Abstract
The amphibian chytrid fungi, Batrachochytrium dendrobatidis (Bd) and B. salamandrivorans (Bsal), pose a major threat to amphibian biodiversity. Recent evidence suggests Southeast Asia as a potential cradle for both fungi, which likely resulted in widespread host-pathogen co-existence. We sampled 583 salamanders from 8 species across Vietnam in 55 locations for Bsal and Bd, determined scaled mass index as a proxy for fitness and collected environmental data. Bsal was found within 14 of the 55 habitats (2 of which it was detected in 2013), in 5 salamandrid species, with a prevalence of 2.92%. The globalized pandemic lineage of Bd was found within one pond on one species with a prevalence of 0.69%. Combined with a complete lack of correlation between infection and individual body condition and absence of indication of associated disease, this suggests low level pathogen endemism and Bsal and Bd co-existence with Vietnamese salamandrid populations. Bsal was more widespread than Bd, and occurs at temperatures higher than tolerated by the type strain, suggesting a wider thermal niche than currently known. Therefore, this study provides support for the hypothesis that these chytrid fungi may be endemic to Asia and that species within this region may act as a disease reservoir.
Globalization has led to the emergence of infectious diseases that threaten biodiversity and contribute to the current 6th mass extinction, a recent example being amphibian chytrid fungi: Batrachochytrium dendrobatidis (Bd), which has caused declines and extinctions within Anura, Urodela and Gymnophiona throughout many parts of the world1,2,3,4,5,6,7 and B. salamandrivorans (Bsal), which is causing massive declines in salamander populations in Europe8,9. Bsal appears to be restricted to urodelan hosts, causing erosive skin lesions with subsequent death in diseased individuals, and the type strain has lower thermal growth tolerance limits than its sister species Bd8. A recent screening for Bsal revealed the presence of the fungus during disease outbreaks in wild salamander populations within the Netherlands, Germany and Belgium9, and in captive animals from the UK and Germany10,11. The presence of Bsal in several urodelan species in Thailand, Vietnam and Japan, coupled with clinical infection trials indicating three Asian salamander species were capable of persisting with the infection or clearing the infection completely, gave rise to the hypothesis that Bsal is endemic to eastern Asia and the salamander species inhabiting this area may act as a reservoir for the fungus12. This hypothesis has been proposed for Bd within Asia as well, due to the fact that a high diversity of lineages is found in eastern Asia13,14 and, throughout this region Bd has a low prevalence with no signs of disease outbreak in many amphibian species15,16,17,18,19,20. A reservoir, in relation to disease, has been defined as “a passive host or carrier that harbors pathogenic organisms without injury to itself and serves as a source from which other individuals can be infected”21. In regards to Bsal and Bd, we thus predict suitable reservoirs to sustain the fungus in the absence of mortality and disease events that negatively affect the host populations. Here we conducted the first large scale screening for Bsal, along with Bd, in Southeast Asia to test this hypothesis. In order to do this, we sampled 583 salamanders belonging to 8 urodelan species of the salamandrid genera Paramesotriton and Tylototriton across 55 localities (ponds or streams) in Vietnam to test for the presence of Bsal and Bd. We then looked into associations of pathogen prevalence and load with potentially influential environmental factors and the presence of associated disease.
Results
Across 11 provinces located in northern Vietnam, 44 ponds and 11 streams were sampled and 583 individuals from 8 species, belonging to the genera Paramesotriton and Tylototriton, were sampled for the presence of Bsal and Bd and their body condition determined (Table 1). A total of 17 individuals belonging to 5 species (T. vietnamensis, T. ziegleri, T. asperrimus, P. deloustali and P. sp.) tested positive for B. salamandrivorans resulting in an overall prevalence of 2.92% (95% CI: 1.80%, 4.65%). Two of the three species which did not contain positive individuals had small sample sizes (T. anguliceps (8), T. sp. (17) and P. guanxiensis (54)) and as the prevalence is low within this area more samples need to be gathered to definitively determine if these populations contain infected individuals. The average prevalence of the 14 positive sites was 17.56% (SD ± 25.27, 95% CI: 3.06%, 32.24%). Four individuals of T. ziegleri, in one pond, tested positive for Bd resulting in an overall prevalence of 0.69% (95% CI: 0.20%, 1.83%) and a prevalence of 30.77% (95% CI: 12.35%, 57.96%) within the positive pond. The Bd samples amplified with the global pandemic lineage (BdGPL) probes indicating a BdGPL-like lineage.
