Abstract
Intracellular calcium [Ca2+]i dysregulation plays an important role in the pathophysiology of iron overload cardiomyopathy. Although either iron chelators or antioxidants provide cardioprotection, a comparison of the efficacy of deferoxamine (DFO), deferiprone (DFP), deferasirox (DFX), N-acetyl cysteine (NAC) or a combination of DFP plus NAC on cardiac [Ca2+]i homeostasis in chronic iron overload has never been investigated. Male Wistar rats were fed with either a normal diet or a high iron (HFe) diet for 4 months. At 2 months, HFe rats were divided into 6 groups and treated with either a vehicle, DFO (25 mg/kg/day), DFP (75 mg/kg/day), DFX (20 mg/kg/day), NAC (100 mg/kg/day), or combined DFP plus NAC. At 4 months, the number of cardiac T-type calcium channels was increased, whereas cardiac sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) was decreased, leading to cardiac iron overload and impaired cardiac [Ca2+]i homeostasis. All pharmacological interventions restored SERCA levels. Although DFO, DFP, DFX or NAC alone shared similar efficacy in improving cardiac [Ca2+]i homeostasis, only DFP + NAC restored cardiac [Ca2+]i homeostasis, leading to restoring left ventricular function in the HFe-fed rats. Thus, the combined DFP + NAC was more effective than any monotherapy in restoring cardiac [Ca2+]i homeostasis, leading to restored myocardial contractility in iron-overloaded rats.
Iron overload cardiomyopathy is the primary cause of death in hereditary hemochromatosis1,2 and transfusion dependent thalassemia (TDT) patients3,4. Non-transferrin bound iron (NTBI) occurs when transferrin becomes saturated and can therefore no longer bind free iron5,6. NTBI is represented in the plasma of iron-overloaded patients when transferrin saturation is more than 70% and which two conditions are associated with iron overload cardiomyopathy in thalassemic patients7,8. In addition, labile plasma iron (LPI) is a redox active, and a labile-chelatable component of NTBI, which is presented in the plasma of TDT and non-transfusion dependent thalassemia (NTDT) patients7,9,10,11. Excess plasma NTBI and LPI leads to iron overload, both systemically and within tissues, resulting in increased reactive oxygen species (ROS) via Haber-Weiss and Fenton’s reactions6,7. Increased ROS is a major cause of tissue and organ damage, particularly in the heart6,12. Plasma NTBI and LPI can rapidly enter cardiomyocytes, resulting in increased free iron or labile cellular iron, which causes increased cardiac oxidative stress and iron overload cardiomyopathy7. L-type calcium (Ca2+) channels (LTCC) and T-type Ca2+ channels (TTCC) are voltage-gated ion channels which are abundantly expressed in the heart, and play an important role in the excitation–contraction coupling of cardiomyocytes13,14. Previous studies found that LTCC and TTCC are major portals for iron entry into the heart in iron overload cardiomyopathy15,16,17,18,19. In addition, TTCC exist and contribute to cardiac electrical activity during the early embryonic state20 and disappear shortly after birth so they cannot be found in adult ventricular cardiomyocytes under physiological conditions21,22. However, TTCC reappear in ventricular myocytes under some pathological conditions such as ventricular hypertrophy23, post myocardial infarction22, and iron overload18,19. Previous studies have demonstrated that an increase in cardiac iron concentration depended on Fe2+ influx through either LTCC or TTCC, leading to abnormal cardiac contractility and cardiac dysfunction under conditions of iron overload15,16,18,19. Increased ROS production mediated by iron overload can directly affect key regulators of excitation-contraction coupling, in particular sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) and/or sodium- Ca2+ exchangers (NCXs), which leads to impaired cardiac intracellular Ca2+ [Ca2+]i transients causing diminished cardiac contractility and heart failure2,24. Additionally, SERCA is very sensitive to oxidative stress leading to protein damage and dysfunction which can be a major cause of increased diastolic Ca2+ level and prolonged Ca2+ decay rate leading to cardiac dysfunction and heart failure under the conditions of iron overload2,25.
There are several iron regulatory proteins which monitor iron metabolism in the heart. Divalent metal transporter 1 (DMT1) has an important role in the uptake of iron into the heart26. However, studies on the function of DMT1 in the iron-overloaded heart have inconsistent findings. A study found that iron overload could suppress the level of cardiac DMT1 protein but it had no significant effect on DMT1 mRNA expression in the heart of the rats27. On the contrary, a few studies found that the level of DMT1 protein did not alter in the hearts of rats26 and thalassemic mice17 under conditions of iron overload. Zrt- and Irt-like protein 14 (Zip14) are abundantly expressed in the liver, pancreas and heart26,28. A previous study found that Zip14 mediated the uptake of zinc and NTBI into the liver29 and it may also mediate the uptake of NTBI into the heart. In addition, hepcidin and ferroportin are iron regulatory proteins which play an important role in iron homeostasis. Previous studies demonstrated that hepcidin and ferroportin are presented in the heart17,30. Ferroportin functions to export iron when iron is too abundant in the heart17,30. On the other hand, hepcidin is a negative regulator of iron metabolism by promoting ferroportin internalization and degradation31,32 leading to reduced cardiomyocyte iron efflux30. Additionally, hepcidin functions in a decreased dietary iron absorption form duodenal enterocyte under iron overload conditions leading to iron homeostasis33,34.
At the present time, three frequently used iron chelators; subcutaneous deferoxamine (DFO), oral deferiprone (DFP) and oral deferasirox (DFX), are used to treat patients with iron overload cardiomyopathy35,36,37. Nevertheless, a head to head comparison of the comparative therapeutic effects between DFO, DFP or DFX on cardiac [Ca2+]i homeostasis as well as calcium cycling and iron regulatory proteins in iron-overloaded rats has never been investigated. In addition, growing evidence indicates that N-acetyl cysteine (NAC) has an iron chelating property38, and is a potent antioxidant which is used to treat children with β-thalassemia39. Moreover, our recent study also found that combined DFP plus NAC had synergistic beneficial effects in restoring function to the brain40,41 and heart42 in iron-overloaded rats. However, the effects of combining an oral iron chelator DFP and an antioxidant NAC on cardiac [Ca2+]i homeostasis as well as the impact of this treatment on calcium cycling and iron regulatory proteins in iron-overloaded rats have never been investigated. The aim of this study was to investigate the hypothesis that DFO, DFP, DFX or NAC alone can improve cardiac [Ca2+]i homeostasis and left ventricular (LV) function in iron-overloaded rats, as well as whether the combined DFP plus NAC treatment can synergistically provide beneficial effects for these conditions.
Results
Effects of the pharmacological interventions on plasma NTBI and MDA levels, and cardiac iron and MDA concentrations
Plasma NTBI level was significantly increased in the HFeV rats when compared with the NDV rats, indicating that an iron overload condition occurred in the HFe-fed rats (Supplementary Table 1). On the other hand, plasma NTBI level was significantly decreased in iron-overloaded rats treated with DFO, DFP, DFX, NAC and combined DFP plus NAC treatment for 2 months (Supplementary Table 1). In consistency with plasma NTBI level, plasma MDA level was significantly increased in the HFeV rats, when compared with the NDV rats, indicating that plasma oxidative stress occurred in the HFe-fed rats (Supplementary Table 1). All pharmacological interventions significantly decreased plasma MDA level in iron-overloaded rats after 2 months of treatment. In addition, the cardiac iron status showed that cardiac iron concentration was significantly increased in the HFeV rats when compared with the NDV rats (Supplementary Table 1). DFO, DFP or DFX alone decreased cardiac iron concentration, and NAC exerted iron chelating properties as it also lowered cardiac iron concentration in iron-overloaded rats (Supplementary Table 1). However, only combined DFP plus NAC significantly decreased cardiac iron concentration to a normal level as shown in the NDV rats (Supplementary Table 1). Also, cardiac MDA concentration was significantly increased in the HFeV rats when compared with the NDV rats (Supplementary Table 1). Although monotherapy alone could reduce cardiac MDA concentration, combined DFP plus NAC reduced cardiac MDA concentration to a normal level as shown in the NDV rats (Supplementary Table 1). These results suggest that a combination therapy of DFP and NAC can effectively restore cardiac iron concentration and oxidative stress in iron-overloaded rats.
