Abstract
Background:
Mesenchymal stem cells (MSCs) are multipotent cells with immunomodulatory effect on immune cells including dendritic cells (DCs). DCs are the most potent antigen-presenting cells (APC). MSCs have been found to modulate both differentiation and function of DCs. DCs express a broad range of Toll-like receptors (TLR), which play a critical role in DCs maturation and function.
Objective:
To evaluate expression level of TLR3 and TLR9 transcripts in DCs following treatment with MSCs supernatant.
Methods:
MSCs and DCs were derived from adult BALB/c mice bone marrow and spleen, respectively. MSCs supernatant was harvested following 24, 48, and 72 hours. Isolated DCs were treated with MSCs supernatant and incubated for 24 and 48 hours. TLR3 and TLR9 transcript levels were evaluated using real-time PCR.
Results:
The results showed that 48 and 72 hours MSCs supernatant significantly decreased the expression of TLR3 in DCs following 24 and 48 hours incubation in comparison with untreated cells (p<0.01). Moreover, 48 hours of treatment with 24, 48 and 72 hours MSCs supernatant significantly decreased TLR9 transcript level (p<0.05).
Conclusion:
TLR3 and TLR9 mRNA expression decreases in DCs after incubation with MSCs culture supernatant. This confirms the immunomodulatory role of MSCs in cell-base therapy.
Key Words: Mesenchymal stromal cells, Dendritic cell, Antigen-presenting cells, Toll-like receptors, Immunomodulation, Receptors, pattern recognition, Pathogen-associated molecular pattern molecules
INTRODUCTION
Toll-like receptors (TLRs) are a family of pattern-recognition receptors (PRRs) that recognize conserved structures among microbial species, known as pathogen-associated molecular patterns (PAMPs), and are expressed by different immune cells such as dendritic cells (DCs), T cells, B cells and macrophages [1, 2]. So far, 13 types of mouse TLRs have been identified. TLRs 1, 2, 4, 5, 6, and 11 are expressed on the cell surface, whereas TLRs 3, 7, 8, and 9 are localized in several intracellular organelles including endosomes [2]. Although, TLRs were initially identified as innate immune mediators, recently, several studies have indicated TLR contribution in adaptive immune response. TLR signaling causes up-regulation of MHCII and co-stimulatory molecules on DCs and other antigen-presenting cells (APC) to cause inflammation and antigen-specific T cell responses [3, 4]. Furthermore, TLRs activation induces secretion of soluble factors promoting maturation and differentiation of immune cells such as DCs, which play a critical role in the adaptive immune response [5, 6]. Endosomal TLRs including TLR3 and TLR9 recognize nucleic acid-based PAMPs and provoke immune responses against pathogen-derived nucleic acids [2]. Stimulation of DCs through TLR3 induces production of remarkable pro-inflammatory cytokines, which leads to a systemic inflammatory response [7]. Moreover, TLR9, which recognizes CpG motifs in DNA, has been shown to promote both maturation and antigen presentation by DCs [8]. According to inflammatory properties of TLR3 and TLR9, modulation of TLRs expression and their signaling pathways might be a promising therapeutic approach for treatment of multiple inflammatory conditions, including various autoimmune diseases, atherosclerosis, type 2 diabetes, and osteoarthritis [9].
Bone-marrow derived mesenchymal stem cells (MSCs) are pluripotent stem cells that can differentiate into osteoblastic, adipocytic and chondrocytic lineages in certain condition [10]. MSCs have been found to modulate the immune responses by DCs, attenuate inflammation and repair damaged tissues either by cell-cell contact or secretory proteins including IL-10, IDO, and PGE-2 [11, 12]. A number of recent studies have aimed at the influence of MSCs on DCs function. For instance, MSCs supernatants inhibits maturation of DCs and down-regulates B7 and MHCII, causing induction of tolerogenic DCs (tol-DC), which expresses high level of IL-10 [13, 14]. However, the molecular mechanisms by which MSCs modulate DCs functions remain to be investigated. For example, murine MSCs inhibit DCs activation through TLR4, resulting in inconsiderable inflammatory cytokines production, and inhibiting their migration to the lymph nodes and antigen presentation to T cells [15]. Moreover, MSCs express functional TLRs that can regulate MSCs proliferation and differentiation [16].