Table 1. Bd and Bsal prevalence per province/district, indicating the number of ponds or streams sampled, the species found, the total number of animals sampled, the number of Bd and Bsal positive individuals and the corresponding prevalences.
| Province, District | Sampling locations | Species | Water temperature range (°C) | Animals swabbed | Bsal positive | Bd positive | Bsal prevalence | Bd prevalence |
|---|---|---|---|---|---|---|---|---|
| Bac Giang | 11 Ponds | T. vietnamensis | 22.7–24.8 | 184 | 3 | 0 | 1.6% | 0.0% |
| Qiang Ninh | 2 Ponds | T. vietnamensis | 23.8–24.9 | 11 | 0 | 0 | 0.0% | 0.0% |
| Lang Son | 1 Pond | T. vietnamensis | 31.9 | 12 | 0 | 0 | 0.0% | 0.0% |
| Cao Bang, Bao Lac | 4 Ponds | T. ziegleri | 16.07–22.53 | 30 | 2 | 4 | 6.7% | 13.3% |
| Cao Bang, | 4 Streams | P. guanxiensis | 20.6–22.97 | 54 | 0 | 0 | 0.0% | 0.0% |
| Nguyen Binh | 1 Pond | T. ziegleri | 19.57 | 8 | 0 | 0 | 0.0% | 0.0% |
| Ha Giang, Bac Me | 2 Ponds | T. ziegleri | 18.7–19.7 | 13 | 0 | 0 | 0.0% | 0.0% |
| Ha Giang, Quan Ba | 9 Ponds | T. ziegleri | 16.37–20.73 | 33 | 2 | 0 | 6.1% | 0.0% |
| Ha Giang, Bac Quang | 2 Ponds | T. ziegleri | 21.6–23.37 | 26 | 0 | 0 | 0.0% | 0.0% |
| Lai Chau | 4 Ponds | T. sp. | 18.53–23.9 | 17 | 0 | 0 | 0.0% | 0.0% |
| Son la | 1 | T. anguliceps | 20.6 | 8 | 0 | 0 | 0.0% | 0.0% |
| Hoa Binh | 7 Ponds | T. asperrimus | 20.07–26.43 | 46 | 3 | 0 | 6.5% | 0.0% |
| Bac Kan | 2 Streams | P. deloustali | 21.2–22.47 | 62 | 1 | 0 | 1.6% | 0.0% |
| Lao Cai | 4 Streams | P. sp. | 20.5–22.07 | 61 | 4 | 0 | 6.6% | 0.0% |
| Tam Dao | 1 Stream | P. deloustali | 18.8 | 18 | 2 | 0 | 11.1% | 0.0% |
| Total | 55 | 8 species | 16.37–31.9 | 583 | 17 | 4 | 2.9% | 0.7% |
Bsal infection prevalence did not differ between provinces or species (Fisher’s exact tests, P = 0.34 and P = 0.25, respectively). There were no correlations between infection intensity of Bsal or Bd and scaled mass index (SMI) for any of the eight species (Spearman’s correlations < ±0.18). Elevation, province, species, scaled mass index, water temperature and ambient temperature were not significant predictors of infection intensity for Bsal (Generalized linear model, P > 0.05).