Effects of the pharmacological interventions on cardiac function
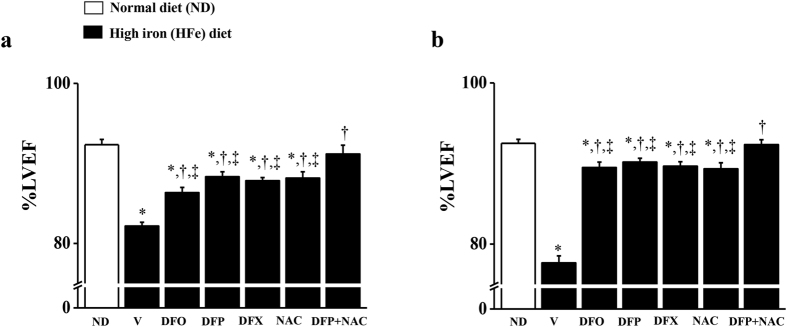
Chronic iron overload led to cardiac dysfunction in iron-overloaded rats. The percentage of LV ejection fraction (%LVEF) decreased markedly in the HFeV rats at 3 months (Fig. 1a), and 4 months (Fig. 1b) when compared with the NDV rats at 3 and 4 months, respectively. At 3 months (after 1 month of treatment with all pharmacological interventions), DFO, DFP, DFX or NAC monotherapy significantly increased %LVEF in the HFe-fed rats, while only the combination therapy of DFP and NAC led to significant increases in %LVEF, attaining a normal level similar to the NDV rats (Fig. 1a). Consistently, %LVEF was similar after 2 months of treatment (at the end of the experiment) with all pharmacological interventions in iron-overloaded rats (Fig. 1b). The results suggest that combined DFP plus NAC can effectively restore cardiac function in iron-overloaded rats which exerts greater cardioprotective effects than either DFO, DFP, DFX or NAC monotherapy after 1 month of treatment.
Figure 1. Combined DFP + NAC restores left ventricular (LV) function in iron-overloaded rats.
The effects of the pharmacological interventions on percentage of LV ejection fraction (%LVEF) at 3 months (a) and 4 months (b), respectively in iron-overloaded rats. *P < 0.05 vs. ND control, †P < 0.05 vs. HFeV, ‡P < 0.05 vs. HFeDFP + NAC.
Effects of the pharmacological interventions on cardiac [Ca2+]i homeostasis
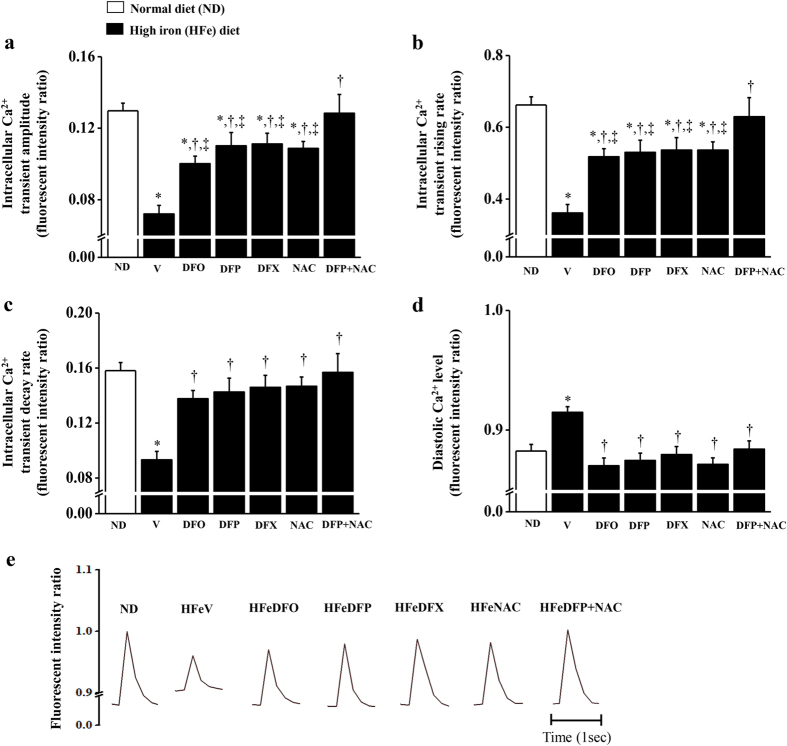
An impaired level of cardiac [Ca2+]i transients occurred in iron-overloaded rats when compared with the NDV rats (Fig. 2a–e). The results found that cardiac [Ca2+]i transient amplitude, rising rate and decay rate were all significantly decreased, whilst diastolic Ca2+ levels were significantly increased in the HFeV rats, indicating cardiac [Ca2+]i dyshomeostasis, when compared with the NDV rats (Fig. 2a–e). After 2 months of treatment, DFO, DFP, DFX or NAC alone caused improved [Ca2+]i transient amplitude and rising rate, while only combined DFP plus NAC led to restored normal [Ca2+]i transient amplitude and rising rate in iron-overloaded rats (Fig. 2a,b). On the other hand, all of the pharmacological intervention treatments led to restored [Ca2+]i transient decay rates and diastolic Ca2+ levels in iron-overloaded rats to normal levels similar to those in the NDV rats (Fig. 2c,d). The results suggest that only combined DFP plus NAC effectively restores all parameters of Ca2+ homeostasis in iron-overloaded rats.
Figure 2. Combined DFP + NAC restores calcium homeostasis in iron-overloaded rats.
The effects of the pharmacological interventions on cardiac intracellular calcium transients: (a) intracellular Ca2+ transient amplitude; (b) intracellular Ca2+ transient rising rate; (c) intracellular Ca2+ transient decay rate; (d) diastolic Ca2+ level, and (e) calcium tracing in iron-overloaded rats. *P < 0.05 vs. ND control, †P < 0.05 vs. HFeV, ‡P < 0.05 vs. HFeDFP + NAC.
