Abstract
Phenylketonuria patients harboring a subset of phenylalanine hydroxylase (PAH) mutations have recently shown normalization of blood phenylalanine levels upon oral administration of the PAH cofactor tetrahydrobiopterin [(6R)-l-erythro-5,6,7,8-tetrahydrobiopterin (BH4)]. Several hypotheses have been put forward to explain BH4 responsiveness, but the molecular basis for the corrective effect(s) of BH4 has not been understood. We have investigated the biochemical, kinetic, and structural changes associated with BH4-responsive mutations (F39L, I65T, R68S, H170D, E178G, V190A, R261Q, A300S, L308F, A313T, A373T, V388M, E390G, P407S, and Y414C). The biochemical and kinetic characterization of the 15 mutants studied points toward a multifactorial basis for the BH4 responsiveness; the mutants show residual activity (>30% of WT) and display various kinetic defects, including increased Km (BH4) and reduced cooperativity of substrate binding, but no decoupling of cofactor (BH4) oxidation. For some, BH4 seems to function through stabilization and protection of the enzyme from inactivation and proteolytic degradation. In the crystal structures of a phenylketonuria mutant, A313T, minor changes were seen when compared with the WT PAH structures, consistent with the mild effects the mutant has upon activity of the enzyme both in vitro and in vivo. Truncations made in the A313T mutant PAH form revealed that the N and C termini of the enzyme influence active site binding. Of fundamental importance is the observation that BH4 appears to increase Phe catabolism if at least one of the two heterozygous mutations has any residual activity remaining.
Most forms of phenylketonuria (PKU) and hyperphenylalaninemia (HPA) are caused by mutations in the phenylalanine hydroxylase (PAH; EC 1.14.16.1) gene, resulting in a nonfunctional enzyme that in turn leads to an accumulation of the l-Phe substrate in blood and brain (1). PAH is a nonheme iron-dependent enzyme that requires (6R)-l-erythro-5,6,7,8-tetrahydrobiopterin (BH4)asan essential cofactor in the hydroxylation of l-Phe and also uses dioxygen as a substrate. The gene for PAH has been cloned and >400 disease-causing mutations identified (www.pahdb.mcgill.ca) (2). An l-Phe-restricted diet can ameliorate the effects of high blood l-Phe levels on cognitive function. However, treatment has to be continued “for life”; otherwise, the effect of high l-Phe levels leads to functional deficits (3).
Recently, certain PKU patients have been responsive to BH4 loading, resulting in a decreased l-Phe level. It was suggested that this response was due to a Km mutant PAH enzyme (4). Kure et al. (5) investigated four patients with HPA who responded to the BH4 loading test using 10 mg of BH4/kg body weight. In all four patients, BH4 defects were excluded, and mutations detected in the PAH gene. After this report, many additional cases have appeared (6–11). These reports emphasize the potential response to BH4 of patients with mild PKU. A database that contains a current listing of the BH4-responsive genotypes has been established at www.bh4.org/biopku.html.
Due to the large potential of response among PKU patients [i.e., up to ≈60% of HPA patients (12)], pilot studies have been undertaken to determine how many patients would respond to BH4. These studies include patients with classical as well as atypical PKU in addition to HPA, and many classical PKU patients were found to be BH4-responsive in addition to the HPA patients (13). In the wake of the clinical studies, we set out on a biochemical and biophysical investigation to study the in vitro mechanisms of BH4 responsiveness in HPA and PKU patients. Several possibilities have previously been put forward to explain the BH4 response in mild PKU (14): (i) decreased affinity of the mutant PAH for BH4; (ii) stabilization of the active tetramer/dimer forms of the mutant proteins and protection from proteolytic cleavage, i.e., BH4 can act as a chemical chaperone preventing misfolding (15) and subsequent ubiquitin-dependent proteosomal degradation; (iii) up-regulation of PAH gene expression (16); (iv) BH4-induced change in BH4 biosynthesis; and (v) PAH mRNA stabilization, as shown for nitric oxide synthase (17). The expression, kinetic, and binding characterization analyses presented here, applied on the mutations in the clinical study plus others (Table 1), contribute to the understanding of BH4 responsiveness in PKU and to a more accurate selection of genotypes that are predictably associated with a positive response to cofactor treatment.
Table 1. PKU patients with BH4-responsive mutations studied and their location/effect on PAH structure.