Considering TLRs critical roles in DCs maturation and function, as well as MSC capacity to modulate DCs, we postulated that MSCs might regulate immune responses through regulation of TLRs expression. Therefore, expression levels of TLR3 and TLR9 transcripts in mouse DCs treated with MSCs supernatant were assessed.
MATERIALS AND METHODS
Mice
Five- to six-week-old BALB/c and C57BL/6 mice were purchased from Razi Institute (Shiraz, Iran). All mice were inbred and maintained under specific pathogen-free and standard conditions.
Isolation and Culture of Bone-marrow-derived MSCs
Bone marrow cells were flashed out from femur and tibia bones of BALB/c mice. Isolated cells were cultured in 25 cm2 flasks in low glucose Dulbecco modified Eagle medium supplemented with 10% heat-activated fetal calf serum, sodium pyruvate (1%), glutamine (1%), and 100 μg/mL penicillin-streptomycin in CO2 incubator at 37 °C. After 24 hrs, non-adherent cells were removed and adherent cells were trypsinized and passaged as described previously [17]. In passage 6, 24, 48, and 72 hrs after trypsinization, supernatants of cells were collected, as MSCs conditioned media, and stored at 20 °C. Purity of the MSCs was determined by flowcytometry using PE-labeled anti-SCA-1, anti-CD45, anti-CD44 and fluorescein-labeled anti-CD34 antibodies.
In Vitro Multilineage Differentiation Analysis
Adipogenesis and osteogenesis of BM-MSCs were assayed in the appropriate induction media. The differentiation phenotype was documented using Oil red O staining for adipocytes and Alizarin red staining for osteocytes.
Isolation of DCs
Gradient media, Nycodenz (Axis Shields, Norway) and magnetic-activated cell sorting were used to isolate splenic DCs as described previously [18]. Briefly, mouse spleens were chopped and digested with 1 mg/mL collagenase D (Roche, Germany) and 2 µg/mL DNase (Roche, Germany), and then meshed with 0.2 µm sieves. Cells were washed with RPMI 1640 culture medium (Sigma, St. Louis, MO, and USA) containing 5 mM EDTA. Cell pellets were re-suspended in RPMI 1640 supplemented with 10% fetal calf serum and 5 mM EDTA. The cell suspension was layered on Nycodenz 12.5% (w/v) (d=1.068) and centrifuged at 1800 rpm at 4 °C for 20 min. The interface layer was collected and purified by anti-CD11c micro-magnetic beads (Miltenyi Biotec, Germany) [18]. The purity was determined based on percentage of CD11c expressing cell population using flowcytometry (BD biosciences, USA).
Primer Design
TLR3 (NM_126166.4), TLR9 (NM_031178.2) and β-actin (NM_007393.3) specific primers were designed by NCBI primer design software (Biosoft, San Diego, CA, USA). The thermodynamic parameters and secondary structures were determined using mfold software. The primer sequences for TLR3, TLR9 and β-actin were as follows:
TLR3 F: 5’-GGGCAAGAACTCACAGGCCAGG-3’
TLR3 R: 5’-AAGGGCCACCCTTCGGAGCA-3’
TLR9 F: 5’-CCTCCACGCATGAGGCCCTG-3’
TLR9 R: 5’-CGGCTGCCGACTTGTCCTT-3’
β-actin F: 5’-ATCTACGAGGGCTATGCTCTCC-3’
β-actin R: 5’-AGCCTCGGTCAGGATCTTCAT-3’
RNA Isolation and Real-time PCR
Isolated splenic DCs were seeded in 6-well plates and treated with conditioned media obtained from 24, 48, and 72 hrs MSCs supernatant of the 6th passage. The treated DCs were incubated for 24 and 48 hrs; then,TLR3 and TLR9 expressions were evaluated. The cells without treatment were considered “negative controls.” Total RNA was isolated from treated and untreated cells according to the manufacturer’s instruction in three independent experiments using RNX-plus™ reagent (CinnaGen, Tehran, Iran). Isolated RNA (1 µg) was reverse-transcribed with random hexamers and oligo d (T) using Vivantis two-steps reverse transcriptase (RT)-PCR kit (Malaysia). Real-time PCR was performed using Takara SYBR Premix Ex taq II in duplicate. The mRNA levels of TLR3 and TLR9 were compared to β-actin mRNA level, as an internal control, by ABI prism 7500 system. Relative gene expression was calculated using ∆∆CT method and β-actin was used as a housekeeping gene for normalization. Finally, expression of each target gene in comparison with reference gene was calculated using 2∆∆CT formula. Reaction with water instead of cDNA template was considered “a non-template control.” Real-time PCR conditions included 95 °C for 2 min as the initial denaturation phase, and 40 cycles of 95°C for 30 sec and 64 °C for 20 sec as elongation. Untreated cells were also considered “negative control.”
