Abstract
Improving early detection and treatment of atrial fibrillation (AF) is critical because untreated AF is a major contributor to stroke and heart failure. We sought to generate knowledge about the feasibility of conducting a randomized controlled trial to test the effect of the Alert for AFib intervention on knowledge, attitudes, and beliefs about treatment-seeking for signs and symptoms of AF. Adults ≥65 years old (96% White) at risk for developing AF were randomized to receive the Alert for AFib intervention (n = 40) or an attention control session (n = 40). Feasibility goals for recruitment, participant retention, adherence, perceived satisfaction and burden, and intervention fidelity were met. From baseline to study completion, knowledge (p = .005) and attitudes (p < .001) about treatment-seeking improved more in the intervention group compared with the control group. Results support testing the effectiveness of the Alert for AFib intervention in a large trial.
Keywords: atrial fibrillation, cognitive behavioral intervention, patient education, randomized controlled trial, self-management, self-monitoring, stroke prevention
Atrial fibrillation (AF) is a chronic illness that affects more than 30 million people worldwide (Chugh et al., 2014). It increases morbidity and mortality rates (Stewart, Hart, Hole, & McMurray, 2002), strains health care resources (Ball, Carrington, McMurray, & Stewart, 2013), and reduces work productivity (Rohrbacker, Kleinman, White, March, & Reynolds, 2010). Improvement in early detection and treatment is critical because untreated AF is a major contributor to disabling embolic strokes, heart failure, and exacerbation of existing cardiac conditions (Benjamin et al., 2009; January et al., 2014). The longer AF goes untreated, the more resistant the atrium is to treatments aimed at converting and maintaining sinus rhythm, leaving many patients with frequent recurrent episodes (Cosio et al., 2008). Because of these recurrent events, AF treatment is more costly (Kirchhof, 2009), and the patients’ quality of life (Thrall, Lane, Carroll, & Lip, 2006) and productivity suffer (Rohrbacker et al., 2010). Delay in seeking treatment of AF at symptom onset results in a missed opportunity for vital early treatment. A recent study (McCabe, Chamberlain, Rhudy, & DeVon, 2016) revealed that 70% of patients with AF symptoms did not seek evaluation within a week of symptom onset. Treatment-seeking for AF is hindered when people do not recognize symptoms that represent AF, attribute those symptoms to alternative causes, or do not believe the symptoms are serious enough to require medical evaluation (McCabe, Rhudy, Chamberlain, & DeVon, 2015; McCabe, Rhudy, & DeVon, 2015). Only a few investigators have reported strategies to engage persons most at risk for AF to self-monitor for the signs and symptoms of AF and seek treatment early if signs and/or symptoms occur (Benito et al., 2015; Virtanen et al., 2014). Yet, such engagement has the potential to reduce treatment-seeking delay.
Gaps in Science for Early Detection of AF
Emerging evidence shows that periodic screening for AF during clinical visits through pulse palpation (Camm et al., 2012), handheld electrocardiography devices, or smartphone applications (Lowres et al., 2015; Svennberg, Engdahl, Al-Khakili, & Friberg, 2015) is effective for detecting undiagnosed AF. In its early stage, AF is often episodic. In such periodic screening, detection relies on AF being present at screening. Engaging at-risk persons in regular self-monitoring that includes symptom awareness and pulse palpation can address this limitation (Benito et al., 2015; Virtanen et al., 2014).
There is a paucity of research pertaining to evaluation of interventions that promote early treatment-seeking behavior through self-monitoring in older adults at risk for developing AF. Although incompletely described, prior interventions appear to have been delivered in lecture format and included content about AF symptoms, the relationship between an irregular pulse and stroke, instruction on radial pulse palpation, and instruction to notify the health care provider when AF symptoms occur or an irregular pulse is detected (Benito et al., 2015; Kallmunzer et al., 2014; Munschauer, Sohocki, Carrow, & Priore, 2004; Virtanen et al., 2014).
The recommended frequency of pulse palpation varied from once a month (Benito et al., 2015; Munschauer et al., 2004) to twice a day (Virtanen et al., 2014). Reported outcomes included number of AF cases diagnosed (Benito et al., 2015; Munschauer et al., 2004; Virtanen et al., 2014), capability to palpate a radial pulse (Kallmunzer et al., 2014; Munschauer et al., 2004; Virtanen et al., 2014), and ability to recognize an irregular pulse (Munschauer et al., 2004; Virtanen et al., 2014). Adherence to daily pulse palpation was reported for a sole study (Virtanen et al., 2014). Although investigations have differed in design and aims, results are suggestive that early AF detection can be fostered when older persons are willing to engage in self-monitoring by pulse palpation and have increased awareness of symptoms.
In summary, previous research suggests that both periodic screening by clinicians and self-monitoring by individuals have the potential to improve early detection of AF. However, studies have not included detailed descriptions of self-monitoring interventions that are critical for replication (Benito et al., 2015; Munschauer et al., 2004; Virtanen et al., 2014). Furthermore, investigators have not reported the interventions’ influence on knowledge attitudes, and beliefs that promote self-monitoring. Information about the effects of the intervention on knowledge, attitudes, and beliefs about AF that promote self-monitoring is crucial to determining the cognitive mechanisms that influence adoption of self-monitoring.
Purpose
The intervention called Alert for AFib was developed by the authors to address the aforementioned gaps and promote early treatment-seeking behavior for AF signs and symptoms. The purpose of the present study was to generate critical practical knowledge about the feasibility of conducting a randomized controlled trial to test the effect of the Alert for Fib intervention on knowledge, attitudes, and beliefs about seeking treatment for signs and symptoms of AF.