| Percentage decrease in l-Phe levels after 24 h in patients with BH4-responsive genotypes*
|
|||||
|---|---|---|---|---|---|
| PAH cDNA nucleotide change | PAH mutation | Structural contacts/comments | Metabolic phenotype* (homozygotes and functional hemizygotes) | Patient genotype | % response |
| c.117C→G | F39L | In the hydrophobic core of RD formed by Leu-37, Leu-41, Val-51, Ile-65, Phe-79, Leu-98, Ile-102, Ala-104, and Leu-106. Substitution to a smaller Leu may change the core structure and destabilize RD. | Mild to classical PKU | F39L/R48W | 44.3 |
| F39L/F55fsdelT | 36.1 | ||||
| F39L/F55fsdelT | 70.7 | ||||
| c.194T→C | I65T | In the hydrophobic core of RD. Substitution to a more polar Thr may distort the hydrophobic packing in the RD core. | Non-PKU HPA to classical PKU | 165T/R68S | 23.2 |
| 165T/R408W | 58.4 | ||||
| 165T/R408W | 40.7 | ||||
| c.204A→T | R68S | H-bonds to Ser-67 and stabilizes secondary structure of Rβ2. In the tetramer model, Arg-68 is close to Tyr-216 from molecule C. Substitution to Ser may disrupt H-bond and dimer/tetramer interactions. | Mild PKU | R68S/R408W | 40.7 |
| R68S/I65T | 23.2 | ||||
| c.311C→A | H170D | On the surface of CD, close to TD and RD. Substitution into an Asp may disrupt a H-bond to Arg-241 at the start of Cβ1. | Non-PKU HPA | H170D/IVS1nt5g→a | 68.2 |
| c.533A→G | E178G | On the surface of CD. Substitution to a small and flexible hydrophobic residue may be very unfavorable, because it can change the fold of the CD core, which is important for maintaining proper catalytic function. | Non-PKU HPA | E178G/IVS10nt-11g→a | 45.8 |
| c.569T→C | V190A | Close to the l-Phe substrate-binding site (7.1 A). Important for proper substrate orientation for catalysis. | - | - | - |
| c.782G→A | R261Q | In the loop between Cα6 and Cβ2. Interacts with Gln-304 and Thr-238 by H-bonds. Close to Tyr-417 in tetrameric model. A substitution would disrupt H-bonds to Gln-304 and Thr-238, which stabilize the secondary structure in the active site and potentially interfere with proper dimer/tetramer formation. | Variant PKU to classical PKU | R261Q/R408W | 17.3 |
| R261Q/L308F | 71.9 | ||||
| c.898C→T | A300S | Close to Thr-238. Not enough room for larger side chain of Ser. May change polarity in the CD core. | Non-PKU HPA | A300S/R408W | 58.7 |
| c.922C→T | L308F | Close to TD Val-412 and Tyr-414, and CD Ala-259 and Glu-305. No room for the Phe side chain. Substitution would push TD away. Mutation may interfere with proper dimer/tetramer formation. | - | L308F/R261Q | 71.9 |
| c.937G→A | A313T | Close to TD Ile-406 and Pro-407. Mutation may interfere with proper dimer/tetramer formation. | Mild PKU | - | - |
| c.1117G→A | A373T | Close to Phe-402 and Lys-320. Mutation may interfere with proper dimer/tetramer formation. | Non-PKU HPA | - | - |
| c.1162G→A | V388M | Val-388 is located to the surface of the monomer, close to the other monomer of the dimer. Mutation to Met may cause disturbances in dimerization. | Mild to moderate PKU | - | - |
| c.1169A→G | E390G | No contacts. On the surface, pointing toward molecule B. Substitution to Gly induces local distortions in CD. | Non-PKU HPA | E390G/IVS12ntlg→a | 70.2 |
| c.1219C→T | P407S | Pro may be important for positioning the TD helix. Mutation may interfere with proper dimer/tetramer formation. | Non-PKU HPA | P407S/R408W | 45.2 |
| c.1241A→G | Y414C | Stacks between Pro-416 (TD) and Phe-260 (CD). Important for keeping TD close to CD. Mutation may interfere with proper dimer/tetramer formation. | Non-PKU HPA to variant PKU | Y414C/R408W | 20.5 |
| Y414C/R408W | 26.3 | ||||
| Y414C/IVS7nt5g→a | 30.1 | ||||
RD, regulatory domain; CD, catalytic domain; TD, tetramerization domain.
Data from ref. 13; PAH mutations database, www.pahdb.mcgill.ca
Materials and Methods
Site-Directed Mutagenesis, Purification. The mutations F39L, I65T, R68S, H170D, E178G, V190A, R261Q, A300S, L308F, A313T, A373T, V388M, E390G, P407S, and Y414C were introduced into the pMAL-c2 plasmid containing the full-length WT PAH (wt-PAH) sequence by using the QuikChange site-directed mutagenesis kit (Stratagene). Measurement of mutant and wt-PAH activity by HPLC and fluorescence detection of l-Tyr for nonactivated (non-l-Phe preincubated) and activated (l-Phe preincubated, 1 mM l-Phe) PAH enzymes were performed as described (18). Isothermal titration calorimetry (ITC) experiments were performed in a VP-ITC titration calorimeter (Microcal, Amherst, MA) at pH 7.0 with the glucose oxidase system (19) and 10-50 μM PAH subunit with ≈0.5 mol ferrous ammonium sulfate/subunit, as described (20). Additional information on the biophysical characterization is available in Table 3, which is which is published as supporting information on the PNAS web site.
Crystallization and Data Collection. Purified protein at a concentration of ≈10 mg/ml of the four mutants (H170D, R261Q, A313T, and Y414C) of double-truncated PAH (dt-PAH) were used for crystallization trials. Only the A313T-dt-PAH mutant crystallized using 5–10% ethylene glycol/20-40 mM Pipes, pH 6.8/8–15% polyethylene glycol 2000. Two data sets were collected on the A313T-dt-PAH and ≈6 mM 7,8-BH2 cocrystallized A313T-dt-PAH crystals (denoted A313T-dt-PAH·7,8-BHs) on an in-house FRD generator (Rigaku/MSC, Tokyo) (wavelength = 1.5418 Å) with an R-axis IV++ image plate detactor, by using Osmic mirrors and an X-stream cryostat set to 100 K. Data were processed with hkl2000 (21) to 2.1 and 2.2 Å for the apo-A313T-dt-PAH and A313T-dt-PAH·7,8-BH2, respectively. The structures were phased by using molecular replacement [PDB ID code 1PAH (22) and PDB ID code 1LRM (23)] for the two structures, respectively. Electron density for a threonine residue at position 313 was clear in the initial maps in both structures, and the entire 7,8-BH2 ligand was readily visible in electron density. Map calculations, energy minimization, and refinement were performed by using the program cns (Ver. 1.1) (24). The program o (Ver. 7) (25) was used to manually fit the differences from the native structures into the experimental electron density. Final agreement statistics are Rcryst 0.21 for both structures and Rfree 0.24 and 0.25 for the apo and BH4 bound structures, respectively. The remaining final model statistics are listed in Table 4, which is published as supporting information on the PNAS web site. Additional information related to the protocols for mutagenesis, expression, purification, and biophysical analysis (isothermal titration calorimetry, circular dichroism, and structure determination) may be found in Supporting Text, which is published as supporting information on the PNAS web site.
