Editorial
Clinical and epidemiological studies have demonstrated that macroenvironmental factors are risk factors for the development and progression of tumor [1]. Macroenvironmental factors include a patient’s physical, social environment and specific psychosocial factors such as chronic stress, depression, and lack of social support. These observations raise intriguing questions on the brain-cancer connection. What are the molecules in brain linking environmental factors to cancer? Through which pathways do these brain molecules modulate the peripheral cancer? How do these molecules impact tumour growth and progression? The effects and mechanisms of the macroenvironment on systemic cancer are much less well defined, because most basic cancer research focuses on microenvironmental factors of tumor.
To study environmental and psychosocial effects on cancer progression, our lab uses environmental enrichment (EE) as a eustress model. EE is a housing environment for laboratory animals, which, in contrast with the standard-environment (SE) cages used in most biomedical research, is socially, physically, and cognitively stimulating. EE has profound impacts on brain structure, function, and progression of neurologic diseases [2].
Our group is the first to report that EE reduces tumor growth and increases remission in both melanoma and colon cancer models of mouse [3]. Reports from others confirm the anticancer effects of an EE in breast and pancreatic cancers [4,5]. Our previous studies have uncovered one underlying mechanism, the activation of a specific neuroendocrine axis: the Hypothalamic-Sympathoneural-Adipocyte (HSA) axis. EE induces hypothalamic Brain-Derived Neurotrophic Factor (BDNF) expression and subsequently activating the sympathetic tone to fat tissues, which suppresses leptin expression and release leading to the reduction of cancer progression.
The story hasn’t ended yet. Recently, we have elucidated a new mechanism of anticancer effect of EE published on Cancer Immunology Research [6]. In this study, we addressed how EE modulated T-cell immunity and its role in the EE-induced anticancer effects. Our data demonstrated that CD8+ T cells were required for the anticancer effects of an EE in an orthotropic model of melanoma. In Secondary Lymphoid Tissue (SLT), an EE induced early changes in the phenotype of T-cell populations, characterized by a decrease in the ratio of CD4+ T helper to CD8+ cytotoxic T lymphocytes. Then we identified hypothalamic BDNF as the brain molecule mediating EE’s effects on T cell immunity. Hypothalamic overexpression of BDNF reproduced EE-induced T-cell phenotypes in SLT, whereas knockdown of hypothalamic BDNF inhibited EE-induced immune modulation in SLT. Furthermore, this study explored the peripheral pathways BDNF employed to regulate T cell immunity. Both the β-blocker propranolol and the anti-corticosteroid compound mifepristone inhibited the EE-associated modulation of CD8+ T cells in SLT, suggesting that both the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis were involved.
To the best of our knowledge, this is the first study to show that hypothalamic BDNF is the regulatory molecule in brain that links an individual’s physical and social environment to T cell immunity and resistance to cancer. Furthermore, it demonstrates that the BDNF-induced tumor inhibition harnesses multiple pathways including systemic metabolic modulation, enhanced T-cell immunity and enhanced NK cell immunity (unpublished data). In summary, the discovery by Xiao et al., not only advances the understanding of the modulation of innate and adaptive immune system by nervous system, but also supports the emerging concept of manipulating a single gene in the brain to improve cancer immunotherapy (Figure 1).
Figure 1.
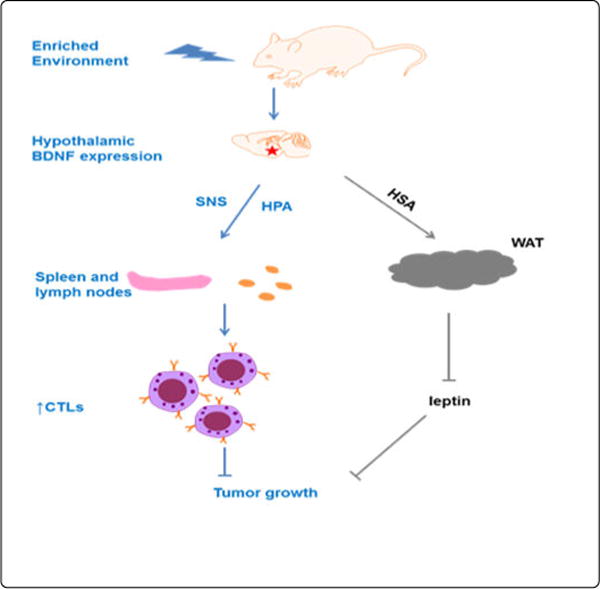
BDNF-induced tumor inhibition
Acknowledgments
The work is supported by NIH grants CA166590, CA178227, and CA163640.
Footnotes
Financial and Competing Interests Disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 3.Cao L, Liu X, Lin EJ, Wang C, Choi EY, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachat-Kappes R, Pinel A, Combe K, Lamas B, Farges MC, et al. Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLoS One. 2012;7:e51525. doi: 10.1371/journal.pone.0051525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Gan Y, Fan Y, Wu Y, Lin H, et al. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci Rep. 2015;5:7856. doi: 10.1038/srep07856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao R, Bergin SM, Huang W, Slater AM, Liu X, et al. Environmental and Genetic Activation of Hypothalamic BDNF Modulates T-cell Immunity to Exert an Anticancer Phenotype. Cancer Immunol Res. 2016;4:488–497. doi: 10.1158/2326-6066.CIR-15-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]


