGFP-injected mouse (left) and LV/luciferase-injected mouse (right). (J) Immunohistochemistry for huCD25 expression on the spleen from D-PBS-injected mouse (left) and LV/α-Gal A-huCD25-injected mouse (right).
We examined various organs for luciferase activity and enGFP expression after euthanizing the animals. Test and control animals were given d-luciferin just before euthanization at 24 weeks. The organs were harvested and immediately imaged. Fig. 1 C–H indicates the absence of bioluminescence in organs harvested from an LV/enGFP-injected control mouse (left-most tissue) and the strong bioluminescence in organs from an LV/luciferase-injected recipient. As could be expected from Fig. 1 A and B, livers and spleens of recipient animals showed the highest levels of bioluminescence. Yet even in hearts, lungs, and kidneys, where the luminescent signals were hardly detected by whole-body in vivo imaging, significant luminescent signals were seen. This result confirms that the data we observed in Fig. 1 A and B are not derived from superficial transgene expression and that the injected LV is penetrating and engineering transgene expression in differential organ systems. Remarkably, we also observed a strong bioluminescent pattern in the brains of recipient animals (Fig. 1H). With regard to enGFP, even though we were able to visualize fluorescence in the liver (Fig. 1I) and lung (data not shown), no distinct fluorescent signal over background autofluorescence was detected in other organs.
Our study demonstrates this type of long-term transgene expression in the brain mediated by injection of recombinant LVs into neonatal animals. This finding provides incentive to develop novel therapeutic schemes for this relatively inaccessible organ. This result can impact not only LSDs but other neurogenetic disorders as well.
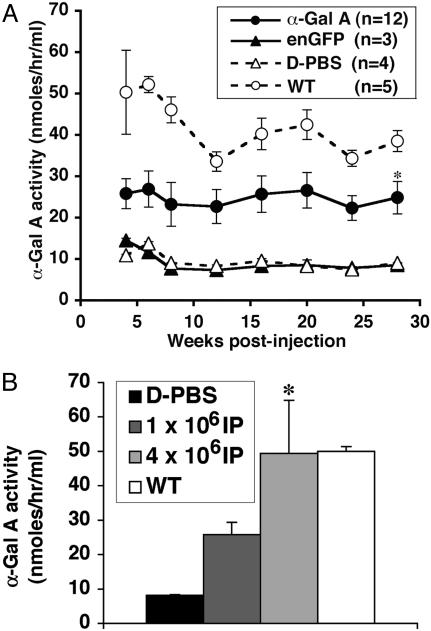
Increased α-Gal A Activity and Soluble huCD25 Levels in the Plasma of LV-Injected Fabry Mice. We examined the systemic effects of injection of a therapeutic LV construct into neonatal Fabry mice. In the first experiment, 12 neonatal Fabry mice were injected with concentrated LV/α-Gal A-huCD25 virions (1 × 107 IP/ml, 3.2 μg/ml p24 antigen). Whole blood was collected, and plasma was isolated from injected and control animals at various time points. Fig. 2A shows the α-Gal A enzyme activity in plasma obtained from each group of experimental animals. α-Gal A activities increased significantly (P < 0.05) in the therapeutically treated group, compared with those of the D-PBS- and LV/enGFP-injected groups. This increase was stable and consistent until termination of the experiment (28 weeks). Indeed, in some individual animals injected with the therapeutic LV plasma α-Gal A, activities were actually greater than those derived from strain-matched wild-type animals (data not shown). In a second experiment, we wanted to determine whether the increases in plasma α-Gal A enzyme activity were dependent on the dose of therapeutic LV injected. After injections in another independent cadre of animals (n = 5 each group), we observed that the plasma levels of α-Gal A activity after 4 weeks were dependent on the dose of recombinant LV (Fig. 2B; P < 0.05).
Fig. 2.
Plasma α-Gal A activity in LV-injected Fabry mice. (A) Plasma from LV/α-Gal A-huCD25-injected mice, LV/enGFP-injected mice, D-PBS-injected mice, and WT control mice was collected at 4, 6, 8, 12, 16, 20, 24, and 28 weeks postinjection. *, P < 0.05 vs. enGFP- and D-PBS-injected groups. (B) LV dose-dependent α-Gal A expression in Fabry mice. Plasma α-Gal A activity was measured 4 weeks after LV injection. Titers of LV/α-Gal A-huCD25 injected into neonatal Fabry mice are shown. D-PBS-injected mice and WT mice were used as controls. *, P < 0.05 vs. 1 × 106 IP-injected group.