Results
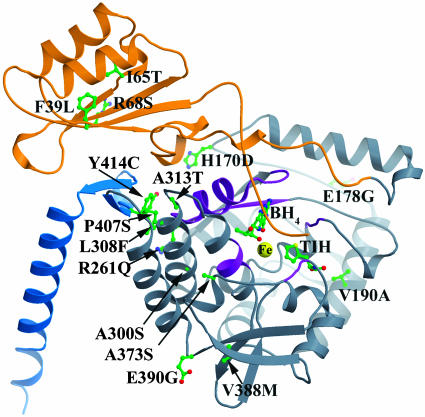
Kinetic Characterization of PKU Mutant Proteins. Fifteen mutations plus WT of full-length human PAH were expressed in E. coli as fusion proteins with maltose-binding protein and purified to homogeneity. The location of mutations characterized in this study is mapped onto the composite model of a monomer of PAH (26) in Fig. 1. The mutations can be found throughout the structure, and not just in the catalytic domain regions involved in the binding of the BH4 cofactor. They are in the regulatory domain (F39L, I65T, and R68S), the catalytic domain (H170D, E178G, V190A, R261Q, A300S, L308F, A313T, A373T, V388M, E390G, and P407S), and the dimerization motif in the oligomerization domain (Y414C). The putative structural effects of the BH4-responsive mutations included in this study and the percent reduction of l-Phe in patient genotypes from the clinical study can be found in Table 1. The relative in vitro activities of purified mutant enzymes are summarized in Table 4. Eight of the mutations studied here have either not been reported previously or to our knowledge, neither been reported expressed in vitro nor activity measured. All mutants studied have >30% residual activity as compared with wt-PAH (except for V388M, which has 23% residual activity).
Fig. 1.
PAH mutations covered in this work mapped onto the monomer of a composite model of full-length PAH. Orange represents the regulatory domain (1–142), gray represents the catalytic domain (143–410), and blue represents the oligomerization domain (411–452). The iron at the active site is displayed as a yellow sphere, whereas the tetrahydrobiopterin (BH4), thienylalanine (TIH) substrate analog, and protein side chains are colored by individual atom colors (green is carbon, blue is nitrogen, red is oxygen, and yellow is sulfur). The purple regions are considered the pterin-binding regions.
Detailed analyses of the steady-state kinetic parameters were performed for all full-length PAH mutations (Table 2). All of the mutants presented various kinetic defects when compared with wt-PAH, and only H170D, V190A, A373T, and P407S showed similar response to l-Phe binding, both as activation fold by preincubation with the substrate and degree of positive binding cooperativity (h, Hill coefficient). Significantly decreased apparent affinity for BH4 was observed for the mutants F39L, I65T, and L308F. Some mutants were obtained in the amounts required to perform isothermal titration calorimetry measurements and the thermodynamic binding parameters for BH4 could be determined (Table 5, which is published as supporting information on the PNAS web site, and ref. 20). Defective BH4 binding was confirmed by this technique for F39L and R68S, whereas I65T and A313T also showed a slightly decreased affinity. In all cases, the decreased affinity appears to be caused by an increased entropic penalization to the enthalpically driven BH4-binding process.
Table 2. Kinetic parameters for studied mutants.
| Specific activity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mutant | Non-l-Phe-activated* | l-Phe-activated† | Activation fold | Km (BH4),‡ μM | Kd (BH4),§ μM | S0.5 (l-Phe),¶ μM | h¶ | Substrate inhibition | Coupling efficiency,∥ % |
| WT | 729 ± 32 | 1,905 ± 12 | 2.6 | 26 ± 3 | 2.7 ± 0.1 | 145 ± 12 | 2.0 | + | 1.03 ± 0.14 |
| F39L | 1,449 ± 24 | 1,703 ± 35 | 1.2 | 44 ± 2 | 8.4 ± 0.8 | 60 ± 8 | 1.4 | + | 1.06 ± 0.08 |
| I65T | 1,600 ± 30 | 2,300 ± 50 | 1.2 | 40 ± 3 | 3.9 ± 0.4 | 80 ± 10 | 1.0 | + | 0.95 ± 0.15 |
| R68S | 1,648 ± 150 | 1,767 ± 134 | 1.1 | 30 ± 3 | 9.0 ± 1.0 | 73 ± 6 | 1.5 | + | 1.18 ± 0.14 |
| H170D | 320 ± 40 | 810 ± 130 | 2.5 | 12 ± 2 | ND | 104 ± 3 | 2.8 | + | ND |
| E178G | 595 ± 119 | 733 ± 74 | 1.2 | 29 ± 5 | ND | 277 ± 38 | 1.1 | + (?) | ND |
| V190A | 500 ± 2 | 2,100 ± 30 | 4.2 | 17 ± 2 | ND | 139 ± 10 | 2.9 | + | ND |
| R261Q | 1,453 ± 56 | 1,485 ± 12 | 1.0 | 25 ± 2 | 2.7 ± 0.1 | 610 ± 60 | 1.1 | - | ND |
| A300S | 500 ± 30 | 590 ± 10 | 1.2 | 26 ± 4 | 2.7 ± 0.1 | 151 ± 25 | 1.1 | + | 0.98 ± 0.22 |
| L308F | 747 ± 9 | 926 ± 28 | 1.2 | 44 ± 8 | ND | 151 ± 13 | 1.9 | + (?) | ND |
| A313T | 650 ± 50 | 1,430 ± 190 | 2.2 | 24 ± 4 | 3.4 ± 0.3 | 165 ± 18 | 1.5 | + | ND |
| A373T | 550 ± 90 | 1,050 ± 110 | 1.9 | 22 ± 3 | ND | 144 ± 14 | 1.8 | + | ND |
| V388M | 280 ± 4 | 440 ± 7 | 1.5 | 24 ± 3 | ND | 1,200 ± 110 | 1.0 | - | ND |
| E390G | 1,370 ± 7 | 1,780 ± 10 | 1.3 | 29 ± 2 | ND | 153 ± 15 | 1.5 | + | ND |
| P407S | 880 ± 60 | 1,800 ± 70 | 2.0 | 17 ± 3 | ND | 140 ± 5 | 2.1 | + | ND |
| Y414C | 653 ± 136 | 1,509 ± 420 | 2.3 | 22 ± 3 | ND | 109 ± 19 | 1.5 | + | ND |