Ethical Considerations
All of the animal care and experimental procedures were approved by the animal Ethical Committee of Shiraz University of Medical Sciences.
Statistics Analysis
Data collected in three independent experiments, were presented as mean±SD. Difference between means of the studied groups was analyzed by one-way ANOVA, followed by Tukey’s HSD, as the post hoc test, using Graph Pad Prism 5 software (Graph-Pad Software Inc, San Diego, CA, USA). A p value <0.05 was considered statistically significant.
RESULTS
DCs and MSCs Characterization
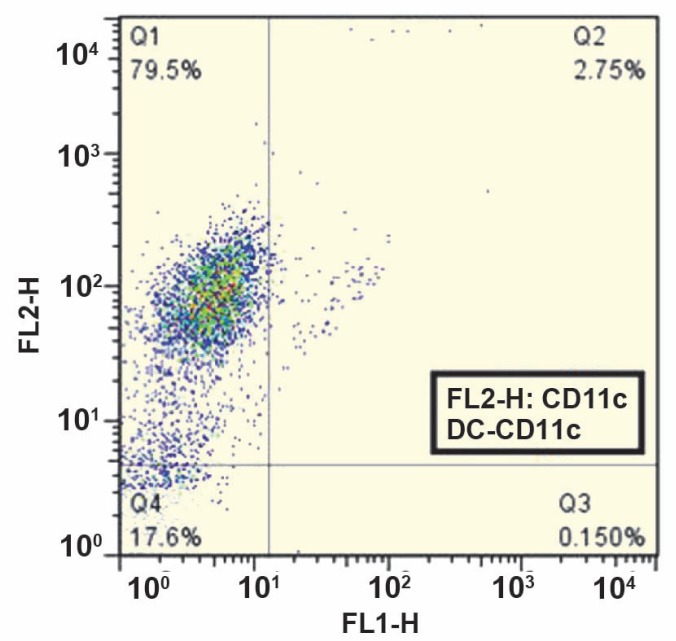
Flowcytometric analysis of MSCs showed that the purity of MSCs was more than 95% based on a pre-dominant population of CD44+, Sca-1+, and CD34–, CD45– cells in passage 5 (Fig 1). Microscopic analysis of MSCs under inductive conditions showed in vitro competence of MSCs to differentiate into adipogenic and osteogenic lineages as confirmed by Oil red O staining (Fig 2A), and Alizarin red staining (Fig 2B), respectively. More than 75% of viable cells were positive for CD11c, a DC-specific antigen (Fig 3).
Figure 1.
Purity of MSCs was determined using PE-labeled anti-SCA-1, CD44, CD45 and fluorescein-labeled antibodies labeled anti-CD34 antibodies. Almost 95% of cells were positive for CD44 and SCA-1and negative for CD34 and CD45 in passage 5 (solid lines) in comparison with cells stained with isotype control antibodies (dash lines). The results are representative of three independent experiments
Figure 2.
Differentiation of bone-marrow-derived MSCs. A) Adipogenic differentiation of MSCs examined by Oil red O staining for intracellular lipid vacuoles (×100). B) Osteogenic differentiation of MSCs examined by Alizarin red staining (×100
Figure 3.