Discussion
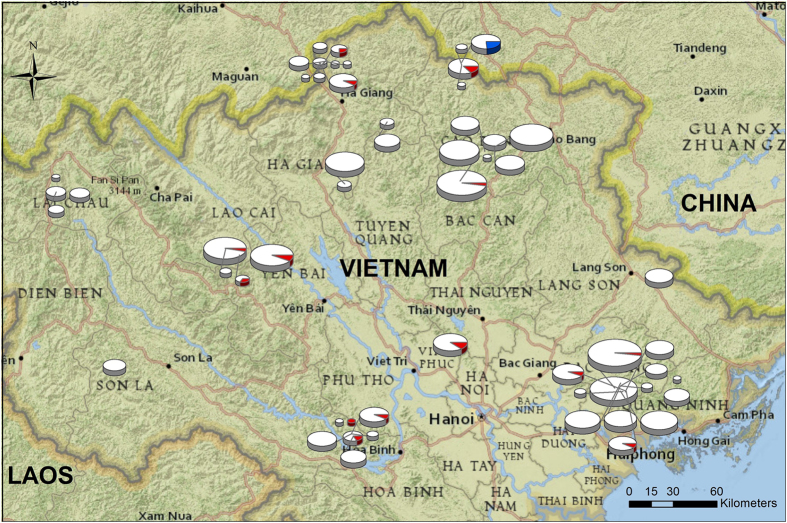
Our study provides the first large scale screening for Batrachochytrium salamandrivorans (Bsal) within Southeast Asia, specifically Vietnam. We found that Bsal was much more widespread throughout salamander populations in Vietnam than its sister species B. dendrobatidis (Bd), occurring in 14 of the 55 locations compared to the one pond in which Bd was detected (Fig. 1), corroborating the predilection of Bsal for urodelan hosts12 and providing further evidence for a Bd prevalence that is low throughout Asia15,16,17,18,19,22. We did not find any signs of Bsal associated disease such as decreased body condition, lesions, or death in the infected populations. The overall low prevalence for both Bsal (2.92%) and Bd (0.69%), despite presence of Bsal in Vietnamese salamander populations at least since 201312, combined with the absence of disease signs indicates endemism of both species of chytrid fungi within Vietnamese salamander populations, adds support to the hypothesis that South East Asian salamanders constitute a Bsal reservoir. Similarly low prevalence but widespread occurrence has been demonstrated for Bd in regions where Bd is considered endemic in the absence of epidemic disease13,23,24. If compensated, co-existence does not exclude an actual cost of Bsal on the Vietnamese salamander populations, as has been demonstrated for Bd23,25, especially given the potential of both pathogens to cause juvenile mortality under experimental conditions12,22.
Figure 1. Map of sampling locations within Northern Vietnam.
The size of the pie charts corresponds to the sample size ranging from 1 to 44 individuals. The pie chart indicates the prevalence of Bsal (Red) or Bd (Blue) within that population. This map was generated in ArcMap 10.1 (http://www.esri.com/software/arcgis/arcgis-for-desktop).
Through the use of lineage specific qPCR, we found that the Bd we detected amplified as global pandemic lineage (BdGPL) indicating it could be the true BdGPL, endemic or non-endemic BdGPL-like, or a hybrid of an endemic and BdGPL cross26. BdGPL is associated with all of the known epizootic events and has experimentally been shown to be more virulent than other lineages27. As Bd seems to occur in an endemic state within Asia and our study site of Vietnam, it is likely an endemic BdGPL-like lineage, further testing should be done to determine the strain as well as the virulence of the Bd within Vietnam.
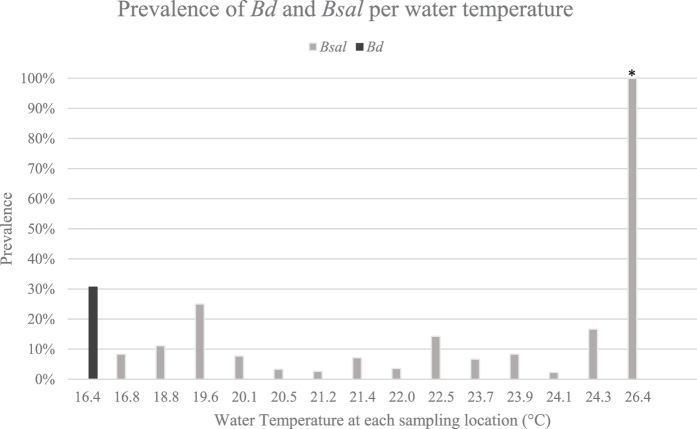
The thermal optimum for the type strain of Bsal is 15–20 °C28, whereas the majority of infected individuals we found in Vietnam were in ponds or streams with water temperatures between 20–25 °C, even reaching 26.43 °C in one positive location (Fig. 2). This might have serious implications as differential thermal preferences of strains could significantly expand the invasive niche if the fungus is vectored into a naive region (Yap et al.29).
Figure 2. Prevalence of Bd and Bsal positive salamanders at each positive location (pond or stream), and its corresponding water temperature.
*Only one individual was sampled at this location.
In conclusion, due to the low prevalence of Bsal and BdGPL and lack of correlation between any of the environmental or physical parameters, we provide evidence supporting the hypothesis that these chytrid fungi may be endemic to Asia and that species within this region may act as a disease reservoir for naive regions. We found that Bsal is much more widespread within northern Vietnam, occurring at temperatures higher than those tolerated by the fungus’ type strain, which suggests a wider thermal niche than currently known. Further screening should be conducted to determine the range of Bsal and which species carry the fungus. These results reinforce the notion that there should be strict biosecurity measures in place, in order to prevent the further spread of Bsal and avoid a global impact similar to that of Bd.