| WT dt-hPAH | - | 1,620 ± 80 | - | 31 ± 1 | ND | 60 ± 5 | 1.0 | + | ND |
| A313T dt-hPAH | - | 1,290 ± 70 | - | 30 ± 3 | ND | 36 ± 3 | 1.2 | + | ND |
Steady-state kinetic parameters of the WT and mutant PAH tetrameric fusion proteins expressed in E. coli. The data include the specific activity with and without prior incubation with l-Phe (activated and non-l-Phe activated), apparent affinity for l-Phe [S0.5 (l-Phe)] and BH4 (Km), and h as a measure of positive cooperativity; equilibrium-binding affinity (Kd) for BH4. ND, not determined.
Obtained with non-l-Phe preincubated enzyme, assayed with 1 mM l-Phe and 75 μM BH4
Obtained with l-Phe preincubated enzyme, assayed with 1 mM l-Phe and 75 μM BH4
Obtained with non-l-Phe preincubated enzyme, assayed with 1 mM l-Phe and variable [BH4] (0-200 μM)
Obtained from equilibrium-binding measurements by isothermal titration calorimetry
Obtained with l-Phe preincubated enzyme, assayed with 75 μM BH4 and variable [l-Phe] (0-4 mM)
Mol l-Tyr produced per mol BH4 oxidized. Data are mean ± SD from five to six independent experiments
Coupling of BH4 Oxidation to l-Tyr Formation. The hydroxylation reaction in wt-PAH is tightly coupled to the oxidation of the natural cofactor BH4. Recently, two severe PKU mutations, R158Q and E280K, were found to display a decoupled oxidation of the cofactor (22). These mutants, which are at locations important for maintaining the correct shape of the active site, both oxidize pterin at more than twice the rate of WT enzyme, and the reactions are only ≈20% coupled to production of l-Tyr. Incorrect formation of the cofactor-binding site would thus seem to be deleterious for coupling efficiency. We therefore analyzed the coupling efficiency of a number of selected BH4 responsive mutations from our study, including some with defective BH4 binding. The changes in stability, activity, binding affinity, and cooperativity seen in the mutants studied here are not reflected in a decoupling of cofactor (BH4) oxidation upon hydroxylation of substrate (Table 2), as would be expected if the binding site for BH4 had been structurally altered to a large extent due to a mutation, with consequent defective formation of a five-coordinate Fe(II)-l-Phe–BH4 complex (27). The decoupling observed in a previous study on the R158Q and E280K mutations was found to be a result of a distortion of the Fe(II) ligation and BH4 binding. Theoretical calculations (28) support the findings on BH4 decoupling in the more severe mutants, because the initial formation of a pterin–peroxy intermediate can occur only if BH4 is properly oriented for formation of an Fe-OO-BH4 bridge and an open coordination position is available on the Fe(II).
The Effect of BH4 on the Stability of PAH Mutants. The effect of BH4 on the stability of the proteins was first studied by measuring the thermostability by circular dichroism spectroscopy of wt-PAH and mutants R68S and A300S in the presence and absence of BH4, essentially as described (29). The thermal denaturation of full-length PAH results in two unfolding transitions with melting temperature (Tm) values at 45–46 and 54°C, corresponding to the N-terminal regulatory and C-terminal catalytic domains of wt-PAH, respectively. The addition of BH4 results in a concentration-dependent increase of Tm for both transitions, with end points at 49°C and 59°C for the regulatory and catalytic domains, respectively, at 250 μMBH4. R68S was found to be similarly stabilized by BH4, with the only difference from wt-PAH being the concentration of BH4 for maximal thermal stabilization (500 μM), a finding that could be related to its reduced affinity (Kd) for BH4 (Table 2). Thus, for the mutant A300S, with an affinity for BH4 similar to wt-PAH (Table 2), the circular dichroism-measured Tm values and the concentration of BH4 for maximal stabilization were found to be similar to wt-PAH. To further analyze the putative chemical chaperone effect of BH4, correcting misfolding and proteolytic degradation of the mutant proteins, we have used pulse–chase analysis after in vitro synthesis in the cell-free in vitro transcription-translation (TnT) system (30, 31). We measured the half-life of the proteins after synthesis in the TnT system and found that all mutants tested except R68S and A300S degraded more rapidly than wt-PAH (Table 4), indicative of folding defects of varying degrees. Interestingly, a significant increase in half-life was observed for V388M and Y414C, whereas for F39L, A373T, and E390G, a slight stabilization in half-life was observed.
