Abstract
Low resting heart rate variability (HRV) is associated with a broad array of negative psychosocial outcomes. Recent theoretical explications of HRV suggest it is an autonomic marker of emotion regulation capacity, but limited research has examined its relationship with emotional information processing indices. The present study utilized eye-tracking methodology to test HRV’s theorized role as a marker of emotion regulation capacity in a non-clinical sample. Attentional biases towards threatening, dysphoric, and positive emotional information as well as affective modulation of pupil size were assessed before and after a stress induction. Low resting HRV marginally predicted larger increases in attentional bias towards positive emotional stimuli from pre to post-stress induction and significantly predicted decreased pupil dilation to positive stimuli after the stress induction only; exploratory analyses suggested that this pattern might reflect an unsuccessful attempt at anxious mood repair. HRV was unrelated to negative emotional information processing. Findings are consistent with existing theories of HRV’s psychological significance and suggest a specific association with altered positive emotional processing under acute stress.
Keywords: attentional bias, eye-tracking, heart rate variability, mood induction, pupil, emotion regulation
Introduction
Low parasympathetically-mediated heart rate variability (HRV) has emerged as a robust correlate of negative psychosocial outcomes across multiple domains (e.g., presence of psychopathology, poor self-regulation, decreased emotion recognition ability; Beauchaine & Thayer, 2015; Quintana, Guastella, Outhred, Hickie, & Kemp, 2012; Segerstrom & Nes, 2007). Resting HRV, a measure of the variability in an individual’s beat-to-beat intervals while in a resting state, is thought to index parasympathetic influence on the heart and has been linked with psychosocial functioning in non-clinical and clinical samples (Beauchaine, 2015; Beauchaine & Thayer, 2015; Ottaviani et al., 2016). Recent reviews of the HRV literature suggest that low HRV may confer risk for poor psychosocial functioning because it is a marker of emotion dysregulation, which is implicated in most forms of psychopathology (Beauchaine, 2015; Beauchaine & Thayer, 2015). Elucidating the specific mechanisms through which low HRV operates is a critical step towards enhancing our understanding of low HRV’s broad associations with negative psychosocial outcomes.
Converging evidence suggests that low HRV is associated with negative psychosocial outcomes because it is a peripheral marker of emotion dysregulation. Specifically, HRV is linked to the connectivity between the PFC and subcortical regions (Beauchaine & Thayer, 2015; Thayer, Hansen, Saus-Rose, & Johnsen, 2009) which plays an important role in adaptive emotional responses to stressors (Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). This hypothesis stems from evidence demonstrating 1) inhibitory pathways from the PFC to the parasympathetic nervous system (PNS), 2) a positive relationship between HRV and executive functioning tasks, and 3) neuroimaging evidence linking greater HRV with greater PFC functioning (see Beachaine & Thayer, 2015 and Thayer et al., 2009 for reviews). Thus, theories of the psychological significance of HRV emphasize the regulation of emotional responding (i.e., PFC-subcortical connectivity) as central to the relationship between HRV and psychopathology (e.g., Appelhans & Luecken, 2006; Beauchaine, 2015; Thayer, Hansen, Saus-Rose, & Johnsen, 2009), but more research is needed to understand the specific mechanisms through which HRV influences maladaptive emotional responding.
HRV may be linked with emotion dysregulation via its influence on emotional information processing. Within cognitive models of affective psychopathology, information processing abnormalities are closely tied to maladaptive emotional responding (Beck & Clark, 1988). Specifically, exaggerated processing of negative emotional information and decreased processing of positive emotional information have been implicated in multiple forms of affective psychopathology (Armstrong & Olatunji, 2012). One well-established behavioral index of maladaptive emotional information processing is attention bias (MacLeod, Mathews, & Tata, 1986; Leppän, 2006). Negative attention bias (NAB) refers to preferential attentional processing of negatively-valenced (i.e., threatening, dysphoric) relative to neutral information, whereas positive attention bias (PAB) refers to preferential processing of positively-valenced relative to neutral information. Increased NAB and decreased PAB are believed to be indices of dysfunctional corticolimbic circuitry (see Gibb, McGeary, & Beevers, 2016 for a review) and have been linked with anxiety/depressive symptoms in non-clinical and clinical samples (Armstrong & Olatunji, 2012; Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & Van Ijzendoorn, 2007; Peckham, McHugh, & Otto, 2010). Given the putative role of corticolimbic circuitry in HRV and attention bias as well as their established associations with emotion dysregulation, low HRV may be related to increased NAB and decreased PAB.
Pupillary responding to emotional stimuli is another index of affective processing that may be associated with HRV. Specifically, stimulus-elicited pupil dilation is thought to reflect arousal and accordingly covaries with sympathetic nervous system activity, with greater increases in pupil dilation to positive and negative relative to neutral emotional stimuli (Bradley, Miccoli, Escrig, & Lang, 2008; Partala & Surakka, 2003). Moreover, multiple studies have demonstrated that pupil dilation is indicative of increased cognitive load and greater allocation of attentional resources independent of the effects of arousal (Stanners, Coulter, Sweet, & Murphy, 1979; Urry, van Reekum, Johnstone, & Davidson, 2009; van Reekum et al., 2007). Consistent with the notion that pupil dilation reflects both emotional (i.e., autonomic arousal) and cognitive (i.e., covert attentional allocation) processes, greater sustained pupil dilation to negative personally-relevant information and dysphoric facial stimuli has been positively associated with individual differences in the propensity to engage in sustained, elaborative processing of negative affect (i.e., trait rumination; Duque, Sanchez, & Vazquez, 2014; Siegle, Steinhauer, Carter, Ramel, & Thase, 2003). Likewise, with regard to pupil dilation to positive emotional information, one study found that greater pupil dilation to high-reward food stimuli was significantly correlated with state food craving (Graham, Hoover, Ceballos, & Komogortsev, 2011), which has been conceptualized as a form of elaborative cognitive processing of reward cues (May, Andrade, Kavanagh, & Hetherington, 2012). Taken together, these data suggest that pupil dilation to emotional stimuli is indicative of sustained allocation of cognitive resources as well as autonomic arousal, and may, as with attention bias, at least partially reflect neural activity in prefrontal regions implicated in emotion regulation (e.g., Siegle, Steinhauer, Friedman, Thompson, & Thase, 2011). As such, resting HRV may be associated with pupillary responding to emotional information.
To our knowledge, no studies to date have investigated if HRV is associated with either attention bias or pupil dilation. However, there is indirect evidence to suggest a relationship between these indices of emotional processing and HRV. For instance, a number of studies have found low HRV to be associated with rumination (Ottaviani, Shapiro, Davydov, Goldstein, & Mills, 2009; Williams et al., 2015; Woody, McGeary, & Gibb, 2014), which has been linked with greater attentional bias towards dysphoric stimuli as well as greater pupil dilation to negative stimuli (Duque, Sanchez, & Vazquez, 2014; Siegle, Steinhauer, Carter, Ramel, & Thase, 2003). Relevant experimental evidence also supports an association between low HRV and increased emotional processing of negative information. Krypotos, Jahfari, van Ast, Kindt, and Forstmann (2011) showed that individuals with low HRV demonstrated greater distractor interference on a response inhibition task only when distractors were of a negative, but not neutral, valence. The association between low HRV and reduced task performance in the context of task-irrelevant negative emotional stimuli suggests that HRV moderates the effects of distracting negative emotional stimuli on attention, potentially by increasing emotional processing of task-irrelevant negative information, resulting in decreased task engagement (Krypotos, Jahfari, van Ast, Kindt, & Forstmann, 2011). Low HRV has also been associated with greater spontaneous and unsuccessful suppression of a negative, personally-relevant intrusive thought, providing further evidence of HRV’s role in maladaptive attentional processing of negative emotional information (Gillie, Vasey, & Thayer, 2015). Relatedly, a recent meta-analysis conducted by Ottaviani et al. (2016) found low HRV to be related to perseverative cognition symptoms which are defined by excessive attention towards repetitive, negatively-valenced thoughts. To summarize, extant indirect evidence suggests that low HRV should predict increased attentional bias towards negative emotional information as well as enhanced pupil dilation while viewing negative emotional stimuli, though to our knowledge this has never been explicitly tested.
