Abstract
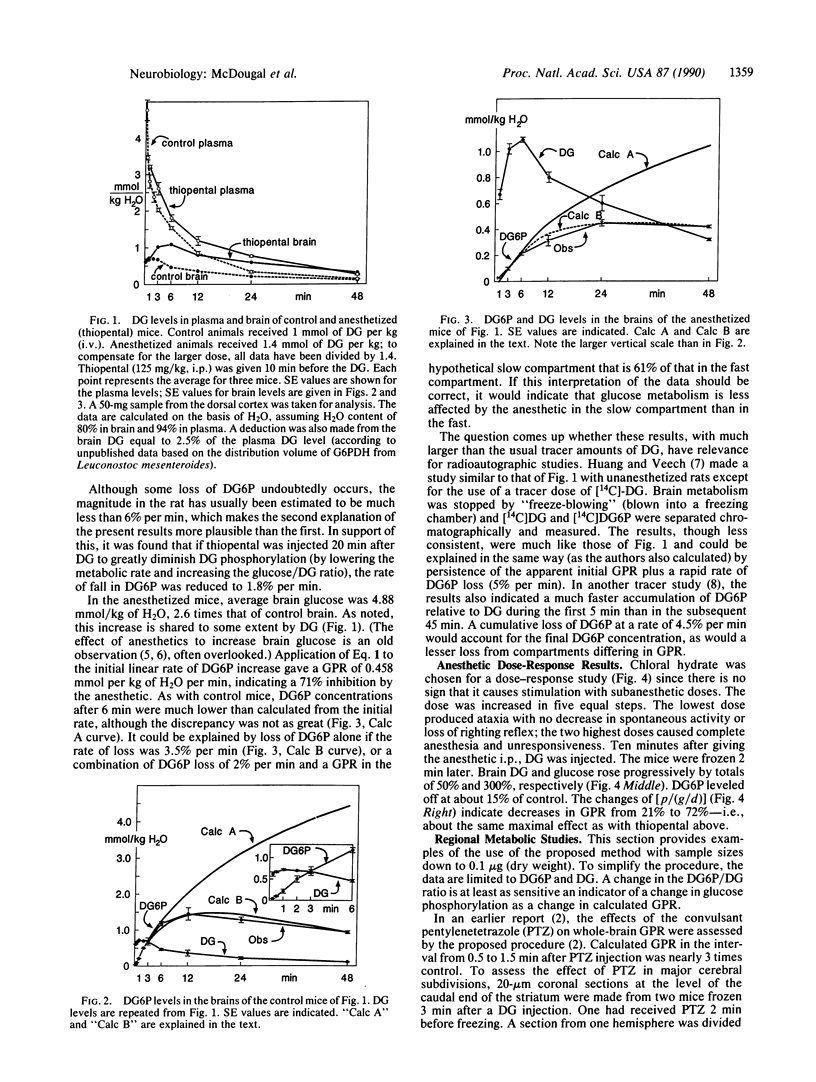
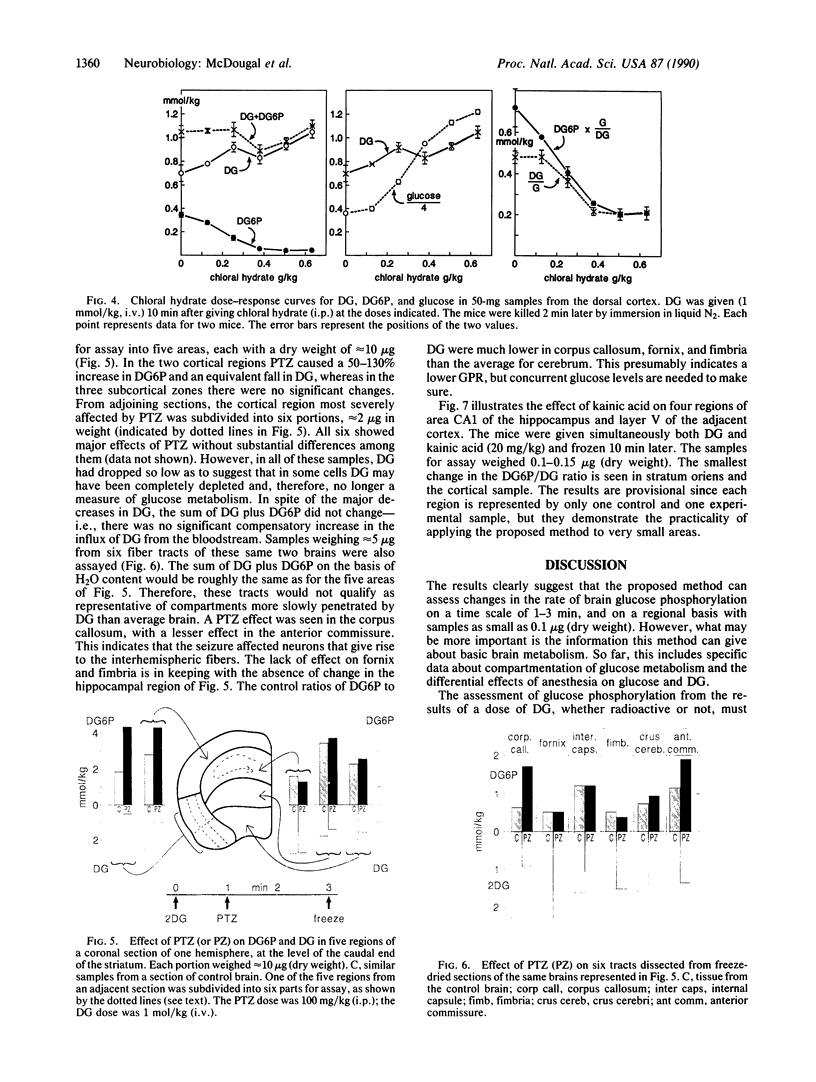
A method is presented for measuring rapid changes in the rate of glucose phosphorylation in mouse brain with nonradioactive 2-deoxyglucose (DG). After times as short as 1 min after DG injection, the mouse is frozen rapidly, and selected brain regions are analyzed enzymatically for DG, 2-deoxyglucose 6-phosphate (DG6P), and glucose. The rate of glucose phosphorylation can be directly calculated from the rate of change in DG6P, the average levels of DG and glucose, and a constant derived from direct comparison of the rate of changes in glucose and DG6P after decapitation. Experiments with large brain samples provided evidence for a 2% per min loss of DG6P and at least two compartments differing in their rates of glucose metabolism, one rapidly entered by DG with glucose phosphorylation almost double that of average brain and another more slowly entered with a much lower phosphorylation rate. The method is illustrated by changes in phosphorylation within 2 min after injection of a convulsant or an anesthetic and over a 48-min time course with and without anesthesia. The sensitivity of the analytical methods can be amplified as much as desired by enzymatic cycling. Consequently, the method is applicable to very small brain samples. Examples are given for regions with volumes of 5 x 10(-4) microliters, but studies with samples as small as single large cell bodies are feasible.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chi M. M., Pusateri M. E., Carter J. G., Norris B. J., McDougal D. B., Jr, Lowry O. H. Enzymatic assays for 2-deoxyglucose and 2-deoxyglucose 6-phosphate. Anal Biochem. 