Effects of the pharmacological interventions on cardiac Ca2+ cycling protein
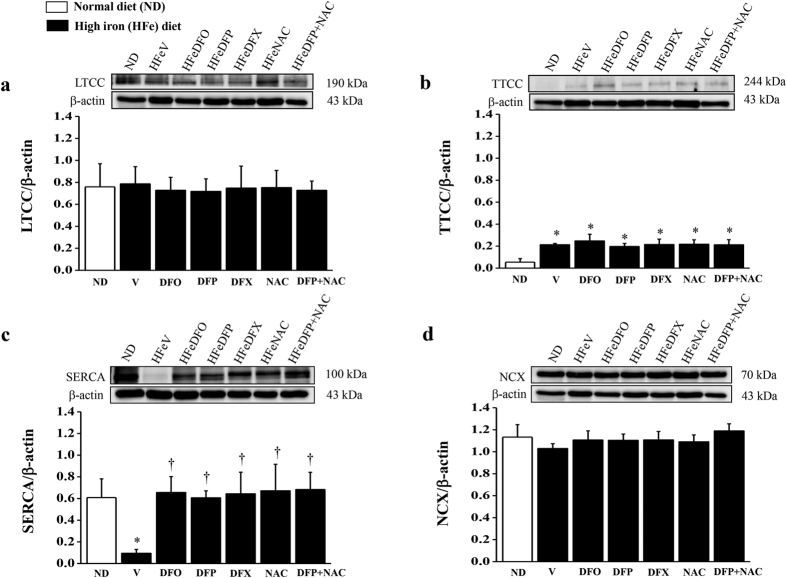
The levels of TTCC were significantly increased and the level of SERCA was significantly decreased in the HFeV rats when compared with the NDV rats, while the levels of LTCC and NCX did not differ between the NDV rats and iron loading groups (Fig. 3a–d). After 2 months of treatment, all pharmacological interventions led to the restoration of SERCA protein expression to a normal level in the HFe-fed rats. On the other hand, the levels of LTCC, TTCC or NCX did not alter in the HFe-fed rats after treatment with either DFO, DFP, DFX, NAC or combined DFP plus NAC for 2 months (Fig. 3a,b,d).
Figure 3. Combined DFP + NAC restores the levels of SERCA protein in iron-overloaded rats.
The effects of the pharmacological interventions on the levels of calcium cycling proteins: (a) L-type Ca2+ channels (LTCC); (b) T-type Ca2+ channels (TTCC); (c) sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA), and (d) sodium- Ca2+ exchangers (NCX) in iron-overloaded rats. *P < 0.05 vs. ND control, †P < 0.05 vs. HFeV. The full-length blots are presented in Supplementary Figures 1, 2 and 3.
Effects of the pharmacological interventions on cardiac iron transporter and regulatory proteins
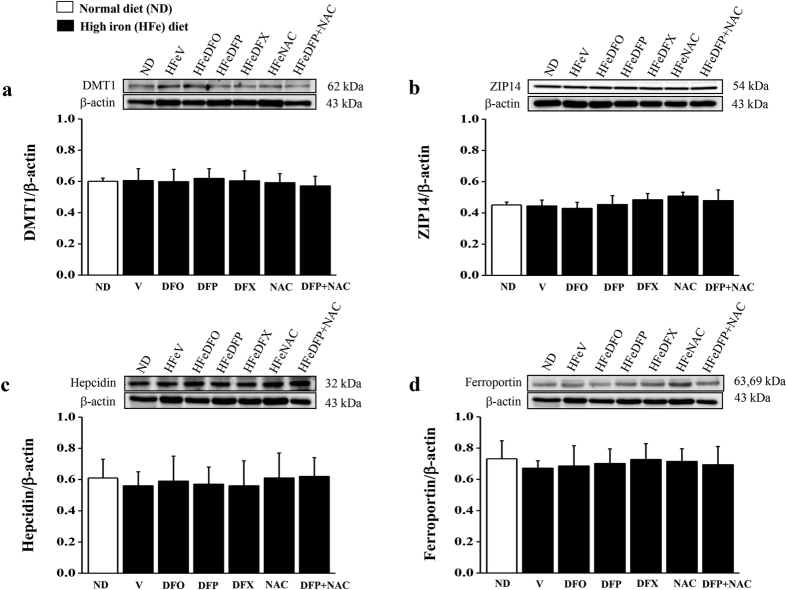
In iron-overloaded heart, the levels of cardiac iron transporter protein, including DMT1 and Zip14 as well as the levels of cardiac iron regulatory protein including ferroportin and hepcidin, did not differ in all groups of iron-overloaded rats when compared with the NDV rats (Fig. 4a–d). Similarly, all protein levels did not alter in the HFe-fed rats after 2 months of treatment with either DFO, DFP, DFX, NAC or combined DFP plus NAC when compared with the HFeV rats (Fig. 4a–d).
Figure 4. All treatments has no effect on the levels of iron transporter and regulatory proteins.
The effects of the pharmacological interventions on the levels of iron transporter protein: (a) divalent metal transporter 1 (DMT1); (b) ZRT/IRT-like protein 14 (ZIP14); the levels of iron regulatory protein (c) hepcidin, and (d) ferroportin in iron-overloaded rats. The full-length blots are presented in Supplementary Figures 1, 2 and 3.
Discussion
The main conclusions which could be drawn from this study were as following: (1) chronic iron overload led to increased levels of TTCC, decreased level of SERCA, and impaired cardiac [Ca2+]i homeostasis, including decreased [Ca2+]i transient amplitude, rising rate, and decay rate as well as increased diastolic Ca2+ levels leading to LV dysfunction; (2) DFO, DFP, DFX or NAC monotherapy could lead to the restoration of the level of SERCA protein, improvement in cardiac [Ca2+]i transient amplitude, rising rate as well as restoring [Ca2+]i transient decay rate and diastolic Ca2+ level leading to improved LV function in iron-overloaded rats; (3) only a combination of DFP plus NAC resulted in a restoration of all parameters of Ca2+ homeostasis leading to restored normal LV function in iron-overloaded rats; (4) chronic iron overload and all pharmacological interventions had no effect on the levels of DMT1, ZIP14, hepcidin, ferroportin, LTCC and NCX in iron-overloaded rats. The summary of major findings of this study is shown in Table 1.
Table 1. Summary the effects of all pharmacological interventions in each cardiac parameter.