With respect to positive emotional processing, multiple studies have found robust cross-sectional and prospective relationships between low HRV and depression (Jandackova, Britton, Malik, & Steptoe, 2016; Kemp et al., 2010; Vazquez et al., 2016), a disorder characterized in part by decreased attention bias towards positive stimuli and blunted reward processing (Armstrong & Olatunji, 2012; Heshmati & Russo, 2015). More pertinently, two recent studies suggest that low HRV might be specific to anhedonia compared to other symptoms of depression and anxious arousal (Sanders & Abaied, 2015; Vazquez et al., 2016). Thus, given data that suggests pupil dilation in response to reward cues reflects reward processing (Graham et al., 2011; Kennerley & Wallis, 2009; O’Doherty, Buchanan, Seymour, & Dolan, 2006; O’Doherty, Dayan, Friston, Critchley, & Dolan, 2003; Sepeta et al., 2012; Steinhauer & Hakerem, 1992), decreased pupil dilation while viewing positive emotional stimuli might be associated with low HRV. Additional evidence supportive of a relationship between low HRV and decreased pupil dilation and attentional bias towards positive emotional stimuli comes from work on emotion regulation and positive emotional information processing. Two separate studies found that more efficient shifts in processing away from neutral and towards positive-valenced stimulus features predicted decreased rumination in daily life and greater ability to downregulate negative affect in response to a negative mood induction (Genet, Malooly, & Siemer, 2013; Malooly, Genet, & Siemer, 2013), suggesting that greater attentional processing of positive emotional information is important for adaptive emotion regulation. Given low HRV’s status as a putative marker of emotion dysregulation (Beauchaine & Thayer, 2015) and its relationship with depression (Jandackova, Britton, Malik, & Steptoe, 2016; Kemp et al., 2010; Vazquez et al., 2016), low HRV may be linked with decreased overt attentional processing of positive emotional information (i.e., indexed by PAB) as well as reduced covert attentional allocation and/or emotional arousal (i.e., indexed by pupil dilation) while viewing positive emotional stimuli.
It is also plausible that the relations between HRV and attention bias/pupil dilation are more pronounced under acute stress. The modulation of emotional processing by mood context is a well-replicated finding (e.g., Bradley, Mogg, & Lee, 1997; Mansell, Clark, Ehlers, & Chen, 1999; Hallion & Ruscio, 2011). A meta-analysis by Hallion and Ruscio (2011) found that modification of attention biases in anxiety were stronger and more robustly linked with symptoms following a stressor. Similarly, Mansell, Clark, Ehlers, and Chen (1999) found attention biases among socially anxious individuals only while under social-evaluative threat. Other research has demonstrated that threat and dysphoric attentional biases are more pronounced among those experiencing high levels of anxiety and dysphoria respectively (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoom 2007; Peckham et al., 2010), suggesting that the experience of negative affective states modulate the emergence of emotional processing biases. Although little research has been conducted on the modulatory role of mood context on pupillary response to emotional information, there is some data to suggest that state mood (e.g., food craving) and related internal contextual variables (e.g., sleep deprivation status) are associated with the degree of pupillary response to affective information (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009; Graham et al., 2011). Given that low HRV is associated with emotion dysregulation (Appelhans & Luecken, 2006; Beauchaine, 2015; Thayer, Hansen, Saus-Rose, & Johnsen, 2009) and emotional information processing is sensitive to affective state (Bradley, Mogg, & Lee, 1997; Graham et al., 2011; Hallion & Ruscio, 2011; Mansell, Clark, Ehlers, & Chen, 1999), evaluating the influence of mood state on the relationship between HRV and indices of maladaptive emotion information processing is critical.
The current study seeks to evaluate the relationship between low HRV and maladaptive emotional information processing. This investigation was designed to test the hypothesis that low resting HRV will be associated with increased negative emotional processing (as indexed by NAB and pupil dilation change) as well as decreased positive emotional processing (as indexed by PAB and pupil dilation change) specifically under acute stress. This hypothesis arises out of recent reviews (Beauchaine, 2015; Beauchaine & Thayer, 2015) and extant theories (Appelhans & Luecken, 2006; Beauchaine, 2015; Thayer, Hansen, Saus-Rose, & Johnsen, 2009) positing that HRV is a proxy for regulated emotional responding, and emotional responding is closely tied to emotional information processing (Beck & Clark, 1988). Extant data suggests that greater attentional bias/pupil dilation towards negative emotional information and decreased attentional bias/pupil dilation towards positive emotional information is related to maladaptive emotional information processing (e.g., rumination, anhedonia) and associated psychopathology (e.g., anxiety/depression) (Armstrong & Olatunji, 2012; Duque et al., 2014; Genet et al., 2013; Graham et al., 2011; Malooly et al., 2013; Sepeta et al., 2012; Siegle et al., 2003). Thus, if low HRV is an autonomic marker of poor PFC functioning, particularly as it relates to inhibition of limbic regions, then it should predict behavioral (i.e., increased NAB, decreased PAB) and physiological (i.e., increased pupil dilation while viewing negative stimuli, decreased pupil dilation while viewing positive stimuli) indices of maladaptive emotional processing under stress. To test these predictions, eye-tracking methodology was utilized to measure PAB and two forms of NAB (i.e., threat, dysphoric) as well as affective modulation of pupil size before and after a laboratory stressor. We hypothesized that lower resting HRV would predict increased NAB and pupil dilation while viewing negative stimuli as well as decreased PAB and pupil dilation while viewing positive stimuli from pre- to post-stress induction.
Method
Participants
To test our hypotheses, we utilized a student sample that was recruited as part of a larger study on emotion dysregulation from the undergraduate psychology population at a large southeastern university (N = 165; 77% female; M age = 19.32, SD = 1.96). The findings presented in this paper have not been previously presented in any other submitted or published work. Given the larger study aims, individuals scoring in the top quartile on a measure of distress intolerance (Distress Intolerance Index; McHugh & Otto, 2012), a construct strongly related to emotion dysregulation (McHugh, Reynolds, Leyro, & Otto, 2013), were oversampled. Thus, the present sample was expected to contain sufficient variability in individuals prone to maladaptive emotional information processing. The sample was predominantly Caucasian (70.9%), although other ethnicities were also represented (Hispanic: 13.3%, African-American: 11.5%, Asian: 1.2%, American Indian or Alaskan Native: 0.6%, Other: 2.4%).
Measures
Questionnaires
State mood
Three visual analog scales (VASs) for each mood state (happy, anxious, sad) were used to assess state mood at three different times during the experiment. Each scale consisted of an emotion word and a line with 11 anchor points ranging from 0 (not at all) to 10 (very much); participants were asked to indicate the extent to which they were experiencing that particular emotion at that moment. Each mood state was assessed using three emotion words (happy mood: optimistic, joyful, happy; anxious mood: nervous, tense, anxious; sad mood: upset, sad, depressed). Mean internal consistencies were excellent for each mood state (happy: α = .95; anxious: α = .93; sad: α = .92; see Table 1 for descriptives).
Table 1.
Change in Affect across Experiment
| Time 1: Pre-Stress | Time 2: Anticipatory Stress | Time 3: Post-stress | |
|---|---|---|---|
|
| |||
| M (SD) | M (SD) | M (SD) | |
| Self-Report | |||
| Happy | 16.27 (7.11) | 16.69 (7.51) | 16.43 (7.74) |
| Depressed | 4.71 (5.69) | 4.23 (5.42) | 3.11 (4.36)*** |
| Anxious | 7.47 (7.19) | 10.43 (8.49)*** | 6.03 (6.53)*** |
| Psychophysiology | |||
| Mean HR | 75.10 (9.30) | 79.31 (10.33)*** | 76.50 (9.94)*** |
Note. HR = Heart Rate.
Significant difference from previous assessment at p < .001.