Method
Design, Sample, and Setting
This pilot randomized controlled trial was conducted at an academic medical center in the U.S. Midwest. Medical records of patients who had previously given permission for their records to be reviewed were screened for eligibility. Patients were eligible if they (a) were ≥65 years of age, (b) had ≥1 risks for AF (e.g., hypertension, coronary artery disease, heart failure, heart valve disease, obstructive sleep apnea, diabetes mellitus, and obesity [body mass index ≥30]), (c) had never received an AF diagnosis, (d) spoke English, and (e) were community dwelling with access and ability to communicate by telephone. Patients were excluded if they (a) had a documented history of palpitations, atrial or ventricular arrhythmias resulting in irregular pulse; (b) had a documented cognitive impairment; (c) had uncompensated hearing or visual deficits; (d) were receiving ongoing active therapy for malignancy, hospice or palliative care; (e) anticipated surgery in the 2 months following study enrollment; (f) had received general anesthesia in the 30 days before enrollment; (g) resided with a person who had diagnosed AF; or (h) had been employed or received training in a health care field where they had been exposed to information about AF.
Measures
Demographic and clinical data form
Demographic data (i.e., age, sex, race, educational level, marital status, and living alone vs. with someone) and data pertinent to the participants’ risk factors for AF were abstracted from medical records.
Knowledge, attitudes, and beliefs about AF
No published instruments were available to measure knowledge, attitudes, and beliefs about AF among people at risk for developing AF. Therefore, we developed the Knowledge, Attitudes, and Beliefs about Atrial Fibrillation Survey (KABAFS). The KABAFS is a 33-item instrument that includes five subscales: eight-item knowledge of AF-related symptoms (response yes/no/not sure); a six-item knowledge of symptoms not related to AF (response yes/no/not sure); five-item general knowledge about AF (response true/false/not sure); five-item attitudes about recognizing AF and help-seeking behaviors for AF symptoms (response from 1 = not at all sure to 4 = very sure); and nine-item beliefs about AF and symptoms (response from 1 = strongly disagree to 4 = strongly agree). Content validity of the KABAFS was evaluated by four advanced-practice registered nurses (RNs) with expertise in AF care and five research experts in the field of treatment-seeking delay. Cognitive interviews with six mock participants were conducted to evaluate the content validity of items and assure items were interpreted as intended. Items of the KABAFS were revised according to experts’ and mock participants’ suggestions. Cronbach’s alpha coefficients of reliabilities for the subscales of this newly developed instrument were the following: AF symptom knowledge, .78; knowledge of symptoms not related to AF, .70; general knowledge about AF, .42; attitudes .72; and beliefs, .69.
For this study, the KABAFS was administered by interview to minimize missing data that may occur with the use of mailed surveys. The subscales AF symptom knowledge and the knowledge of symptoms not related to AF were combined to produce a single symptom knowledge score. General Knowledge, Attitudes, and Beliefs subscales were scored separately. Higher scores reflected knowledge, attitudes, or beliefs more favorable to early treatment-seeking behavior.
Feasibility
Data were collected on the number of records screened, number of patients excluded and the reason for exclusion, number of eligible patients, number of patients who declined, number of patients unable to contact, attrition, reason for attrition, number of data collections completed, number of pulse logs returned, and percentage of total possible pulse checks completed. An intervention script and a protocol fidelity checklist were used to evaluate audio recordings of selected intervention sessions. Average time to deliver content was recorded for the intervention group and the attention control group.
Acceptability
A seven-item study participation acceptability rating survey was used to measure participants’ opinions about the value and burden of study participation. Participants received the survey by mail within 1 week of study completion and returned it in the provided self-addressed stamped envelope. Participants rated the burden, effort, and value of study participation on a 5-point scale (from 1 = strongly disagree to 5 = strongly agree). Intervention participants were asked to respond to items related to the value, time burden, and difficulty of daily pulse palpation.
Conceptual Model
The Alert for AFib intervention, designed by the coauthors, was guided by Leventhal’s Common Sense Model. The components of the intervention and link to concepts of the Common Sense Model are illustrated in Figure 1 (Leventhal et al., 2012). Leventhal et al. (2012) proposed that when persons have a symptom or symptoms, they have perceptions about the symptom’s cause, seriousness, timeline (intermittent vs. chronic), and controllability that form the schema of or an explanation for the symptoms. The schema evolving from the perceptions drives the strategies to manage the symptoms (Leventhal et al., 2012). Previous research findings suggest that inability to develop an accurate schema for AF symptoms was associated with treatment-seeking delay (McCabe, Rhudy, et al., 2015). Investigators discovered that lack of knowledge about AF and its consequences, lack of awareness about personal risk of AF, and inability to recognize the seriousness of AF symptoms were related to delay in seeking treatment of the symptoms (McCabe, Rhudy, et al., 2015; McCabe, Rhudy, & DeVon, 2015).
Figure 1.
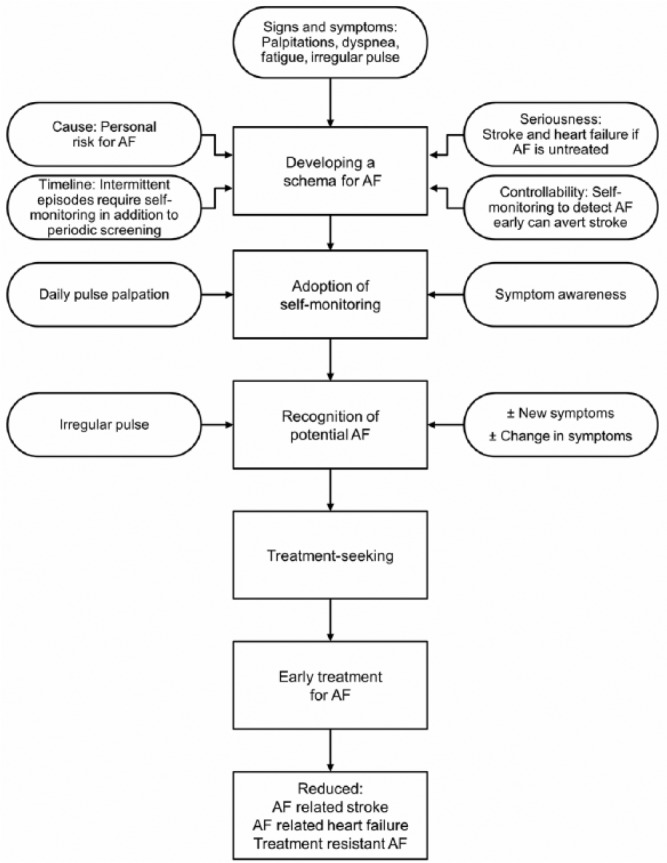
Conceptual model for the Alert for AF Intervention.