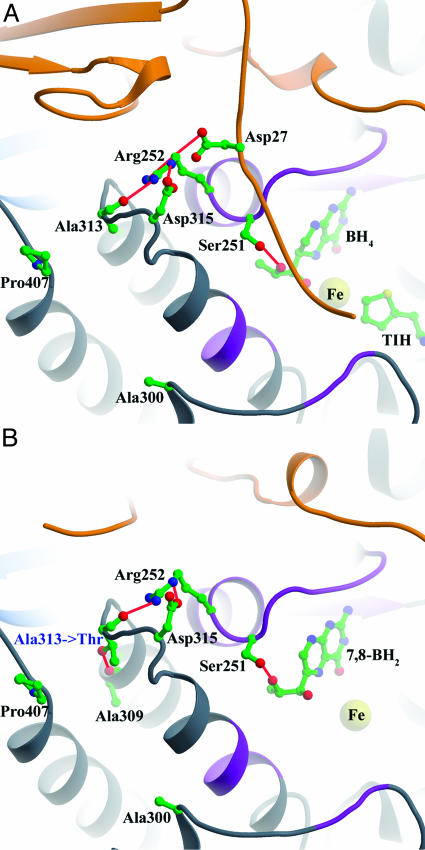
Structural Determination of the A313T-PAH BH4-Responsive Mutant. To investigate the effects of the BH4-responsive mutations on the structure of PAH, we tried to crystallize several mutant forms from this study. Double-truncated (N- and C-terminal) and single-truncated (C-terminal) PAH does crystallize, and many structures of these forms of human and rat PAH have been determined in our lab (22, 32–35). In this study, only one mutant crystallized: the A313T dt-PAH mutant. X-ray diffraction data were collected for both ligand-free and 7,8-BH2-bound A313T dt-PAH (Table 6, which is published as supporting information on the PNAS web site). The structure of mutant A313T-dt-PAH is shown in Fig. 2B. A superposition of the WT dt-hPAH structures with and without 7,8-BH2, and A313T-dt-PAH structures determined in the current study, with and without 7,8-BH2, and a plot showing the residual differences (rms deviation, RMSD) among the same structures are shown in Figs. 3 and 4, which are published as supporting information on the PNAS web site.
Fig. 2.
(A) Ala-313 environment in wt-PAH composite full-length model. (B) Thr-313 environment in the A313T-dt-PAH·7,8-BH2 structure. The color scheme is as in Fig. 1.
Small individual differences were observed between the mutant and WT, ligand-bound, and nonligand-bound dt-PAH structures. The overall RMSD of the WT dt-hPAH structure (PDB ID code 1PAH) versus the WT dt-PAH structure with 7,8-BH2 bound (32) is 0.26 Å. The pterin-binding region between amino acids 245 and 250 was found to move ≈1.3 Å in the direction of the iron, thus allowing several important hydrogen bonds to the pterin ring to be formed upon cofactor binding in the WT dt-PAH enzyme (32). This induced fit-type of cofactor binding is believed to be necessary to form an active site that is properly arranged for l-Phe and O2 binding (32–34). In the A313T dt-PAH mutant structure, only four regions appear to have shifted as compared with the WT dt-PAH structure (1PAH) (Figs. 3 and 4). These are located in the pterin-binding region (amino acids 247 and 248) and regions 336–339, the 380s loop (residues 377–379), and residues 411–414. No large differences are seen when comparing the A313T-PAH and A313T-PAH·7,8-BH2 structures (the overall RMSD for A313T dt-hPAH·7,8-BH2 versus A313T dt-PAH is 0.13 Å). Thus, the conformational changes observed in WT dt-PAH upon cofactor binding (32), as mentioned above, seem to already have been induced in the A313T dt-PAH mutant, even without cofactor bound. The overall RMSD for A313T dt-PAH·7,8-BH2 versus wt-PAH·7,8-BH2 is 0.20 Å.
Discussion
Most BH4-responsive patients have a mild HPA or moderate PKU phenotype and harbor at least one associated missense mutation, although some classic PKU patients have been found to be BH4 responsive (13). This observation suggests that some residual PAH activity (in vivo) is a prerequisite for BH4 responsiveness, and that mutations causing severe structural distortion in the expressed protein leading to undetectable PAH activity are not likely to be stimulated by BH4. In our kinetic characterization of 15 BH4-responsive PAH mutants, we have found that all mutants studied have >30% residual activity as compared with wt-PAH (except V388M, which has 23% residual activity). In most of the mutations studied, some kinetic defects can be detected. A reduced apparent binding affinity for BH4, originally hypothesized as the reason for BH4-responsive PKU (5), however, was found in only a few mutants: F39L, I65T, and L308F. A clearly increased Kd was observed by isothermal titration calorimetry only for the mutants F39L and R68S, both at the N-terminal domain of the enzyme.