1987 Mar;161(2):508–513. doi: 10.1016/0003-2697(87)90481-7. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., Cremer J. E. A method for the simultaneous estimation of regional rates of glucose influx and phosphorylation in rat brain using radiolabeled 2-deoxyglucose. Brain Res. 1981 Sep 28;221(2):319–330. doi: 10.1016/0006-8993(81)90781-2. [DOI] [PubMed] [Google Scholar]
- Hawkins R., Hass W. K., Ransohoff J. Measurement of regional brain glucose utilization in vivo using [2(-14)C] glucose. Stroke. 1979 Nov-Dec;10(6):690–703. doi: 10.1161/01.str.10.6.690. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Veech R. L. Metabolic fluxes between [14C]2-deoxy-D-glucose and [14C]2-deoxy-D-glucose-6-phosphate in brain in vivo. J Neurochem. 1985 Feb;44(2):567–573. doi: 10.1111/j.1471-4159.1985.tb05450.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MAYMAN C. I., GATFIELD P. D., BRECKENRIDGE B. M. THE GLUCOSE CONTENT OF BRAIN IN ANAESTHESIA. J Neurochem. 1964 Jun;11:483–487. doi: 10.1111/j.1471-4159.1964.tb11607.x. [DOI] [PubMed] [Google Scholar]
- Manchester J. K., Chi M. M., Carter J. G., Pusateri M. E., McDougal D. B., Lowry O. H. Measurement of 2-deoxyglucose and 2-deoxyglucose 6-phosphate in tissues. Anal Biochem. 1990 Feb 15;185(1):118–124. doi: 10.1016/0003-2697(90)90265-b. [DOI] [PubMed] [Google Scholar]
- Nelson T., Lucignani G., Goochee J., Crane A. M., Sokoloff L. Invalidity of criticisms of the deoxyglucose method based on alleged glucose-6-phosphatase activity in brain. J Neurochem. 1986 Mar;46(3):905–919. doi: 10.1111/j.1471-4159.1986.tb13057.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]