Attention Task
Stimuli materials
Stimuli were pairs of face images consisting of an emotional and neutral facial expression of the same person. The same image set used by Sanchez and colleagues (2013) was used in the present study. The images were derived from the Karolinska Directed Emotional Faces (KDEF) database (Lundqvist, Flykt, & Ohman, 1998). Images were modified to fit into an oval window, and the hair, neck, and surrounding parts of the pictures were darkened to remove irrelevant aspects of the faces. KDEF images were chosen based upon emotional intensity and prototypicality of the corresponding facial emotion (see Sanchez & Vazquez, 2013 for validation data). The final stimuli set consisted of 36 happy, angry, and sad facial expressions (18 men and 18 women for each emotion), together with a corresponding neutral expression by the same person.
Experimental set-up and attention indices
The attention task consisted of six practice trials and 108 experimental trials in which an emotional and neutral face were presented on opposite sides of the screen (36 happy – neutral, 36 sad – neutral, 36 angry – neutral). Each trial contained one emotion-neutral face pair and trial order was randomized. Emotional and neutral faces were presented equally often on the left and right sides of the screen.
Stimuli were presented on a 41 cm (width) x 30.5 cm (height) screen. Each face was 9 cm (width) x 12.7 cm (height). Facial stimuli were centered on the screen, 18.1 cm apart (measured from their centers). Participants were seated approximately 90 cm from the screen’s center, resulting in a visual angle of approximately 5.7 degrees between each picture’s center and the screen’s center. These dimensions replicate the stimuli size/position ratios used by Sanchez and colleagues (2013).
The attention task design used in the present study was developed by Sanchez and colleagues (2013). Each trial started with a blank black screen for 500ms, followed by a centered white fixation cross for 500ms. A random number then appeared on the center of the screen for 1,000ms. Participants were instructed to fixate on the number and silently name it as quickly as possible; note that participants did not say the number out loud because of concerns that vocalization would affect the positioning of the participant’s head in the mount. This procedure has been used in prior studies to ensure the participant’s gaze is focused on the center of the screen prior to facial stimuli presentation (Calvo & Avero, 2005). After the offset of the random number, an emotion-neutral face pair (happy – neutral, sad – neutral, angry – neutral) was presented for 3,000ms during which time participants were instructed to look at the screen freely. Fixation data recorded during the 3,000ms period was used to calculate an index of emotional information processing (e.g., Kellough, Beevers, Ellis, & Wells, 2008) for each emotion-neutral category. Total dwell time on the emotion face during the 3,000ms period was divided by the sum of the dwell time on the emotion face and neutral face to compute a proportion of time spent fixating on the emotion face (relative to the neutral face) for that trial. The average proportion of time spent fixating on the emotion face across all trials for that particular emotion-neutral category was computed to derive an overall index of attentional bias towards emotional information. At the end of the 3,000ms free viewing period, participants completed a gaze-contingent paradigm on two-thirds of the trials that is not relevant to the present study’s hypotheses. Note that the paradigm involved identification of randomly appearing shape stimuli that were not contingent upon the affective characteristics of the facial stimuli; thus, fixation patterns during the 3,000ms free-viewing period should not be influenced by task demands.
Emotional modulation of pupil size was computed using a procedure suitable for free-viewing paradigms characterized by affective-neutral stimulus pairs (Graham, Hoover, Ceballos, & Komogortsev, 2011). In line with Graham and colleagues’ (2011) rationale, we decided to use pupil size while viewing the paired neutral facial stimulus instead of pupil size during the pre-stimulus onset period (i.e., when the fixation cross and number stimuli are serially presented) as a baseline for the following reasons: 1) In contrast to the fixation cross and number stimuli, the paired neutral facial stimulus is matched with the corresponding emotional facial stimulus across relevant visual properties (e.g., visual complexity, luminosity, color) and both stimuli belong to the salient category of human faces; 2) The paired neutral facial stimulus is matched with the corresponding emotional facial stimulus with regard to screen position given that both stimuli are presented horizontally equidistant from the center of the screen; further, the paired neutral and emotional stimuli are presented an equal number of times on the left and right sides of the screen, creating equivalent degrees of pupil occlusion due to viewing angle for both stimuli. These features of the paired neutral face stimulus make it an ideal baseline from which to compare relative changes in pupil size while viewing the corresponding emotional face because their well-matched visual properties/screen position allow us to confidently interpret changes in pupil size as attributable to the emotionality aspect of the face specifically. The average pupil size during each fixation on the neutral and emotional facial stimuli on a given trial were averaged to create trial-level averaged pupil sizes for the affective and corresponding neutral stimulus. Trial-level averages were themselves averaged to create task-level measures of grand averaged pupil size while viewing the emotional and its corresponding neutral stimulus. Because pupil size is measured in arbitrary units, change scores are considered appropriate for analysis of pupil size data. Emotional modulation of pupil size was computed as a percent change relative to pupil size while viewing the corresponding neutral stimulus (e.g., (Task-Level Average Pupil Size while viewing Angry Facial Stimulus – Task-Level Average Pupil Size while viewing the corresponding Neutral Facial Stimulus) / Task-Level Average Pupil Size while viewing the corresponding Neutral Facial Stimulus x 100).
Eye-tracking device
The desktop mounted EyeLink 1000 (SR Research Ltd, Ottawa, Ontario, Canada) was used to record participants’ eye movements. The EyeLink 1000 is a video camera-based infrared eye-tracking system that uses a velocity-based event-detection algorithm to filter raw gaze samples into saccade, fixation, and blink events (Stampe, 1993). Velocity thresholds for saccade detection were set to the recommended values for cognitive research (30 degrees/second; EyeLink 1000 User Manual, Version 1.5.2). Gaze data were acquired from the right eye at a sampling rate of 1000 Hz. A 9-point calibration and validation procedure was conducted prior to starting the attention task to configure the system such that the spatial accuracy error was below 0.5 degrees on average. Further, calibration accuracy was re-checked after every trial. Participants were required to fixate on a central fixation point; if error was greater than 1 degree, the calibration validation procedure was conducted again to obtain a spatial accuracy of less than 0.5 degrees of average error. OpenSesame was used to control stimuli presentation and its synchronization with the eye-tracking system (Mathot, Schreij, & Theeuwes, 2012). The participant’s head was kept stable using an adjustable head mount, with a distance between the participant’s eyes and the camera of approximately 60 cm. All participants had normal to corrected-to-normal vision and were allowed to wear contact lenses or glasses during the attention tasks if necessary; further, participants were required to remove eye make-up before completing the attention tasks.
Stress Induction
Speech Anticipation Task
To assess attentional bias and affective modulation of pupil size under stress, a speech anticipation procedure developed by Waugh, Panage, Mendes, and Gotlib (2010) was utilized. After participants completed the baseline attention task, they rested for five minutes (i.e., the pre-stress period) and then rated their current mood. The experimenter then placed a camera in the room and told participants that they would have two minutes to prepare a five-minute speech during which they would be recorded and judged by evaluators on their clarity, coherence, and persuasiveness. The speech topic was “Why are you a good friend?”, a topic that has been used successfully in prior studies to elicit anticipatory stress responses (Sanchez et al., 2013; Waugh et al., 2010). Participants were told that there would be two coin flips that would determine if and when the participants gave the speech. They were informed that the first coin would be flipped immediately after the two minute speech preparation period and would determine if they gave the speech immediately, or waited for the second coin flip after the second attention task, which would determine whether they gave the speech at that time or not at all. The experimenter then informed the participant to remain still and silent so as not to disturb the heart rate recording and left the participant alone for two minutes to prepare their speech (i.e., the anticipatory-stress period). After the two minute speech preparation period, the first coin was flipped and participants were told that a computer randomizer would determine if the result of the coin flip indicated that they would give the speech now or wait until the second attention task was completed. All participants were told that they would not give the speech immediately and that the second coin flip, taking place after the second attention task, would determine whether they gave the speech. After participants rated their mood, they completed the second attention task and then the second coin flip was conducted. All participants were told that they would not be giving the speech, and they rested for five minutes (i.e., the post-stress recovery period) before completing a final mood rating.