More relevant for interpreting BH4 responsiveness are the results on the in vitro transcription-translation expression system, which suggests that the cofactor has a significant role as a chemical chaperone, preventing misfolding, degradation, or inactivation of mutant PAH. For the mutants F39L, A373T, V388M, E390G, and Y414C, an increase in the half-life (T1/2) of mutant PAH was observed upon expression with BH4 in the expression media. However, only for Y414C was the half-life restored to WT levels by BH4. The metabolic availability of BH4 in vivo has been shown in hepatocytes to be sequestered by forming a PAH·BH4 complex, which has much less activity and is less readily l-Phe-activated than the uncomplexed enzyme (35). The less-active complex between BH4 and PAH may play an important physiological role in that it can stabilize some mutants and wt-PAH and thus prevent the ubiquitin-dependent degradation (36) in resting situations in the absence of high concentrations of l-Phe. A nonspecific role for the cofactor in protecting not only PAH protein integrity, but also the activity of essentially all mutants, by preventing a chemical inactivation has recently been put forward (20). Thus, the binding of BH4 at saturating concentrations might additionally prevent peroxide formation due to uncoupled reactions [hydrogen peroxide is known to inactivate PAH (37, 38)] and protect the right configuration of the active site in PKU mutants, independent of the location of the mutation.
Previous crystal structure determinations of truncated forms of PAH (22, 39–41) have shown that the monomer has a three-domain structure. The substrate l-Phe, which activates the tetrameric enzyme by cooperative homotropic binding, is proposed to induce a conformational change that displaces an N-terminal autoregulatory sequence from its position in the active site (40, 42). The cofactor and cosubstrate BH4, on the contrary, acts as a negative regulator, blocking the l-Phe-binding site (43). Recent structural and mutational studies of hinge-bending regions (41, 42, 44) have revealed further evidence that global activating conformational changes caused by l-Phe binding have the epicenter at the active site and are transmitted throughout the enzyme through hinge-bending motions. A reduction or loss of the cooperative substrate-dependent activation has been seen for most of the kinetically characterized PKU mutations to date (20, 45, 46), severe as well as mild forms, suggesting that the enzyme is quite susceptible to mutations that destroy the cooperative activation mechanism probably by hindering the transmission of the conformational change. The kinetic Hill coefficient (h) shows that some of the mutants tested in our study displayed a complete loss of cooperativity, in particular the I65T, E178G, R261Q, A300S, and V388M mutants (h = 1.1 or 1.0) (Table 2). The other mutants have a slightly lowered h of between 1.4 and 2.0, as compared with 2.0 in wt-PAH. Three mutations stand out with increased h: H170D, V190A, and P407S (2.8, 2.9, and 2.1, respectively). The reason for this high cooperativity may be in the relatively high BH4-binding affinity [lower than WT Km(BH4)] observed for these three mutants (12 ± 2, 17 ± 2, and 17 ± 3), as compared with wt-PAH [Km(BH4) = 26 ± 3], whereas the substrate affinity is practically the same as for WT. These mutants also retain close to or higher than WT activation, with 2.5-, 4.2-, and 2.0-fold l-Phe activation. Thus, there must exist other, still unexplored, mechanisms for BH4 responsiveness, in addition to a correction of catalytic defects (notably for the mutants with lower affinity for BH4) and stabilization, in particular because these mutations with high affinity for BH4, which putatively would inhibit the enzyme reaction, are responsive in vivo.
Very recently, Kure et al. (47) presented in vivo results on BH4 supplementation in PKU mice, showing an ≈35% increase in activity for normal (WT) animals upon treatment. Their concluding hypothesis was that the responsiveness to BH4 in patients with PAH deficiency was due to the suboptimal physiological concentrations of BH4 normally present in hepatocytes and to the enhancement of the residual activity upon supplementation to higher than physiological concentrations. A hepatic concentration of BH4 of 5–10 μM has been measured (48–50). Michaelis–Menten kinetics tells us that the enzyme is not saturated at this concentration (Km = 12–44 μM; Table 2), and thus the activity will increase with increasing concentration of BH4. However, the concentration of BH4 that is functional in both treated and nontreated animals during catalysis was not determined by Kure et al. (47), and this issue remains to be investigated. Moreover, the observed modest increase in activity does not appear to be enough to explain the positive response and the rescue in most human BH4-responsive mutants and, more importantly, our results show specific effects of BH4 on the mutant PAH protein and specific activity (ref. 20 and this work). Thus, after in vitro transcription translation synthesis in the presence of BH4, the activity measured at a fixed concentration of BH4 (75 μM, which is the standard used by most research groups) increases both in mutant and wt-PAH (20).
Conclusion
The molecular mechanisms for BH4 responsiveness potentially increase the amount of active enzyme in vivo, as well as mutant PAH activity. The BH4-responsive patients would therefore reach the lower-limit value of l-Phe hydroxylation, allowing the catabolism of l-Phe amounts usually present in normal diets. Based on the relatively high residual activity and generally small kinetic and structural defects of the responsive mutations studied here, it appears that this lower-limit or threshold value would be close to that of the heterozygous state, with a WT gene copy in combination with a functionally hemizygous null mutation. Importantly, we have been able to discern that the BH4 response is mainly a result of a correction by BH4 of PAH mutant kinetic defects and/or stabilization defects, distributed throughout the PAH structure. However, all patients with mutations associated with any residual activity should be tested for BH4 responsiveness to improve the current models.
Supplementary Material
Acknowledgments
We thank Mary Straub, Ali Javier Sepulveda, Albert E. Beuscher, and Randi M. Svebak for expert technical assistance. This work received funding from the National Institutes of Health (HD038718), the Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo [REDEMETH (G03/054), RECGEN C03/07, and PI020117], the Comisión Interministerial de Ciencia y Tecnología, Spain (SAF20010544), and the Research Council of Norway. An institutional grant from Fundación Ramón Areces to Centro de Biología Molecular is gratefully acknowledged.