The purity of DCs isolated from spleen. CD11c expression was determined using PE conjugated CD11c antibody. Positive cell population was considered “purified DCs.” The result is representative of three independent experiments
TLR3 and TLR9 Gene Expression
TLR3 and TLR9 transcript levels in DCs treated with MSCs supernatant were compared to untreated cells following 24 and 48 hrs; 24 and 48 hrs treatments of DCs with 48 and 72 hrs conditioned media of MSCs resulted in down-regulation of TLR3 transcripts in comparison with untreated cells (p<0.01) (Fig 4). Moreover, 24 and 48 hrs treatment with 48 and 72 hrs conditioned media of MSCs caused significant (p<0.05) decrease in mean±SD TLR3 transcript (0.099±0.00 and 0.24±0.14, respectively) compared to 24 hrs conditioned media of MSCs (0.67±0.21) (Fig 4). TLR9 expression was also decreased due to treatment with MSCs supernatant. DCs treatment with 24, 48, and 72 hrs conditioned media of MSCs for 48 hrs, resulted in significant (p<0.05) down-regulation of mean±SD TLR9 mRNA level (0.44±0.06, 0.42±0.01, and 0.41±0.12, respectively) (Fig 5). TLR9 transcript levels of DCs treated with MSCs (24, 48, and 72 hrs MSCs supernatant) conditioned media for 48 hrs were significantly down-regulated comparing to 24 hrs treatment.
Figure 4.
Expression of TLR3 transcript in DCs treated with MSCs supernatant. DCs were treated with MSCs supernatant collected at 24 h, 48 h, and 72 h intervals, and the mRNA level of TLR3 was measured following 24 h and 48 h treatments. 24 h and 48 h treatment with 48 h and 72 h MSCs conditioned media caused significant down-regulation of TLR3 expression compared to untreated cells. Data represent mean±SD of three independent experiments. (**p<0.01, ***p<0.001) (Sup: MSCs supernatant
Figure 5.
Expression of TLR9 transcript in DCs treated with MSCs supernatant. DCs were treated with MSCs supernatant, collected at 24 h, 48 h and 72 h intervals, and mRNA level of TLR9 was measured after 24 h and 48 h treatment. TLR9 expression was decreased significantly with 24 h, 48 h, and 72 h MSCs supernatant after 48 h incubation compared to untreated cells. Data represent mean±SD of three independent experiments. (*p<0.05) (Sup: Supernatant, h: hours
DISCUSSION
Stimulation through TLRs provides a vital signaling for DCs activation in response to antigenic stimuli [19, 20]. TLR3 and TLR9 functional expressions on DCs have been reported earlier [21]. Activated and matured DCs by TLRs are able to present pathogen-derived peptides to T cells, inducing T cell activation, and differentiation and cell-mediated adaptive immune responses [20]. Some studies demonstrated the importance of TLR9 signaling for Th1-mediated immune responses [22]. Absence of TLR9 inhibits the development of protective Th1 response transiently [23, 24]. TLRs also trigger T cell stimulatory function and attenuate the differentiation into T-regulatory cells [25]. It has been shown that MSCs, as the immunomodulatory agent, strongly inhibit the maturation and functioning of DCs via soluble inhibitory mediators [26].
We assumed that immunomodulatory properties of MSCs could be partially accomplished by regulation of TLRs expressions and functions. Therefore, we evaluated the expression levels of TLR3 and TLR9 transcripts in mouse DCs treated with MSCs conditioned media.
Our findings indicated that MSCs supernatant, harvested at 24–72-hr intervals, could significantly down-regulate TLR3 and TLR9 expressions at the mRNA level in DCs. In keeping with our results, a recent study has shown that MSCs inhibit TLR4 expression and signaling as well as DCs downstream molecular events, which impairs T cell priming and activation [15]. Several reports have demonstrated that many factors change TLR 2, 3, and 4 expression and signaling pathways [27-29]. However, the effect of MSCs on endosomal TLRs including TLR 3, 7, and 9 remained unidentified. Further investigations are needed to find the exact soluble mediators of MSCs responsible for TLR down-regulation of DCs.
Down-regulation of TLR9 on DCs causes immature DCs not to differentiate into mature DCs; they represent tolerogenic DCs with suppressive effects on immune response [30, 31]. Likewise, TLR3 down-regulated DCs produce ineffective amount of pro-inflammatory cytokines and fail to initiate antigen-specific inflammatory responses.
In conclusion, MSCs may induce tolerogenic DCs phenotype by down-regulating TLR3 and TLR9 that culminate in ineffective adaptive immunity. Further analyses are needed to explore potential inhibition of mitogen-activated protein kinase and nuclear factor-κB activation as a downstream of TLRs for a better understanding of how DCs maturation and activation are regulated by MSCs. A functional study is also demanded to link TLR3 and TLR9 down-regulation to the inability of priming and activating T cells and mounting an effective antigen-specific immune response. Although the possible application of MSCs to treat immune-mediated disorders is currently under scrutiny, our findings confirm MSCs profound inhibitory effect on TLRs in DCs. Therefore, it may be considered a novel molecular inhibitory mechanism by which MSCs modulate DCs activation and maturation.