Psychophysiology Recording
Polar RS800CX
Participants wore heart rate monitors (i.e., Polar Electro RS800CX wristwatch monitors; Anderson & Hope, 2009) to record heart rate at a sampling rate of 1000 Hz from the pre-stress relaxation period through the end of the post-stress recovery period. The heart rate monitor consisted of a wristwatch and a dampened two-lead elastic band worn underneath the clothing around the sternum, and has been used in prior work on cardiovascular stress reactivity (Gouin, Deschenes, & Dugas, 2014). Timestamps were recorded to mark the beginning of the five minute pre-stress period, the two minute anticipatory-stress period, and the five minute post-stress period.
Procedure
After providing consent, participants completed questionnaires and the baseline attention task. Next, the heart rate monitor was attached and participants sat quietly for a five minute pre-stress relaxation period, after which they rated their current mood. Participants then completed the speech-anticipation period, rated their current mood, and completed the attention task again. After completing the post-stress attention task, participants sat quietly for a five minute post-stress recovery period, after which they rated their current mood.
Results
Data Preprocessing
Attention Task
Of the 165 participants consented, 134 completed the baseline attention task and 126 completed the post-stress elicitation attention task. 123 participants completed both the baseline and post-stress elicitation attention task (see Supplemental material for more information on reasons for exclusion). Scripts were developed to parse the event data generated by the EyeLink system. The EyeLink generates a paired saccade-blink event to demarcate recording periods during which the pupil could not be found (e.g., due to a blink or technical error). Paired saccade-blink events that occurred during the free-viewing portion of a trial were identified and removed by parsing scripts (% of total free-viewing samples removed for baseline and post-stress tasks, respectively; M = 8.0, SD = 6.6, range: 0.3–33.4; M = 9.8, SD = 6.6, range: 0.4–31.5). Prior work has used a 40% criterion for participant exclusion due to excessive signal loss (Graham, Hoover, Ceballos, & Komogortsev, 2011); thus, no participants were excluded for this reason. Next, individual trials were removed if the participant made a saccade away from the center of the screen before facial stimuli onset or if a fixation was not made on either facial stimulus during the 3,000 ms free-viewing period (1.6% and 2.2% of all trials in the baseline and stress tasks, respectively). One outlier had 26.9% and 64.8% of their trials removed on the baseline and post-stress attention tasks, respectively; this participant was excluded from all analyses, resulting in 133 participants with valid baseline attention task data, 125 participants with valid post-stress elicitation task data, and 122 participants with valid data for both attention tasks. Finally, fixations on either facial stimulus during the 3,000ms free-viewing period with durations >= 100 ms were extracted and used in the computation of the attentional bias indices. Further, the average pupil size for each fixation was also extracted and used in the computation of the pupil size modulation indices. A 100ms minimum fixation duration was utilized for two reasons: 1) Prior work on free-viewing paradigms has identified 100ms as an optimal threshold for discriminating genuine fixations from other oculomotor activity (Manor & Gordon, 2003), and 2) Fixations of less than 100ms are more likely to be artifacts (e.g., due to partial pupil occlusion during a blink) (EyeLink 1000 User Manual, version 1.5.2, SR Research Ltd, Mississauga, Ontario, Canada, 2005–2010). In line with recent recommendations (Gibb et al., 2016), split-half reliabilities were computed for attention bias across the three emotion-neutral categories for the baseline (Positive: .76; Threat: .64; Dysphoric: .59) and post-stress attention tasks (Positive: .79; Threat: .62; Dysphoric: .69).
Heart Rate
Of the 136 participants with at least one valid attention task data point, 90, 93, and 107 had physiological data for the pre-stress, anticipatory-stress, and post-stress periods, respectively; data loss occurred primarily because of technical errors and transmission signal loss during the experiment. Heart rate data were cleaned and extracted using a five step process. First, we used the Polar Pro Trainer 5TM software to export the collected IBI data to a text file for preprocessing before analysis. Second, the IBI series was loaded into HRV analysis software (HRVAS; Ramshur, 2010) and visually inspected for ectopic intervals. Third, an automated filter was utilized to confirm visually identified ectopic intervals by marking IBIs that differed more than 20% from the previous interval; these intervals were then corrected using cubic spline interpolation (% of IBIs interpolated; pre-stress: M = 2.0, SD = 2.7, range: 0–14.9; anticipatory-stress: M = 2.0, SD = 3.6, range: 0–30; post-stress: M = 2.8, SD = 3.9, range: 0–24.2). Fourth, the corrected IBI series was detrended using the discrete wavelet packet transform (Shafqat, Pal, & Kyriacou, 2007). Lastly, HRVAS was used to compute the natural log of the Root Mean Square of the Successive Differences (RMSSD) during the pre-stress period to index resting parasympathetically-mediated HRV. The RMSSD metric is a time-domain measure of HRV thought to primarily represent high frequency (i.e., parasympathetically-mediated) heart rate variability (Task Force, 1996); further, in contrast to other time-domain (i.e., pNN50) and time-frequency (i.e., HF-HRV) measures, changes in breathing rate have been found to not significantly affect RMSSD (Penttila et al., 2001), an important consideration given that respiration was not measured in the current study. Mean heart rate for the pre-stress, anticipatory-stress, and post-stress periods were also computed to ensure that the social-evaluative stressor successfully elicited physiological arousal.
Data Analytic Strategy
To account for the lack of independence inherent to repeated measurements, multilevel marginal models were constructed to test hypotheses. Although repeated measures ANOVA analyses are commonly used for these kind of data, only multilevel models can accommodate missing within-subject observations and model correlated within-subject residuals without imposing assumptions (e.g., sphericity, compound symmetry) (Quené & van der Bergh, 2004). These advantages were particularly relevant to the present study given the prevalence of missing data for attention bias/pupil indices (see above section for more detail on missing attention task data) and RMSSD (see above section for more detail on missing HRV data). Thus, multilevel marginal models were used for all analyses to maximize inclusion of all valid observations. Further, in line with recent recommendations (Gurka, Edwards, & Muller, 2011), no assumptions were imposed on the within-subject covariances and they were modeled using an unstructured matrix.
For the stress induction manipulation check, 114 participants had mean HR data and 135 participants had state mood data for at least one time point (i.e., pre-stress, anticipatory-stress, post-stress), leaving 114 and 135 participants available for the mean HR (see above section for more detail on missing HR data) and subjective mood manipulation check analyses, respectively. Note that missing self-report mood data points were primarily due to participant error. Of the 136 subjects with at least one valid attention task data point for attention bias/pupil measures, 90 had valid pre-stress RMSSD data, leaving 90 subjects available for testing a priori hypotheses.
Statistical Analyses
Stress Induction Manipulation Check
Descriptives for state mood and mean HR at each time point are presented in Table 1.
In the first model, the effect of the speech anticipation stressor on self-reported negative affect was evaluated. State mood was entered as the dependent variable. Emotion was entered as a three-level within-subject factor (happy mood, anxious mood, dysphoric mood) and Time was also entered as a three-level within-subject factor (pre-stress, anticipatory-stress, post-stress). Both factors were entered as fixed effects. Tests of fixed effects revealed a significant main effect of Mood, F(2, 133.50) = 146.45, p < .001; Bonferroni-corrected follow-up comparisons on the estimated marginal means revealed that participants endorsed significantly greater levels of happy mood relative to anxious, t(133.19) = 8.50, p < .001, and dysphoric mood, t(133.85) = 14.27, p < .001, across the entire experiment. Further, anxious mood was also endorsed to a greater extent than dysphoric mood across the entire experiment, t(133.05) = 8.83, p < .001. Results also revealed a significant main effect of Time, F(2, 128.78) = 37.07, p < .001; Bonferroni-corrected follow-up comparisons revealed that participants’ mood ratings were significantly greater during the anticipatory-stress period relative to the pre-stress, t(130.90) = 3.99, p < .001, and post-stress periods, t(129.35) = 8.31, p < .001. Further, mood ratings were significantly greater during the pre-stress relative to the post-stress period, t(126.86) = 6.03, p < .001. Finally, a significant Emotion*Time interaction effect emerged, F(4, 128.71) = 12.90, p < .001; to decompose the interaction, three follow-up models were constructed in which state mood for a particular emotion was entered as the dependent variable and Time was entered as a three-level within-subject factor.