Author contributions: H.E., R.K., S.S., S.T., R.M., C.R.S., M.U., A.M., and R.S. designed research; A.L.P., A.G., B.P., L.R.D., C.A., A.M., L.R.D., B.P., and R.S. performed research; A.L.P., A.G., B.P., L.R.D., C.A., A.M., and R.S. contributed new reagents or analytic tools; H.E., A.L.P., A.G., B.P., L.R.D., C.A., R.K., S.S., S.T., R.M., C.R.S., M.U., A.M., and R.S. analyzed data; H.E., A.M., and R.S. wrote the paper.
Abbreviations: BH4,(6R)-l-erythro-5,6,7,8-tetrahydrobiopterin; HPA, hyperphenylalaninemia; PAH, phenylalanine hydroxylase; wt-PAH, WT PAH; dt-PAH, double-truncated PAH; PKU, phenylketonuria; RMSD, rms deviation; h, Hill coefficient.
Data deposition: The protein structures have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 1TDW and 1TG2 for the PKU mutation A313T and the A313T 7,8-BH2-bound form, respectively).
References
- 1.Scriver, C. R. & Kaufman, S. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), pp. 1667-1724.
- 2.Scriver, C. R., Hurtubise, M., Konecki, D., Phommarinh, M., Prevost, L., Erlandsen, H., Stevens, R., Waters, P. J., Ryan, S., McDonald, D., et al. (2003) Hum. Mutat. 21, 333-344. [DOI] [PubMed] [Google Scholar]
- 3.de la Cruz, F. & Koch, R. (2001) Clin. Perinatol. 28, 419-424. [DOI] [PubMed] [Google Scholar]
- 4.Niederwieser, A. & Curtius, H. C. (1985) in Inherited Diseases of Amino Acid Metabolism, eds. Bickel, H. & Wachtel, U. (Georg Thieme, Stuttgart), pp. 104-121.
- 5.Kure, S., Hou, D. C., Ohura, T., Iwamoto, H., Suzuki, S., Sugiyama, N., Sakamoto, O., Fujii, K., Matsubara, Y. & Narisawa, K. (1999) J. Pediatr. 135, 375-378. [DOI] [PubMed] [Google Scholar]
- 6.Trefz, F. K., Aulela-Scholz, C. & Blau, N. (2001) Eur. J. Pediatr. 160, 315. [DOI] [PubMed] [Google Scholar]
- 7.Lindner, M., Haas, D., Mayatepek, E., Zschocke, J. & Burgard, P. (2001) Mol. Genet. Metab. 73, 104-106. [DOI] [PubMed] [Google Scholar]
- 8.Blau, N. & Trefz, F. K. (2002) Mol. Genet. Metab. 75, 186-187. [DOI] [PubMed] [Google Scholar]
- 9.Weglage, J., Grenzebach, M., von Teeffelen-Heithoff, A., Marquardt, T., Feldmann, R., Denecke, J., Godde, D. & Koch, H. G. (2002) J. Inherit. Metab. Dis. 25, 321-322. [DOI] [PubMed] [Google Scholar]
- 10.Muntau, A. C., Roschinger, W., Habich, M., Demmelmair, H., Hoffmann, B., Sommerhoff, C. P. & Roscher, A. A. (2002) N. Engl. J. Med. 347, 2122-2132. [DOI] [PubMed] [Google Scholar]
- 11.Spaapen, L. J. & Rubio-Gozalbo, M. E. (2003) Mol. Genet. Metab. 78, 93-99. [DOI] [PubMed] [Google Scholar]
- 12.Bernegger, C. & Blau, N. (2002) Mol. Genet. Metab. 77, 304-313. [DOI] [PubMed] [Google Scholar]
- 13.Matalon, R., Koch, R., Michals-Matalon, K., Moseley, K., Surendran, S., Tyring, S., Erlandsen, H., Gamez, A., Stevens, R. C., Romstad, A., et al. (2004) Genet. Med. 6, 27-32. [DOI] [PubMed] [Google Scholar]
- 14.Blau, N. & Erlandsen, H. (2004) Mol. Genet. Metab. 82, 101-111. [DOI] [PubMed] [Google Scholar]
- 15.Scriver, C. R. & Waters, P. J. (1999) Trends Genet. 15, 267-272. [DOI] [PubMed] [Google Scholar]
- 16.Hyland, K., Gunasekara, R. S., Munk-Martin, T. L., Arnold, L. A. & Engle, T. (2003) Ann. Neurol. 54, S46-S48. [DOI] [PubMed] [Google Scholar]
- 17.Linscheid, P., Schaffner, A. & Schoedon, G. (1998) Biochem. Biophys. Res. Commun. 243, 137-141. [DOI] [PubMed] [Google Scholar]
- 18.Knappskog, P. M., Flatmark, T., Aarden, J. M., Haavik, J. & Martinez, A. (1996) Eur. J. Biochem. 242, 813-821. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan, P. T. & Pei, D. (1998) J. Biol. Chem. 273, 22305-22310. [DOI] [PubMed] [Google Scholar]
- 20.Pey, A. L., Pérez, B., Desviat, L. R., Martínez, M. A., Erlandsen, H., Gámez, A., Stevens, R. C., Ugarte, M. & Martínez, A. (2004) Hum. Mutat. 24, 388-399. [DOI] [PubMed] [Google Scholar]
- 21.Otwinowski, Z. & Minor, W. (1997) in Methods in Enzymology, eds. Carter, C. W., Jr. & Sweet, R. M. (Academic, New York), Vol. 276, pp. 307-326. [DOI] [PubMed] [Google Scholar]