Note. AF = atrial fibrillation.
The Alert for AFib intervention
Intervention components
The cognitive portion of the Alert for AFib intervention was designed to create an accurate schema for AF by improving participants’ knowledge about AF and its symptoms and promoting accurate beliefs about AF (Figure 1). Major components of the intervention included information about AF and its consequences when not treated; personal risks for developing AF; recognition of AF symptoms; palpation of radial pulse or auscultation of apical pulse with a stethoscope; recognition of an irregular pulse; and an action plan for responding to symptoms of AF or an irregular pulse. The content was presented using a handheld paper flip chart and a 5-min video produced by the medical center.
The flip chart content, created by the authors was written for a sixth-grade reading level with only 4 to 5 points per page and illustrations or graphs as appropriate to the content. Pages included content about (a) a simple explanation of AF; (b) importance of treating AF (preventing stroke and heart failure); (c) risks for developing AF with emphasis on the participant’s own risks such as hypertension, diabetes, obstructive sleep apnea, and obesity; (d) recognition of AF symptoms, differentiating AF symptoms from symptoms of other medical conditions, and emphasis that AF may not be accompanied by symptoms; and (e) action planning for self-monitoring and seeking treatment for signs and symptoms of AF. The video included information about AF, with animation comparing normal cardiac electrical conduction with abnormal conduction in AF, AF symptoms, and how untreated AF can lead to stroke and heart failure. To increase participants’ awareness of their risk of AF, the investigators invited them to enter their personal risks into an online calculator that produced an estimate of low, medium, or high risk of AF (Heart Rhythm Society AFib Risk Assessment, 2015).
The Alert for AFib behavioral component was modeled from the intervention reported by Munschauer et al. (2004), in which participants received group education for pulse palpation and recognition of an irregular pulse and were asked to palpate their pulse once per month. Our intervention was delivered individually, and participants were asked to palpate their pulses and record their pulse rhythms daily. We also provided a face-to-face individualized follow-up session 2 weeks after the baseline session, to assess participant adherence and the challenges to performing daily pulse palpation.
Prestudy test of intervention protocol
We performed a test of the intervention protocol with six volunteers before enrolling study participants. The volunteers evaluated relevance and clarity of session content, approach to content delivery, time required for the session, effectiveness of pulse palpation instruction, readability and value of handouts, and overall perceived benefits. The six volunteers expressed high satisfaction with the content and the approach of intervention, believed it provided new and meaningful information, was delivered within an acceptable time frame, and reported that handout material was helpful and easy to understand. On the basis of feedback from some volunteers, we removed the presentation components that were perceived as unclear and redundant and made minor changes to several KABAFS items.
Intervention procedure
The science of behavior change and habit formation suggests considerable variability in the time required to build a habit (Lally, van Jaarsveld, Potts, & Wardle, 2010) such as daily pulse palpation. To make sure that we were delivering an intervention that was consistent with the National Institutes of Health behavior change guidelines (Bellg et al., 2004) which requires time to demonstrate participant receipt and enactment of the intervention, we chose an 8-week intervention time frame. We chose this time frame to balance fidelity to treatment with participant burden and cost of conducting the study.
The intervention group participated as individuals in a face-to-face, 45-min interactive session delivered by one of two RNs who had no clinical relationship with the participants (one Bachelor’s prepared RN and one clinical nurse specialist, henceforth both are referred to as RNs) trained to follow a specific protocol regarding the intervention’s content and approach. The intervention was delivered in a private room in the patient education center of the medical center. Family members of participants were welcomed to attend but were asked not to respond to questions. The session was conducted in a dialog format using an Ask-Tell-Ask approach (Boxer & Snyder, 2009).
At the beginning of each component, participants were asked to share their perceptions about the topic. They were asked, for example, “What have you heard about AF?”; “What are your thoughts about stroke?”; “What medical conditions do you have that you think increase your chance of getting AF?”; and “How do you feel about taking your pulse?” The discussion then was tailored to the participant’s level of knowledge and the accuracy of perceptions. To assess participant comprehension of the content for each component, the RN asked participants to tell in their own words what they thought were the important points to remember and reinforced the content as needed.
For the pulse palpation instruction, the RN described an irregular pulse, differentiated between a regular pulse and an irregular one, and instructed how an irregular pulse could signify AF and discussed the benefit of regular pulse palpation to identify an irregular pulse. The RN used illustration and demonstration to instruct participants in radial pulse palpation. Participants were then asked to perform a return demonstration to assess their technique. The goal was to identify the pulse rhythm as regular or irregular, not to count the rate. If participants were unable to palpate the pulse, they received a stethoscope for home use and the RN instructed them in use of a stethoscope to auscultate the apical pulse. The RN validated the participant’s ability to auscultate the apical pulse accurately by placing her stethoscope in an adjacent location to auscultate simultaneously with the participant and both counted the heartbeat for 30 s. To validate ability to palpate the radial pulse, both the RN and the participant palpated the pulse simultaneously on each wrist and both counted for 30 s. Ability to palpate the pulse or auscultate the heartbeat was considered satisfactory when the participant’s count was within five beats of the RN’s count. To aid recognition of an irregular pulse, participants listened to an audio recording created by the institution’s media services that produced sounds similar to an irregular and regular heartbeat. Participants were asked to identify the irregular heartbeat.