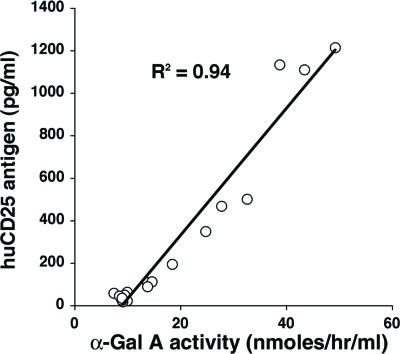
Our therapeutic LV that engineers expression of human α-Gal A was bicistronic with huCD25 as a coexpressed cell surface marker. This marker allowed us to measure functional viral titer in vitro as we have done previously with recombinant oncoretroviruses (8). Beyond this direct application, we also examined the possibility of using the expression of this secondary transgene product as a surrogate assay for vector persistence in vivo and as a marker to assess the biodistribution of therapeutically transduced cells. This opportunity is because of the fact that huCD25 has a soluble form with very low background expression levels in mice, rhesus macaques, and humans (unpublished data). Having a reliable and independent surrogate read-out for functional vector persistence would be invaluable when testing therapeutic strategies in normal large animal models, for example, because endogenous proteins are often indistinguishable from transgene products. As shown in Fig. 3, there was a direct correlation between soluble huCD25 antigen levels and plasma α-Gal A activity (R2 = 0.94). Immunohistochemistry results also demonstrate huCD25 expression in the spleen (Fig. 1J) at the 28-week termination point from the first cadre of LV/α-Gal A-huCD25-injected neonatal animals.
Fig. 3.
The correlation between plasma α-Gal A activity and plasma soluble huCD25 antigen level in LV-injected Fabry mice.
Increased α-Gal A Activity and Reduced Gb3 Levels in Organs of Treated Fabry Mice. We determined α-Gal A activity in the extracts of various organs from each of the treated and control mice from the first experimental cohort at the 28-week termination point and analyzed the Gb3 levels in these organs by using HPLC. In the LV/α-Gal A-huCD25-treated group, α-Gal A activity in the liver increased significantly to ≈14% of normal, and with these levels of activity, Gb3 levels decreased significantly (Tables 1 and 2). In the spleen, α-Gal A activities increased up to 8% of normal levels, but Gb3 levels decreased 88%, compared with D-PBS-injected Fabry mice. α-Gal A activities also increased, and Gb3 levels decreased significantly in the heart. Even though we observed what appeared to be a significant increase in α-Gal A activity in the kidney, statistically significant Gb3 reduction was not observed (Table 2). We also observed a significant increase in α-Gal A activity in the brain. This potentially therapeutic outcome is especially important if we wish to apply this treatment to other LSDs that have extensive CNS involvement.
Table 1. α-Gal A specific activity in organs from Fabry mice euthanized 28 weeks after neonatal injection.
| Specific Activity (nmol/hr per mg of protein)
|
|||||||
|---|---|---|---|---|---|---|---|
| Injection | Liver | Spleen | Lung | Heart | Kidney | BM | Brain |
| α-Gal A (n = 12) | 13.2 ± 2.0** | 91.6 ± 8.1* | 9.2 ± 1.4 | 4.5 ± 0.3** | 3.9 ± 0.1* | 43.5 ± 0.4* | 6.2 ± 0.1* |
| enGFP (n = 3) | 3.0 ± 0.1 | 51.7 ± 0.6 | 7.6 ± 0.3 | 3.0 ± 0.1 | 3.1 ± 0.1 | 40.4 ± 0.0 | 5.4 ± 0.0 |
| D-PBS (n = 4) | 3.1 ± 0.1 | 52.8 ± 0.9 | 7.3 ± 0.5 | 2.9 ± 0.1 | 3.0 ± 0.1 | 39.6 ± 0.7 | 5.4 ± 0.1 |
| WT (n = 5) | 92.9 ± 4.1 | 1211.3 ± 88.6 | 139.4 ± 10.0 | 16.8 ± 1.7 | 52.9 ± 2.9 | 551.0 ± 40.8 | 253.4 ± 7.5 |
α-Gal A, LV/α-Gal A-huCD25-injected group; enGFP, LV/enGFP-injected group; BM, bone marrow. *, P < 0.05 vs. enGFP group; **, P < 0.01 vs. enGFP group.