- 22.Erlandsen, H., Fusetti, F., Martinez, A., Hough, E., Flatmark, T. & Stevens, R. C. (1997) Nat. Struct. Biol. 4, 995-1000. [DOI] [PubMed] [Google Scholar]
- 23.Andersen, O. A., Flatmark, T. & Hough, E. (2001) J. Mol. Biol. 314, 279-291. [DOI] [PubMed] [Google Scholar]
- 24.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 25.Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. (1991) Acta Crystallogr. A 47, 110-119. [DOI] [PubMed] [Google Scholar]
- 26.Erlandsen, H. & Stevens, R. C. (1999) Mol. Genet. Metab. 68, 103-125. [DOI] [PubMed] [Google Scholar]
- 27.Kemsley, J. N., Wasinger, E. C., Datta, S., Mitic, N., Acharya, T., Hedman, B., Caradonna, J. P., Hodgson, K. O. & Solomon, E. I. (2003) J. Am. Chem. Soc. 125, 5677-5686. [DOI] [PubMed] [Google Scholar]
- 28.Bassan, A., Blomberg, M. R. & Siegbahn, P. E. (2003) Chemistry 9, 106-115. [DOI] [PubMed] [Google Scholar]
- 29.Thorolfsson, M., Fojan, P., Petersen, S. B. & Martinez, A. (2000) Pteridines 11, 29-31. [Google Scholar]
- 30.Waters, P. J., Parniak, M. A., Akerman, B. R. & Scriver, C. R. (2000) Mol. Genet. Metab. 69, 101-110. [DOI] [PubMed] [Google Scholar]
- 31.Desviat, L. R., Pérez, B. & Ugarte, M. (2003) in Protein Misfolding and Disease. Principles and Protocols, eds. Bross, P. & Gregersen, N. (Humana, Totowa, NJ), pp. 57-264.
- 32.Erlandsen, H., Bjørgo, E., Flatmark, T. & Stevens, R. C. (2000) Biochemistry 39, 2208-2217. [DOI] [PubMed] [Google Scholar]
- 33.Erlandsen, H., Flatmark, T., Stevens, R. C. & Hough, E. (1998) Biochemistry 37, 15638-15646. [DOI] [PubMed] [Google Scholar]
- 34.Andersen, O. A., Flatmark, T. & Hough, E. (2002) J. Mol. Biol. 320, 1095-1108. [DOI] [PubMed] [Google Scholar]
- 35.Mitnaul, L. J. & Shiman, R. (1995) Proc. Natl. Acad. Sci. USA 92, 885-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Døskeland, A.P. & Flatmark, T. (1996) Biochem. J. 319, 941-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milstien, S., Dorche, C. & Kaufman, S. (1990) Arch. Biochem. Biophys. 282, 346-351. [DOI] [PubMed] [Google Scholar]
- 38.Kappock, T. J. & Caradonna, J. P. (1996) Chem. Rev. 96, 2659-2756. [DOI] [PubMed] [Google Scholar]
- 39.Fusetti, F., Erlandsen, H., Flatmark, T. & Stevens, R.C. (1998) J. Biol. Chem. 273, 16962-16967. [DOI] [PubMed] [Google Scholar]
- 40.Kobe, B., Jennings, I. G., House, C. M., Michell, B. J., Goodwill, K. E., Santarsiero, B. D., Stevens, R. C., Cotton, R. G. & Kemp, B. E. (1999) Nat. Struct. Biol. 6, 442-448. [DOI] [PubMed] [Google Scholar]
- 41.Andersen, O. A., Stokka, A. J., Flatmark, T. & Hough, E. (2003) J. Mol. Biol. 333, 747-757. [DOI] [PubMed] [Google Scholar]
- 42.Thorolfsson, M., Teigen, K. & Martinez, A. (2003) Biochemistry 42, 3419-3428. [DOI] [PubMed] [Google Scholar]
- 43.Teigen, K. & Martinez, A. (2003) J. Biomol. Struct. Dyn. 20, 733-740. [DOI] [PubMed] [Google Scholar]
- 44.Stokka, A. J., Carvalho, R. N., Barroso, J. F. & Flatmark, T. (2004) J. Biol. Chem. 279, 26571-26580. [DOI] [PubMed] [Google Scholar]
- 45.Bjørgo, E., Knappskog, P. M., Martinez, A., Stevens, R. C. & Flatmark, T. (1998) Eur. J. Biochem. 257, 1-10. [DOI] [PubMed] [Google Scholar]
- 46.Leandro, P., Rivera, I., Lechner, M. C., de Almeida, I. T. & Konecki, D. (2000) Mol. Genet. Metab. 69, 204-212. [DOI] [PubMed] [Google Scholar]
- 47.Kure, S., Sato, K., Fujii, K., Aoki, K., Suzuki, Y., Kato, S. & Matsubara, Y. (2004) Mol. Genet. Metab. 83, 150-156. [DOI] [PubMed] [Google Scholar]
- 48.Fukushima, T. & Nixon, J. C. (1980) Anal. Biochem. 102, 176-188. [DOI] [PubMed] [Google Scholar]
- 49.Duch, D. S., Bowers, S. W., Woolf, J. H. & Nichol, C. A. (1984) Life Sci. 35, 1895-1901. [DOI] [PubMed] [Google Scholar]
- 50.Harding, C. O., Neff, M., Wild, K., Jones, K., Elzaouk, L., Thony, B. & Milstien, S. (2004) Mol. Genet. Metab. 81, 52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.