For happy mood, the fixed effect of Time was non-significant, F(2, 131.19) = 0.94, p = .39. For anxious mood, the fixed effect of Time was significant, F(2, 128.18) = 27.21, p < .001; Bonferroni-corrected follow-up comparisons revealed that self-reported anxious mood was significantly greater during the anticipatory-stress period relative to the pre-stress, t(128.54) = 5.11, p < .001, and post-stress, t(128.37) = 7.32, p < .001, periods. Further, anxious mood was significantly greater during the pre-stress relative to the post-stress period, t(129.39) = 4.71, p < .001. For depressed mood, the fixed effect of Time was significant, F(2, 133.47) = 20.48, p < .001; Bonferroni-corrected follow-up comparisons revealed that self-reported depressed mood was significantly greater during the anticipatory-stress relative to the post-stress period, t(131.61) = 4.09, p < .001, though the difference was non-significant relative to the pre-stress period, t(133.37) = −1.78, p = .08. Further, depressed mood was significantly greater during the pre-stress relative to the post-stress period, t(133.35) = 6.01, p < .001.
In the second model, the effect of the speech anticipation stressor on mean HR was evaluated. Mean HR was entered as the dependent variable. Time was entered as a three-level within-subject factor (pre-stress, anticipatory-stress, post-stress) fixed effect. Results revealed a significant effect of Time, F(2, 94.07) = 21.27, p < .001; Bonferroni-corrected follow-up comparisons revealed that mean HR was significantly greater during the anticipatory-stress relative to the pre-stress, t(94.02) = 6.51, p < .001, and post-stress, t(95.11) = 4.48, p < .001, periods. However, mean HR was non-significantly different during the pre-stress relative to the post-stress period after Bonferroni correction, t(93.03) = 2.24, p = .027.
The results demonstrate that the uncertain speech anticipation manipulation successfully elicited a subjective and physiological stress response that subsequently decreased after the possible speech threat was removed.
Test of the Moderating Effect of Resting HRV on Stress-Elicited Change in Attentional Bias Towards Negative and Positive Emotional Information
Descriptives and bivariate correlations for all study variables are presented in Table 2.
Table 2.
Descriptive statistics and bivariate correlations.
| Measures M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Resting RMSSD 3.79 (0.48) | 1 | ||||||||||||
| Attention Bias (Baseline) | |||||||||||||
| 2. Positive 0.57 (0.07) | −.03 | 1 | |||||||||||
| 3. Threat 0.53 (0.06) | −.17 | .33** | 1 | ||||||||||
| 4. Dysphoric 0.52 (0.05) | −.19 | .29** | .62* | 1 | |||||||||
| Attention Bias (Stress) | |||||||||||||
| 5. Positive 0.57 (0.07) | −.27* | .67** | .33** | .33** | 1 | ||||||||
| 6. Threat 0.53 (0.05) | −.15 | .36** | .64** | .48** | .34** | 1 | |||||||
| 7. Dysphoric 0.52 (0.06) | −.09 | .21* | .56** | .56** | .22* | .65** | 1 | ||||||
| Pupil Change (Baseline) | |||||||||||||
| 8. Positive 0.24 (1.53) | .06 | −.01 | .05 | −.04 | .05 | .12 | .08 | 1 | |||||
| 9. Threat 0.05 (1.55) | −.02 | .02 | .02 | .09 | .06 | .11 | .16 | −.03 | 1 | ||||
| 10. Dysphoric −0.08 (1.38) | −.08 | −.12 | −.13 | −.03 | −.09 | −.04 | −.08 | −.03 | .09 | 1 | |||
| Pupil Change (Stress) | |||||||||||||
| 11. Positive −0.02 (1.78) | .37** | .06 | .00 | .03 | −.01 | .17 | .12 | −.01 | −.02 | −.01 | 1 | ||
| 12. Threat 0.25 (1.63) | −.09 | .10 | .06 | .05 | .03 | .09 | .03 | −.07 | −.04 | .01 | .05 | 1 | |
| 13. Dysphoric 0.23 (1.51) | .15 | −.09 | .03 | −.06 | −.16 | −.06 | −.09 | −.05 | .00 | −.06 | .06 | .18* | 1 |
Note: Resting RMSSD = pre-stress Root Mean Squared Successive Differences; Attention Bias = Average proportion of gaze dwell time on the emotional relative to the neutral facial stimulus; Pupil Change = Average change in pupil size while viewing the emotional relative to the neutral stimulus (expressed as a %).
p < .05;
p < .01
To test the hypothesis that resting HRV would moderate the effect of acute stress on attention bias, attention bias was entered as the dependent variable. Emotion was entered as a three-level within-subject factor (positive, threat, dysphoric) and Time was entered as a two-level within-subject factor (baseline, stress). Resting RMSSD during the pre-stress period was entered as a between-subjects predictor. All variables were entered as fixed effects.
The main effect of RMSSD was significant, F(1, 88.75) = 3.93, p = .05, revealing an inverse association between RMSSD and attention bias towards all emotional stimuli collapsed across the baseline and post-stress elicitation tasks. The RMSSD*Emotion, F(2, 87.13) = 0.03, p = .98, and RMSSD*Time, F(1, 86.41) = 0.55, p = .46, interactions were also non-significant, and, in contrast to hypotheses, the RMSSD*Emotion*Time interaction, F(2, 86.39) = 2.92, p = .059, was also non-significant. Because the hypothesized three-way interaction did not reach significance, post-hoc analyses were not conducted. However, visual inspection of the RMSSD*Emotion*Time interaction suggested that, in contrast to hypotheses, the effect was driven by an inverse association between RMSSD and change in positive bias from pre to post-stressor such that low RMSSD predicted increased attentional bias towards positive stimuli from pre to post-stressor (see Supplemental material).
To determine if the trend-level relationship between HRV and stress-elicited change in attention bias towards positive emotional stimuli reflected an emotion regulation attempt, Bonferroni-corrected (p = .05/3) exploratory correlations were conducted to determine if change in attentional bias towards positive emotional stimuli predicted anxious mood reactivity to and recovery from the stressor. Only change in anxious mood was examined given that only anxious mood significantly increased from the pre-stress to anticipatory-stress period. Based upon Burt and Obradovic’s guidelines (2013) for evaluating the relative reliability of difference vs residualized change scores, residualized change scores were chosen to measure change in attentional bias towards positive emotional stimuli and anxious mood (see Supplemental material for more detail). Residualized change scores were created by regressing anxious mood at one time point on the prior time point. Three anxious mood change scores were computed: 1) Anxious mood reactivity (i.e., anticipatory-stress anxious mood regressed on pre-stress anxious mood; more positive scores indicate greater reactivity; 2) Anxious mood recovery relative to peak activation (i.e., post-stress anxious mood regressed on anticipatory-stress anxious mood; more positive scores indicate worse recovery); 3) Anxious mood recovery relative to pre-stress anxious mood (i.e., post-stress anxious mood regressed on pre-stress anxious mood; more positive scores indicate worse recovery).
Results revealed non-significant associations between change in positive attention bias and all three anxious mood change scores (anxious mood reactivity: r = −.16, p = .083; anxious mood recovery relative to peak activation: r = .16, p = .097; anxious mood recovery relative to baseline: r = .14, p = .12). However, the pattern of findings is inconsistent with an emotion regulation account as increased attention bias towards positive emotional information from pre to post-stress elicitation was generally associated with decreased anxious mood reactivity to and worse recovery from the laboratory stressor.
Test of the Moderating Effect of Resting HRV on Stress-Elicited Change in Pupillary Response to Negative and Positive Emotional Information
To test the hypothesis that resting HRV would moderate the effect of acute stress on pupillary responding to emotional stimuli, affective modulation of pupil size was entered as the dependent variable in a multilevel marginal model. Emotion was entered as a three-level within-subject factor (positive, threat, dysphoric) and Time was entered as a two-level within-subject factor (baseline, stress). Resting RMSSD during the pre-stress period was entered as a between-subjects predictor. All variables were entered as fixed effects.