Table 2. Gb3 levels in organs from Fabry mice euthanized 28 weeks after neonatal injection.
| Gb3, nmol/mg of protein
|
|||||
|---|---|---|---|---|---|
| Injection | Liver | Spleen | Lung | Heart | Kidney |
| α-Gal A (n = 12) | 0.8 ± 0.5* | 7.5 ± 2.1* | 2.2 ± 0.2 | 1.0 ± 0.2* | 24.0 ± 2.7 |
| D-PBS (n = 4) | 4.3 ± 1.2 | 64.2 ± 9.6 | 2.5 ± 0.2 | 2.8 ± 0.4 | 26.4 ± 5.2 |
| WT (n = 5) | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 1.8 ± 0.5 |
α-Gal A, LV/α-Gal A-huCD25-injected group. *, P < 0.05 vs. D-PBS group.
Absence of Anti-α-Gal A Antibody as Measured by ELISA. One of the main limitations to applying gene therapy in the clinic is the immune response often generated in recipients against transgene products. Invasive procedures such as myelo-ablation or the use of immunosuppressive drugs to diminish immune response is unacceptable for severely affected patients. Because our transgene product is a foreign protein to Fabry (null) mice, we evaluated the immune response against human α-Gal A post-LV injection over time. Compared with above-mentioned negative and positive controls (see Materials and Methods), the level of anti-α-Gal A IgG antibody was below the detectable range (<100 ng/ml, data not shown) at 28 weeks postinjection in the LV/α-Gal A-huCD25-treated mice and the various control cohorts.
Discussion
We have demonstrated that direct injection of recombinant LVs into neonatal mice appears to have significant long-term therapeutic potential. This minimally invasive delivery method leads to sustained systemic expression of marking and therapeutic transgenes in a dose-dependent manner without any immune-based inhibitor formation. As evidenced by our data employing the luciferase construct, we observed a broad distribution of marking gene expression in many organs, including the brain, that did not diminish within the time frame of this investigation. With regard to our therapeutic construct that was engineered for the correction of Fabry disease, we see increased plasma and organ levels of α-Gal A activity along with a reduction of Gb3 in many organs. We also demonstrate that soluble human CD25, a secondary product of our bicistronic LV, can be an effective surrogate marker to measure therapeutic vector persistence and expression in vivo.
A major advantage of the luciferase marking system with BLI using a CCCD camera is the capacity to evaluate gene transfer outcomes sequentially over time in the same living animal. This ability obviates the need for multiple cohorts of animals to be killed for analysis at each desired time point. This approach reduces potential animal-to-animal variance and allows long-term studies of vector persistence and transgene expression. Moreover, we found that the sensitivity of the luciferase detection system is much greater than that for analysis of enGFP expression, and there is no background signal in control animals.
In our present studies, an obvious bioluminescent signal was observed in areas close to the main arterial supply when organs were imaged directly. For example, we saw strong BLI patterns around the hilar regions of the lung and kidney and the circle of Willis in the brain (Fig. 1 E, G, and H). Notably, in our study, the injected LV also was able to transit to the CNS and engineer functional expression of the transgene for at least 24 weeks. This is an important finding because one of the critical obstacles to developing any type of therapy for most LSDs is the limited access of the corrective agent to the CNS. In many pathological conditions, this location is most severely affected.
Other investigators have observed correction of LSDs mediated by gene transfer into neonatal recipients by using adenoviruses (21), adeno-associated viruses (22), and oncoretroviruses (23). LVs themselves also have been injected directly into the brain to effect correction of LSDs (24). The data that we present demonstrates long-term systemic correction by recombinant LVs of an LSD mediated by direct injection into neonatal animals.
LVs have some inherent properties that may offer unique advantages over some of the other aforementioned delivery systems. Adenovirus and adeno-associated virus initiate immune responses and integrate at low frequencies, which can limit long-term transgene expression. On the other hand, because LVs integrate into nondividing cells, their tropism differs from that of oncoretroviral vectors. Moreover, LVs can accommodate larger cDNA inserts, compared with oncoretroviral vectors (25), and transcriptional silencing of this complex retroviral vector appears to be less pronounced than with oncoretroviral vectors during cell differentiation (26). Our minimally invasive neonatal delivery method also has advantages over stereotactic injection (24), including ease and safety of delivery.