Methods
Study Species
Eight salamander species were sampled for the presence of B. salamandrivorans: Tylototriton vietnamensis (207 individuals), T. ziegleri (110 indv.), T. sp. (17 indv.), T. anguliceps (8 indv.), T. asperrimus (46 indv.), Paramesotriton deloustali (80 indv.), P. guangxiensis (54 indv.) and P. sp. (61 indv.). Nets were utilized to capture salamanders within the stream or pond. Each salamander was weighed, using a digital scale (MS-Series,G&G GmbH), and measured for snout to vent and tail length, using a 0–150 mm digital caliper (Fixpoint). A scaled mass index was calculated following the method developed by Peig and Green30.
Data collection
Since the Bsal type strain has a low tolerance for high environmental temperatures, these were predicted to drive Bsal infection. Therefore, at each pond or stream water temperature and ambient temperature were measured using an HHC201 thermocouple thermometer, with a hermetically sealed thermocouple (OMEGA®). Prevalence and infection loads of Bsal and Bd in the captured amphibians was determined by collecting non-invasive skin swabs29. All methods were carried out in accordance with Vietnamese guidelines and regulations. Experimental procedures were approved and research was carried out under permits obtained for each province of Vietnam through the corresponding provincial government and the Ministry of Agriculture and Rural Development (MARD) (Lang son: 224/SNgV-LS, Bac Giang: 866/UBND-XD, Quang Ninh: 1781/UBND-MT, Cao Bang: 998/UBND-NC, Lai Chau: 663/UBND-NC, Hoa Binh: 1444/VPUBND-TH, Ha Giang: 107/SNgV-NVLS, Bac Kan: 1293/UBND-NV, Vinh Phuc: 104/BTTNVN, Lao Cai: 1887/UBND-NC and Son La: 304/BTTNVN).
Sample processing
DNA was extracted from the swabs using 100 μl of Prepman Ultra DNA extraction buffer. Extracted DNA samples were diluted 1/10 with HPLC water to reduce PCR inhibition31 and then stored at −20 °C until processing. Samples were processed using the B. salamandrivorans and B. dendrobatidis specific duplex real-time PCR procedures described by Blooi et al.32 on CFX96 real-time system (Bio-Rad Laboratories, Hercules, CA). Real-time PCR results (genomic equivalents (GE) of B. salamandrivorans and B. dendrobatidis zoospores) were corrected for the applied dilution factor. Lineage specific qPCR was conducted utilizing Taqman MGB probes that are able to discriminate single nucleotide polymorphisms in the Bd mitochondrial genome which are diagnostic for the globalized pandemic lineage (BdGPL), following the protocol of Bletz et al.26.
Data analysis
The modified Wald method used to calculate the 95% CI for proportion of infected individuals. Fisher exact tests were used to compare prevalence between species and provinces. We examined how GE load was affected by species, SMI, SVL, water temperature and elevation using generalized linear models (Gaussian functions) and spearman correlations in R (version 3.2.3), due to the non-normal distribution of the data.
Additional Information
How to cite this article: Laking, A. et al. Batrachochytrium salamandrivorans is the predominant chytrid fungus in Vietnamese salamanders. Sci. Rep. 7, 44443; doi: 10.1038/srep44443 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This project is supported by (GOF3816N) of the Research Foundation – Flanders (FWO) and Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number FWO.106-NN.2015.02. We are grateful to A. V. Pham, C. T. Pham, D. K. Pham, M. van Schingen, local Vietnamese park rangers and guides for their support in the field. We thank M.T. Nguyen and L.V. Vu, T. Q. Nguyen, for support of our work in Vietnam. S. We thank S. Van Praet for the technical work.
Footnotes
The authors declare no competing financial interests.
Author Contributions A.L., F.P., A.M., H.N.N. and T.T.N. conceived and designed the study. A.L., T.T.N. and H.N.N. coordinated and conducted field sampling. A.L., F.P. and A.M. analysed the results. All authors wrote the manuscript.