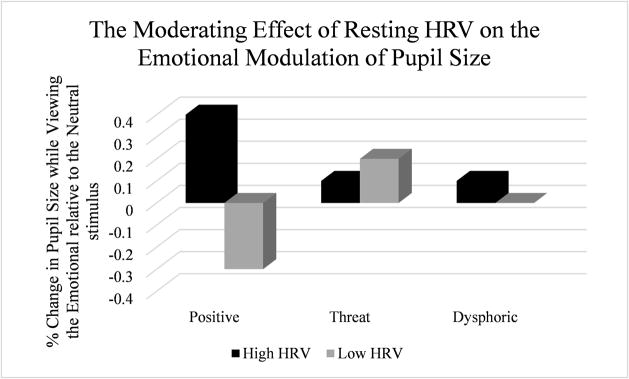
The main effect of RMSSD, F(1, 86.89) = 2.96, p = .09, as well as the RMSSD*Time, F(1, 86.28) = 2.93, p = .09, interaction effect were non-significant. Unexpectedly, the RMSSD*Emotion interaction was significant, F(2, 88.44) = 3.92, p = .02 (see Figure 1). To evaluate this relationship, baseline and corresponding post-stress elicitation pupil modulation indices were averaged for each emotion to create variables reflecting the main effect of the Emotion within-subject factor. Correlational analyses revealed a significant positive association between RMSSD and pupil size enhancement to positive, r = .29, p = .007, but not dysphoric, r = .12, p = .27, or threat, r = −0.05, p = .64, stimuli. However, the relationship between RMSSD and pupillary responding to positive emotional stimuli appeared to be driven by the former’s association with pupillary response to positive stimuli after stress elicitation, r = .37, p = .001, rather than at baseline, r = .06, p = .60. Contrary to hypotheses, the RMSSD*Emotion*Time interaction was non-significant, F(2, 87.65) = 2.82, p = .065; however, given the trend-level effect, the interaction was inspected and appeared to be driven by decreases in pupillary responding to positive emotional stimuli from pre to post-stress elicitation for individuals with lower RMSSD (see the Supplemental material).
Figure 1.
Estimated marginal means for change in pupil size while viewing emotional relative to neutral stimuli (collapsed across the baseline and post-stress elicitation tasks) are presented at high (+1 SD) and low (−1 SD) resting HRV. Follow-up analyses revealed that only the association between HRV and pupillary responding to positive emotional stimuli is statistically significant and that the relationship is driven by pupillary responding measured after stress elicitation but not at baseline.
To determine if the trend-level relationship between HRV and stress-elicited change in pupillary responding to positive stimuli reflected an emotion regulation attempt, Bonferroni-corrected (p = .05/3) exploratory correlations were conducted to determine if change in pupillary responding towards positive emotional stimuli predicted anxious mood reactivity to and recovery from the stressor. Change in pupillary responding to positive emotional stimuli and anxious mood reactivity/recovery indices were computed in the same manner as the exploratory positive attention bias correlations (see Supplemental material for more detail).
As with the attention bias analyses, results revealed non-significant associations between change in pupil dilation towards positive emotional information from pre to post-stress elicitation and all three anxious mood change indices (anxious mood reactivity: r = −.15, p = .12; anxious mood recovery relative to peak activation: r = −.01, p = .90; anxious mood recovery relative to baseline: r = −.15, p = .10). Because the significant RMSSD*Emotion interaction appeared to be driven by the relationship between RMSSD and pupillary responding to positive stimuli after stress elicitation but not at baseline, we also conducted correlations between just pupillary responding to positive stimuli measured under stress (rather than change from baseline to post-stress elicitation) and all three anxious mood change indices (anxious mood reactivity: r = −.14, p = .14; anxious mood recovery relative to peak activation: r = −.04, p = .66; anxious mood recovery relative to baseline: r = −.18, p = .044). Results were non-significant after Bonferroni correction, though, consistent with an emotion regulation account, the relationship between larger pupil size while viewing positive stimuli under stress and greater recovery of anxious mood to baseline did reach significance using a p-value threshold of .05. Thus, the overall pattern of findings suggest increased pupil dilation while viewing positive stimuli predicted adaptive regulation of anxious responding to the laboratory stressor.
Discussion
The results of the present study were partially consistent with predictions. In contrast with hypotheses, resting HRV was not a significant moderator of stressor-elicited change in NAB or pupillary response to negative emotional information. A marginal effect was found for PAB, but the relationship was in the opposite direction as expected such that low HRV appeared to be related to increased PAB in the context of acute stress. In contrast, low HRV was significantly associated with pupillary responding in the hypothesized direction, such that low HRV predicted decreased pupil size while viewing positive emotional stimuli; however, the hypothesized relationship between low HRV and greater decreases in pupillary responding to positive stimuli from pre- to post-stress elicitation was marginal and did not reach significance. Examination of the significant relationship between HRV and pupillary responding to positive emotional information revealed that the association was primarily driven by low HRV’s link with decreased pupil size while viewing positive stimuli in the context of acute stress but not at baseline. Overall, the present results provide partial empirical support for theoretical frameworks positing that low HRV’s association with negative psychosocial outcomes is attributable to its relationship with emotion dyregulation (Appelhans & Luecken, 2006; Beauchaine, 2015; Beauchaine & Thayer, 2015).
Although unexpected, the marginal association between low HRV and greater increases in PAB from pre- to post-stress elicitation may reflect an attempt to decrease negative affect. Indeed, a prior eye-tracking study found that greater attentional deployment to happy faces after a negative mood induction predicted greater subjective mood recovery at the end of the study (Sanchez, Vazquez, Gomez, & Joormann, 2014). Similarly, Newman and Sears (2015) found that individuals without a depression history attended more to positive emotional stimuli after a negative mood induction, whereas individuals with a depression history did not. These data suggest that increased PAB in response to acute stress may reflect an adaptive attempt to repair a negative mood state. However, though the exploratory correlational analyses were non-significant, the observed pattern of anxious mood reactivity and recovery with respect to stress-elicited change in PAB suggests anxious mood rigidity characterized by decreased initial reactivity and worse mood recovery, a pattern inconsistent with adaptive emotion regulation (Kashdan & Rottenberg, 2010). Given existing evidence of low HRV’s relationship with emotion dysregulation and negative psychosocial outcomes (Beauchaine & Thayer, 2015), the observed association between low HRV and a putative marker (i.e., increased stressor-elicited PAB) of adaptive emotional information processing (Newman & Sears, 2015; Sanchez et al., 2014) is difficult to explain. This unexpected finding may be more comprehensible when considered in the context of the corresponding pupillary response data.
In the present study, low HRV marginally predicted larger increases in attention bias towards positive emotional stimuli from pre- to post-stress elicitation but significantly decreased pupil size while fixating on these stimuli under acute stress. This pattern of effects may reflect an ineffective attempt at negative mood regulation such that positive emotional stimuli are being preferentially attended but insufficiently processed. Indeed, pupil dilation is considered to be a highly sensitive psychophysiological index of cognitive load (Paas et al., 2003; Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003) and has been linked with individual differences in sustained processing of emotional information (Graham et al., 2011; Siegle et al., 2003; Siegle, Granholm, Ingram, & Matt, 2001). Low HRV’s relationship with increased PAB and decreased pupillary responding to positive stimuli under stress may reflect greater utilization of less effortful behavioral emotion regulation strategies (e.g., behavioral avoidance/overt attentional distraction) relative to regulation strategies that require greater cognitive control resources (e.g., cognitive reappraisal) (Sheppes & Meiran, 2008) given that low HRV is thought to index decreased prefrontal cortex functioning (Beachaine & Thayer, 2015). Indeed, prior findings demonstrating a relationship between low HRV and less effective utilization of covert emotion regulation strategies (i.e., thought suppression; Gillie et al., 2015) may reflect the greater difficulty low HRV individuals experience when attempting to utilize cognitive resources to regulate negative affect. Thus, individuals with low HRV may have attempted to adaptively respond to the social-evaluative stressor by increasing overt attention towards positive emotional information, but failed to maintain such information in working memory and/or process the happy facial stimuli as emotionally arousing reward cues. Indeed, prior work has demonstrated a positive relationship between greater covert processing of the rewarding features of stimuli and enhanced emotion regulation in naturalistic and laboratory settings (Genet et al., 2013; Malooly et al., 2013). Although the correlation between the degree of anxious mood recovery to pre-stress levels and pupillary responding to positive stimuli under acute stress was not significant after Bonferroni correction, findings were consistent with the notion that increased pupil size while viewing positively-valenced stimuli under stress is linked with more adaptive emotion regulation. Given the more robust relationship between HRV and pupillary response to positive emotional stimuli compared to corresponding attentional behavior, low HRV may be primarily linked with emotion dysregulation via decreased covert processing of positive emotional information in acutely stressful contexts. However, this hypothesis is speculative and replication is required before firmer conclusions can be made.