One concern of this integrating LV-mediated delivery strategy is the potential of germ-line transmission of the transgene. Previous studies with oncoretroviruses showed no detectable vector sequences in the testis after neonatal gene transfer into mice (27) or dogs (28). However, similar detailed analyses will have to be performed with the LV system because of its different vector tropism and integrative capacity. To begin to address this possibility directly, we bred some of the LV-injected animals that were treated neonatally. We examined 25 offspring pups for LV-specific sequences by PCR on genomic DNA and for functional transgene expression by measuring plasma enzyme activity. We did not observe any LV-specific amplicons or increased α-Gal A activity levels above background values (data not shown).
We observed metabolic correction of a number of organs relevant to Fabry disease by using this LV approach. Another advantage of the present strategy is that systemic transduction by the injected LV may generate differential posttranslationally modified forms of α-Gal A that may be more physiologically useful for alternative physiological niches (29). In this study, we were not able to reduce Gb3 to therapeutic levels in the kidney and lungs. Methods to improve outcomes in these organs are needed. As we showed by the LV dose-dependent augmentation of α-Gal A activity in plasma, it is possible that the lack of lipid reduction in the kidney and lung can be overcome by increasing the injection dose or the frequency of injections within an appropriate interval with careful monitoring of toxicity. Alternative routes of administration also may have a favorable effect on the therapeutic outcome. It should also be possible to dissect out the exact cell types that are expressing the marking and therapeutic transgenes and determine what effects on systemic correction may be mediated by alternative promoters or alternatively pseudotyped virions.
In conclusion, minimally invasive LV-mediated neonatal gene delivery has the potential for long-term therapy because of efficient transduction and expression. Although the safety of this approach needs to be further investigated in large animals, our findings prompt studies in such models to verify the therapeutic potential for the treatment of Fabry disease and other LSDs.
Acknowledgments
This work was supported in part by National Institutes of Health Grant HL70569-03 (to J.A.M.) and a Roscoe Brady Genetic Diseases Fellowship (to M.Y.) from the National Organization for Rare Disorders (Danbury, CT).
Author contributions: M.Y., L.W., and J.A.M. designed research; M.Y., T.S., K.T., J.S.W., V.I.R., G.T.S., G.J.M., A.G.P., and J.U. performed research; M.Y., T.S., J.S.W., V.I.R., G.J.M., R.O.B., and J.A.M. analyzed data; and M.Y., R.O.B., and J.A.M. wrote the paper.
Abbreviations: LSD, lysosomal storage disorder; LV, lentiviral vector; CCCD, cooled charged-coupled device; α-Gal A, α-galactosidase A; Gb3, globotriaosylceramide; huCD25, human CD25; BLI, bioluminescent imaging; EF, elongation factor; RT, room temperature; IP, infectious particles; enGFP, enhanced GFP.
References
- 1.Brady, R. O., Gal, A. E., Bradley, R. M., Martensson E., Warshaw, A. L. & Laster, L. (1967) N. Engl. J. Med. 276, 1163-1167. [DOI] [PubMed] [Google Scholar]
- 2.Desnick, R. J., Ioannou, Y. A. & Eng, C. M. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), pp. 3733-3774.
- 3.Schiffmann, R., Kopp, J. B., Austin, H. A., Sabnis, S., Moore, D. F., Weibel, T., Balow, J. E. & Brady, R. O. (2001) J. Am. Med. Assoc. 285, 2743-2749. [DOI] [PubMed] [Google Scholar]
- 4.Eng, C. M., Guffon, N., Wilcox, W. R., Germain, D. P., Lee, P., Waldek, S., Caplan, L., Linthorst, G. E. & Desnick, R. J. (2001) N. Engl. J. Med. 345, 9-16. [DOI] [PubMed] [Google Scholar]
- 5.Medin, J. A., Tudor, M., Simovitch, R., Quirk, J. M., Jacobson, S., Murray, G. J. & Brady, R. O. (1996) Proc. Natl. Acad. Sci. USA 93, 7917-7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takenaka, T., Qin, G., Brady, R. O. & Medin, J. A. (1999) Hum. Gene Ther. 10, 1931-1939. [DOI] [PubMed] [Google Scholar]
- 7.Takenaka, T., Murray, G. J., Qin, G., Quirk, J. M., Oshima, T., Qasba, O., Clark, K., Kulkarni, A. B., Brady, R. O. & Medin, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 7515-7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin, G., Takenaka, T., Telsch, K., Kelly, L., Howard, T., Levade, T., Deans, R., Howard, B. H., Malech, H. L., Brady, R. O., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 3428-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siatskas, C., Yoshimitsu, M. & Medin, J. A. (2004) in Focus on Stem Cell Research, ed. Greer, E. V. (Nova Science Publishers, Hauppauge, NY), in press.