| Cardiac parameters | ND | HFeV | HFeDFO | HFeDFP | HFeDFX | HFeNAC | HFeDFP + NAC |
|---|---|---|---|---|---|---|---|
| Echocardiography | |||||||
| - % LVEF | +++ | + | ++ | ++ | ++ | ++ | +++ |
| Intracellular Ca2+ transients | |||||||
| - Amplitude | +++ | + | ++ | ++ | ++ | ++ | +++ |
| - Rising rate | +++ | + | ++ | ++ | ++ | ++ | +++ |
| - Decay rate | +++ | + | +++ | +++ | +++ | +++ | +++ |
| - Diastolic Ca2+ level | +++ | ++++ | +++ | +++ | +++ | +++ | +++ |
| The levels of Ca2+ cycling protein | |||||||
| - LTCC | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| - TTCC | + | ++ | ++ | ++ | ++ | ++ | ++ |
| - SERCA | +++ | + | +++ | +++ | +++ | +++ | +++ |
| - NCX | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| The levels of iron transporter and regulatory proteins | |||||||
| - DMT1 | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| - ZIP14 | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| - Hepcidin | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| - Ferroportin | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
This is the first study to demonstrate the cardioprotective effects of DFO, DFP, DFX, NAC or combined DFP plus NAC treatment on iron overload-induced cardiac [Ca2+]i dysregulation in rats. It is also significant that our results show that combined DFP plus NAC exerted synergistically beneficial effects on cardiac [Ca2+]i transients, and the combination therapy was more effective than monotherapy in iron-overloaded rats. Ca2+ function as secondary messengers and play an important role in many signaling pathways in all cell types43,44,45. In cardiomyocytes, [Ca2+]i regulate electrical signals which play a role in cardiac rhythm and excitation–contraction coupling leading to cardiac contraction and relaxation43. During cardiac action potentials, LTCCs are activated and then an influx of Ca2+ triggers Ca2+ release from the sarcoplasmic reticulum through ryanodine receptor2 (RyR2), which is called Ca2+ -induced Ca2+ release leading to directly activated cardiomyocyte contraction43,46. During cardiac relaxation, Ca2+ is removed from the cytosol by four types of Ca2+ transporters: (1) Ca2+ is taken up into the sarcoplasmic reticulum by SERCA; (2) Ca2+ is removed from cytosol by NCX, and (3) sarcolemmal Ca2+-ATPase; (4) Ca2+ is taken up into mitochondria by mitochondrial Ca2+ uniporters46. Under conditions of iron overload, LTCC and TTCC are the major pathways for iron entry into the heart leading to disturbance in cardiac contractility and iron overload cardiomyopathy15,16,18,19. Previous studies found that at a high concentration of ferrous iron (Fe2+), Ca2+ current could decrease, indicating that Fe2+ might compete with Ca2+ influx into the heart through LTCC which may contribute to systolic dysfunction in cases of iron overload cardiomyopathy47,48. Additionally, we found that the decay of the Ca2+ current was slower when Fe2+ concentration was increased in isolated ventricular cardiomyocytes of rats, which might possibly contribute to impaired diastolic function47,48. However, the mechanism of extracellular Fe2+ entry into the cardiomyocytes through TTCC in iron overload conditions may be similar to LTCC. As expected, a previous study found that CaV3.1 TTCC was a significant portal for Fe2+ entry into cells leading to an iron overload condition49. Consistent with a recent study indicated that at a high concentration of free Fe2+ may compete with a Ca2+ influx into the myocardium through Ca2+ channels by both LTCC and TTCC50. Our previous study also found that TTCC is an important pathway for Fe2+ entry into the heart of thalassemic mice under iron overload conditions17,18,19. Hence, elevated TTCC expression in this study indicates that it may be an important portal for iron uptake into the cardiomyocytes and causes an increased cardiac iron concentration in rats suffering from iron-overload. Although iron overload did not alter the level of cardiac LTCC protein in this study, LTCC activity might be altered in the hearts of the HFe-fed rats and this needs to be investigated further. In addition, our previous study found that rats with iron-overload for 4 months had both increased systemic iron (plasma NTBI) and cardiac iron concentrations42 which were consistent with an increase in TTCC protein expression in this study. Plasma NTBI levels were used to determine an iron status in iron-overloaded patients including hereditary hemochromatosis, β-thalassemia, myelodysplastic syndrome and sickle cell disease51. However, the mere presence of NTBI in plasma of patients with iron overload might not be sufficient for causing heart iron overload as found in hypertransfused sickle cell anemia52, and in most cases of myelodysplastic syndrome53. A previous study found that heart disease was not present in patients with thalassemia major and intermedia who showed negative plasma NTBI and had a plasma transferrin saturation of less than 70%8. On the other hand, all thalassemic patients with heart disease found that they were plasma NTBI positive and had a plasma transferrin saturation above 70%8. However, several previous studies noted that LPI is found in iron-overloaded patients, which is used as a marker of iron toxicity7,9,10,11. An increase in LPI leads to increased labile cellular iron in the heart, resulting in increased cardiac oxidative stress and cardiomyopathy7. In addition, glutathione (GSH) system is an endogenous antioxidant that plays an important role in scavenging cellular oxidative stress, particularly in an iron overload condition54. Oxidative stress can occur in systemic iron overload, caused by a decrease in the levels of antioxidants, such as GSH and glutathione peroxidase (GPX), and an increase in highly toxic free radicals such as hydroxyl radical and superoxide anion7,54. Therefore, LPI and those of GSH levels should be further determined to warrant the levels of free iron and antioxidant as well as oxidative stress in plasma and heart tissues in our iron-overloaded animals.
Iron-catalyzed Haber-Weiss and Fenton’s reactions led to increased ROS production in iron overloaded rat’s plasma and heart tissue42. Iron overloading mediated oxidative stress and altered cardiac excitation-contraction coupling which contributed to decreased systolic and increased diastolic Ca2+ levels, which resulted in inhibited systolic and diastolic functions; a characteristic of iron overload cardiomyopathy2,48. Defects of calcium cycling protein including LTCC, RyR2, SERCA2a and NCX were the result of impaired calcium signaling and led to heart failure43. Importantly, a previous study indicated that chronic iron overload led to reduced levels of SERCA2a in both its mRNA and protein form, resulting in abnormal Ca2+ cycling and decreased cardiac function2. Consistent with this line of reasoning the level of cardiac SERCA protein was significantly decreased in iron-overloaded rats which could have led to the resulting impaired cardiac [Ca2+]i transients and cardiac contractility in the present study. Impaired cardiac SERCA function could prolong [Ca2+]i transient decay rates, leading to reduced Ca2+ reuptake into the sarcoplasmic reticulum and resulting in increased diastolic Ca2+ level in the present study. In addition, a previous study found that Fe2+ could directly inhibit the binding of [3H] ryanodine to its high affinity sites on RyR2 which led to reduced sensitivity of cardiac ryanodine receptors towards activation by Ca2+ 55. This same study indicated that Fe2+ competed with Ca2+ at the activating sites on RyR2 leading to decreased Ca2+-induced Ca2+ release in rat hearts which might contribute to impaired cardiac [Ca2+]i homeostasis, and cardiac dysfunction under conditions of iron overload55. Therefore, iron overload might subsequently result in reduced Ca2+ release by the sarcoplasmic reticulum leading to decreased [Ca2+]i transient amplitude and rising rate, and caused impaired cardiac contractility in the present study. Finally, %LVEF was significantly decreased in the HFe-fed rats leading to LV dysfunction in our study. In addition, our previous study and others demonstrated that an increase in cardiac iron store led to increased cardiac oxidative stress which caused increased mitochondrial dysfunction, resulting in cardiac dysfunction and iron overload cardiomyopathy7,42. In consistency, a previous study found that iron overload-evoked production of cardiac oxidative stress contributed to damage to mitochondrial DNA, reduced complex I (NADH dehydrogenase) and complex IV (cytochrome c oxidase) activities, and decreased cardiac mitochondrial respiration, leading to mitochondrial and cardiac dysfunctions in iron-overloaded mice56. Hence, cardiac mitochondrial dysfunction evoked by chronic iron overload might cause reduced ATP production in cardiomyocytes7. In addition, SERCA uses the free energy of ATP to transport Ca2+ into the sarcoplasmic reticulum through a concentration gradient which leads to intracellular Ca2+ homeostasis57. This may lead to a decrease in ATP levels in cardiac iron overload and cause reduced SERCA function and/or activity, which results in impaired Ca2+ homeostasis as shown in our present study. Therefore, the restoration of Ca2+ homeostasis in iron-overloaded heart could be an indicator of functional restoration, which might have resulted from improved ATP production and respiration, due to both iron detoxification and an improved reductive capacity by either iron chelators or antioxidant(s). Nevertheless, the effects of iron chelators and/or antioxidant(s) on cardiac mitochondrial ATP production and respiration in iron-overloaded rats should be further investigated.
However, in our present study the levels of DMT1 and ZIP14 did not alter in the HFe-fed rats when compared with the NDV rats. The results support the findings in previous studies which demonstrated that DMT1 and ZIP14 were not the common pathways for iron entry into the heart under iron overload conditions18,26. In addition, the levels of cardiac ferroportin and hepcidin also did not alter in the HFe-fed rats when compared with the NDV rats. This result was consistent with our previous study in which cardiac ferroportin and hepcidin did not alter in the wild type mice after 4 months of iron loading when compared with the ND control groups17. However, previous studies demonstrated that hepcidin is primarily produced and secreted by the liver leading to the regulation of iron exporter ferroportin, all of which play a major role in controlling systemic iron concentrations in conditions of iron overload58,59. Hence, ferroportin and hepcidin may not play an important role in the heart in the iron overload conditions induced in this present study.