After demonstrating satisfactory pulse palpation or auscultation technique and ability to differentiate an irregular from a regular heartbeat recording, participants were instructed on how to complete the pulse-checking log and were asked to log the rhythm (regular vs. irregular) daily for 2 months. The RN asked participants to rate their self-confidence in their ability to palpate their pulse, to perform palpation or auscultation daily, and to identify barriers to performance and strategies to overcome barriers. To promote habit formation, participants were encouraged to perform the pulse palpation at the same time each day and combine it with another routine activity (Gardner, Lally, & Wardle, 2012).
For home use and reinforcement of content covered in the face-to-face session, participants were given printed resources. These included a two-page handout on AF, published by the American College of Cardiology (2015) that described AF, listed symptoms, discussed causes and consequences of AF, and briefly described treatment options and an institutionally produced handout describing signs and symptoms of stroke and seeking care for stroke. Participants were given a pulse-checking log that was produced by the investigators for recording the recording the daily pulse palpation (regular or irregular) over the 2-month study period. The log contained an illustration and instructions for palpating the pulse and advice to notify their health care provider when they noted an irregular pulse.
Two weeks after study enrollment, intervention participants returned to the center for an individualized, 15- to 20-min, face-to-face follow-up session to assess their comprehension of content covered in the initial session, to answer participant questions, to review the pulse log, and to address any challenges that had arisen related to pulse palpation or auscultation. Content from the initial session was reinforced as needed, and participants were commended for pulse-checking performance.
Intervention fidelity
To ensure intervention fidelity, the investigators created a script to guide the open-ended questions asked of participants, content presentation in each page of the flip chart, introduction to the video and risk assessment, instruction regarding pulse palpation/auscultation, and recognition of irregular pulse. The script included an approximate time frame for each component. The investigators developed a checklist that contained elements of the intervention to evaluate the RNs’ adherence to the protocol. In addition to training for content of the protocol, the RNs were trained in the Ask-Tell-Ask approach (Boxer & Snyder, 2009). Before enrolling participants, the two RNs who delivered the interventions were observed by members of the research team to ensure consistency in their approach of content delivery and their adherence to the protocol. During the study, team members Drs.Vickers Douglas and Barton evaluated the fidelity of selected, audio recorded sessions for adherence to the script and approach to intervention delivery. Regular meetings with the RNs were held to evaluate participants’ responses to the intervention and to address any challenges of adherence to protocol.
Attention control session
After the baseline interview to collect KABAFS data, a study coordinator (not the RN providing the intervention) delivered an individual face-to-face, 45-min interactive session about healthy sleep to the control group to control for the influence of time and attention. This session was designed to be similar to the intervention in terms of the dialog approach and media used to present the content. The session included a video presentation on the importance of sleep, assessment of perceptions about sleep, and use of a handheld paper flip chart to present strategies to promote healthy sleep. Control participants did not receive a follow-up session. At the end of the study, they were given the option to receive the same AF education materials provided for the intervention group.
Procedures
The study was approved by the institutional review board of the study site. Medical records of patients from primary care and cardiology clinics who gave permission to review their records for research purposes were screened for eligibility. Invitation letters containing information about the study and participation requirements were sent to eligible patients. Letters contained an interest page where patients could decline to be contacted about the study or indicate an interest in hearing more about the study. Patients were instructed to return the interest page with their response in the self-addressed stamped envelope. The study coordinator called the interested patients to explain the study eligibility requirements and arrange a visit to the study site. Staff reinforced information about the study and obtained written consent at the in-person visit.
After providing written consent, participants were randomly assigned to the intervention group or the control group through the randomization function in Excel software (Microsoft). The KABAFS was administered during face-to-face interview before beginning the Alert for AFib or the healthy sleep content. At the close of the intervention session, participants were scheduled to return in 2 weeks for a face-to-face follow-up session with the RN who conducted their initial session. The study coordinators administered the KABAFS by telephone interview to both intervention and control participants at 1 and 2 months after enrollment. During the 1- and 2-month data collection interviews for intervention participants, the study coordinators also inquired about any challenges to pulse palpation and asked participants to report any missed days of pulse palpation. On completion of the study, the Study Participation Acceptance Rating Survey was mailed to all participants and returned by mail in a provided self-addressed stamped envelope. Intervention participants were asked to return their pulse log by mail. All participants received US$25 remuneration for each visit to the study site.
Data Analysis
Frequencies and percentages were used to describe feasibility and acceptability outcomes, as well as demographic and clinical characteristics of participants. Feasibility was supported if (a) recruitment of the target number of participants (n = 80) was accomplished within 12 months of beginning the study, (b) 90% of intervention participants attended the 2-week follow-up session, (c) attrition was ≤10%; (d) the intervention could be delivered within 60 min, (e) fidelity to the intervention protocol was maintained at 90%, and (f) at least 80% of intervention participants returned their pulse log with 80% of all possible pulse checks recorded. The intervention was considered acceptable to intervention participants when >80% reported low burden and ≥80% rated the intervention moderately to extremely helpful. Change in mean scores from baseline to study completion for symptom knowledge, AF knowledge, attitudes, and beliefs were analyzed by independent t tests to explore effect sizes for a future multisite effectiveness trial. For the purposes of exploring the responsiveness of the KABAFS to measure change in knowledge, attitudes, and beliefs about AF prior to and after the intervention, paired t tests to compare baseline with 2-month KABAFS scores of the intervention group were performed. Significance was set at p ≤ .05 (two-sided). Data were analyzed using IBM SPSS for Windows (Version 21).