- 10.Sarzotti, M. (1997) Curr. Opin. Hematol. 4, 48-52. [DOI] [PubMed] [Google Scholar]
- 11.Kim, D. W., Uetsuki, T., Kaziro, Y., Yamaguchi, N. & Sugano, S. (1990) Gene 91, 217-223. [DOI] [PubMed] [Google Scholar]
- 12.Contag, C. H. & Bachmann, M. H. (2002) Annu. Rev. Biomed. Eng. 4, 235-260. [DOI] [PubMed] [Google Scholar]
- 13.Wu, J. C., Sundaresan, G., Iyer, M. & Gambhir, S. S. (2001) Mol. Ther. 4, 297-306. [DOI] [PubMed] [Google Scholar]
- 14.Follenzi, A., Ailles, L. E., Bakovic, S., Geuna, M. & Naldini, L. (2000) Nat. Genet. 25, 217-222. [DOI] [PubMed] [Google Scholar]
- 15.Naldini, L., Blömer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M. & Trono, D. (1996) Science 272, 263-267. [DOI] [PubMed] [Google Scholar]
- 16.Chang, L.-J., Urlacher, V., Iwakuma, T., Cui, Y. & Zucali, J. (1999) Gene Ther. 6, 715-728. [DOI] [PubMed] [Google Scholar]
- 17.Ohshima, T., Murray, G. J., Swain, W. D., Longenecker, G., Quirk, J. M., Cardarelli, C. O., Sugimoto, Y., Pastan, I., Gottesman, M., Brady, R. O., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 2540-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sands, M. S. & Barker, J. E. (1999) Lab. Anim. Sci. 49, 328-330. [PubMed] [Google Scholar]
- 19.Ohshima, T., Schiffmann, R., Murray, G. J., Kopp, J., Quirk, J. M., Stahl, S., Chan, C. C., Zerfas, P., Tao-Chen, J. H., Ward, J. M., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 6423-6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung, S. C., Han, I. P., Limaye, A., Xu, R., Gelderman, M. P., Zerfas, P., Tirumalai, K., Murray, G. J., During, M. J., Brady, R. O., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 2676-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaji, A., Kosuga, M., Li, X.-K., Fukuhara, Y., Tanabe, A., Kamata, Y., Azuma, N., Yamada, M., Sakamaki, T., Toyama, Y. & Okuyama, T. (2003) Mol. Ther. 8, 718-725. [DOI] [PubMed] [Google Scholar]
- 22.Daly, T. M., Vogler, C., Levy, B., Haskines, M. E. & Sands, M. S. (1999) Proc. Natl. Acad. Sci. USA 96, 2296-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponder, K. P., Melniczek, J. R., Xu, L., Weil, M. A., O'Malley, T. M., O'Donnell, P. A., Knox, V. W., Aguirre, G. D., Mazrier, H., Ellinwood, N. M., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13102-13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosch, A., Peret, E., Desmaris, N., Trono, D. & Heard, J. M. (2000) Hum. Gene Ther. 11, 1139-1150. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, M., Keller, B., Makalou, N. & Sutton, R. E. (2001) Hum. Gene Ther. 12, 1893-1905. [DOI] [PubMed] [Google Scholar]
- 26.Lois, C., Hong, E. J., Pease, S., Brown, E. J. & Baltimore, D. (2002) Science 295, 868-872. [DOI] [PubMed] [Google Scholar]
- 27.Xu, L., Mango, R. L., Sands, M. S., Haskins, M. E., Ellingwood, N. M. & Ponder, K. P. (2002) Mol. Ther. 6, 745-758. [DOI] [PubMed] [Google Scholar]
- 28.Xu, L., Haskins, M. E., Melniczek, J. R., Gao, C., Weil, M. A., O'Malley, T. M., O'Donnell, P. A., Mazrier, H., Ellinwood, N. M., Zweigle, J., et al. (2002) Mol. Ther. 5, 141-153. [DOI] [PubMed] [Google Scholar]
- 29.Bishop, D. F. & Desnick, R. J. (1981) J. Biol. Chem. 256, 1307-1316. [PubMed] [Google Scholar]