Interestingly, our recent studies found that the combination of an oral iron chelator DFP and an antioxidant NAC could improve and restore normal brain and heart function effectively and provided more robust results than either DFO, DFP, DFX or NAC alone in the iron-overloaded rat model40,42. Consistently in the present study, a combination of DFP plus NAC provided more effective results than either DFO, DFP, DFX, or NAC alone in restoring cardiac [Ca2+]i homeostasis leading to restored normal cardiac contractility and LV function by increased %LVEF to a normal condition in iron-overloaded rats. Although previous studies reported that neither DFO nor DFX have shown to be efficient in improving %LVEF in thalassemia major or TDT patients60,61, DFP has effectively improved % LVEF in these patients60. On the other hand, a recent study found that although % LVEF was not altered, cardiac iron concentration was continuously reduced, and end-diastolic and end-systolic left ventricular volume were significantly diminished, leading to improved cardiac function in TDT patients after 5 years of treatment with DFX62. In an animal study, it has been shown that %LVEF was improved by chronic treatment with DFO, leading to improve cardiac function in iron-overloaded gerbils63. In consistency, our present study found that long-term treatment with DFO or DFX for 2 months could ameliorate cardiac function by increasing %LVEF, as well as improve hemodynamic parameters, including left ventricular end-systolic pressure, maximum pressure, cardiac output and stroke volume, contributing to improved cardiac function in iron-overloaded rats as shown in our previous study42. However, the dose and duration of treatment with DFX or DFO in TDT patients and iron-overloaded gerbils were different from our present study. Consequently, the present study indicate that chronic and continuous treatments with DFO or DFX might help to ameliorate cardiac function in patients with iron overload cardiomyopathy. The mechanisms regarding the combination of DFP and NAC in restoring cardiac [Ca2+]i transients could be clarified by the following reasons. First, DFP has the lowest molecule weight of iron chelators when compared with DFO and DFX, and has a high lipophilicity which means it can rapidly penetrate into cells64 leading to DFP chelating intracellularly effectively free irons from heart tissue42, and may contribute to attenuating impaired cardiac [Ca2+]i homeostasis. Second, NAC is an antioxidant that can decrease ROS levels, increase SERCA2a activity as well as enhance cytosolic Ca2+ removal65. In addition, NAC provides an iron chelating activity38 as it can remove excessive free irons out of the heart42 which may lead to a decrease in iron-mediated cardiac [Ca2+]i dysregulation in this study. Moreover, NAC has been considered and used as a promoter of GSH synthesis and thereby cell reductive power66. As such, it could have facilitated iron removal from iron-overloaded cells or tissues by chelators, by rendering the accumulated iron more accessible (indirectly via its reduction). Therefore, these two conditions have shown the synergistic effects of combined DFP plus NAC on cardiac [Ca2+]i homeostasis in the present study. The effects of all pharmacological interventions on cardiac [Ca2+]i homeostasis and LV function in iron-overloaded rats are summarized in Fig. 5. However, the usefulness of combined DFP plus NAC needs clinical trials to warrant its cardioprotective effects in human patients with iron overload cardiomyopathy. In conclusion, all pharmacological interventions had no effect on the levels of DMT1, ZIP14, hepcidin, ferroportin, LTCC, TTCC or NCX in the heart. However, only cardiac SERCA levels were restored by all pharmacological intervention treatments in rats with iron-overload. In addition, DFO, DFP, DFX or NAC alone exerted similar effects in improving [Ca2+]i homeostasis leading to improved LV function. Although these monotherapy treatments showed similar efficacy to combined DFP plus NAC in restoring [Ca2+]i transient decay rate and diastolic Ca2+ level, only combined DFP plus NAC could restore all parameters of [Ca2+]i transients, including [Ca2+]i transient amplitude, rising rate, decay rate as well as diastolic Ca2+ levels. These factors led to a restored normal LV function in rats with iron-overload. For this reason, combined DFP plus NAC therapy may provide these similar cardioprotective results in patients with iron overload and so may be used as a future therapeutic strategy for treatment of iron overload cardiomyopathy particularly in hemochromatosis and TDT patients.
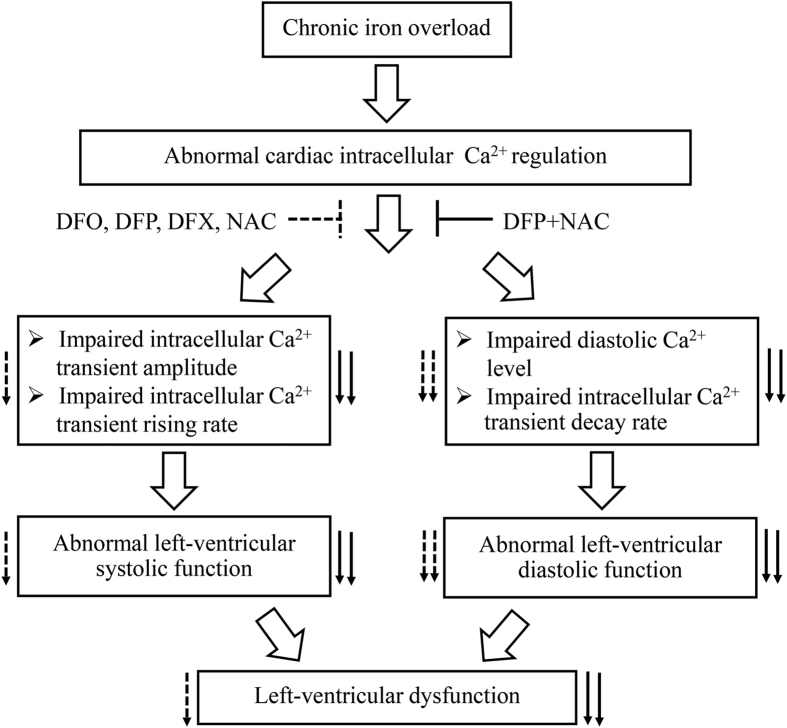
Figure 5. The effects of the pharmacological interventions on calcium homeostasis in iron-overloaded rats.
Diagram demonstrating the suggested mechanisms of chronic iron overload on cardiac intracellular Ca2+ homeostasis, and left ventricular function as well as the effects of the pharmacological interventions on cardiac intracellular Ca2+ homeostasis, and left ventricular dysfunction mediated by chronic iron overload. Dashed arrows indicate the effects of either DFO, DFP, DFX or NAC treatment; solid arrows indicate the effects of combined DFP plus NAC treatment. Ca2+ = calcium; DFO = deferoxamine; DFP = deferiprone; DFX = deferasirox; NAC = N-acetyl cysteine.
Methods
Animal preparation
The Institutional Animal Care and Use Committee (IACUC) at the Faculty of Medicine, Chiang Mai University authorized the animal study protocols (Permit number: 21/2557), in compliance with NIH guidelines, and in accordance with the ARRIVE guidelines for reporting experiments involving animals67. The National Laboratory Animal Center, Mahidol University, Bangkok, Thailand supplied all adult male Wistar rats which had a body weight of 180–200 g An animal holding room was used to house all rats in a controlled environment of 23 ± 2 °C, 50 ± 10% humidity and 12 h light/dark cycles. Throughout the entire experiment all rats were given drinking water ad libitum and were acclimatized for 7 days prior to the experimental protocols.