Results
Sample
The patient sample was 63% male (n = 50) with mean (SD) age of 71.8 (9.8) years (range = 65-90 years). The sample contained 96% White participants, and 54% held a 4-year college degree or a graduate degree. The most common risks of AF were hypertension, diabetes, and coronary artery disease. No significant differences were found between intervention and control groups in demographic or clinical characteristics (Table 1).
Table 1.
Demographic and Clinical Characteristics of Participants (N = 80).
| Control (n = 40) n (%) | Intervention (n = 40) n (%) | p | χ2 | |
|---|---|---|---|---|
| Sex | .64 | 0.21 | ||
| Male | 24 (60) | 26 (65) | ||
| Female | 16 (40) | 14 (35) | ||
| Race | 1.00 | a | ||
| White | 39 (98) | 38 (95) | ||
| Black | 1 (2) | 2 (5) | ||
| Education | .71 | 2.90 | ||
| ≤High school | 7 (18) | 9 (23) | ||
| Some college/vocational | 9 (23) | 11 (28) | ||
| 4-year college | 10 (25) | 10 (25) | ||
| Graduate school | 13 (29) | 10 (25) | ||
| Lives with someone | 29 (73) | 34 (85) | .17 | 1.90 |
| Comorbidity | ||||
| Hypertension | 34 (85) | 33 (83) | .76 | 0.09 |
| Diabetes mellitus | 18 (45) | 17 (43) | .82 | 05 |
| Coronary artery disease | 12 (30) | 18 (45) | .16 | 1.90 |
| Obstructive sleep apnea | 12 (30) | 12 (30) | >.99 | 0.00 |
| Obesity | 11 (28) | 11 (28) | >.99 | 0.00 |
Fisher’s exact test.
Feasibility Outcomes
Recruitment
Of 1,247 records screened, 680 patients (55%) were excluded, most commonly because of an existing or prior diagnosis of AF or having no medical risk factors for AF. When interested patients were contacted, a secondary screening eliminated an additional 52 patients who did not meet eligibility criteria. The response rate to invitations was 15% (Figure 2), yet with the large pool of available patients, we were able to recruit 80 participants within 6 months, well ahead of the target of 12 months.
Figure 2.
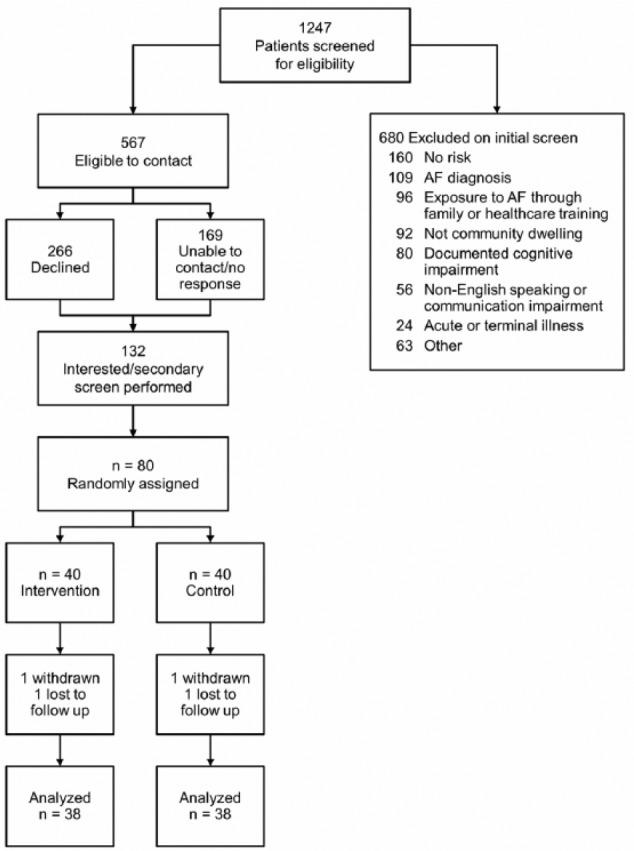
Flow diagram of screening, enrollment, and retention of study participants.
Note. AF = atrial fibrillation.
Attrition
One participant from the control group died, and another was withdrawn from the intervention group because of unanticipated surgery. One control and one intervention participant were lost to follow-up after the 1-month data collection. One intervention participant did not complete the 1-month data collection but did complete the 2-month collection.
Missing data
Using the interview method to administer the KABAFS resulted in a low rate of missing data. Complete data were available for baseline, 1- and 2-month data collection points for 37 (93%) of the intervention group and 38 (95%) of the control group.
Adherence to study protocol
Only one intervention participant did not attend the 2-week intervention booster session. The pulse log was returned by 87% of the intervention group, and all but two participants completed ≥88% of the possible daily entries for the 2 months following study enrollment.
Participant burden, effort, and value of participation
Intervention and control participants alike reported that the burden and effort required to participate in the study were low. Intervention participants rated the Alert for AFib intervention as very helpful (49%) to extremely helpful (35%), and 87% reported that daily pulse taking was easy and worthwhile (Table 2). On completion of the study, a majority (89%) of control group participants opted to receive printed material about AF that was provided to intervention participants.
Table 2.