References
- Wake D. B. & Vredenburg V. T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 105, 11466–11473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P. et al. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 5, 735–48 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerratt L. F. et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4, 125–134 (2007). [Google Scholar]
- Berger L. et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 95, 9031–9036 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcore J. E., Pessier A. P. & Nichols D. K. Batrachochytrium dendrobatidis gen. et sp. nov., a Chytrid Pathogenic to Amphibians. 91, 219–227 (1999). [Google Scholar]
- Vredenburg V. T., Knapp R. A., Tunstall T. S. & Briggs C. J. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl. Acad. Sci. USA 107, 9689–9694 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A. et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 110, 15325–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzen-van der Sluijs A. et al. Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in europe. Emerg. Infect. Dis. 22, 4–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. A. et al. Emerging disease in UK amphibians. Vet. Rec. 176, 468 (2015). [DOI] [PubMed] [Google Scholar]
- Sabino-Pinto J. et al. First detection of the emerging fungal pathogen Batrachochytrium salamandrivorans in Germany. Amphibia-Reptilia, doi: 10.1163/15685381-00003008 (2015). [DOI] [Google Scholar]
- Martel A. et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346, 630–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille A. et al. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol. Ecol. 22, 4196–4209 (2013). [DOI] [PubMed] [Google Scholar]
- Goka K. et al. Amphibian chytridiomycosis in Japan: Distribution, haplotypes and possible route of entry into Japan. Mol. Ecol. 18, 4757–4774 (2009). [DOI] [PubMed] [Google Scholar]
- Rowley J. J. L. et al. Low prevalence or apparent absence of Batrachochytrium dendrobatidis infection in amphibians from sites in Vietnam and Cambodia. Herpetol. Rev. 44, 466–469 (2013). [Google Scholar]
- Thien T. N. et al. A Survey for Batrachochytrium dendrobatidis in endangered and highly susecptible Vietnamese salamanders (Tylotototrition spp.). J. Zoo Wildl. Med. 44, 627–633 (2013). [DOI] [PubMed] [Google Scholar]
- Swei A. et al. Is chytridiomycosis an emerging infectious disease in Asia? PLoS One 6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C., Liu X., Fisher M. C., Garner T. W. J. & Li Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers. Distrib. 18, 307–318 (2012). [Google Scholar]
- Vörös J., Satasook C., Bates P. & Wangkulangkul S. First record of the amphibian chytrid fungus, Batrachochytrium dendrobatidis in Thailand. Herpetol. Notes 5, 519–521 (2012). [Google Scholar]
- Gilbert M. et al. Amphibian pathogens in Southeast Asian frog trade. Ecohealth 9, 386–398 (2012). [DOI] [PubMed] [Google Scholar]
- Keane M. & O’Toole M. T. Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health. (Saunders, an imprint of Elsevier Inc, 2003). [Google Scholar]
- Thien T. N. et al. A survey for Batrachochytrium dendrobatidis in endangered and highly susceptible Vietnamese salamanders (Tylototriton spp.). J. Zoo Wildl. Med. 44, 627–633 (2013). [DOI] [PubMed] [Google Scholar]
- Spitzen-van der Sluijs A. et al. Environmental determinants of recent endemism of Batrachochytrium dendrobatidis infections in amphibian assemblages in the absence of disease outbreaks. Conserv Biol, doi: 10.1111/cobi.12281 (2014). [DOI] [PubMed] [Google Scholar]
- Bai C., Liu X., Fisher M. C., Garner T. W. J. & Li Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers. Distrib. 18, 307–318 (2012). [Google Scholar]
- Pasmans F. et al. Chytridiomycosis related mortality in a midwife toad (Alytes obstetricans) in Belgium Sterfte door chytridiomycose bij een vroedmeesterpad (Alytes obstetricans) in België. Vlaams Diergeneeskd. Tijdschr. 79, 461–463 (2010). [Google Scholar]
- Bletz M. C. et al. Widespread presence of the pathogenic fungus Batrachochytrium dendrobatidis in wild amphibian communities in Madagascar. PLoS One 10, 1–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer R. A. et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl. Acad. Sci. USA 108, 18732–18736 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blooi M. et al. Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Sci. Rep. 5, 8037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap T. A., Koo M. S., Ambrose R. F., Wake D. B. & Vredenburg V. T. Averting a North American biodiversity crisis. Science 349, 481–482 (2015). [DOI] [PubMed] [Google Scholar]
- Peig J. & Green A. J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 118, 1883–1891 (2009). [Google Scholar]
- Hyatt A. D. et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 73, 175–192 (2007). [DOI] [PubMed] [Google Scholar]
- Blooi M. et al. Duplex real-Time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 51, 4173–4177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]