Experimental protocols
A standard laboratory rat diet containing 0.2% of ferrocene (C10H10Fe; Sigma-Aldrich, Co., St. Louis, USA) (w/w) was prepared as previously outlined42. Adult male Wistar rats were divided into 2 groups and were given either a normal diet (a chow diet, ND) (n = 12), or a high iron diet (0.2% ferrocene w/w, HFe) (n = 72) for 4 months. At 2 months, ND-fed rats had a vehicle (normal saline solution, NSS) (NDV or ND control group) administered once a day via either subcutaneous injection (n = 6) or gavage feeding (n = 6) and a normal chow diet was maintained for 2 months. The HFe-fed rats were divided into 6 groups (n = 12/group) and were given: (1) NSS (HFeV) once a day via either subcutaneous injection (n = 6) or gavage feeding (n = 6), (2) deferoxamine (DFO; Desferal®, Novartis Pharma Stein AG, Stein, Switzerland), (HFeDFO) 25 mg/kg/ day via subcutaneous injection, (3) deferiprone (DFP; Ferriprox®, Apotex Inc., Toronto, Ontario, Canada), (HFeDFP) 75 mg/kg/day, (4) deferasirox (DFX; Exjade®, ICL670, Novartis Pharma Stein AG, Stein, Switzerland), (HFeDFX) 20 mg/kg/day, (5) N-acetyl cysteine (NAC, Sigma-Aldrich, Co., St. Louis, USA), (HFeNAC) 100 mg/kg/day, or (6) combined DFP 75 mg/kg/day plus NAC 100 mg/kg/day (HFeDFP + NAC) through gavage feeding for 2 months, and all iron loading groups were regularly fed with the high iron diet. Since DFO is a parenteral iron chelator, it may cause suffering in patients with cardiac iron overload who are continuously treated with this drug. Previous studies have shown that DFP was significantly more potent than DFO in diminishing cardiac iron overload in TDT patients35,60. Although DFP and DFX are oral iron chelators, the cost of DFX is more expensive than DFP. Additionally, DFP was found to exert greater efficacy than DFX in reducing severe cardiac iron and improving cardiac function in TDT patients35,68. DFX also failed to reduce cardiac iron concentration in TDT patients with severe hepatic iron loading69. Hence, DFP was chosen in combination with NAC in this study for determining its effects on chronic iron-mediated the impairment of cardiac [Ca2+]i homeostasis. Dosage of all iron chelators are the lowest therapeutic range that can be used to treat patients with iron overload36. At the end of 3 and 4 months of the experiment, all rat groups had their cardiac function determined by echocardiography. After 4 months of the experimental period, half of the rats in each group (n = 6/group) were deeply anesthetized by intraperitoneal injections of Zolitil (ZolazepamTiletamine) 50 mg/kg combined with Xylazine 3 mg/kg70, then the hearts were rapidly removed. The heart tissues were frozen in liquid nitrogen immediately, and stored at −80 °C in preparation for western blot analysis. In addition, the other half of the rats from each group (n = 6/group) were deeply anesthetized with thiopental (0.5 mg/kg; Research institute of antibiotics and biotransformations, Roztoky, Czech Republic), and then the hearts of the rats were rapidly removed for single ventricular myocyte isolation71. Cardiomyocyte cells were isolated by using an enzymatic technique71,72. In brief, rats were injected intraperitoneally with 0.2 mL heparin for 10 minutes. After the initiation of deep anesthesia using 100 mg/kg thiopental, the heart was excised and the aorta was cannulated rapidly to enable cardiomyocyte isolation. Then, isolated cardiomyocytes from the hearts of the rats in each group (n = 5–8 cells/rat) were used for the measurement of [Ca2+]i transients.
Echocardiographic studies
Cardiac function was determined using echocardiography (GE Vivid I)42. Echocardiographic study was performed using a method described previously42. The parameters from the echocardiography including LV end-systolic volume (LVESV), and end-diastolic volume (LVEDV) were determined. The percentage of ejection fraction was calculated by using the following formula: %EF (Teich) = (LVEDV-LVESV) × 100/ LVEDV42,73.
Experimental protocol for studying cardiac [Ca2+]i transients
The Ca2+ transient level in rat ventricular myocytes was measured using a fluorimetric ratio technique71,72. The fluorescent Ca2+ indicator Fura-2 was loaded by incubating the cardiomyocytes at room temperature for 30 minutes with 25 μM of Fura-2-AM (Sigma Chemical, St. Louis, MO, USA). Ultraviolet light wavelengths of 340 and 387 nm were used for the excitation of the Fura-2 from a xenon arc lamp controlled by a microfluorometry system (CellR, MT 20, Olympus Soft Imaging Solutions GmbH, Germany) and the excitation light beam was directed into an inverted microscope (IX-81, Olympus Tokyo, Japan). The ratio of emitted fluorescence signals from the Fura-2/AM loaded cardiomyocytes at 510 nm was recorded. The Ca2+ transient parameters including the [Ca2+]i transient amplitude, rising rate, and decay rate, as well as the diastolic Ca2+ level were recorded during electrical pacing (1 hertz field-stimulation, 10 millisecond duration and 15 volts). The ratio of the emission wavelengths is directly related to the amount of cardiac [Ca2+]i74. The fluorescence ratio data was processed and stored in a computer using Xcellence imaging software (Olympus, Tokyo, Japan)71,72.
Western blot analysis
The heart tissues were extracted in a homogenizer in a lysis buffer (20 mM Tris–HCl, 1 mM Na3VO4, 5 mM NaF, and 1% proteinase inhibitor) at 4 °C, and subsequently centrifuged at 13,500 rpm for 10 minutes. The total protein of 40 mg was mixed with the loading buffer (1 mg/mL), boiled for 10 minutes and loaded onto 10% gradient sodium dodecyl sulfate (SDS)-polyacrylamide gels. Proteins were transferred to a nitrocellulose blotting membrane (GE Healthcare Life Sciences, Freiburg, Germany). Immunoblots were blocked with either 5% of bovine serum albumin or 5% of skimmed milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 (TBST) for 1 hour at room temperature. Then, immunoblots were exposed to the primary antibodies including the iron transporter protein DMT1 (ab55812; Abcam, Cambridge, MA, USA) and Zip14 (ab106568; Abcam, Cambridge, MA, USA), iron regulatory protein ferroportin (ab85370; Abcam, Cambridge, MA, USA) and hepcidin (ab75883; Abcam, Cambridge, MA, USA), Ca2+ cycling protein LTCC (C1603; Sigma-Aldrich Co. LLC., Saint Louis, MO, USA.), TTCC (sc25691; Santa Cruz Biotechnology Inc., Dallas, Texas, USA), SERCA (sc30110; Santa Cruz Biotechnology Inc., Dallas, Texas, USA) and NCX (sc32881; Santa Cruz Biotechnology Inc., Dallas, Texas, USA), and β-actin (sc47778; Santa Cruz Biotechnology Inc., Dallas, Texas, USA) for a loading control at 4 °C overnight. After that, the membrane was washed five times with TBST and then incubated with the horseradish peroxidase-conjugated secondary antibody at room temperature for 1 hour. After washing the membrane again five times with TBST, the blots were visualized with an enhanced chemiluminescence (ECL) reagent and exposed by using ChemiDocTM Touch Imaging System (Life science AP, Bio-Rad, CA, USA). The images of the western blots were analysed using the ImageJ (NIHimage) analysis software17,75.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). The data were processed using SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) release 22.0 for Windows. Differences between groups were tested for via One-way ANOVA analyses. Significant difference between groups was assumed if P values < 0.05.