In contrast to hypotheses, resting HRV was non-significantly associated with attentional and pupillary indices of negative emotional information processing. Examination of bivariate relationships revealed trend-level associations between HRV and baseline NAB in the hypothesized direction, but the associations became non-significant in the stressor context. Relationships between HRV and pupillary responding to negative emotional stimuli were non-significant across the baseline and post-stress attention tasks. The null results are inconsistent with prior studies demonstrating a relationship between low HRV and greater deleterious influence of negative emotional stimuli on cognitive control functioning (Gillie et al., 2015; Krypotos et al., 2011; Ottaviani et al., 2015). However, these prior investigations did not explicitly assess negative emotional information processing, instead relying upon indirect measures such as reaction time (Krypotos et al., 2011) and self-reported difficulties dismissing negative intrusive thoughts (Gillie et al., 2015; Ottaviani et al., 2015). The observed null relationships between HRV and direct indices of negative emotional information processing in the present study (i.e., NAB, pupillary response to negative stimuli) suggests that alternative mechanisms may underlie HRV’s established relationship with mood/anxiety psychopathology (Beauchaine & Thayer, 2015). However, given the limited number of studies investigating HRV and emotional information processing mechanisms, further research is required.
Although not hypothesized, the observed specificity between HRV and altered positive, but not negative, emotional information processing has been found in prior work. In a longitudinal study in adolescents, Vazquez and colleagues (2016) found a significant, prospective association between resting HRV and anhedonia, but not negative mood or interpersonal problem symptoms of depression assessed one year later. Further, Sanders and Abaied (2015) found a significant relationship between low HRV and greater anhedonic depression, but not anxious arousal symptoms. Interestingly, in two recent studies high HRV demonstrated specific associations with manic symptoms, suggesting that HRV may generally index disturbances in positive valence and reward processing neural systems (Faurholt-Jepsen, Brage, Kessing, & Munkholm, 2017; Gruber, Mennin, Fields, Purcell, & Murray, 2015). Given that anhedonia symptoms have been proposed to arise from dysfunctional interactions between stress and reward neural systems (Pizzagalli, 2014), low HRV may specifically predict anhedonia via altered processing of positive emotional information during acute stress. Although speculative, increased PAB in conjunction with decreased pupillary response to positively-valenced stimuli in the context of acute stress may reflect risk for development of anhedonia symptoms. More research is needed to explicitly test the hypothesis that attentional and pupillary indices of positive emotional information processing in stressful contexts mediate the prospective relationship between low HRV and anhedonia symptoms.
The present study has some limitations. First, in contrast to attentional bias, affective modulation of pupillary response during the baseline attention task was not related to the corresponding measure during the post-stress attention task, suggesting that our measure of pupil size modulation may be less reliable or it may not be indexing a trait-like construct. Limited research has been conducted on psychometric properties of pupillary response to emotional stimuli and split-half reliabilities were not computed in the present study due to variability in fixations on the neutral stimulus during each trial, precluding calculation of trial-level pupil modulation values. Thus, future studies should consider using separate tasks to assess attention bias and affective modulation of pupil size (e.g., instructed central fixation on a single stimulus per trial; Bradley et al., 2008). It is also possible that affective modulation of pupil size is less trait-like than attentional bias and more influenced by state-level variables, a possibility supported by the observed significant association between pupillary responding to threat and dysphoric emotional stimuli after the stress induction but not at baseline. Future research is needed on the psychometric properties and role of mood state on affective modulation of pupil size. Second, respiration was not assessed, which is recommended when measuring HRV (Berntson et al., 1997). Although we used a measure of HRV that has been found to be robust to change in respiration (Penttila et al., 2001), future studies should ensure the present results are robust to respiration effects. Third, although the stress induction utilized in the present study elicited physiological arousal and subjective feelings of anxiety, it is unclear if similar findings would emerge in the context of other commonly used laboratory mood inductions. Future studies should determine if the present effects emerge under different laboratory stress inductions. Finally, though the sampling strategy ensured representation of emotionally dysregulated individuals, the present sample was uncharacterized with regard to psychopathology; future investigations should determine if HRV is similarly related to emotional processing indices in clinical samples.
Overall, the results support the notion that resting HRV is an autonomic marker of emotion regulation capacity (Appelhans & Luecken, 2006; Beauchaine & Thayer, 2015). The emergence of significant effects of HRV on emotional processing indices only during acute stress is consistent with neuroimaging data on HRV’s relationship with corticolimbic circuitry (Thayer et al., 2009) known to be central to regulation of negative affect (Li & Sinha, 2008). The specific effect of HRV on stressor-elicited positive emotional processing was robust, especially for pupil modulation, and should be explored in future research, particularly as it pertains to symptom dimensions theoretically related to stress-induced alterations in positive emotional processing (e.g., anhedonia). Finally, the utilization of behavioral and physiological measures of emotional information processing in conjunction with a theoretically-relevant mood state manipulation provided a more complete picture of HRV’s relationship with affective processing than if context was ignored or a single emotional processing measure was used, providing support for recent research initiatives (e.g., NIMH’s RDoC; Insel et al., 2010) emphasizing multi-method approaches to understanding transdiagnostic biobehavioral constructs relevant to mental health.