Participant Study Burden and Satisfaction Perceptions.
| Perception | Intervention (n = 37), n (%) | Control (n = 34), n (%) |
|---|---|---|
| The amount of burden required to participate | ||
| Very burdensome | 0 (0) | 0 (0) |
| Somewhat burdensome | 2 (5) | 5 (15) |
| Not at all burdensome | 35 (95) | 29 (85) |
| The effort required to participate | ||
| Too much effort | 0 (0) | 0 (0) |
| Some effort | 1 (3) | 3 (9) |
| Not much effort | 18 (49) | 16 (47) |
| No effort at all | 18 (49) | 15 (44) |
| How helpful was the Alert for AFib educational program? | ||
| Not helpful at all | 0 (0) | NA |
| Mostly not helpful | 0 (0) | NA |
| Moderately helpful | 6 (16) | NA |
| Very helpful | 18 (49) | NA |
| Extremely helpful | 13 (35) | NA |
Note. AFib = atrial fibrillation.
Intervention fidelity
Audio recordings of selected sessions were evaluated by Dr. Vickers Douglas and Barton of the research team. Fidelity to the intervention was high, and both RNs demonstrated >95% adherence to the fidelity checklist.
Determination of Effect Size
Because the purpose of this study was to test the feasibility of delivering the Alert for AFib intervention, the study was not powered to detect statistically significant differences in KABAFS scores between groups from baseline to study completion. However, to inform decisions regarding sample size for an efficacy trial, we sought to explore the effect of exposure to the intervention on KABAFS scores. Mean change difference between baseline scores and 2-month scores of the intervention and control groups were analyzed through independent t tests, and the results were used to determine effect sizes. The range of effect sizes was from 0.4 for the Beliefs subscale to 1.2 for the Attitudes subscale (Table 3).
Table 3.
Comparison of Mean Difference in KABAFS Scores From Baseline to Study Completion by Group.
| KABAFS Subscale | Control (n = 38) | Intervention (n = 38) | Mean Difference | t | 95% CI | p | d |
|---|---|---|---|---|---|---|---|
| Symptom knowledge | 0.71 (3.5) | 3.03 (3.4) | 2.32 | 2.91 | [0.73, 3.90] | .005 | 0.67 |
| Knowledge | 0.66 (1.3) | 1.29 (1.1) | 0.632 | 2.23 | [0.07, 1.19] | .03 | 0.52 |
| Attitude | 1.97 (2.8) | 6.45 (3.5) | 4.47 | 6.16 | [3.02, 5.92] | <.001 | 1.40 |
| Beliefs | 0.08 (3.3) | 1.34 (2.9) | 1.26 | 1.77 | [−0.16, 2.69] | .08 | 0.41 |
Note. KABAFS = Knowledge, Attitudes, and Beliefs about Atrial Fibrillation Survey; CI = confidence interval.
KABAFS Scores From Baseline to 2 Months for Intervention Group
Change scores of the intervention group from baseline to 2 months following the intervention were analyzed to determine responsiveness to the KABAFS. The analysis revealed significant changes in all subscale scores for the intervention group between baseline and 2 months (Table 4). Except for the Knowledge subscale (M = 0.27, SD = 0.65; t = 2.5; p = .02), no significant changes were found in KABAFS subscale scores between 1 and 2 months.
Table 4.
Comparison of KABAFS Scores From Baseline to Study Completion for the Participants in the Intervention Group (n = 38).
| KABAFS Subscale | Baseline M (SD) |
2-month M (SD) |
t | 95% CI | p | d |
|---|---|---|---|---|---|---|
| Symptom knowledgea | 7.76 (2.95) | 10.76 (2.08) | 5.57 | [1.90, 4.09] | <.001 | 1.20 |
| AF knowledgeb | 3.07 (1.22) | 4.37 (.79) | 6.99 | [0.92, 1.66] | <.001 | 1.20 |
| Attitudesc | 9.47 (2.9) | 15.92 (2.29) | 11.46 | [5.31, 7.58] | <.001 | 2.50 |
| Beliefsd | 24.66 (2.71) | 26.00 (2.48) | 2.83 | [0.38, 2.30] | .008 | 0.52 |
Note. Higher scores reflect knowledge, attitudes, and beliefs more favorable to early treatment-seeking. KABAFS = Knowledge, Attitudes, and Beliefs about Atrial Fibrillation Survey; CI = confidence interval; AF = atrial fibrillation.
Possible score range = 0-14.
Possible score range = 0-5.
Possible score range = 5-20.
Possible score range = 9-36.
Discussion
The Alert for AFib intervention was feasible to deliver in a cohort of older adults recruited from primary care and cardiac clinics. The majority of participants completed the study and were adherent to study protocol. Participants perceived the study burden to be low, despite the required visit to the study site and performance of daily pulse palpation and recording. The majority of intervention participants reported that the Alert for AFib intervention was very to extremely helpful. Intervention participants reported that pulse palpation was easy to do, worth the time and effort, were confident that they could do it well, and that they would be likely to very likely to continue pulse palpation after study completion.
Study findings add to the body of knowledge about older adults’ willingness to participate in studies to improve treatment-seeking behavior through self-monitoring for AF signs and symptoms and adoption of daily pulse palpation. The present study’s recruitment rate of 15% was lower than the 23% and 33% rates reported by Benito et al. (2015) and Virtanen et al. (2014), respectively. The time commitment of 45 min and an additional visit to the medical center may have limited interest in participating in the study. The 93% retention rate for intervention participants reflects the effectiveness of the training and protocols and the participant interest. Participant retention during the 2-month study was consistent with the 90% and 93% retention rates of Benito et al. (2015) and Virtanen et al. (2014), respectively.
Results of the present investigation support prior findings about older adults’ ability to perform pulse palpation and recognize an irregular pulse. Kallmunzer et al. (2014) reported that pulse palpation to detect AF by older adults was performed with a sensitivity of 54% and a specificity of 96%. One month after an educational session, 81% of participants in the study by Virtanen et al. (2014) were capable of pulse palpation and recognition of irregular heartbeat. All intervention participants in the present study were able to either palpate or auscultate their pulse, to differentiate an audio recording of an irregular heartbeat from a regular heartbeat, and to describe the characteristics of an irregular pulse.