Additional Information
How to cite this article: Wongjaikam, S. et al. Restoring the impaired cardiac calcium homeostasis and cardiac function in iron overload rats by the combined deferiprone and N-acetyl cysteine. Sci. Rep. 7, 44460; doi: 10.1038/srep44460 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We would like to thank Miss Maria Love for her editorial assistance, and Miss Sasiwan Kerdphoo and Miss Thidarat Jaiwongkam for their technical assistance. This work was supported by the NSTDA Research Chair Grant from the National Science and Technology Development Agency Thailand (NC) and grants from the Thailand Research Fund BRG5780016 (SC), MRG5980222 (SK), the Royal Golden Jubilee program (SC and SW), Chiang Mai University Center of Excellence Award (NC), and the Faculty of Medicine Chiang Mai University Fund (SW and NC).
Footnotes
The authors declare no competing financial interests.
Author Contributions S.W. and J.K. performed the research; N.C. and S.C. designed research study; N.C. and S.C. contributed essential materials and reagents; S.W., S.K. and J.K. analysed the data; S.W., S.K., S.C. and N.C. wrote and revised the manuscript; S.C. and N.C. critically reviewed and approved of the submitted and the final version.
References
- von Herbay A. et al. -Cardiomyopathy as the cause of death in genetic hemochromatosis. Z Gastroenterol 34, 178–182 (1996). [PubMed] [Google Scholar]
- Das S. K. et al. Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy. Sci Rep 5, 18132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo M. G. et al. Survival and causes of death in thalassaemia major. Lancet 2, 27–30 (1989). [DOI] [PubMed] [Google Scholar]
- Kremastinos D. T. et al. Beta-thalassemia cardiomyopathy: history, present considerations, and future perspectives. Circ Heart Fail 3, 451–458 (2010). [DOI] [PubMed] [Google Scholar]
- Hider R. C., Silva A. M., Podinovskaia M. & Ma Y. Monitoring the efficiency of iron chelation therapy: the potential of nontransferrin-bound iron. Ann N Y Acad Sci 1202, 94–99 (2010). [DOI] [PubMed] [Google Scholar]
- Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Ann NY Acad Sci 1202, 1–9 (2010). [DOI] [PubMed] [Google Scholar]
- Berdoukas V., Coates T. D. & Cabantchik Z. I. Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic Biol Med 88, 3–9 (2015). [DOI] [PubMed] [Google Scholar]
- Piga A. et al. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol 84, 29–33 (2009). [DOI] [PubMed] [Google Scholar]
- Le Lan C. et al. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood 105, 4527–4531 (2005). [DOI] [PubMed] [Google Scholar]
- Pootrakul P. et al. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalassemia/HbE patients treated with an oral chelator. Blood 104, 1504–1510 (2004). [DOI] [PubMed] [Google Scholar]
- Lal A. et al. Combined chelation therapy with deferasirox and deferoxamine in thalassemia. Blood Cells Mol Dis 50, 99–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongjaikam S., Kumfu S., Chattipakorn S. C., Fucharoen S. & Chattipakorn N. Current and future treatment strategies for iron overload cardiomyopathy. Eur J Pharmacol 765, 86–93 (2015). [DOI] [PubMed] [Google Scholar]
- Das S. K. & Oudit G. Y. Voltage-gated Ca2+ channels as key mediators of iron-transport and iron-overload cardiomyopathy: L-type vs. T-type Ca+ channels. Eur J Haematol 88, 476–477 (2012). [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16, 521–555 (2000). [DOI] [PubMed] [Google Scholar]
- Oudit G. Y., Trivieri M. G., Khaper N., Liu P. P. & Backx P. H. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med (Berl) 84, 349–364 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G. Y. et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 9, 1187–1194 (2003). [DOI] [PubMed] [Google Scholar]
- Kumfu S., Chattipakorn S. C., Fucharoen S. & Chattipakorn N. Dual T-type and L-type calcium channel blocker exerts beneficial effects in attenuating cardiovascular dysfunction in iron-overloaded thalassaemic mice. Exp Physiol 101, 521–539 (2016). [DOI] [PubMed] [Google Scholar]
- Kumfu S. et al. T-type calcium channel as a portal of iron uptake into cardiomyocytes of beta-thalassemic mice. Eur J Haematol 86, 156–166 (2011). [DOI] [PubMed] [Google Scholar]
- Kumfu S., Chattipakorn S., Chinda K., Fucharoen S. & Chattipakorn N. T-type calcium channel blockade improves survival and cardiovascular function in thalassemic mice. Eur J Haematol 88, 535–548 (2012). [DOI] [PubMed] [Google Scholar]
- Niwa N. et al. Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol 286, H2257–2263 (2004). [DOI] [PubMed] [Google Scholar]
- Yasui K. et al. Pathophysiological significance of T-type Ca2+ channels: expression of T-type Ca2+ channels in fetal and diseased heart. J Pharmacol Sci 99, 205–210 (2005). [DOI] [PubMed] [Google Scholar]
- Huang B., Qin D., Deng L., Boutjdir M. & Nabil El-Sherif Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc Res 46, 442–449 (2000). [DOI] [PubMed] [Google Scholar]
- Martinez M. L., Heredia M. P. & Delgado C. Expression of T-type Ca(2+) channels in ventricular cells from hypertrophied rat hearts. J Mol Cell Cardiol 31, 1617–1625 (1999). [DOI] [PubMed] [Google Scholar]
- Xie L. H., Doye A. A., Conley E. & Gwathmey J. K. Sickle cell anemia: the impact of discovery, politics, and business. J Health Care Poor Underserved 24, 147–158 (2013). [DOI] [PubMed] [Google Scholar]
- Li X., Li W., Gao Z. & Li H. Association of cardiac injury with iron-increased oxidative and nitrative modifications of the SERCA2a isoform of sarcoplasmic reticulum Ca(2+)-ATPase in diabetic rats. Biochimie 127, 144–152 (2016). [DOI] [PubMed] [Google Scholar]
- Nam H. et al. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica 98, 1049–1057 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y. et al. Post-transcriptional expression of DMT1 in the heart of rat. J Cell Physiol 196, 124–130 (2003). [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Morgan H. E., Johnson A. & Nicholson R. I. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett 579, 427–432 (2005). [DOI] [PubMed] [Google Scholar]
- Liuzzi J. P., Aydemir F., Nam H., Knutson M. D. & Cousins R. J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103, 13612–13617 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal-Littleton S. et al. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc Natl Acad Sci USA 112, 3164–3169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao B. et al. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab 15, 918–924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004). [DOI] [PubMed] [Google Scholar]
- Hansen S. L. et al. High dietary iron reduces transporters involved in iron and manganese metabolism and increases intestinal permeability in calves. J Dairy Sci 93, 656–665 (2010). [DOI] [PubMed] [Google Scholar]
- Brasse-Lagnel C. et al. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 140, 1261–1271 e1261 (2011). [DOI] [PubMed] [Google Scholar]
- Pepe A. et al. Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: cardiac iron and function comparison determined by quantitative magnetic resonance imaging. Haematologica 96, 41–47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski J. L. Management of transfusional iron overload - differential properties and efficacy of iron chelating agents. J Blood Med 2, 135–149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbrand A. V., Taher A. & Cappellini M. D. How I treat transfusional iron overload. Blood 120, 3657–3669 (2012). [DOI] [PubMed] [Google Scholar]