Supplementary Material
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Anderson ER, Hope DA. The relationship among social phobia, objective and perceived physiological reactivity, and anxiety sensitivity in an adolescent population. Journal of Anxiety Disorders. 2009;23(1):18–26. doi: 10.1016/j.janxdis.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of general psychology. 2006;10(3):229–240. [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. 2012;32(8):704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Current opinion in psychology. 2015;3:43–47. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98(2):338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Clark DA. Anxiety and depression: An information processing perspective. Anxiety research. 1988;1(1):23–36. [Google Scholar]
- Berntson GG, Bigger T, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, Van Der Molen MW. Heart rate variability: Origins, methods, and interpretative caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour research and therapy. 1997;35(10):911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt KB, Obradovic J. The construct of psychophysiological reactivity: Statistical and psychometric issues. Developmental Review. 2013;33(1):29–57. [Google Scholar]
- Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: Gaze direction and duration. Cognition and Emotion. 2005;19:433–451. doi: 10.1080/02699930441000157. [DOI] [PubMed] [Google Scholar]
- Duque A, Sanchez A, Vazquez C. Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition and Emotion. 2014;28(8):1347–1366. doi: 10.1080/02699931.2014.881327. [DOI] [PubMed] [Google Scholar]
- Faurholt-Jepsen M, Brage S, Kessing LV, Munkholm K. State-related differences in heart rate variability in bipolar disorder. Journal of Psychiatric Research. 2017;84:169–73. doi: 10.1016/j.jpsychires.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological psychology. 2009;80(3):300–305. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genet JJ, Malooly AM, Siemer M. Flexibility is not always adaptive: Affective flexibility and inflexibility predict rumination use in everyday life. Cognition & emotion. 2013;27(4):685–695. doi: 10.1080/02699931.2012.733351. [DOI] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2016;171(1):65–80. doi: 10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillie BL, Vasey MW, Thayer JF. Individual differences in resting heart rate variability moderate thought suppression success. Psychophysiology. 2015;52(9):1149–1160. doi: 10.1111/psyp.12443. [DOI] [PubMed] [Google Scholar]
- Gouin J, Deschênes SS, Dugas MJ. Respiratory sinus arrhythmia during worry forecasts stress-related increases in psychological distress. Stress: The International Journal On The Biology Of Stress. 2014;17(5):416–422. doi: 10.3109/10253890.2014.949666. [DOI] [PubMed] [Google Scholar]
- Graham R, Hoover A, Ceballos NA, Komogortsev O. Body mass index moderates gaze orienting biases and pupil diameter to high and low calorie food images. Appetite. 2011;56(3):577–586. doi: 10.1016/j.appet.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Gruber J, Mennin DS, Fields A, Purcell A, Murray G. Heart rate variability as a potential indicator of positive valence system disturbance: A proof of concept investigation. International Journal of Psychophysiology. 2015;98(2):240–248. doi: 10.1016/j.ijpsycho.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Gurka MJ, Edwards LJ, Muller KE. Avoiding bias in mixed model inference for fixed effects. Statistics in medicine. 2011;30(22):2696–2707. doi: 10.1002/sim.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Current behavioral neuroscience reports. 2015;2(3):146–153. doi: 10.1007/s40473-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. The American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jandackova VK, Britton A, Malik M, Steptoe A. Heart rate variability and depressive symptoms: a cross-lagged analysis over a 10-year period in the Whitehall II study. Psychological medicine. 2016:1–11. doi: 10.1017/S003329171600060X. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clinical psychology review. 2010;30(7):865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellough J, Beevers CG, Ellis A, Wells TT. Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy. 2008;46:1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Reward-dependent modulation of working memory in lateral prefrontal cortex. The Journal of neuroscience. 2009;29(10):3259–3270. doi: 10.1523/JNEUROSCI.5353-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krypotos AM, Jahfari S, Ast VA, Kindt M, Forstmann BU. Individual differences in heart rate variability predict the degree of slowing during response inhibition and initiation in the presence of emotional stimuli. Frontiers in psychology. 2011;2:278. doi: 10.3389/fpsyg.2011.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Current opinion in psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Li CR, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psychostimulant addiction. Neuroscience & Biobehavioral Reviews. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces - KDEF, CD ROM. Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; 1998. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of abnormal psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Malooly AM, Genet JJ, Siemer M. Individual differences in reappraisal effectiveness: The role of affective flexibility. Emotion. 2013;13(2):302. doi: 10.1037/a0029980. [DOI] [PubMed] [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of neuroscience methods. 2003;128(1):85–93. doi: 10.1016/s0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mansell W, Clark DM, Ehlers A, Chen YP. Social anxiety and attention away from emotional faces. Cognition & Emotion. 1999;13(6):673–690. [Google Scholar]
- Mathôt S, Schreij D, Theeuwes J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behavior Research Methods. 2012;44(2):314–324. doi: 10.3758/s13428-011-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J, Andrade J, Kavanagh DJ, Hetherington M. Elaborated Intrusion theory: A cognitive-emotional theory of food craving. Current Obesity Reports. 2012;1(2):114–121. [Google Scholar]
- McHugh RK, Reynolds EK, Leyro TM, Otto MW. An examination of the association of distress intolerance and emotion regulation with avoidance. Cognitive Therapy and Research. 2013;37(2):363–367. doi: 10.1007/s10608-012-9463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Otto MW. Refining the measurement of distress intolerance. Behavior Therapy. 2012;43:641–651. doi: 10.1016/j.beth.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KR, Sears CR. Eye gaze tracking reveals different effects of a sad mood induction on the attention of previously depressed and never depressed women. Cognitive Therapy and Research. 2015;39(3):292–306. [Google Scholar]
- O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Shahabi L, Tarvainen M, Cook I, Abrams M, Shapiro D. Cognitive, behavioral, and autonomic correlates of mind wandering and perseverative cognition in major depression. Frontiers in neuroscience. 2015;8:433. doi: 10.3389/fnins.2014.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D, Davydov DM, Goldstein IB, Mills PJ. The autonomic phenotype of rumination. International Journal of Psychophysiology. 2009;72(3):267–275. doi: 10.1016/j.ijpsycho.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF. Physiological Concomitants of Perseverative Cognition: A Systematic Review and Meta-Analysis. Psychological Bulletin. 2016;142(3):231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Paas F, Tuovinen JE, Tabbers H, Van Gerven PW. Cognitive load measurement as a means to advance cognitive load theory. Educational psychologist. 2003;38(1):63–71. [Google Scholar]
- Partala T, Surakka V. Pupil size variation as an indication of affective processing. International journal of human-computer studies. 2003;59(1):185–198. [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Penttilä J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, … Scheinin H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clinical Physiology. 2001;21(3):365–376. doi: 10.1046/j.1365-2281.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- Quené H, Van den Bergh H. On multi-level modeling of data from repeated measures designs: A tutorial. Speech Communication. 2004;43(1):103–121. [Google Scholar]
- Quintana DS, Guastella AJ, Outhred T, Hickie IB, Kemp AH. Heart rate variability is associated with emotion recognition: direct evidence for a relationship between the autonomic nervous system and social cognition. International Journal of Psychophysiology. 2012;86(2):168–172. doi: 10.1016/j.ijpsycho.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Ramshur JT. Doctoral dissertation, Master’s thesis. The University of Memphis; Memphis, TN: 2010. Design, evaluation, and application of heart rate variability software (HRVAS) Master’s thesis. [Google Scholar]
- Sanchez A, Vazquez C. Prototypicality and intensity of emotional faces using an anchor-point method. Spanish Journal of Psychology. 2013;16:1–11. doi: 10.1017/sjp.2013.9. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C, Gomez D, Joormann J. Gaze-fixation to happy faces predicts mood repair after a negative mood induction. Emotion. 2014;14(1):85. doi: 10.1037/a0034500. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C, Marker C, LeMoult J, Joormann J. Attentional disengagement predicts stress recovery in depression: An eye-tracking study. Journal of Abnormal Psychology. 2013;122(2):303–313. doi: 10.1037/a0031529. [DOI] [PubMed] [Google Scholar]
- Sanders W, Abaied J. Motivational systems and autonomic functioning: Overlapping and differential contributions to anhedonic depression and anxious arousal. Motivation and Emotion. 2015;39(4):602–612. [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological science. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Sepeta L, Tsuchiya N, Davies MS, Sigman M, Bookheimer SY, Dapretto M. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. Journal of neurodevelopmental disorders. 2012;4(1):1. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafqat K, Pal SK, Kyriacou PA. Evaluation of two detrending techniques for application in heart rate variability. Proceedings of the 29th Annual International Conference of the IEEE EMBS, Cite Internationale; Lyon, France. 2007. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8(6):870. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological psychiatry. 2001;49(7):624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27(3):365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biological psychiatry. 2011;69(8):726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Stampe DM. Heuristic filtering and reliable calibration methods for video-based pupil-tracking systems. Behavior Research Methods. 1993;25(2):137–142. [Google Scholar]
- Stanners RF, Coulter M, Sweet AW, Murphy P. The pupillary response as an indicator of arousal and cognition. Motivation and Emotion. 1979;3(4):319–340. [Google Scholar]
- Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophreniaa. Annals of the New York Academy of Sciences. 1992;658(1):182–204. doi: 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology, Task Force of the European Society of Cardiology. The North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47(3):852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36(3):1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vazquez L, Blood JD, Wu J, Chaplin TM, Hommer RE, Rutherford HJV, Potenza MN, Mayes LC, Crowley MJ. High frequency heart-rate variability predicts adolescent depressive symptoms, particularly anhedonia, across one year. Journal of Affective Disorders. 2016;196:243–247. doi: 10.1016/j.jad.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Panage S, Mendes WB, Gotlib IH. Cardiovascular and affective recovery from anticipatory threat. Biological Psychology. 2010;84:169–175. doi: 10.1016/j.biopsycho.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, Thayer JF. Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in Psychology. 2015;6:261. doi: 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.