Older adults’ motivation to perform pulse palpation is further demonstrated in the finding that 87% of intervention participants returned their 2-month pulse-checking log and all but two of those 87% completed >88% of possible checks that could be recorded. In comparison, Virtanen et al. (2014) observed that 1 month after enrollment, 82% of participants completed 80% of their pulse diary. Munschauer et al. (2004) did not ask participants to record pulse checks, but when participants were asked whether they had checked their pulse following their education session, 70% stated they had. Participants of the Munschauer study and the study by Benito et al. (2015) were instructed to palpate their pulses once a month; in comparison, participants in the Virtanen et al. (2014) study palpated their pulses twice a day and participants in the present study palpated their pulses daily.
The present study’s intervention session was longer (45 min) than the 10 min reported by Virtanen et al. (2014) and Benito et al. (2015). It was difficult to determine what approach was used in previous studies, but the sessions focused on pulse palpation. The Ask-Tell-Ask approach (Boxer & Snyder, 2009) used in the present study is likely to require more time because the participant is actively engaged in the dialog. In this study, instructing the participant on pulse palpation and validating performance often required >5 min for participants unfamiliar with pulse palpation. The content and approach of the Alert for AFib intervention may have contributed to the observed adherence rate of 87% over 2 months compared with 82% observed by Virtanen et al. (2014) over 1 month.
Few, if any, investigators have conducted randomized controlled trials to evaluate the influence of a cognitive behavioral intervention on knowledge, attitudes, beliefs, and behaviors to promote early detection of AF. Although Benito et al. (2015) randomly assigned participants to receive instruction about pulse palpation and recognition of warning signs, the outcome of interest was the number of new AF diagnoses, not adherence to pulse palpation or changing knowledge of AF symptoms or attitudes, and beliefs about AF that support early treatment-seeking. We observed that scores for knowledge of symptoms, knowledge of AF, and attitudes toward seeking treatment in the intervention group improved to a greater degree than the control group in this cohort of older adults.
Sampling bias is a limitation to our study. This feasibility study was conducted at a single site in the Midwestern United States and enrolled patients who had given prior consent to be contacted for research purposes. Our study likely included participants who value research and may have been economically advantaged. Therefore, responses on the KABAFS may be biased in favor of healthy attitudes and beliefs. The fact that only 15% of eligible participants enrolled in the study introduces additional sampling bias, and we do not know whether individual characteristics varied between participants and non-participants. Our findings are only generalizable to older, White adults. Control group participants (38%) reported that they sought information about AF from Internet resources or their health care provider following enrollment in the study which may have influenced their responses on the 1- and 2-month KABAFS scores. Our findings may also be limited by using the new KABAFS that was evaluated for content validity, but not construct validity at the time it was administered to participants. The responsiveness of the KABAFS to change in knowledge, attitudes, and beliefs after the Alert for AFib intervention was demonstrated.
Additional investigations in larger and more diverse samples are needed to determine whether our findings can be replicated in populations that vary by race, ethnicity, sex, sexual identity, age, disability, socioeconomic status, and geographic location (U.S. Department of Health and Human Services, 2014). Prior research has demonstrated that social, economic, and environmental disadvantage are associated with risk for cardiovascular disease (Yancy et al., 2011).Testing of the Alert for AFib intervention in diverse populations may help to reduce patterns of known health disparities in AF (Naderi, Rodrguez, Wang, & Foody, 2014).
The Alert for AFib intervention is a theoretically and empirically based intervention that was pilot tested using a rigorously controlled protocol. The Alert for AFib intervention was feasible to deliver and was acceptable to older, well-educated White adults in an academic medical center setting. Results of this feasibility study suggest that the efficacy of this intervention can be tested in a large sample across multiple sites.
Acknowledgments
The authors wish to acknowledge Wendi Lytle, M Ed for assistance with manuscript preparation
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number K23NR0114253. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- American College of Cardiology. (2015). CardioSmart—Atrial fibrillation. Retrieved from https://www.cardiosmart.org/heart-conditions/atrial-fibrillation
- Ball J., Carrington M. J., McMurray J. J., Stewart S. (2013). Atrial fibrillation: Profile and burden of an evolving epidemic in the 21st century. International Journal of Cardiology, 167, 1807-1824. doi: 10.1016/j.ijcard.2012.12.093 [DOI] [PubMed] [Google Scholar]
- Bellg A. J., Resnick B., Minicucci D. S., Ogedegbe G., Ernst D., Borelli B., . . . Czajkowski S. (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH behavior change consortium. Health Psychology, 23, 443-451. [DOI] [PubMed] [Google Scholar]
- Benito L., Coll-Vinent B., Gomez E., Marti D., Mitjavila J., Torres F., . . . Mont L. (2015). EARLY: A pilot study on early diagnosis of atrial fibrillation in a primary healthcare centre. Europace. Advance online publication. doi: 10.1093/europace/euv146 [DOI] [PubMed] [Google Scholar]