- Hjortso E., Fomsgaard J. S. & Fogh-Andersen N. Does N-acetylcysteine increase the excretion of trace metals (calcium, magnesium, iron, zinc and copper) when given orally? Eur J Clin Pharmacol 39, 29–31 (1990). [DOI] [PubMed] [Google Scholar]
- Ozdemir Z. C., Koc A., Aycicek A. & Kocyigit A. N-Acetylcysteine supplementation reduces oxidative stress and DNA damage in children with beta-thalassemia. Hemoglobin 38, 359–364 (2014). [DOI] [PubMed] [Google Scholar]
- Sripetchwandee J., Wongjaikam S., Krintratun W., Chattipakorn N. & Chattipakorn S. C. A combination of an iron chelator with an antioxidant effectively diminishes the dendritic loss, tau-hyperphosphorylation, amyloids-beta accumulation and brain mitochondrial dynamic disruption in rats with chronic iron-overload. Neuroscience 332, 191–202 (2016). [DOI] [PubMed] [Google Scholar]
- Sripetchwandee J., Pipatpiboon N., Chattipakorn N. & Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One 9, e85115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongjaikam S. et al. Combined iron chelator and antioxidant exerted greater efficacy on cardioprotection than monotherapy in iron-overloaded rats. PLoS One 11, e0159414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho C., Lee A. & Hajjar R. J. Altered sarcoplasmic reticulum calcium cycling–targets for heart failure therapy. Nat Rev Cardiol 9, 717–733 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell 80, 259–268 (1995). [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Lipp P. & Bootman M. D. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1, 11–21 (2000). [DOI] [PubMed] [Google Scholar]
- Bers D. M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res 87, 275–281 (2000). [DOI] [PubMed] [Google Scholar]
- Tsushima R. G. et al. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res 84, 1302–1309 (1999). [DOI] [PubMed] [Google Scholar]
- Murphy C. J. & Oudit G. Y. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card Fail 16, 888–900 (2010). [DOI] [PubMed] [Google Scholar]
- Lopin K. V., Gray I. P., Obejero-Paz C. A., Thevenod F. & Jones S. W. Fe(2)(+) block and permeation of CaV3.1 (alpha1G) T-type calcium channels: candidate mechanism for non-transferrin-mediated Fe(2)(+) influx. Mol Pharmacol 82, 1194–1204 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila R. A. et al. Mechanisms involved in the in vitro contractile dysfunction induced by different concentrations of ferrous iron in the rat myocardium. Toxicol In Vitro 36, 38–45 (2016). [DOI] [PubMed] [Google Scholar]
- Maas R. P., Voets P. J., de Swart L. & Swinkels D. W. [Non-transferrin-bound iron: a promising biomarker in iron overload disorders]. Ned Tijdschr Geneeskd 157, A6258 (2013). [PubMed] [Google Scholar]
- Koren A., Fink D., Admoni O., Tennenbaum-Rakover Y. & Levin C. Non-transferrin-bound labile plasma iron and iron overload in sickle-cell disease: a comparative study between sickle-cell disease and beta-thalassemic patients. Eur J Haematol 84, 72–78 (2010). [DOI] [PubMed] [Google Scholar]
- Konen E. et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 82, 1013–1016 (2007). [DOI] [PubMed] [Google Scholar]
- Kalpravidh R. W. et al. Glutathione redox system in beta -thalassemia/Hb E patients. ScientificWorldJournal 2013, 543973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Giri S. N. & Pessah I. N. Iron(II) is a modulator of ryanodine-sensitive calcium channels of cardiac muscle sarcoplasmic reticulum. Toxicol Appl Pharmacol 130, 57–66 (1995). [DOI] [PubMed] [Google Scholar]
- Gao X. et al. Mitochondrial dysfunction may explain the cardiomyopathy of chronic iron overload. Free Radic Biol Med 49, 401–407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horakova L., Strosova M. K., Spickett C. M. & Blaskovic D. Impairment of calcium ATPases by high glucose and potential pharmacological protection. Free Radic Res 47 Suppl 1, 81–92 (2013). [DOI] [PubMed] [Google Scholar]
- Gardenghi S. et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood 109, 5027–5035 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. & Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 62, 347–360 (2011). [DOI] [PubMed] [Google Scholar]
- Pennell D. J. et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood 107, 3738–3744 (2006). [DOI] [PubMed] [Google Scholar]
- Pennell D. J. et al. A 1-year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in beta-thalassemia major (CORDELIA). Blood 123, 1447–1454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinerio E. et al. A 5-year follow-up in deferasirox treatment: improvement of cardiac and hepatic iron overload and amelioration in cardiac function in thalassemia major patients. Ann Hematol 94, 939–945 (2015). [DOI] [PubMed] [Google Scholar]
- Walker E. M. Jr. et al. Acetaminophen combinations protect against iron-induced cardiac damage in gerbils. Ann Clin Lab Sci 39, 378–385 (2009). [PubMed] [Google Scholar]
- Glickstein H., El R. B., Shvartsman M. & Cabantchik Z. I. Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood 106, 3242–3250 (2005). [DOI] [PubMed] [Google Scholar]
- Balderas-Villalobos J. et al. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol 305, H1344–1353 (2013). [DOI] [PubMed] [Google Scholar]
- Arakawa M. & Ito Y. N-acetylcysteine and neurodegenerative diseases: basic and clinical pharmacology. Cerebellum 6, 308–314 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Browne W., Cuthill I. C., Emerson M. & Altman D. G. Animal research: reporting in vivo experiments–the ARRIVE guidelines. J Cereb Blood Flow Metab 31, 991–993 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoukas V. et al. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: a clinical observational study. J Cardiovasc Magn Reson 11, 20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. C. et al. The effect of deferasirox on cardiac iron in thalassemia major: impact of total body iron stores. Blood 116, 537–543 (2010). [DOI] [PubMed] [Google Scholar]
- Chinda K., Sanit J., Chattipakorn S. & Chattipakorn N. Dipeptidyl peptidase-4 inhibitor reduces infarct size and preserves cardiac function via mitochondrial protection in ischaemia-reperfusion rat heart. Diab Vasc Dis Res 11, 75–83 (2014). [DOI] [PubMed] [Google Scholar]
- Palee S., Apaijai N., Shinlapawittayatorn K., Chattipakorn S. C. & Chattipakorn N. Acetylcholine Attenuates Hydrogen Peroxide-Induced Intracellular Calcium Dyshomeostasis Through Both Muscarinic and Nicotinic Receptors in Cardiomyocytes. Cell Physiol Biochem 39, 341–349 (2016). [DOI] [PubMed] [Google Scholar]
- Weerateerangkul P., Palee S., Chinda K., Chattipakorn S. C. & Chattipakorn N. Effects of Kaempferia parviflora Wall. Ex. Baker and sildenafil citrate on cGMP level, cardiac function, and intracellular Ca2+ regulation in rat hearts. J Cardiovasc Pharmacol 60, 299–309 (2012). [DOI] [PubMed] [Google Scholar]
- Pongkan W., Chattipakorn S. C. & Chattipakorn N. Chronic testosterone replacement exerts cardioprotection against cardiac ischemia-reperfusion injury by attenuating mitochondrial dysfunction in testosterone-deprived rats. PLoS One 10, e0122503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M. & Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260, 3440–3450 (1985). [PubMed] [Google Scholar]
- Tanajak P. et al. Fibroblast growth factor 21 (FGF21) therapy attenuates left ventricular dysfunction and metabolic disturbance by improving FGF21 sensitivity, cardiac mitochondrial redox homoeostasis and structural changes in pre-diabetic rats. Acta Physiol (Oxf) 217, 287–299 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.