ACKNOWLEDGEMENTS
The present article was financially supported by the Transplant Research Center (Grant number 90/154) affiliated to Shiraz University of Medical Sciences, Shiraz, Iran. The authors are grateful to Mrs. Farzaneh Taki from Autoimmune Diseases Research Center, Ms. Salimeh Ebrahim-Nejad and Ms. Maryam Babaei from Transplant Research Center for technical assistance.
References
- 1.Gay NJ, Symmons MF, Gangloff M, et al. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2000;14:546–58. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- 2.Seledtsov VI, Seledtsova GV. A balance between tissue-destructive and tissue-protective immunities: a role of toll-like receptors in regulation of adaptive immunity. Immunobiology. 2012;217:430–5. doi: 10.1016/j.imbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Lenert PS. Targeting Toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus. Arthritis Res Ther. 2006;8:203. doi: 10.1186/ar1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werling D, Jungi TW. Toll like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 5.Klinman DM, Beaucage SL, Conover J, et al. CpG motifs present in bacteria DNA rapidly induces lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 7.Kaisho T. Pathogen sensors and chemokine receptors in dendritic cell subsets. Vaccin. 2012;30:7652–57. doi: 10.1016/j.vaccine.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Kindrachuk J, Potter J, Wilson HL, et al. Activation and regulation of toll-like receptor 9: CpGs and beyond. Mini Rev Med Chem. 2008;8:590–600. doi: 10.2174/138955708784534481. [DOI] [PubMed] [Google Scholar]
- 9.Dunne A, Marshall NA, Mills KH. TLR based therapeutics. Curr Opin Pharmacol. 2011;11:404–11. doi: 10.1016/j.coph.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu WH, Liu JJ, Wu J, et al. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS One. 2013;8:e55487. doi: 10.1371/journal.pone.0055487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Sun B, Wang D, et al. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol. 2008;68:607–15. doi: 10.1111/j.1365-3083.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 14.Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–79. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiesa S, Morbelli S, Morando S, et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108:17384–89. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pevsner-Fischer M, Morad V, Cohen-Sfady , et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–32. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 17.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 18.Soltani MH, Kalantari T, Karimi MH, et al. Evaluation of the immunomodulatory effect of curdlan on maturation and function of mouse spleen-derived dendritic cells. Iran J Immunol. 2012;9:168–74. [PubMed] [Google Scholar]
- 19.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 20.Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–85. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- 21.Shirota H, Sano K, Hirasawa N, et al. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol. 2001;167:66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]
- 22.Shi G, Vistica BP, Nugent LF, et al. Differential involvement of Th1 and Th17 in pathogenic autoimmune processes triggered by different TLR ligands. J Immunol. 2013;191:415–23. doi: 10.4049/jimmunol.1201732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou Fakher FH, Rachinel N, Klimczak M, et al. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J Immunol. 2009;182:1386–96. doi: 10.4049/jimmunol.182.3.1386. [DOI] [PubMed] [Google Scholar]
- 24.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–27. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin B, Sun T, Yu XH. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 27.Tolouei Semnani R, Venugopal PG, Leifer CA. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood. 2008;112:1290–8. doi: 10.1182/blood-2008-04-149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fragale A, Stellacci E, Ilari R, et al. Critical Role of IRF8 in Negative Regulation of TLR3 Expression by Src Homology 2 Domain Containing Protein Tyrosine Phosphatase2 Activity in Human Myeloid Dendritic Cells. J Immunol. 2011;186:1951–62. doi: 10.4049/jimmunol.1000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E, Hong H, Cho D. Enhancement of Toll like receptor 2 mediated immune responses by AIMP1, a novel cytokine, in mouse dendritic cells. Immunology. 2011;134:73–81. doi: 10.1111/j.1365-2567.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonuleit H, Schmitt E, Steinbrink K. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 31.Mahnke K, Schmitt E, Bonifaz L. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–83. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]