- Benjamin E. J., Chen P. S., Bild D. E., Mascette A. M., Albert C. M., Alonso A., . . . Wyse D. G. (2009). Prevention of atrial fibrillation: Report from a National Heart, Lung, and Blood Institute Workshop. Circulation, 119, 606-618. doi: 10.1161/CIRCULATIONAHA.108.825380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer H., Snyder S. (2009). Communication strategies to promote self-management of chronic illness. Family Practice Management, 16(5), 12-16. [PubMed] [Google Scholar]
- Camm A. J., Lip G. Y., De Caterina R., Savelieva I., Atar D., Hohnloser S. H., . . . Kirchhof P. (2012). 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation. European Heart Journal, 33, 2719-2747. [DOI] [PubMed] [Google Scholar]
- Chugh S. S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E. J., . . . Murray C. J. (2014). Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation, 129, 837-847. doi: 10.1161/circulationaha.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio F. G., Aliot E., Luca Botto G., Heidbuchel H., Geller C., Kirchhof P., . . . Crijns H. (2008). Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: Reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace, 10, 21-27. [DOI] [PubMed] [Google Scholar]
- Gardner B., Lally P., Wardle J. (2012). Making health habitual: The psychology of “habit-formation” and general practice. British Journal of General Practice, 62, 664-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart Rhythm Society AFib Risk Assessment. (2015). Available from http://www.afibrisk.org/
- January C. T., Wann L. S., Alpert J. S., Calkins H., Cleveland J. C., Jr., Cigarroa J. E., . . . Yancy C. W. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation, 130, 2071-2104. doi: 10.1161/cir.0000000000000040 [DOI] [PubMed] [Google Scholar]
- Kallmunzer B., Bobinger T., Kahl N., Kopp M., Kurka N., Hilz M., . . . Kohrmann M. (2014). Peripheral pulse measurement after ischemic stroke: A feasibility study. Neurology, 83, 598-603. [DOI] [PubMed] [Google Scholar]
- Kirchhof P. (2009). Can we improve outcomes in AF patients by early therapy? BMC Medicine, 7, Article 72. doi: 10.1186/1741-7015-7-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally P., van Jaarsveld C., Potts J., Wardle J. (2010). How are habits formed: Modeling habit formation in the real world. European Journal of Social Psychology, 40, 998-1009. [Google Scholar]
- Leventhal H., Bodnar-Deren S., Breland J. Y., Hash-Converse J., Phillips L. A., Leventhal E. A. (2012). Modeling health and illness behavior: The approach of the commonsense model. In Baum A., Revenson T. A., Singer J. (Eds.), Handbook of health psychology (2nd ed., pp. 3-25). New York, NY: Psychology Press. [Google Scholar]
- Lowres N., Krass I., Neubeck L., Redfern J., McLachlan A., Bennet A., Freedman S. B. (2015). Atrial fibrillation screening in pharmacies using an iPhone ECG: A qualitative review of implementation. International Journal of Clinical Pharmacy, 37, 111101120. [DOI] [PubMed] [Google Scholar]
- McCabe P. J., Chamberlain A., Rhudy L., DeVon H. A. (2016). Symptom representation and treatment-seeking prior to diagnosis of atrial fibrillation. Western Journal of Nursing Research, 38, 200-215. doi: 10.1177/0193945915570368 [DOI] [PubMed] [Google Scholar]
- McCabe P. J., Rhudy L., Chamberlain A., DeVon H. A. (2015, August 28). Fatigue, dyspnea, and intermittent symptoms are associated with treatment-seeking delay for symptoms of atrial fibrillation before diagnosis. European Journal of Cardiovascular Nursing. Advance online publication. doi: 10.1177/1474515115603901 [DOI] [PubMed] [Google Scholar]
- McCabe P. J., Rhudy L., DeVon H. A. (2015). Patients’ experiences from symptom onset to initial treatment for atrial fibrillation. Journal of Clinical Nursing, 24, 786-796. doi: 10.1111/jocn.12708 [DOI] [PubMed] [Google Scholar]
- Munschauer F. E., Sohocki D., Carrow S., Priore R. L. (2004). A community education program on atrial fibrillation: Implications of pulse self-examination on awareness and behavior. Journal of Stroke & Cerebrovascular Diseases, 13, 208-213. [DOI] [PubMed] [Google Scholar]
- Naderi S., Rodrguez F., Wang Y., Foody J. (2014). Racial disparities in hospitalizations, procedural treatments, and mortality of patients hospitalized with atrial fibrillation. Ethnicity & Disease, 24, 144-149. [PubMed] [Google Scholar]
- Rohrbacker N. J., Kleinman N. L., White S. A., March J. L., Reynolds M. R. (2010). The burden of atrial fibrillation and other cardiac arrhythmias in an employed population: Associated costs, absences, and objective productivity loss. Journal of Occupational and Environmental Medicine, 52, 383-391. [DOI] [PubMed] [Google Scholar]
- Stewart S., Hart C. L., Hole D. J., McMurray J. (2002). A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley Study. American Journal of Medicine, 113, 359-364. [DOI] [PubMed] [Google Scholar]
- Svennberg E., Engdahl J., Al-Khakili F., Friberg L. (2015). Mass screening for untreated atrial fibrillation: The STROKESTOP study. Circulation, 131, 2176-2184. [DOI] [PubMed] [Google Scholar]
- Thrall G., Lane D., Carroll D., Lip G. Y. (2006). Quality of life in patients with atrial fibrillation: A systematic review. American Journal of Medicine, 119, 448.e1-448.e1.19. doi: 10.1016/j.amjmed.2005.10.057 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2014). Healthy People 2020 (Foundation health measures/disparities). Retrieved from https://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities
- Virtanen R., Kryssi V., Vasankari T., Salminen M., Kivela S., Airaksinen J. (2014). Self-detection of atrial fibrillation in an aged population: The LietoAF Study. European Journal of Preventive Cardiology, 21, 1437-1442. [DOI] [PubMed] [Google Scholar]
- Yancy C. W., Wang T., Ventura H., Pina I., Vijayaraghavan K., Ferdinand K., Hall L. (2011). The coalition to reduce racial and ethnic disparities in cardiovascular disease outcomes (credo): Why credo matters to cardiologists. Journal of the American College of Cardiology, 57, 245-252. [DOI] [PubMed] [Google Scholar]


